Abstract
Objective
The impact of systemic inflammation on clinical outcomes after CABG surgery is still controversial. In this study, we evaluated the impact of the markers of inflammation, endothelial damage and platelet activation on clinical outcomes after on- and off-pump CABG.
Methods
A group of 191 consecutive on- and off-pump CABG patients were prospectively studied. Blood samples were drawn before surgery, 18–36 h after the procedure and 5–7 days postoperatively and analyzed for 8-iso-prostaglandin F2α (8-iso-PGF2α), asymmetric dimethylarginine (ADMA) and β-thromboglobulin (β-TG). White blood count and C-reactive protein were measured twice, first before and then during the first 18–36 h after CABG. The primary clinical end-points were: low cardiac output syndrome (LCOS), postoperative myocardial infarction (PMI) and in-hospital cardiovascular death.
Results
Elevation of 8-iso-PGF2α, ADMA and β-TG before surgery was associated with an increased risk of morbidity and mortality after CABG. There were no differences in analyzed markers and clinical outcomes between the on- and off-pump groups. Even during the uncomplicated postoperative course the inflammatory response was enhanced and still remained higher than baseline 5–7 days after surgery.
Conclusion
Links between preoperative 8-iso-PGF2α, ADMA and β-TG and unfavorable early post-CABG outcomes suggest that these markers could be useful in identifying patients with increased risk of LCOS, PMI and in-hospital cardiovascular death following elective CABG.
Keywords: Cardiopulmonary bypass, Coronary artery bypass grafting, Inflammation
What is already known?
-
•
The influence of systemic inflammatory markers on clinical outcomes after CABG surgery remains a matter of scientific debate.
What this study adds?
-
•
High levels of inflammatory markers before surgery could be helpful in prediction of unfavorable early post-CABG clinical outcomes. This study also confirms the concept that CABG is associated with systemic inflammatory response regardless of the use of CPB.
1. Introduction
Systemic inflammatory response together with microembolization is responsible for most of the morbidity after on-pump coronary artery bypass graft surgery (CABG). In spite of improvements in anesthesia as well as surgical techniques, CABG is still associated with enhanced inflammation.1 To reduce these effects, reintroduction of off-pump coronary artery bypass surgery (OPCAB) has become increasingly more common in recent years.2 The influence of OPCAB on reduction of systemic inflammation remains a matter of scientific debate. A small number of trials have correlated the biochemical findings of systemic inflammation with the clinical outcomes of patients after CABG.2, 3, 4, 5
In this prospective, cohort study we evaluated the impact of CABG and OPCAB regarding: (1) oxidative stress, (2) endothelial cells, (3) platelets, (4) C-reactive protein (CRP) and (5) white blood count (WBC).
Oxidative stress is caused by an imbalance between free radical generation and elimination. Evidence indicates that oxidative stress contributes to myocardial ischemia-reperfusion injury.6 8-iso-prostaglandin F2α (8-iso-PGF2α) was analyzed as a marker of oxidative stress.7
By the production of various substances, the vascular endothelium is a significant regulator of cardiovascular function. Nitric oxide (NO) is produced in endothelial cells and plays an important role in various cardioprotective effects including: inhibition of superoxide radicals and inhibition of platelet activation.8 Oxidative stress during myocardial reperfusion after cardiac surgery reduces the bioavailability of NO. For the assessment of endothelial injury we chose asymmetric dimethylarginine (ADMA) as a natural inhibitor of NO synthase.9, 10
A variety of agonists secreted by platelets and other cells contribute to activation of platelets during surgery. Platelet secreted cytokines may be involved in the inflammatory response to coronary surgery because of the strong activation of platelets.11 β-thromboglobulin (β-TG) stands for a group of platelet proteins, which are released in large amounts subsequent to platelet activation.12
Therefore, in this study we evaluated the influence of cardiopulmonary bypass (CPB) on systemic inflammatory response, endothelial damage and platelet activation. In addition, we correlated the pre- as well as postoperative values of inflammatory markers with the clinical outcomes after CABG surgery.
2. Materials and methods
Study protocol was approved by the local Research Ethics Board and all participating patients signed an informed consent. The study was performed in accordance with the Declaration of Helsinki and with the consensus guidelines expressed by the STROBE statement.
2.1. Patients
The study was compromised of 191 consecutively recruited patients undergoing isolated, elective on- or off-pump CABG procedure between January and March 2008. Indications for CABG were consistent with the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization.13 Exclusion criteria were history of previous cardiac surgery, or percutaneous coronary intervention (PCI) within 1 month prior to surgery, renal insufficiency and liver dysfunction. All data was collected prospectively. The primary clinical endpoints were low cardiac output syndrome (LCOS) without postoperative myocardial infarction (PMI), PMI and in-hospital cardiovascular death.
LCOS was diagnosed if the patient required an intra-aortic balloon pump to be weaned from CPB or because of hemodynamic compromise while in the intensive care unit (ICU). LCOS was also diagnosed if the patient required inotropic therapy (dopamine, dobutamine, milnirone, or epinephrine) to maintain the systolic blood pressure >90 mm Hg and cardiac output >2.2 L/min/m2 for ≥30 min in the ICU, after correction of all of the electrolyte and blood gas abnormalities and after adjusting the preload and afterload to its optimal value. Patients who received a low dose of dopamine (≤3 μg/kg/min) were not considered to have LCOS. Patients who required vasoconstricting medications because of a high cardiac output (≥2.5 L/min/m2) and low peripheral resistance were also not considered to have LCOS.14
PMI was defined as a serum creatine kinase-myocardial band (CK-MB) value 5 times greater than the upper limit of normal (>85 U/L) with new Q waves present, or a CK-MB level 10 times greater than the upper limit of normal (<170 U/L), (with or without Q waves), or new regional wall motion abnormalities demonstrated by transesophageal echocardiography.15
In-hospital cardiovascular death was defined as death occurring during the same hospitalization period as the CABG surgery due to heart failure, PMI, stroke, cardiovascular hemorrhage or sudden cardiac death.
2.2. CABG procedure
All patients underwent standardized anesthesia. CABG was carried out through median sternotomy. All patients were operated on by consecutive surgeons according to their own preference, including use of CPB and the type of cardioplegia. CPB was carried out with a 40 μm arterial blood filter (Jostra Medizintechnik AG, Hirrlingen, Germany) and a nonpulsatile roller pump (Jostra Medizintechnik AG, Hirrlingen, Germany). Besides heparin coated CPB circuit we did not make any special measures to reduce inflammation during surgery. Mean arterial pressure was maintained between 40 and 60 mm Hg and blood flow was kept at 2.0–2.4 L/min per m2. Moderate systemic hypothermia (esophageal temperature, 32–34 °C) was applied. Myocardial preservation was achieved with aortic root infusion of intermittent warm blood or cold crystalloid cardioplegia, according to the surgeon's preference. Anticoagulation was achieved by administration of heparin (500 IU/kg) before the onset of CPB and was monitored by means of the activated clotting time (>400 s). After all anastomoses were completed, anticoagulation was reversed by protamine chloride to a normal activated clotting time. All patients received standardized postoperative care.
OPCAB procedures were performed by the same heparinization protocol as described above. The revascularization in the OPCAB patients was performed on the beating heart using the Octopus IV system (Medtronic Inc., Minneapolis, USA) and a coronary flow-shunt was always passed into the coronary arteriotomy.
2.3. Laboratory investigations
Blood samples were drawn from an anticubital vein with minimal stasis after an overnight fast, before the start of any anesthesia and surgery procedures, 18–36 h after the procedure and 5–7 days postoperatively and analyzed for 8-iso-PGF2α, ADMA and β-TG. Commercially available enzyme-linked immuno sorbent assays were used to measure plasma 8-iso-PGF2α (Cayman Chemicals, Ann Arbor, MI) and plasma β-TG (Diagnostica Stago, Asnieres, France). ADMA measurement was performed by isocratic high-performance liquid chromatography as described by Teerlink et al.16 Routine laboratory variables, including blood cell count, CRP and myocardial necrosis markers, were determined using standard laboratory techniques. WBC and CRP were measured preoperatively and 18–36 h after CABG. Normal values of WBC and CRP were 3.8–10.0 × 109/L and below 5 mg/L respectively. CK-MB values were measured at 6, 12, 18, and 24 h after surgery. The CK-MB level of 17 U/L was considered the upper normal value. All measurements were performed by technicians blinded to the origin of the samples. Intra- and inter-assay coefficients of variation were <7%.
2.4. Statistical analysis
Continuous variables were presented as mean and standard deviation (±) or as median with 25th, 75th percentiles inter-quartile range (IQR) as it concerned postoperative drainage and CRP. Categorical variables were presented as numbers and percentages. The Shapiro–Wilk test was used to test the normality of continuous variables. In order to examine the differences between the two independent groups, Student's t-test (for continuous normally distributed variables) or the Mann–Whitney U-test (for non-normally distributed variables) were used. The χ2 test or Fisher's exact test were used to compare distributions of categorical variables between independent groups. To assess the linear correlation between variables, the Pearson correlation coefficient for normally distributed variables or Spearman's rank correlation coefficient for non-normally distributed variables were calculated. The association between the 8-iso-PGF2α, ADMA, β-TG, WBC, CRP levels and LCOS, PMI or in-hospital death was evaluated by logistic regression analysis. No multivariate logistic regression modeling was performed owing to a low number of LCOS events (13 cases), PMI events (19 cases) and in-hospital deaths (7 cases). Statistical analysis was performed with Statistica 10.0 (StatSoft, Tulsa, OK, USA). Two-sided p-values of <0.05 were considered statistically significant.
3. Results
3.1. Peri- and postoperative data
Table 1 shows patient's demographic data. Baseline variables such as age, gender, risk factors for coronary artery disease, preoperative ejection fraction, EuroSCORE I and medications were comparable among groups. 158 patients underwent on-pump CABG and 33 were operated without CPB. The groups did not differ in terms of total transfusion as well as LCOS, PMI, ICU length of stay and in-hospital death, whereas postoperative drainage was slightly higher for patients with CPB (Table 2). Mean baseline values of studied parameters are shown in Table 3.
Table 1.
Baseline characteristics of CABG patients.
| Variable | All patients n = 191 | Patients with CPB n = 158 | Patients without CPB n = 33 | p |
|---|---|---|---|---|
| Age (years) | 64.9 ± 8.1 | 65.2 ± 8.2 | 63.5 ± 7.4 | 0.282 |
| Female, n (%) | 43 (22.5) | 35 (22.2) | 8 (24.2) | 0.794 |
| Peripheral vascular disease, n (%) | 31 (16.2) | 25 (15.8) | 6 (18.2) | 0.738 |
| Diabetes mellitus, n (%) | 59 (30.9) | 46 (29.1) | 13 (39.4) | 0.245 |
| Insulin, n (%) | 28 (14.7) | 22 (13.9) | 6 (18.2) | 0.529 |
| Hypertension, n (%) | 158 (82.7) | 127 (80.4) | 31 (93.9) | 0.061 |
| Hypercholesterolemia, n (%) | 126 (66.0) | 101 (63.9) | 25 (75.8) | 0.192 |
| Previous myocardial infarction, n (%) | 154 (80.6) | 128 (81.0) | 26 (78.8) | 0.769 |
| Previous PCI, n (%) | 23 (12.0) | 19 (12.0) | 4 (12.1) | 0.998 |
| Current smoking, n (%) | 54 (34.2) | 44 (32.6) | 10 (43.5) | 0.309 |
| COPD, n (%) | 11 (5.8) | 9 (5.7) | 2 (6.1) | 0.942 |
| Atrial fibrillation, n (%) | 11 (6.8) | 10 (7.2) | 1 (4.3) | 0.610 |
| BMI, kg/m2 | 28.0 ± 3.9 | 28.0 ± 3.9 | 28.0 ± 4.0 | 0.791 |
| LVEF, % | 52.0 ± 9.3 | 52.1 ± 9.4 | 51.3 ± 9.2 | 0.648 |
| EuroSCORE I | 2.9 ± 1.9 | 3.0 ± 1.9 | 2.6 ± 2.0 | 0.329 |
| Aspirin, n (%) | 38 (20.1) | 31 (19.9) | 7 (21.2) | 0.862 |
| ACE inhibitors, n (%) | 168 (88.0) | 137 (86.7) | 31 (93.9) | 0.246 |
| β-blockers, n (%) | 172 (90.1) | 141 (89.2) | 31 (93.9) | 0.412 |
| Lipid-lowering statin, n (%) | 171 (89.5) | 141 (89.2) | 30 (90.9) | 0.776 |
Values are displayed as mean ± standard deviation or number (percentage).
ACE, angiotensin-converting enzyme; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary interventions.
Table 2.
Operative and postoperative characteristics of CABG patients.
| Variable | All patients n = 191 | Patients with CPB n = 158 | Patients without CPB n = 33 | p |
|---|---|---|---|---|
| Aortic cross-clamp time, minutes | 40.1 ± 14.0 | 40.1 ± 14.0 | – | – |
| CPB time, minutes | 89.2 ± 32.2 | 89.2 ± 32.2 | – | – |
| Transfusion (red cells/platelets/plasma) ≥2 units, n (%) | 126 (66) | 105 (66.5) | 21 (63.6) | 0.137 |
| Postoperative drainage, mL | 700 (480–975) | 850 (540–1150) | 685 (480–920) | 0.017 |
| LCOS, n (%) | 13 (6.8) | 10 (6.3) | 3 (9.1) | 0.567 |
| PMI, n (%) | 19 (9.9) | 13 (8.2) | 6 (18.2) | 0.082 |
| ICU length of stay ≥2 days, n (%) | 82 (43.2) | 62 (39.2) | 20 (62.5) | 0.511 |
| In-hospital cardiovascular death, n (%) | 7 (3.7) | 6 (3.8) | 1 (3.0) | 0.831 |
Values are displayed as mean ± standard deviation, number (percentage) or median with 25th–75th percentiles inter-quartile range.
CPB, cardiopulmonary bypass; ICU, intensive care unit; LCOS, low cardiac output syndrome; PMI, postoperative myocardial infarction.
Table 3.
Changes of studied inflammatory response parameters in patients with analyzed clinical end-points.
| Variable | All patients n = 191 | Patients with LCOS n = 13 | Patients without LCOS n = 178 | p | Patients with PMI n = 19 | Patients without PMI n = 172 | p | In-hospital death n = 7 | Survivors n = 184 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline 8-iso-PGF2α, (pg/mL) | 358.6 ± 39.3 | 392.1 ± 56.2 | 356.1 ± 36.8 | 0.001 | 386.5 ± 52.5 | 355.5 ± 36.5 | <0.001 | 385.8 ± 53.9 | 357.0 ± 38.1 | 0.011 |
| 18–36 h post CABG 8-iso-PGF2α, (pg/mL) | 468.1 ± 43.4 | 526.2 ± 75.1 | 463.4 ± 36.4 | 0.015 | 505.3 ± 66.4 | 463.4 ± 37.4 | 0.017 | 543.4 ± 50.2 | 464.7 ± 40.4 | 0.021 |
| 5–7 days post CABG 8-iso-PGF2α, (pg/mL) | 417.0 ± 48.2 | 528.9 ± 58.9 | 409.3 ± 36.8 | 0.001 | 500.7 ± 70.7 | 409.5 ± 37.9 | 0.005 | 549.3 ± 10.6 | 412.2 ± 43.2 | 0.004 |
| Baseline ADMA, (μmol/L) | 0.56 ± 0.06 | 0.65 ± 0.08 | 0.56 ± 0.05 | 0.001 | 0.64 ± 0.09 | 0.55 ± 0.09 | <0.001 | 0.62 ± 0.11 | 0.56 ± 0.06 | 0.041 |
| 18–36 h post CABG ADMA, (μmol/L) | 0.95 ± 0.10 | 1.13 ± 0.17 | 0.93 ± 0.09 | 0.002 | 1.06 ± 0.16 | 0.93 ± 0.08 | 0.002 | 1.17 ± 0.14 | 0.93 ± 0.10 | 0.031 |
| 5–7 days post CABG ADMA, (μmol/L) | 0.75 ± 0.12 | 1.04 ± 0.12 | 0.73 ± 0.09 | <0.001 | 0.96 ± 0.15 | 0.73 ± 0.10 | 0.002 | 1.10 ± 0.06 | 0.74 ± 0.10 | 0.003 |
| Baseline β-TG, (IU/mL) | 58.8 ± 10.0 | 70.4 ± 12.6 | 56.9 ± 9.2 | 0.002 | 68.2 ± 12.5 | 56.7 ± 9.1 | <0.001 | 64.8 ± 15.3 | 57.5 ± 9.7 | 0.025 |
| 18–36 h post CABG β-TG, (IU/mL) | 84.6 ± 21.5 | 109.3 ± 28.2 | 82.6 ± 19.7 | 0.007 | 100.4 ± 26.9 | 82.6 ± 20.0 | <0.001 | 108.0 ± 24.2 | 83.5 ± 20.7 | <0.001 |
| 5–7 days post CABG β-TG, (IU/mL) | 68.5 ± 17.3 | 107.6 ± 16.7 | 65.8 ± 13.8 | <0.001 | 94.9 ± 24.1 | 66.1 ± 14.5 | 0.006 | 114.0 ± 15.0 | 66.7 ± 14.7 | 0.005 |
| Baseline WBC count, (×109/L) | 7.3 ± 2.1 | 8.8 ± 3.9 | 7.2 ± 1.9 | 0.009 | 8.2 ± 3.5 | 7.2 ± 1.9 | 0.252 | 8.2 ± 1.5 | 7.3 ± 2.2 | 0.215 |
| 18–36 h post CABG WBC count, (×109/L) | 10.6 ± 3.9 | 15.5 ± 7.2 | 10.1 ± 3.1 | 0.032 | 14.1 ± 6.3 | 10.0 ± 3.1 | 0.016 | 15.7 ± 5.9 | 10.5 ± 3.7 | 0.039 |
| Baseline CRP, (mg/L) | 2.5 (1.5–4.8) | 4.0 (1.8–9.1) | 2.4 (1.5–4.7) | 0.342 | 3.9 (1.7–8.9) | 2.4 (1.5–4.5) | 0.748 | 2.6 (2.4–8.2) | 2.4 (1.5–4.7) | 0.356 |
| 18–36 h post CABG CRP, (mg/L) | 160.5 (125.9–188.5) | 180.7 (170.9–258.8) | 152.9 (124.0–187.1) | 0.009 | 175.8 (170.6–230.2) | 152.8 (123.8–187.5) | 0.025 | 254.8 (201.4–256.2) | 153.1 (124.5–186.3) | 0.030 |
Values are displayed as mean ± standard deviation or median with 25th–75th percentiles inter-quartile range.
8-iso-PGF2α, 8-iso-prostaglandin F2α; ADMA, asymmetric dimethylarginine; β-TG, β-thromboglobulin; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; CRP, C-reactive protein; WBC, white blood counts.
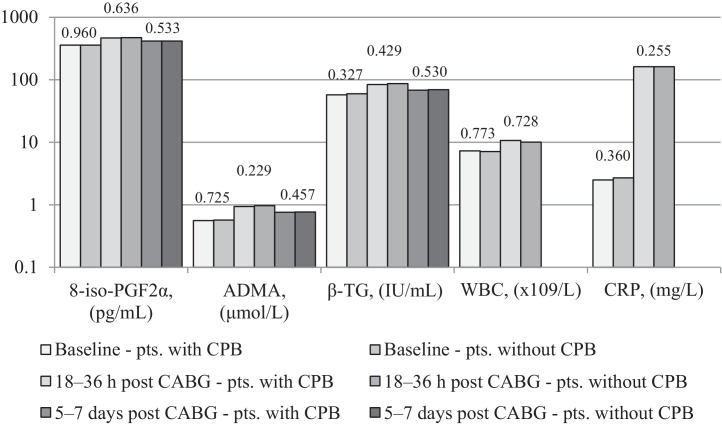
In all patients 8-iso-PGF2α increased by 30% at 18–36 h post-CABG (p = 0.05) and then decreased by 11% 5–7-days after surgery (p < 0.0001). Similarly, ADMA increased by 70% 18–36 h after CABG (p = 0.0001) and then decreased by 21% in 5–7 postoperative days (p = 0.0001). β-TG increased by 44% 18–36 h post-CABG (p < 0.001) and decreased by 19% 5–7days after the procedure (p = 0.001) (Table 3). There was a positive correlation between ADMA and 8-iso-PGF2α at all-time points (r = 0.71 at baseline, r = 0.73 at 18–36 h and r = 0.70 in 5–7 postoperative days, p < 0.001 for all comparisons) and between β-TG and 8-iso-PGF2α (r = 0.62 at baseline, r = 0.68 at 18–36 h and r = 0.61 in 5–7 postoperative days, p < 0.001 for all comparisons). WBC increased by 45% and CRP increased by 6320% 18–36 h after CABG (p = 0.016 and p = 0.024 respectively) (Table 3). No differences, in terms of analyzed markers, were noted for the different types of CABG (on-pump CABG versus OPCAB) (Fig. 1). There were no correlations between postoperative 8-iso-PGF2α, ADMA, β-TG, WBC or CRP and cross-clamp time or CPB time.
Fig. 1.
Level changes of studied parameters in patients with and without CPB. No statistical differences, in terms of analyzed markers, were noted for the different types of CABG (on-pump CABG versus OPCAB). p Values are shown above the columns of the diagram.
8-iso-PGF2α, 8-iso-prostaglandin F2α; ADMA, asymmetric dimethylarginine; β-TG, β-thromboglobulin; CABG, coronary artery bypass grafting; OPCAB, off-pump coronary artery bypass surgery; CPB, cardiopulmonary bypass; CRP, C-reactive protein; pts., patients; WBC, white blood counts.
3.2. Low cardiac output syndrome without postoperative myocardial infarction
Of the 191 patients, 6.8% developed LCOS without PMI, 6.3% patients from the on-pump CABG group and 9.1% from the OPCABG patients (p = 0.567) (Table 2). There were no differences in the baseline demographic or clinical variables between the LCOS subgroup and remaining subjects (data not shown). All studied parameters before CABG, except for the baseline CRP, were statistically higher in patients with LCOS (Table 3). Postoperative values of analyzed markers were significantly higher in the LCOS subgroup, regardless of the use of CPB. Regression analysis showed a significant correlation between preoperative 8-iso-PGF2α, ADMA and β-TG levels, and LCOS (p = 0.024, p = 0.017 and p = 0.021 respectively).
3.3. Postoperative myocardial infarction
9.9% patients developed PMI, 8.2% patients from the on-pump CABG group and 18.2% from the OPCABG group (p = 0.082) (Table 2). There were no significant differences between the PMI patients and the remaining subjects with regard to demographic data (data not shown). Baseline 8-iso-PGF2α, ADMA and β-TG levels were significantly higher in the PMI subgroup (Table 3). After surgery, all analyzed markers were statistically higher in the PMI subgroup, irrespective of use of CPB. Regression analysis showed a significant correlation between preoperative 8-iso-PGF2α, ADMA and β-TG levels, and PMI (p = 0.045, p = 0.012 and p = 0.001 respectively).
3.4. In-hospital cardiovascular death
3.7% patients died during the early postoperative period due to extensive PMI. These were 3.8% patients from the on-pump CABG group and 3.0% from the OPCABG group (p = 0.831), (Table 2). These patients did not differ from the remaining subjects with respect to demographic and clinical variables (data not shown). As in the case of LCOS and PMI, this small subgroup was characterized by higher 8-iso-PGF2α, ADMA, and β-TG levels at baseline (Table 3). After CABG all studied markers were significantly higher in this subgroup of patients, regardless of the use of CPB. Regression analysis showed a significant correlation between preoperative 8-iso-PGF2α, ADMA and β-TG levels, and in-hospital cardiovascular death (p = 0.037, p = 0.043 and p = 0.014 respectively).
4. Discussion
We demonstrated for the first time in a large number of CABG patients the simultaneous level changes of 8-iso-PGF2α, ADMA, β-TG, WBC and CRP. We showed that clinical outcomes in early postoperative course are associated with these markers of inflammation, oxidative stress and platelet activation. The most important finding of our study is that elevation of 8-iso-PGF2α, ADMA and β-TG before surgery is associated with an increased risk of morbidity (LCOS and PMI) and mortality after CABG, which expands our previous observations made in smaller groups of patients that suggested a prognostic role of these processes in patients undergoing CABG.17, 18 Postoperatively all studied parameters, including WBC and CRP, increased statistically higher in patients with primary clinical endpoints, regardless of use of CPB. Even during the uncomplicated postoperative course the inflammatory response was enhanced and still remained higher than baseline 5–7 days after surgery. On-pump as well as off-pump surgery were associated with a similar increase of studied markers. Additionally there was no correlation between analyzed postoperative markers and time of CPB or even with aortic cross-clamp time. Our findings are consistent with other studies that demonstrated that cardiac surgery results in extensive oxidative stress response irrespective of whether CABG was performed with or without CPB.19, 20 In contrast to our results several studies have shown that with OPCAB, the degree of inflammatory response is significantly less expressed.2 Because of the small number of patients in these trials, no significant difference in clinical outcomes between groups was observed.
We have already reported that oxidative stress, measured by 8-iso-PGF2α, is associated with ADMA increase after on-pump CABG and pre-procedure high levels of these markers are associated with PMI and in-hospital cardiovascular death.17 In this study we demonstrate that also in OPCAB, baseline ADMA as well as 8-iso-PGF2α are predictors of unfavorable postoperative outcomes in the same degree as in on-pump CABG. Also baseline ADMA elevation cannot be explained by renal dysfunction, as renal insufficiency was an exclusion criterion in this report. These results corroborate findings obtained by Karu et al. who observed that cardiac surgery results in extensive oxidative stress response and a marked increase in ADMA, regardless of the method or type of surgical procedure used.19, 21
This study demonstrates that the occurrence of LCOS, PMI and in-hospital death after elective, isolated CABG is also associated with increased platelet activation, measured by β-TG. We reported previously that platelet activation and oxidative stress are significantly enhanced in patients undergoing CABG surgery and preoperative elevated levels of β-TG are associated with an increased risk of PMI.18 Our current study shows that the activation of platelets increase to the same degree in on-pump CABG as in OPCAB and increased level of β-TG at baseline is associated with unfavorable early postoperative outcomes, including as well PMI, LCOS and in-hospital cardiovascular death. Moller et al. observed that platelets after OPCAB were more easily activated in the early postoperative period than in on-pump CABG.22
Inflammatory markers such as CRP and WBC were raised similarly after on-pump CABG as well as after OPCAB. There are wide number of studies showing controversial results of inflammatory markers in comparison of OPCAB versus on pump CABG. As confirmed in our study, Karu et al. showed that CRP concentrations still remained higher than baseline by the end of the first postoperative week. This CRP response was irrespective of whether CABG surgery was performed with or without CPB.19 On the other hand, Raja et al. published a few studies in which the increases of CRP levels and WBC were greater and persisted for a longer time after on-pump CABG with respect to OPCAB.2 Nevertheless in our study preoperative levels of CRP and WBC were not associated with any clinical endpoints.
We reported previously that the inflammatory response during cardiac surgery is at least in part related to the genetic characteristics of any given individual and the candidates are genes for tumor necrosis factor alpha and interleukin 6 as evidenced previously.23, 24 These findings are consistent with a study by Lehmann et al. who also demonstrated a genetic influence on systemic inflammatory response to CABG.25 In our study, elevation of 8-iso-PGF2α, ADMA and β-TG before surgery is associated with an increased risk of morbidity and mortality after CABG and high baseline levels of these markers are likely of genetic origin. The data regarding inflammatory response to CPB are still inconsistent. Our findings suggest that 8-iso-PGF2α, ADMA and β-TG determined before on-pump CABG as well as OPCAB, could be helpful in identifying patients at risk of LCOS, PMI and in-hospital death. In addition, this study validate the concept that other mechanisms rather than CPB and myocardial ischemia during aorta cross-clamp are responsible for increased postoperative inflammatory response.
This study has some limitations. The most important limitation of our study is the lack of control group. Comparison of systemic inflammatory response, endothelial damage and platelet activation between patients having classical full sternotomy cardiac surgery to the same surgery performed by minithoracotomy or to the simple sternotomy in non cardiac surgery could help to answer the question of how important a role in inflammatory response plays range of surgical trauma. It is a retrospective analysis of prospectively collected data at a single center. The sample size of the study group was limited and restricted to CABG patients. 8-iso-PGF2α, ADMA and β-TG levels were measured only at three time points. Windows of postoperative measurements were wide. Using a stricter measurement regimen could also give less variation in the results. It is probable that measurements performed more often, especially during the first 24 h after the procedure, could provide new information on the effect of CABG on inflammatory response, endothelial damage and platelet activation markers formation and elimination. EuroSCORE I values were low as only elective, isolated CABG patients, without previous history of cardiac surgery and without renal function impairment were recruited. The results of this study cannot be extrapolated to high-risk patients. Other factors such as diet might also affect preoperative levels of the variables studied especially oxidative stress.26, 27 The low incidence of the primary clinical endpoints may impact the power available for this study and could introduce unexpected bias into the outcomes. At last, our study was limited to the hospital stay. It would be of interest to assess analyzed markers and clinical endpoints, within the first postoperative months.
5. Conclusion
Links between preoperative levels of 8-iso-PGF2α, ADMA and β-TG and unfavorable early post-CABG outcomes suggest that these markers could be useful in identifying patients at increased risk of LCOS, PMI and in-hospital cardiovascular death following elective surgery. Our results validate the concept that CABG surgery is associated with systemic inflammatory response, endothelial damage and platelet activation regardless of the use of CPB.
Conflicts of interest
The authors have none to declare.
References
- 1.Edmunds L.H., Jr. Extracorporeal circulation. In: Cohn L.H., Edmunds L.H. Jr., editors. Cardiac surgery in the adult. 2nd ed. McGraw-Hill; New York: 2003. p. 316. [Google Scholar]
- 2.Raja S.G., Berg G.A. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Card Surg. 2007;22:445–455. doi: 10.1111/j.1540-8191.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 3.Dorman B.H., Kratz J.M., Multani M.M. A prospective, randomized study of endothelin and postoperative recovery in off-pump versus conventional coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2004;18:25–29. doi: 10.1053/j.jvca.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Wan I.Y., Arifi A.A., Wan S. Beating heart revascularization with or without cardiopulmonary bypass: evaluation of inflammatory response in a prospective randomized study. J Thorac Cardiovasc Surg. 2004;127:1624–1631. doi: 10.1016/j.jtcvs.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Tatoulis J., Rice S., Davis P., Goldblatt J.C., Marasco S. Patterns of postoperative systemic vascular resistance in a randomized trial of conventional on-pump versus off-pump coronary artery bypass graft surgery. Ann Thorac Surg. 2006;82:1436–1444. doi: 10.1016/j.athoracsur.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 6.Raedschelders K., Ansley D.M., Chen D.D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Roberts L.J., Morrow J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 8.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 9.Sibal L., Agarwal S.C., Home P.D., Boger R.H. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwasny-Krochin B., Gluszko P., Undas A. Plasma asymmetric dimethylarginine in active rheumatoid arthritis: links with oxidative stress and inflammation. Pol Arch Med Wewn. 2012;122:270–276. doi: 10.20452/pamw.1277. [DOI] [PubMed] [Google Scholar]
- 11.Menasche P., Edmunds L.H., Jr. Extracorporeal circulation. In: Cohn L.H., Edmunds L.H. Jr., editors. Cardiac surgery in the adult. 2nd ed. McGraw-Hill; New York: 2003. pp. 349–360. [Google Scholar]
- 12.Bergseth G., Lappegard K.T., Videm V., Mollnes T.E. A novel enzyme immunoassay for plasma thrombospondin. Comparison with betathromboglobulin as platelet activation marker in vitro and in vivo. Thromb Res. 2000;99:41–50. doi: 10.1016/s0049-3848(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 13.ESC/EACTS Guidelines on myocardial revascularization. Available at http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/ESC-EACTS-Guidelines-in-Myocardial-Revascularisation-Guidelines-for.
- 14.Algarni K.D., Maganti M., Yau T.M. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011;92:1678–1684. doi: 10.1016/j.athoracsur.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K., Alpert J.S., White H.D. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 16.Teerlink T., Nijveldt R.J., de Jong S., van Leeuwen P.A. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem. 2002;303:131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 17.Plicner D., Mazur P., Sadowski J., Undas A. Asymmetric dimethylarginine and oxidative stress following coronary artery bypass grafting: associations with postoperative outcome. Eur J Cardiothorac Surg. 2014;45:136–141. doi: 10.1093/ejcts/ezt646. [DOI] [PubMed] [Google Scholar]
- 18.Plicner D., Ziętkiewicz M., Mazur P., Stąpor R., Sadowski J., Undas A. Beta-thromboglobulin as a marker of perioperative myocardial infarction in patients undergoing coronary artery bypass grafting following aspirin discontinuation. Platelets. 2014;25:603–607. doi: 10.3109/09537104.2013.854877. [DOI] [PubMed] [Google Scholar]
- 19.Karu I., Taal G., Zilmer K., Pruunsild C., Starkopf J., Zilmer M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand Cardiovasc J. 2010;44:119–124. doi: 10.3109/14017430903490981. [DOI] [PubMed] [Google Scholar]
- 20.Suleiman M.S., Zacharowski K., Angelini G.D. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karu I., Zilmer K., Starkopf J., Zilmer M. Changes of plasma asymmetric dimethylarginine levels after coronary artery bypass grafting. Scand Cardiovasc J. 2006;40:363–367. doi: 10.1080/14017430600983648. [DOI] [PubMed] [Google Scholar]
- 22.Møller C.H., Steinbrüchel D.A. Platelet function after coronary artery bypass grafting: is there a procoagulant activity after off-pump compared with on-pump surgery? Scand Cardiovasc J. 2003;37:149–153. doi: 10.1080/14017430310001456. [DOI] [PubMed] [Google Scholar]
- 23.Boehm J., Hauner K., Grammer J. Tumor necrosis factor-α-863 C/A promoter polymorphism affects the inflammatory response after cardiac surgery. Eur J Cardiothorac Surg. 2011;40:50–54. doi: 10.1016/j.ejcts.2011.01.084. [DOI] [PubMed] [Google Scholar]
- 24.Wypasek E., Undas A., Sniezek-Maciejewska M. The increased plasma C-reactive protein and interleukin-6 levels in patients undergoing coronary artery bypass grafting surgery are associated with the interleukin-6-174G > C gene polymorphism. Ann Clin Biochem. 2010;47:343–349. doi: 10.1258/acb.2010.090305. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann L.E., Schroeder S., Hartmann W. A single nucleotide polymorphism of macrophage migration inhibitory factor is related to inflammatory response in coronary bypass surgery using cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;30:59–63. doi: 10.1016/j.ejcts.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 26.Zujko M.E., Witkowska A.M., Górska M., Wilk J., Krętowski A. Reduced intake of dietary antioxidants can impair antioxidant status in type 2 diabetes patients. Pol Arch Med Wewn. 2014;124:599–607. doi: 10.20452/pamw.2497. [DOI] [PubMed] [Google Scholar]
- 27.Grygiel-Górniak B., Marcinkowska J., Szczepanik A., Przysławski J. Nutritional habits and oxidative stress in postmenopausal age. Pol Arch Med Wewn. 2014;124:298–305. doi: 10.20452/pamw.2293. [DOI] [PubMed] [Google Scholar]