Abstract
We previously demonstrated that serotonin (5-HT) and 5-HT2A receptor (5-HT2AR) levels in platelets were up- or down-regulated after myocardial infarction (MI) associated with depression. In this study, we further evaluated the effects of pretreatment with ginseng fruit saponins (GFS) on the expression of 5-HT and 5-HT2AR in MI with or without depression. Eighty Sprague-Dawley (SD) rats were treated with saline and GFS (n=40 per group). The animals were then randomly divided into four subgroups: sham, MI, depression, and MI + depression (n=10 per subgroup). Protein levels of 5-HT and 5-HT2AR in the serum, platelets and brain tissues were determined with ELISA. The results demonstrated that serum 5-HT levels was significantly increased by GFS pretreatment in all subgroups (except the sham subgroup) when compared with saline-treated counterparts (p<0.01). In platelets, GFS pretreatment significantly increased 5-HT levels in all subgroups when compared with their respective saline-treated counterparts (p<0.01). Brain 5-HT levels also declined with GFS pretreatment in the MI-only and depression-only subgroups (p<0.05 vs. saline pretreatment). With respect to 5-HT2AR levels, platelet 5-HT2AR was decreased in GFS pretreated MI, depression and MI + depression subgroups (p<0.01 vs. saline pretreatment). Similarly, brain 5-HT2AR levels decreased in all four subgroups pretreated with GFS (p<0.01 vs. saline pretreatment). We conclude that GFS plays a clear role in modulating 5-HT and 5-HT2AR expressions after MI and depression. Although the effects of GFS on brain 5-HT remain to be elucidated, its therapeutic potential for comorbidities of acute cardiovascular events and depression appears to hold much promise.
Keywords: serotonin, ginseng, myocardial infarction, depression, brain
Coronary heart disease and clinical depression are commonly associated; patients worldwide have more often than not experienced symptoms of depression while recovering from myocardial infarction (MI). The incidence of coronary heart disorder among depression cases ranges from 5% to 50% depending on the location and scope of study [1-4]. There is a substantial amount of evidence that depression is an independent risk factor for coronary heart disease, while patients with heart disease also have a high risk of depression [5]. Patients with multiple cardiovascular disease-associated depression and/or anxiety disorder have been recognized at home and abroad [6,7]. In 2013, a survey of general hospitals in five major Chinese cities found 2123 such cases. The current prevalence of depression in China is 10.55% with a lifetime prevalence is 13.75% while anxiety disorders have a current prevalence of 7.77% and a lifetime prevalence of 8.53% [8]. Studies show that depression and/or anxiety can increase the risk of cardiovascular disease by a factor of 1.5 to 2.7 [9]. The specific mechanism is unclear, but depression and cardiovascular disease may reinforce each other in comorbidity [10,11]. Thrombosis and increased platelet activity are important pathological features of atherosclerosis and acute coronary events, but they are also found in patients with depression. Platelet activation may play an important role in either MI or depression, and increase the vulnerability of depressed MI patients to cardiac events due to endothelium damage, dyslipidemia and elevation in circulating substances such as thromboxane [12,13].
Of the factors that could affect both MI and depression, the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) is one of the more intriguing culprits. Platelets contain approximately 99% of all the 5-HT in the bloodstream and have been known to release 5-HT at vascular injury sites [14]. 5-HT is critical to thrombus formation, platelet aggregation, vascular wall repair and proliferation of endothelial cells, but elevated 5-HT in blood is associated with increased risk of cardiac events in MI patients [15]. 5-HT imbalance is also associated with depression. In depression, platelet reactive activity is elevated with platelets presenting increased levels of the serotonin 5-HT2A receptor (5-HT2AR) [16]. Therefore, 5-HT may be involved in the pathogenesis of comorbid coronary heart disease and depression by affecting both platelet function and mental state [17].
Clinical work suggests that various traditional Chinese medicine (TCM) remedies may affect this interaction [18]. Ginseng fruit saponins (GFS) extracted from the ginseng fruit are particularly promising candidates for effective therapeutic interventions [19]. Ginseng, the rhizome of the Panax ginseng plant, has been used for thousands of years in TCM for a multitude of purposes. Indeed, the word “panax” is derived from “panacea,” meaning “cure-all” in Greek [20]. Ginsenosides, the active ingredients of GFS, are triterpene saponins composed of a dammarane skeleton with various sugars attached at the C-3 and C-20 positions [21]. Over 30 different ginsenosides in GFS have been identified and classified into two categories: 20(S)-protopanaxadiols (PPD) and 20(S)-protopanaxatriols (PPT). PPTs differ from PPDs in that they possess an additional hydroxyl moiety or sugar residue at the C6 position [22]. It has been reported that GFS have beneficial effects on both the nervous and circulatory systems [23,24].
In the present study, we aimed to establish the effects of GFS pretreatment on the comorbidity of MI and depression by quantifying levels of 5-HT and 5-HT2AR in the serum, platelets and brain. Platelets were chosen because their demonstrated similarity to neurons suggested a potential for diagnostic use, considering that a blood test is much less invasive and expensive than nervous tissue biopsy [16]. 5-HT2AR was not measured in the serum because it is membrane-bound and not found in serum.
MATERIALS AND METHODS
Subjects
In this study, we used 80 Sprague Dawley (SD) rats, both male and female, weighing 180-200 grams (Pharmaceutical Base, Jiangsu Province). The rats were randomly divided into two pre-treated groups with GFS (Jilin Ji’an Yisheng Pharmaceutical Co. Ltd.) or placebo saline (n=40 per group). After pretreatment (4 weeks), both groups were further divided into four subgroups (n=10 per subgroup): 1) control/sham operation without MI and depression; 2) depression; 3) MI; 4) combined MI and depression (MI + depression). Animals were then sacrificed after 3 days to observe the effects of GFS on levels of 5-HT and 5-HT2AR in the rat serum, platelets, and brain tissues.
Pretreatment
The GFS-pretreated rats received GFS by gavages at 20 mg/kg dissolved in 2.5 ml saline once a day for 4 weeks while the saline-pretreated rats received an equivalent volume of placebo saline for the same period.
Various Pathological States
After the 4 weeks of pretreatment, 4 different pathological conditions were induced. 1) MI was performed with Akbay’s approach [28]. Rats, anesthetized with ketamine (40 mg/kg) and xylazine (1 mg/kg) via intra-muscular injection, were placed in the supine position. After disinfecting, the thorax was opened in the fourth intercostal space. The left anterior descending artery (LAD) was cauterized at the midpoint through the starting point and the cardiac apex. After cauterization, the air in the thorax was squeezed out by the fore finger and the thorax was closed with the suture. 2) Depression was induced with the Modified Forced Swimming Test (FST) described previously by Porsolt [25-27]. Rats were plunged individually in a cylinder (40 cm height, 20 cm diameter) containing 30 cm water maintained at 25°C. After 15 min in the cylinder, they were removed and allowed to dry for 15 min in a heated enclosure (32°C) before returning to their individual cages. This procedure involved long periods of immobility in the water (10-12 min total) and hypoactivity for 30 min. After 24 h, the FST was repeated except this time, the rats were placed in the cylinder for only 5 min. 3) MI + Depression was induced by first performing MI surgery then FST 3 days after the surgery, following the procedures described above. 4) Sham (no induction of MI and depression) rats were anesthetized but did not receive thoracotomy or cauterization of the left anterior artery. FST was also not performed.
ELISA
Protein levels were determined using ELISA kits specific for 5-HT (product #: MEXN-M136) and 5-HT2AR (product #: MEXN-M142) obtained from Shanghai Meixuan Biological Science and Technology Ltd. Platelet-rich plasma (PRP) was prepared by centrifuging blood for 15 minutes at room temperature, 2,000 RPM, within 30 minutes of blood collection [29]. Platelets were then separated by centrifuging PRP for 10 minutes at 4 °C, 2,100 RPM [30]. 5-HT levels were measured using the standardized ELISA method (Instructions for use: Serotonin ELISA. IB89546 [DB/OL]; http://www.ibl-america.com/pdf/elisa/IB89546.pdf) [31, 32]. 5-HT2AR levels were measured according to Jurado’s receptor assay [33]. The unit used in all cases was pg/ml.
Statistical Analysis
We used SPSS 19.0 for all data processing and analysis. Data was represented as mean ± standard deviation (SD). Comparisons between two subgroups were assessed using the independent t-test. P values less than 0.05 were considered statistically significant.
RESULTS
Effect of GFS on 5-HT
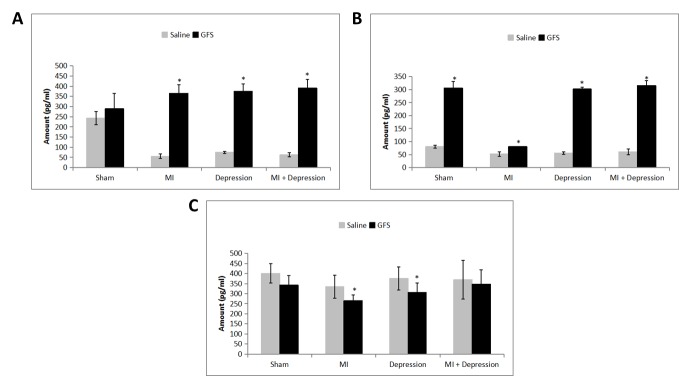
At the end of the experiments, we measured 5-HT levels in the rat serum (Fig. 1A). When we compared 5-HT expression in the serum between saline- and GFS-pretreated animals for the four subgroups: GFS pretreatment increased 5-HT levels in the sham, but this did not reach a significant level (p=0.184). Instead, we report significant increases in 5-HT levels in the MI, depression, and MI + depression subgroups (all p<0.01 vs. respective saline pretreated counterparts). Figure 1B shows 5-HT expression in the platelet lysate between saline and GFS-treated animals for the four subgroups. GFS pretreatment significantly increased 5-HT levels for all subgroups when compared with saline pretreatment (all p<0.01). In the brain (Fig. 1C), GFS pretreatment caused 5-HT levels to decline in all four subgroups when compared with saline-pretreated animals. The declines for the MI (p=0.025) and depression subgroups (p=0.044) were significant, while the differences did not reach significance for the sham (p=0.060) and MI + depression subgroups (p=0.663).
Figure 1.
Animals were first randomly divided into two groups: saline and GFS (n=40 per group). The rats were pretreated with GFS (20 mg/kg) or with an equivalent volume of saline once daily via oral gavage for a period of 4 weeks. Rats were then equally divided randomly into four subgroups (n=10 per subgroup) and the appropriate surgeries and tests were performed: Sham, MI, Depression, MI + Depression. After 3 days, animals were sacrificed and 5-HT levels measured in the serum, platelets, and brain tissues using an ELISA kit. Data are presented as mean ± SD. A) Quantification of 5-HT level in serum. As compared with saline-treated animals, GFS pretreatment increased 5-HT levels in the sham group although it did not achieve significance (p=0.184). However, there was a significant increase in 5-HT levels in the GFS-pretreated MI, depression, and MI + depression subgroups. *p<0.01, n=10 per subgroup. B) Quantification of 5-HT level in platelets. GFS pretreatment significantly increased 5-HT levels when compared with saline pretreatment for all subgroups: sham, MI, depression, and MI + depression. *p<0.01, n=10 per subgroup. C) Quantification of 5-HT level in the brain. With GFS pretreatment, 5-HT levels declined for all four animal subgroups. The declines for the MI-only (*p=0.025) and depression-only (*p=0.044) subgroups were significant, while the decreases did not achieve significance for the sham (p=0.060) and MI + depression subgroups (p=0.663).
Effect of GFS on 5-HT2AR
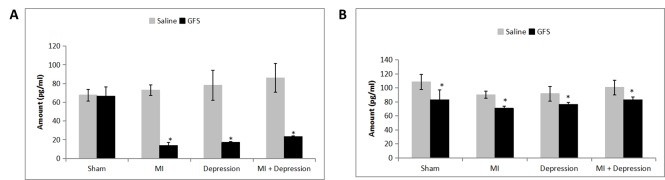
5-HT2AR was not measured in the serum because it is membrane-bound and not found in serum. There was no significant difference in platelet 5-HT2AR levels (Fig. 2A) between the saline and GFS pretreatment in the sham model (p=0.838). It was further indicated that GFS pretreatment decreased 5-HT2AR for the MI, depression, and MI + depression subgroups when compared with saline pretreatment subgroups (all p<0.01). In the brain (Fig. 2B), there was a significant decrease in 5-HT2AR levels in GFS-treated animals for all four subgroups (p<0.01) when compared with saline-pretreated animals.
Figure 2.
5- HT2AR levels were measured in the platelets and brain tissues using an ELISA kit. Data are presented as mean ± SD. A) Platelet 5-HT2AR quantification. There was no significant difference in 5-HT2AR levels between the saline- and GFS-pretreated groups in the sham subgroup (p=0.838). GFS pretreatment induced significant decreases in the MI, depression and MI + depression subgroups. *p<0.01 vs. saline, n=10 per subgroup. B) Brain 5-HT2AR quantification. All four subgroups demonstrated significant decreases in 5-HT2AR levels after GFS pretreatment. *p<0.01 vs. saline, n=10 per subgroup.
DISCUSSION
In the present study, we found that GFS have significant effects on expression of 5-HT and 5-HT2AR in MI, depression or both. GFS intervention reverses 5-HT declines in the serum of MI, depression and MI + depression rats. GFS pretreatment also elevates 5-HT protein levels in platelet from sham, MI, depression or MI + depression rats, reversing the decline seen in the saline-treated disease model. Further, GFS lowered 5-HT levels in the brains of MI-only and depression-only, but not MI + depression or sham rats. Surprisingly, 5-HT levels in the brain were decreased in GFS-pretreated MI-only and depression-only rats, which were not consistent with results obtained from the serum and platelet lysate. The implications of this finding however, are still unclear. Nonetheless, GFS did have a clear effect on 5-HT2AR levels in both the platelet and brain. To further evaluate the viability of platelets as a proxy for brain tissue in assessing 5-HT levels, the use of more sensitive techniques may be warranted.
It has been confirmed that 5-HT and its related pathway play important roles in the activation of platelets. 5-HT dysfunction is a key factor in the pathogenesis of depression [16, 34]. In the course of coronary heart disease, a variety of predisposing factors can lead to 5-HT secretion after platelet activation. 5-HT secretion mediated by 5-HT2AR induces platelet aggregation and coronary artery contraction. After 5-HT binds to 5-HT2AR on the platelet membrane, it is transported into the platelet through the serotonin transporter (SERT) and stored in dense granules. On the other hand, 5-HT levels decrease while platelet 5-HT2AR expressions increase during depression. Therefore, activation of the platelet 5-HT2AR could cause a more than usual increase in 5-HT signaling to result in increased platelet aggregation as well as alteration of platelet reactive activity. Such changes are similar to the platelet response in atherosclerotic disease. Patients with depression experience a decrease in 5-HT levels, an up-regulation in the 5-HT receptor, and functional decline of SERT resulting in reduced 5-HT re-uptake rate and inter-synaptic 5-HT concentration imbalance [35-37]. Our results suggest that GFS contributes to 5-HT stabilization by decreasing 5-HT2AR density.
In the clinical setting, serum levels of 5-HT are affected by many factors. This, along with other reasons makes the clinical feasibility of using cerebrospinal fluid to detect 5-HT relatively poor; brain biopsy is even more difficult in practice. However, the mechanisms for 5-HT uptake and release are similar for platelets and neurons in the CNS. Neurons and platelets also have significant structural and functional similarities thus, platelet 5-HT2AR has been used as a plausible proxy for CNS 5-HT2AR. Considering all these, we were led to choose platelet and brain tissue homogenates of 5-HT and 5-HT2AR as targets for our research. Our previous study found that rats with acute myocardial infarction, depression or a combination of the two showed significant elevation in platelet 5-HT2AR levels compared with the control group, with the combined myocardial infarction and depression group displaying the greatest increase [38]. This association of platelet 5-HT2AR with acute myocardial infarction showing symptoms of depression suggests a potential diagnostic tool for coronary heart disease and depression disorders, a comparatively sensitive and specific biomarker of the outer periphery [38]. Our results suggest, though, that the uptake and release of 5-HT between platelets and neurons is not equivalent.
Integrative Chinese and Western medical treatment has a therapeutic effect on coronary heart disease and depression. In Chinese medicine, ginseng has long been regarded as the king of herbs, nourishing vitality, improving stamina and generally contributing to well-being. Modern medical research is consistent with these traditional beliefs. Studies have shown that ginseng may improve cardiovascular blood flow, correct blood viscosity, enhance left ventricular function, and also significantly improve mental stress induced by myocardial ischemia [39]. Comorbidities of cardiovascular and psychological disorders are receiving increasing attention. Psycho-cardiology has become one of the hot spots of medical research. There is great promise that combination therapies may be proven to have certain advantages and efficacies. Our current study has shown the stabilizing effect of GFS on the serotonin system after depression, as well as myocardial infarction with depression, but not with acute myocardial infarction alone. One limitation of our study is that because we used total GFS, we were unable to identify the active ingredient with the largest effect as well as its related mechanism of action. For future studies, we plan on investigating ginsenoside monomers.
In conclusion, GFS has a clear role in modulating 5-HT expression and is effective in restoring 5-HT levels in MI and depression. Our findings warrant further in-depth study of the molecular mechanisms governing the body's response to GFS in various pathological states.
Acknowledgments
Thanks go to Professor Yingbin Ge, who made several contributions to the animal experiments.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- [1].Carney RM, Freedland KE (2003). Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry, 54: 241-247. [DOI] [PubMed] [Google Scholar]
- [2].Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, et al. (2006). Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med, 21: 30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cooney MT, Kotseva K, Dudina A, De Backer G, Wood D, Graham I (2013). Determinants of risk factor control in subjects with coronary heart disease: a report from the EUROASPIRE III investigators. Eur J Prev Cardiol, 20: 686-691. [DOI] [PubMed] [Google Scholar]
- [4].Carro A, Kaski JC (2011). Myocardial infarction in the elderly. Aging Dis, 2: 116-137. [PMC free article] [PubMed] [Google Scholar]
- [5].Dickens C (2015). Depression in people with coronary heart disease: prognostic significance and mechanisms. Curr Cardiol Rep, 17: 83. [DOI] [PubMed] [Google Scholar]
- [6].Alcántara C, Davidson KW (2014). Mental disorders and coronary heart disease risk: could the evidence elude us while we sleep? Circulation, 129:139-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zuidersma M, Conradi HJ, van Melle JP, Ormel J, de Jonge P (2013). Self-reported depressive symptoms, diagnosed clinical depression and cardiac morbidity and mortality after myocardial infarction. Int J Cardiol, 167: 2775-2780. [DOI] [PubMed] [Google Scholar]
- [8].Li G, Jiang RH, Guo CJ, Liu MY, Zhang LJ (2014). Prevalence of depressive and anxiety disorders in cardiovascular outpatients from 14 tertiary general hospitals of 5 Chinese cities. Chin J Cardiol, 42:1035-1038. [PubMed] [Google Scholar]
- [9].Steptoe A, Whitehead DL (2005). Depression, stress, and coronary heart disease: the need for more complex models. Heart, 91: 419-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Glassman A (2008). Depression and cardiovascular disease. Pharmacopsychiatry, 41: 221-225. [DOI] [PubMed] [Google Scholar]
- [11].Glassman AH, Bigger JT, Gaffney M (2009). Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of sadhart participants. Arch of Gen Psychiatry, 66:1022-1029. [DOI] [PubMed] [Google Scholar]
- [12].Wagner DD, Burger PC (2003). Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol, 23: 2131-2137. [DOI] [PubMed] [Google Scholar]
- [13].Hansson GK, Libby P, Schonbeck U, Yan ZQ (2002). Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res, 91: 281-291. [DOI] [PubMed] [Google Scholar]
- [14].McCloskey DJ, Postolache TT, Vittone BJ, Nghiem KL, Monsale JL, Wesley RA, et al. (2008). Selective serotonin reuptake inhibitors: measurement of effect on platelet function. Transl Res, 151: 168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schins A, Hamulyak K, Scharpe S, Lousberg R, Van Melle J, Crijns H, et al. (2004). Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients. Life Sci, 76: 637-650. [DOI] [PubMed] [Google Scholar]
- [16].Nemeroff CB, Musselman DL (2000). Are platelets the link between depression and ischemic heart disease?. Am Heart J, 140:57-62. [DOI] [PubMed] [Google Scholar]
- [17].Vikenes K, Farstad M, Nordrehaug JE (1999). Serotonin is associated with coronary artery disease and cardiac events. Circulation, 100:483-489. [DOI] [PubMed] [Google Scholar]
- [18].Mashour NH, Lin GI, Frishman WH (1998). Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med, 158: 2225-2234. [DOI] [PubMed] [Google Scholar]
- [19].Kiefer D, Pantuso T (2003). Panax ginseng. Am Fam Physician, 68: 1539-1542. [PubMed] [Google Scholar]
- [20].Cheng Y, Shen LH, Zhang JT (2005). Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin, 26: 143-149. [DOI] [PubMed] [Google Scholar]
- [21].Lee DG, Lee AY, Kim KT, Cho EJ, Lee S (2015). Novel Dammarane-Type Triterpene Saponins from Panax ginseng Root. Chem Pharm Bull (Tokyo), 63: 927-934. [DOI] [PubMed] [Google Scholar]
- [22].Leung KW, Wong AS (2010). Pharmacology of ginsenosides: a literature review. Chin Med, 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim HJ, Kim P, Shin CY (2013). A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res, 37: 8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee CH, Kim JH (2014). A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res, 38: 161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Porsolt RD, Le Pichon M, Jalfre M (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature, 266: 730-732. [DOI] [PubMed] [Google Scholar]
- [26].Detke MJ, Lucki I (1996). Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res, 73: 43-46. [DOI] [PubMed] [Google Scholar]
- [27].Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F(2002). Forced swimming test and fluoxetine treatment: in vivo evidence that peripheral 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes.Exp Brain Res, 143:191-197. [DOI] [PubMed] [Google Scholar]
- [28].Akbay E, Onur MA (2013). A new modified myocardial infarction animal model. Journal-CVS, 1:69-71. [Google Scholar]
- [29].Rao ML, Hawellek B, Papassotiropoulos A, Deister A, Frahnert C (1998). Upregulation of the platelet Serotonin2A receptor and low blood serotonin in suicidal psychiatric patients. Neuropsychobiology, 38: 84-89. [DOI] [PubMed] [Google Scholar]
- [30].Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F (2002). Forced swimming test and fluoxetine treatment: in vivo evidence that peripheral 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes. Exp Brain Res, 143:191-197. [DOI] [PubMed] [Google Scholar]
- [31].Sanner JE, Frazier L, Udtha M (2013). The role of platelet serotonin and depression in the acute coronary syndrome population. Yale J Biol Med, 86: 5-13. [PMC free article] [PubMed] [Google Scholar]
- [32].Adam H, Omar E, Changya P, et al. (2014). Reduced Apoptosis by Ethanol and Its Association with PKC-δ and Akt Signaling in Ischemic Stroke. Aging Dis, 2014, 5: 366-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jurado N, Torner C, Heinze G, Lopez G, Mendoza-Sotelo J, Lazo-Langner A, et al. (2003). Methodologic pitfalls in measurement of 5-hydroxytriptamine uptake transporters in human platelets by [3H]-paroxetine binding assay. Arch Med Res, 34: 422-427. [DOI] [PubMed] [Google Scholar]
- [34].Arora RC, Meltzer HY (1989). Increased serotonin 2, (5-HT2) receptor binding as measured by 3 h-lysergic acid diethylamide (3H-LSD) in the blood platelets of depressed patients. Life Sci, 44:725-734. [DOI] [PubMed] [Google Scholar]
- [35].Davi G, Patrono C (2007). Platelet activation and atherothrombosis. N Engl J Med, 357: 2482-2494. [DOI] [PubMed] [Google Scholar]
- [36].Shelton RC, Sanders-Bush E, Manier DH, Lewis DA (2009). Elevated 5-HT2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience, 158:1406-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schins A, Honig A, Crijns H, Baur L, Hamulyák K (2003). Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link?. Psychosom Med, 65:729-737. [DOI] [PubMed] [Google Scholar]
- [38].Liu MY, Ren YP, Wei WL, Tian GX, Li G (2015). Changes of Serotonin (5-HT), 5-HT2A Receptor, and 5-HT Transporter in the Sprague-Dawley Rats of Depression, Myocardial Infarction and Myocardial Infarction Co-exist with Depression. Chin Med J (Engl), 128: 1905-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li Q, Liu MY, Liu MC, Guo CJ (2014). Study on effect of combined treatment of traditional Chinese medicine and western medicine on myocardial ischemia induced by mental stress. Chin Med, 9:62 [Google Scholar]