Abstract
Background
Recently, the value of various structural body components have been proposed for predicting cardio-metabolic risk. The present study aimed to assess the wrist circumference (WrC) as an alternative measure for differentiating patients with CAD and METs from those without CAD and METs.
Methods
We studied 228 consecutive subjects who underwent coronary angiography. Those with and without evidence of coronary artery involvement at angiography were considered as the coronary artery disease (CAD) group (n = 139) and the non-CAD group (n = 89), respectively. WrC was measured; and metabolic syndrome (METs) was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria.
Results
WrC was significantly higher in CAD compared to non-CAD patients (17.85 ± 1.29 mm vs 17.43 ± 1.29 mm, P = 0.017). The overall prevalence of METs was significantly different between the CAD and non-CAD subjects (74.3% vs 58.8%, P = 0.016). Although there was a tendency for association, no statistically significant association between the mean of the WrC and the severity of CAD was found (P = 0.065). WrC had a weak positive correlation with triglyceride (r = 0.172, P = 0.011) and cholesterol (r = 0.141, P = 0.038) level and a weak negative association with high-density lipoprotein level (r = −0.279, P < 0.001). In multivariate logistic regression models, WrC could predict neither presence of CAD nor METs.
Conclusion
Although correlated with METs-type lipid profile, WrC may not be a valuable index for predicting the presence of CAD or METs.
Keywords: Wrist circumference, Coronary artery disease, Metabolic syndrome
1. Introduction
Cardiovascular diseases (CVDs) are now clearly identified as a major cause of mortality worldwide at both developed and developing countries.1, 2 Because of the multifactorial risk profile of CVDs, reducing the incidence of those diseases has been a fundamental problem of healthcare systems in each community.3, 4 Whereas preventive programs in developed countries resulted in downward trend of CVDs over the past decade,5, 6 lack of such programs made the CVDs even more prevalent in low-income countries during the recent years.7, 8 Therefore, nowadays, early diagnosis and control of cardiovascular risk factors is more essential among developing communities.
Of various potential risk factors for CVDs, the role of anthropometric and metabolic indicators, with some causal and synergistic effects,9 is obvious. On epidemiologic studies, it has been clearly found that obesity has a role to develop insulin resistance, diabetes, and metabolic syndrome (METs).10 In fact, a cluster of anthropometric parameters are proposed as obesity-equivalent for identifying individuals with higher risk for CVDs. Initially, body weight and body mass index (BMI) were identified as main indicators for obesity; but those indices could not specifically distinguish fat from muscle mass, nor represent the distribution pattern of one's body fat.11, 12 Therefore, some other anthropometric parameters such as increased waist circumference and waist to hip ratio have been proposed and have been demonstrated to be closely associated with higher cardiovascular risk.12, 13 The values of these parameters, however, are significantly affected by various environmental and hereditary factors, making their specificity lowered for cardiovascular risk estimation.14 Also, it is practically difficult to get the accurate measurement of such anthropometric indices especially in different seasons and different sites of measurements.15
Recently, some other structural components of the body have been introduced for predicting cardio-metabolic risk. In some studies, increased neck or wrist circumference (WrC) could well discriminate high-risk groups with morbid obesity, diabetes mellitus, dyslipidemia, or METs.16, 17 In a pathophysiologic point of view, increased bone mass has been proved to be associated with hyperinsulinemia18; and the insulin receptor signaling pathway has been demonstrated to communicate the bone remodeling process with the body metabolic control.19 Therefore, WrC in this context could be considered as an indirect determinant of hyperinsulinemia and insulin resistance. Assuming there is no comprehensive data in the literature focusing on the eligibility of WrC as a potential risk indicator for CVD, the present study aimed to assess the WrC as an alternative tool for differentiating patients with CAD and METs from those without CAD and METs.
2. Materials and methods
2.1. Study population
This case control association study was conducted on consecutive patients aged >30 years who were underwent coronary angiography at Shahid Rajaei Heart Center, Tehran, Iran during 2014. Among participants, those with evidence of meaningful coronary involvement in angiography (≥50% stenosis) were considered as the coronary artery disease (CAD) group and others without any evidences of significant coronary stenosis were considered as the non-CAD group. Those with previous history of CVDs or cardiovascular diagnostic or therapeutic interventions were all excluded. Also, those with the history of upper extremities’ major trauma, fractures or deformities, acquired or inherited skeletal disorders, stroke, or malignancy were excluded from the study. The study protocol was approved by the Research Ethics Committee of the Iran University of Medical Sciences, and signed informed consents were collected from all participants. All authors agreed on adherence to the Helsinki Declaration.
2.2. Study measurements
Baseline demographic characteristics, cardiovascular risk factors, and medications were collected by interviewing, physical examination, and lab tests. In this regard, hypertension was defined as concurrent use of antihypertensive agents or systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg; obesity was defined according to the World Health Organization definition as a BMI ≥ 30 kg/m2; diabetes mellitus was diagnosed either from concurrent use of oral hypoglycemic agents or insulin, or if fasting blood sugar (FBS) was ≥126 mg/dl; current smoking history was defined as regular smoking a tobacco product, or a smoking history during the 30 days prior to admission; and hyperlipidemia was defined as a total cholesterol ≥ 200 mg/dl, high-density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dl (men) or ≤50 mg/dl (women), and triglycerides ≥ 150 mg/dl. To assess anthropometric parameters, body weight was measured by Seca scales (Germany) with minimal clothing and no shoes (recorded as rounded to the nearest 100 g); height was measured in a standing position, without shoes while the shoulders were in a normal position (recorded as rounded to the nearest 0.5 cm); waist circumference was measured at top of iliac crest in mid axillary line without applying any pressure (recorded as rounded to the nearest 0.1 cm); BMI was estimated as weight (kg) divided by height (m) squared; waist to hip ratio was measured as waist circumference (cm) divided by height (cm); and WrC was measured using an un-stretchable tape measure positioned over the Lister tubercle of the distal of radius and over the distal of ulna when subjects were in a sitting position. All measurements were performed by a single person to minimize inter-observer variability.
METs was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria as the presence of at least 3 of the followings:
-
1)
waist circumferences ≥ 90 cm for men and ≥80 cm for women
-
2)
SBP ≥ 130 mmHg or DBP ≥ 85 mmHg
-
3)
serum triglyceride level ≥ 150 mg/dl
-
4)
HDL-C ≥50 mg/dl and ≥40 mg/dl for women and men, respectively
-
5)
FBS ≥ 100 mg/dl
A qualified physician measured blood pressure two times in a sitting position after 15 min of rest using a standard mercury sphygmomanometer; and the mean of the two measurements was recorded as the participant's blood pressure. To measure laboratory parameters, blood samples were obtained in the early morning after an overnight fast at the subject's home. Samples were assayed for FBS, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and HDL-C using standard techniques on a Cobas autoanalyzer system.
2.3. Statistical analysis
Results were presented as mean ± standard deviation (SD) for quantitative variables and were summarized by frequency (percentage) for categorical variables. Continuous variables were compared using t test or Mann–Whitney U test whenever the data did not appear to have normal distribution or when the assumption of equal variances was violated across the study groups. Categorical variables were, on the other hand, compared using Chi-square test.
The multivariate logistic regression analysis model was used to assess the difference in WrC between CAD and non-CAD groups with the presence of confounders. Namely, the presence of CAD was considered as the dependent variable, wrist circumference as the independent variable, and the baseline parameters including gender, age, family history for CAD, current smoking, history of diabetes mellitus, METs, high SBP and/or DBP measurements, lipid profiles (triglyceride, cholesterol, LDL-C, HDL-C), and serum uric acid level as the potential confounders. In such model, we included 13 baseline variables as the independents to assess the association between WrC and CAD. In other word, with the suspicion to potential confounding impact of gender, advanced age, cardiovascular risk factors and laboratory indices, all of these parameters were included into the model for adjusting.
To determine the value of anthropometric indices to discriminate CAD from non-CAD and also METs from non-METs conditions, the receiver-operating characteristic (ROC) curve was analyzed defining a value >0.80 as good discriminative value. For the statistical analysis, the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL) was used. P values of 0.05 or less were considered as statistically significant.
3. Results
By strict consideration of the exclusion criteria, no subjects were secondarily excluded after first being included; and a total of 228 subjects were recruited to this study, 139 of whom were categorized as CAD and 89 as non-CAD. Comparing the two groups (Table 1), those with CAD showed higher male gender frequency, higher mean age, as well as higher prevalence of diabetes mellitus and METs. Also, the mean level of FBS and SBP were significantly higher in CAD group. The overall prevalence of METs was significantly different between the CAD and non-CAD groups (74.3% vs 58.8%, P = 0.016). Of anthropometric indices, only the mean ± SD for WrC was significantly higher in CAD compared to non-CAD patients (17.43 ± 1.29 mm vs 17.85 ± 1.29 mm, P = 0.017). Considering the achieved mean (SD) of WrC in the individuals with normal coronary status as 17.43 (1.29) mm, in patients with single-vessel disease as 17.79 (1.33) mm, in patients with 2-vessel disease as 17.92 (1.41) mm, and in those with 3-vessel disease as 17.93 (1.18) mm, there was no statistically significant association between the mean WrC and the severity of CAD (P = 0.065). As shown in Table 2, the value of WrC found to have a weakly positive correlation with the METs score (r = 0.207, P = 0.002), serum triglyceride (r = 0.172, P = 0.011), and serum cholesterol level (r = 0.141, P = 0.038); and a weakly negative correlation with serum HDL-C level (r = −0.279, P < 0.001).
Table 1.
Baseline characteristics in coronary artery disease (CAD) and non-CAD groups.c
| Item | None CAD group (n = 89) |
CAD group (n = 139) |
P-value |
|---|---|---|---|
| Male gendera | 45 (51) | 101 (73) | 0.001 |
| Age, yearb | 53 ± 11 | 61 ± 1 | <0.001 |
| Family history for CADa | 23 (26) | 43 (31) | 0.408 |
| Current smokinga | 16 (18) | 37 (27) | 0.132 |
| Diabetes mellitusa | 21 (24) | 56 (40) | 0.009 |
| Metabolic syndromea | 50 (59) | 101 (74) | 0.016 |
| SBP (mmHg)b | 127 ± 17 | 135 ± 22 | 0.003 |
| DBP (mmHg)b | 76 ± 10 | 76 ± 10 | 0.985 |
| BMI (kg/m2)b | 27.8 ± 5.4 | 28.2 ± 4.9 | 0.623 |
| Waist to hip ratiob | 0.58 ± 0.09 | 0.59 ± 0.08 | 0.405 |
| Waist circumference (cm)b | 95 ± 15 | 97 ± 11 | 0.178 |
| Wrist circumferences (mm)b | 17 ± 1 | 18 ± 1 | 0.017 |
| Serum triglyceride (mg/dl)b | 107 (85, 172) | 130 (91, 192) | 0.218 |
| Serum cholesterol (mg/dl)b | 158 (133, 178) | 146 (125, 174) | 0.075 |
| Serum LDL (mg/dl)b | 92 (73.5, 108) | 85 (71, 103) | 0.157 |
| Serum HDL (mg/dl)b | 36 (35, 42) | 36 (33, 38) | 0.029 |
| Serum uric acid (mg/dl)b | 5.2 ± 1.5 | 5.4 ± 1.4 | 0.461 |
| FBS (mg/dl)b | 93 (85, 108) | 110 (94, 152) | <0.001 |
Values are number (%).
Values are mean ± standard deviation, or median (interquartile range) where appropriate.
SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, LDL: low-density lipoprotein, HDL: high-density lipoprotein, FBS: fasting blood sugar.
Table 2.
Correlation between anthropometric indices and risk factors of coronary artery disease.a
| Item | R coefficient | P value |
|---|---|---|
| Age | 0.056 | 0.405 |
| BMI | 0.363 | <0.001 |
| Metabolic syndrome score | 0.207 | 0.002 |
| SBP | 0.013 | 0.851 |
| DBP | 0.102 | 0.125 |
| Serum triglyceride | 0.172 | 0.011 |
| Serum cholesterol | 0.141 | 0.038 |
| Serum LDL | 0.128 | 0.060 |
| Serum HDL | −0.279 | <0.001 |
| Serum uric acid | 0.033 | 0.635 |
| FBS | 0.066 | 0.333 |
BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, LDL: low-density lipoprotein, HDL: high-density lipoprotein, FBS: fasting blood sugar.
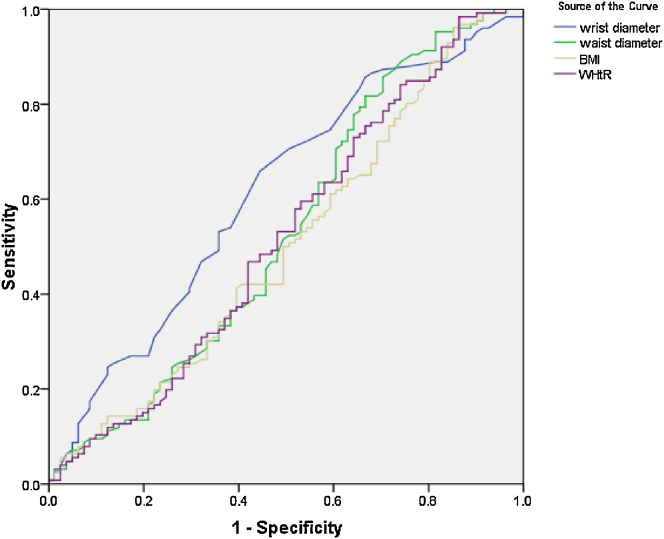
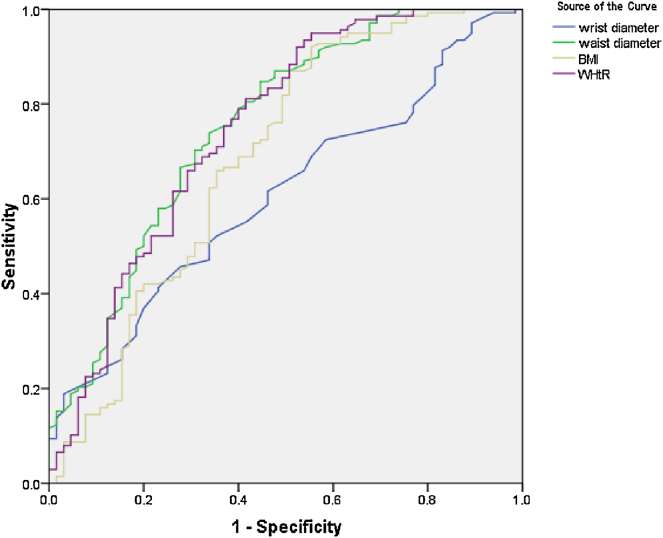
On multivariable logistic regression model (Table 3), the main determinants of CAD included male gender, advanced age, and higher SBP; while higher WrC could not predict the presence of CAD. In similar multivariate regression model (Table 4), it was shown that the value of WrC could not predict presence of METs. Using the ROC curve analysis (Fig. 1, Fig. 2), none of the anthropometric indices, including wrist diameter, waist circumference, waist to hip ratio and BMI, could discriminate CAD from non-CAD (Fig. 1) or METs from non-METs subjects (Fig. 2).
Table 3.
Main predictors of coronary artery disease (CAD) in multivariable logistic regression model.a
| Factor | B | SE | Wald | P-value | OR | 95.0% CI for OR |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Wrist diameter | .144 | .165 | .754 | .385 | 1.155 | .835 | 1.597 |
| Male gender | 1.027 | .466 | 4.852 | .028 | 2.793 | 1.120 | 6.967 |
| Age | .063 | .017 | 13.080 | .000 | 1.065 | 1.029 | 1.102 |
| Family history of CAD | .410 | .402 | 1.040 | .308 | 1.507 | .685 | 3.313 |
| Smoking | .575 | .448 | 1.649 | .199 | 1.777 | .739 | 4.274 |
| Diabetes mellitus | .433 | .446 | .942 | .332 | 1.542 | .643 | 3.693 |
| SBP | .027 | .011 | 5.852 | .016 | 1.028 | 1.005 | 1.050 |
| DBP | −.003 | .020 | .029 | .864 | .997 | .958 | 1.037 |
| Serum triglyceride | .001 | .003 | .069 | .793 | 1.001 | .996 | 1.006 |
| Serum cholesterol | −.004 | .006 | .484 | .487 | .996 | .984 | 1.008 |
| Serum LDL | −.009 | .028 | .103 | .748 | .991 | .937 | 1.048 |
| Serum HDL | .053 | .125 | .180 | .671 | 1.055 | .825 | 1.349 |
| Serum uric acid | .003 | .005 | .576 | .448 | 1.003 | .995 | 1.012 |
Hosmer and Lemeshow goodness of fit: Chi-square = 17.274, P = 0.027.
SBP: systolic blood pressure, DBP: diastolic blood pressure, LDL: low-density lipoprotein, HDL: high-density lipoprotein.
Table 4.
Main predictors of metabolic syndrome in multivariable logistic regression model.b
| Factor | B | SE | Wald | P-value | OR | 95.0% CI for OR |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Wrist diameter | .068 | .259 | .069 | .792 | 1.071 | .644 | 1.780 |
| Male gender | −1.770 | .780 | 5.146 | .023 | .170 | .037 | .786 |
| Age | .032 | .025 | 1.577 | .209 | 1.032 | .982 | 1.084 |
| Family history for coronary artery disease | −.479 | .560 | .732 | .392 | .620 | .207 | 1.856 |
| Smoking | .586 | .690 | .722 | .395 | 1.797 | .465 | 6.942 |
| Diabetes mellitus | −.495 | .666 | .553 | .457 | .609 | .165 | 2.248 |
| SBP | .090 | .022 | 16.204 | .000 | 1.094 | 1.047 | 1.143 |
| DBP | .026 | .032 | .635 | .425 | 1.026 | .963 | 1.093 |
| Serum triglyceride | .046 | .010 | 19.618 | .000 | 1.047 | 1.026 | 1.068 |
| Serum cholesterol | −.022 | .011 | 4.009 | .045 | .978 | .957 | 1.000 |
| Serum LDL | −.079 | .043 | 3.379 | .066 | .924 | .849 | 1.005 |
| Serum HDL | −.196 | .187 | 1.105 | .293 | .822 | .570 | 1.185 |
| Serum uric acid | .036 | .011 | 10.859 | .001 | 1.036 | 1.015 | 1.059 |
a Hosmer and Lemeshow goodness of fit: Chi-square = 16.038, P = 0.042.
SBP: systolic blood pressure, DBP: diastolic blood pressure, LDL: low-density lipoprotein, HDL: high-density lipoprotein.
Fig. 1.
The receiver-operating characteristic analysis curve to determine value of anthropometric indices to discriminate CAD from non-CAD status (the area under the curve for wrist diameter, waist diameter, body mass index, and waist to hip ratio are 0.615, 0.531, 0.505, and 0.522 respectively).
Fig. 2.
The receiver-operating characteristic analysis curve to determine the value of anthropometric indices for discriminating metabolic syndrome (METs) from non-METs status (the area under the curve for wrist diameter, waist diameter, body mass index, and waist to hip ratio are 0.607, 0.755, 0.689, and 0.748 respectively).
4. Discussion
Anthropometric parameters have been well proved as decisive measures related to cardio-metabolic risk in large cohort studies such as the well-known Framingham survey20 and the World Health Organization guidelines.21 In this regard, increased BMI, increased waist circumference, and increased waist to hip ratio have all been shown to be associated with increased risk for both CAD and METs. However, in this study, those parameters were not different among CAD and non-CAD groups, nor could discriminate CAD subjects. One explanation might be our ‘point’ measurement of those indices rather than measuring them during a time course. Moreover, the role of such parameters is best considered in the context of other known risk factors as a whole rather than in parts.
Some recent evidences have been focused on the usefulness of measuring WrC for predicting risk of CVDs.22, 23 In fact, these evidences emphasize more on association between WrC and metabolic risk subgroups such as obesity, insulin resistance, and METs; and the independent role of WrC in predicting CAD risk has not been clearly determined. The present study attempted to examine whether more WrC might predict higher risk for CAD and METs. At the first step and univariately, those with CAD were shown to have more WrC. However, using multivariate regression modeling or by ROC curve analysis, that univariate relationship was not proved as predictive. Besides, we could find significant association between WrC and patients’ atherosclerotic lipid profile; namely higher triglyceride and cholesterol, and lower HDL-C level. Therefore, the relationship between the presence of CAD and higher WrC might be indirectly mediated by higher levels of triglyceride and cholesterol and lower levels of HDL-explaining non-significant weight of WrC for predicting CAD while being slightly associated with the severity of CAD. Additionally, as another description for why WrC was not found to be a predictor of CAD, we may have involved lower risk CAD patients (with lower Framingham risk scores) in our study; and a good issue for future studies could be looking for the possible role of WrC among higher risk patients with CAD.
On the other hand, according to the recent literature, it has been demonstrated by Tatar BT24 that WrC is positively correlated with the level of insulin resistance, weight, and BMI, as well as neck, waist and hip circumferences, and waist to hip ratio; but negatively correlated with HDL-C level. Similar associations have also been indicated among Iranian CAD patients in a study by Amini et al.22 where the WrC was positively associated with such cardio-metabolic risk factors as waist circumference, BMI, and LDL-C, but inversely associated with HDL-C. Capizzi et al.25 also found a close relationship between WrC, its bone component, and insulin resistance among overweight/obese children and adolescents. More importantly, there were some other interesting results: WrC was only independent factor to explain the variance of triglyceride levels among children; in fact, WrC was a better predictor than BMI for insulin resistance measures and for triglyceride levels in overweight/obese children and adolescents.25 In a study by Aykan et al.,26 WrC and patients’ occupation were reported as independent predictors of radial artery diameter which is important for transradial approach for coronary angiography. They found those patients with higher WrC or active outdoor job (compared to office workers) have higher radial artery diameter. Unfortunately, in the present study we did not ask about the participants’ occupation.
Those recent studies, along with the present investigation, attract attention to WrC as a possible novel associated index for developing dyslipidemia, obesity, or even insulin resistance, opening new perspectives for better understanding of CVDs. Point measurement of WrC and small number of the control subjects might be considered as limitations of this study. We also think ethnical differences might have a role and could be considered as a possible confounder for the association between the WrC and CAD. Moreover, by applying more quantitative measures for evaluation of CAD, more exact results might be achieved. In this regard, Aykan et al.27 recently used the Syntax score for grading the complexity of CAD and found a moderate correlation between the METs score and the Syntax score. Novel imaging modalities such as intravascular ultrasonography could also be used for better quantification of the amount of atheromatous coronary artery plaques. Finally, the study population is composed of relatively young patients, and we did not employ risk stratification by using any cardiovascular risk scoring system. Inclusion of older patients with higher risk scores may have yielded different results. Those issues might be addressed in further studies.
5. Conclusion
In conclusion, WrC as a novel anthropometric parameter is positively associated with level of serum triglyceride and cholesterol and negatively associated with HDL-C level. However, it cannot directly predict increased risk of CVDs. The association between WrC and CAD risk may be significant in some especial demographic and racial subgroups which requires more investigations.
Conflicts of interest
The authors have none to declare.
References
- 1.Oliveira G.B., Avezum A., Roever L. Cardiovascular disease burden: evolving knowledge of risk factors in myocardial infarction and stroke through population-based research and perspectives in global prevention. Front Cardiovasc Med. 2015;2(August):32. doi: 10.3389/fcvm.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth G.A., Huffman M.D., Moran A.E. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(October (17)):1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 3.Barquera S., Pedroza-Tobías A., Medina C. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46(July (5)):328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gaziano T.A., Bitton A., Anand S. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(February (2)):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreatsoulas C., Anand S.S. The impact of social determinants on cardiovascular disease. Can J Cardiol. 2010;26(August–September (suppl C)):8C–13C. doi: 10.1016/s0828-282x(10)71075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgers A., Ezzati M., Vander Hoorn S. Distribution of major health risks: findings from the Global Burden of Disease study. PLoS Med. 2004;1(October (1)):e27. doi: 10.1371/journal.pmed.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreira T.V., Staiano A.E., Harrington D.M. Anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in abiracial sample of men and women. Mayo Clin Proc. 2012;87(May (5)):452–460. doi: 10.1016/j.mayocp.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galassi A., Reynolds K., He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(October (10)):812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Lichtash C.T., Cui J., Guo X. Body adiposity index versus body mass index and other anthropometric traits as correlates of cardiometabolic risk factors. PLOS ONE. 2013;8(June (6)):e65954. doi: 10.1371/journal.pone.0065954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansilal S., Castellano J.M., Fuster V. Global burden of cardiovascular disease: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(December (suppl 1)):S1–S7. doi: 10.1016/S0167-5273(15)31026-3. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A. Accuracy of body mass index to diagnose obesity in the US adult population. Int J Obes (Lond) 2008;32(June (6)):959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czernichow S., Kengne A.P., Stamatakis E. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(September (9)):680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble R.E. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. West J Med. 2001;174(April (4)):240–241. doi: 10.1136/ewjm.174.4.240-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder S.J., Lichtenstein A.H., Pittas A.G. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res. 2009;50(September (9)):1917–1926. doi: 10.1194/jlr.P900033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visscher T.L., Seidell J.C. Time trends (1993–1997) and seasonal variation in body mass index and waist circumference in the Netherlands. Int J Obes Relat Metab Disord. 2004;28(October (10)):1309–1316. doi: 10.1038/sj.ijo.0802761. [DOI] [PubMed] [Google Scholar]
- 16.Aswathappa J., Garg S., Kutty K. Neck circumference as an anthropometric measure of obesity in diabetics. N Am J Med Sci. 2013;5(January (1)):28–31. doi: 10.4103/1947-2714.106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Noun L.L., Laor A. Relationship between changes in neck circumference and cardiovascular risk factors. Exp Clin Cardiol. 2006;11(Spring (1)):14–20. [PMC free article] [PubMed] [Google Scholar]
- 18.Kinjo M., Setoguchi S., Solomon D.H. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based US sample. J Clin Endocrinol Metab. 2007;92:4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- 19.Ferron M., Wei J., Yoshizawa T. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preis S.R., Massaro J.M., Hoffmann U. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95(August (8)):3701–3710. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Non Communicable Disease Country Profile 2011, World Health Organization report. Available from: http://www.who.int/nmh/publications/ncd_profiles2011/en/index.html.
- 22.Amini A., Soltanian N., Iraj B. Association of wrist circumferences with cardio metabolic risk factors. J Pak Med Assoc. 2012;62(March (3 suppl 2)) S34–6. [PubMed] [Google Scholar]
- 23.Mohebi R., Mohebi A., Sheikholeslami F. Wrist circumference as a novel predictor of hypertension and cardiovascular disease: results of a decade follow up in a West Asian cohort. J Am Soc Hypertens. 2014;8(November (11)):800–807. doi: 10.1016/j.jash.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Tatar B.T. Neck and wrist circumferences propose a reliable approach to qualify obesity and insulin resistance. Med-Sci. 2014;3(1):1013–1025. [Google Scholar]
- 25.Capizzi M., Leto G., Petrone A. Wrist circumferences is a clinical marker of insulin resistance in overweight and obese children and adolescents. Circulation. 2011;123(April (16)):1757–1762. doi: 10.1161/CIRCULATIONAHA.110.012898. [DOI] [PubMed] [Google Scholar]
- 26.Aykan A.C., Hatem E., Kalaycıoğlu E. Prediction of radial artery diameter in candidates for transradial coronary angiography: is occupation a factor? Turk Kardiyol Dern Ars. 2015;43(July (5)):450–456. doi: 10.5543/tkda.2015.75002. [DOI] [PubMed] [Google Scholar]
- 27.Aykan A.C., Gül İ., Kalaycıoğlu E. Is metabolic syndrome related with coronary artery disease severity and complexity: an observational study about IDF and AHA/NHLBI metabolic syndrome definitions. Cardiol J. 2014;21(3):245–251. doi: 10.5603/CJ.a2013.0126. [DOI] [PubMed] [Google Scholar]