Abstract
Historically, higher levels of serum testosterone were presumed deleterious to the cardiovascular system. In the last two decades, studies have suggested that low testosterone levels are associated with increased prevalence of risk factors for cardiovascular disease (CVD), including dyslipidemia and diabetes. This is a cross sectional study. The aim of our study was to determine the relationship between serum testosterone levels and angiographic severity of coronary artery disease (CAD). Serum testosterone levels were also correlated with flow mediated dilation of brachial artery (BAFMD) – an indicator of endothelial function. Consecutive male patients, aged 40–60 years, admitted for coronary angiography (CAG) with symptoms suggestive of CAD, were included in the study. Out of the 92 patients included in the study, 32 patients had normal coronaries and 60 had CAD on coronary angiography. Severity of CAD was determined by Gensini coronary score. The group with CAD had significantly lower levels of total serum testosterone (363 ± 147.1 vs 532.09 ± 150.5 ng/dl, p < 0.001), free testosterone (7.1215 ± 3.012 vs 10.4419 ± 2.75 ng/dl, p < 0.001) and bioavailable testosterone (166.17 ± 64.810 vs 247.94 ± 62.504 ng/dl, p < 0.001) when compared to controls. Adjusting for the traditional risk factors for CAD, a multiple linear regression analysis showed that low testosterone was an independent predictor of severity of CAD (β = −0.007, p < 0.001). This study also showed that levels of total, free and bioavailable testosterone correlated positively with BAFMD %.
Abbreviations: FBS, fasting blood sugar; BMI, body mass index; BAFMD, brachial artery flow mediated dilatation; CAD, coronary artery disease; CAG, coronary angiography; CVD, cardiovascular disease; CLIA, chemiluminescence; DALYS, disability adjusted life years; HDL-C, high density lipoprotein cholesterol; IHD, ischemic heart disease; LDL-C, low density lipoprotein cholesterol; PP, post-prandial blood sugar; SHBG, sex hormone binding globulin; TG, triglyceride
Keywords: Coronary artery disease, Gensini coronary score, Testosterone
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of death world wide and coronary artery disease (CAD) is the most significant contributor to this mortality.1 In addition, CVD contributes to significant morbidity and loss of disability adjusted life years (DALYS).1 A wealth of information exists on the association of traditional coronary risk factors with CAD.2, 3, 4 In the last two decades, there has been an emergence of several non-traditional risk factors which are associated with an inflammatory and pro-coagulant states in patients with CAD.5 Studies in hypogonadal males have shown an increased prevalence of the traditional coronary risk factors and CAD.6, 7 Furthermore, androgen replacement therapy has been shown to improve risk factor profile and symptoms of ischemia in hypogonadal males.8, 9, 10 Phillips et al.11 was the first to report an inverse correlation between angiographically proven CAD and testosterone levels after adjustment for adiposity and age. Yeap et al.12 found that the highest quartile for serum testosterone was associated with the lowest mortality and the lowest 2 quartiles with higher mortality. Additional studies too indicated an association between incidence of CAD and testosterone concentrations.13, 14, 15, 16, 17, 18, 19, 20 However, a recent study showed no significant correlation between serum testosterone levels and CAD severity.21 Nevertheless, testosterone levels were lower in CAD patients as compared to those with normal coronaries in this study. Above evidences suggest involvement of testosterone in the pathogenesis of CAD; that of a potentially protective role against development and progression of CAD. Additionally, patients with low testosterone levels have been found to have impaired endothelial function which may contribute to the increased cardiovascular risk in them.22, 23
In this cross sectional observational study, we aimed to determine the relationship between serum testosterone levels and angiographic severity of CAD in middle-aged Indian men. Secondary objective was to evaluate the association between serum testosterone levels and flow mediated dilation of brachial artery (BAFMD) – an indicator of endothelial function.
2. Materials and methods
This cross sectional study was performed in consecutive male patients aged 40–60 years admitted for coronary angiogram, in the department of Cardiology from 1/10/2013 to 30/9/2014. The exclusion criteria were: previous revascularization procedure, patients not giving consent, history of hypogonadism, patients of prostate cancer taking anti-androgens, liver/renal dysfunction, recent myocardial infarction, and recent or current infection. Written informed consent was obtained from all patients before sample collection and angiography. The study was approved by the Institutional Review Board and the Ethics committee of Christian Medical College Vellore. The study conformed to Good Clinical Practice and the ethical principles guiding human research. Out of all the patients who had angiography, 92 middle-aged males were recruited, and were divided into two groups according to coronary angiography (CAG) findings – 32 patients had normal coronaries and 60 had presence of CAD on CAG. Coronary artery disease was defined by the presence/absence of atherosclerotic plaques, regardless of the degree of diameter stenosis.
2.1. Baseline data
Detailed socio-demographic and clinical characteristics were recorded for each patient including age, gender, lifestyle, hypertension, diabetes, dyslipidemia, smoking, history of ischemic heart disease (IHD) and family history of IHD. Smoking habits were categorized as current smoker-individuals who currently smoked or quit <3 months prior to CAG or non-smoker. Weight and height were recorded after admission and body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
2.2. Sample collection and laboratory measurements
Blood samples were drawn in the morning, prior to the coronary angiogram and after 8 h overnight fasting. The blood glucose, serum lipid and creatinine levels were measured by enzymatic methods on an automated analyser. Serum fasting testosterone was measured by automated chemiluminescence method, using Siemens Immulite 2000 Xpi machine (Siemens, Munich, Germany) (normal levels for men aged 20–49 years: 270–1030 ng/dl; >50 years: 212–755 ng/dl).
Serum sex hormone binding globulin (SHBG) was measured by automated electro-chemiluminescence method, using Roche E170 Modular machine (Roche Diagnostics, Basel, Switzerland). Free and bio-available testosterone levels were calculated from serum total testosterone and SHBG using an online calculator.
2.3. Coronary angiography and Gensini coronary score
Severity and extent of CAD was determined by the Gensini scoring system, an index that assesses severity based on the number of vessels affected, localization of segment, and grading of the stenosis.24 Gensini score has been shown to correlate with CAD progression and overall as well as CVS mortality.25 The Gensini score was calculated as follows: grading of narrowing of a coronary artery determined by eyeballing as; 1 for ≤25% narrowing, 2 for 26–50% narrowing, 4 for 51–75% narrowing, 8 for 76–90% narrowing, 16 for 91–99% narrowing, and 32 for total occlusion. Next, this primary score is multiplied by a factor that takes into account the importance of position of lesion in the coronary arterial tree: five for the left main, 2.5 for proximal left anterior descending or proximal left circumflex artery and 1.5 for mid-region, 1 for the distal left anterior descending and 1 for mid-distal region of the left circumflex or right coronary artery. Gensini score was expressed as the sum of the scores for all three coronary arteries to evaluate the entire extent of coronary artery disease. Angiographic scoring was done by cardiologist who was unaware of biochemistry values in order to avoid bias.
2.4. BAFMD assessment
The examination was carried out in a quiet air conditioned room with patient in a supine position. A longitudinal section of the brachial artery was analyzed with B mode ultrasound using a Philips iE 33 Ultrasound machine and a linear array transducer (Philips Medical Systems, Andover, MA, USA). After baseline measurement, a cuff which was placed above the transducer position, was inflated to supra systolic pressure to produce ischemia in the forearm. The cuff was deflated after 5 min. BAFMD was calculated as percentage increase in diameter from baseline to maximum value which is obtained after cuff deflation, BAFMD % = [(diameter, maximum-diameter, baseline)/diameter, baseline] × 100.
2.4.1. Data collection and statistical analysis
Continuous variables were expressed as mean value ± standard deviation (SD). Categorical variables were presented as absolute values (percentages). The study population was divided into two groups according to angiographic finding: CAD subjects and normal coronaries (controls). Baseline characteristics of the two groups were noted and compared. Comparisons between the two groups pertaining to categorical data were done by chi-squared test or Fisher's exact test, wherever appropriate. Comparisons pertaining to quantitative data were done by t-tests. Correlations between serum total, free and bio-available testosterone levels and Gensini scores as well as BAFMD were assessed using Pearson's correlation test. Similarly, correlations between serum testosterone and traditional risk factors for CAD were also assessed. A multivariate regression analysis was done to assess whether serum testosterone was independently associated with CAD after adjusting for age, BMI, smoking history, hypertension, diabetes mellitus, dyslipidemia and history and treatment of IHD. A two sided p values <0.05 were considered significant for all tests. All statistical analyses were performed using SPSS 19.0 (IBM SPSS, Chicago, IL, USA).
3. Results
3.1. Baseline, clinical and biochemical characteristics
Baseline and clinical characteristics of the two study groups are displayed in Table 1. Age, diabetes mellitus and history of ischemic heart disease (IHD) differed among the two study groups significantly (all p < 0.05). As expected, CAD group had greater age, higher incidence of diabetes mellitus and history of IHD than controls. However, there were no statistically significant difference between the two groups for hypertension, obesity, smoking and dyslipidemia (p > 0.05). The values of fasting (FBS) and post-prandial sugars (PPBS) were significantly higher in patients with CAD when compared to those with normal coronaries (Table 2). Serum lipid levels did not differ between the two groups significantly.
Table 1.
Baseline and clinical characteristics of study population.
| Variables | CAD (N = 60) | Normal coronaries (N = 32) | p value |
|---|---|---|---|
| Age (mean ± standard deviation) | 54 ± 6 | 51 ± 7 | 0.014 |
| Obesity | 32 (53.3%) | 16 (50%) | 0.760 |
| Diabetes | 36 (60%) | 7 (21.9%) | <0.001 |
| Hypertension | 38 (63.3%) | 18 (56.2%) | 0.507 |
| Smoking | 25 (41.7%) | 10 (31.2%) | 0.327 |
| Dyslipidemia | 22 (36.7%) | 11 (34.4%) | 0.827 |
| History of ischemic heart disease | 11 (18.3%) | 1 (3.125%) | 0.007 |
p value is statistically significant as mentioned under Section 2.4.1.
Table 2.
Results of Lab values in patients with CAD and normal coronaries.
| Variables | CAD (N = 60) (mean ± S.D.) |
Normal coronaries (N = 32) (mean ± S.D.) |
p value |
|---|---|---|---|
| Creatinine (mg/dl) | 0.9742 ± 0.169 | 0.9928 ± 0.1888 | 0.63 |
| Total cholesterol (mg/dl) | 157.12 ± 38.483 | 155.22 ± 40.401 | 0.825 |
| Triglyceride (mg/dl) | 173.48 ± 95.162 | 140.78 ± 63.653 | 0.086 |
| HDL (mg/dl) | 37.15 ± 7.655 | 37.41 ± 7.987 | 0.880 |
| LDL (mg/dl) | 100.4 ± 36.851 | 97.97 ± 33.299 | 0.756 |
| Fasting blood sugar (mg/dl) | 136.42 ± 50.316 | 103.41 ± 15.206 | <0.001 |
| Post-prandial blood sugar (mg/dl) | 194.33 ± 85.291 | 130.88 ± 30.650 | <0.001 |
| Total testosterone (ng/dl) | 363 ± 147.174 | 532.09 ± 150.553 | <0.001 |
| Sex hormone binding globulin (nmol/l) | 35.5467 ± 11.442 | 37.3509 ± 10.502 | 0.450 |
| Free testosterone (ng/dl) | 7.1215 ± 3.012 | 10.4419 ± 2.75 | <0.001 |
| Bioavailable testosterone (ng/dl) | 166.17 ± 64.810 | 247.94 ± 62.504 | <0.001 |
| Brachial artery flow mediated dilation (%) | 11.660 ± 4.4 | 18.143 ± 3.301 | <0.001 |
p value is statistically significant as mentioned under Section 2.4.1.
The mean values of serum total, free and bioavailable testosterone were significantly lower inpatients with CAD when compared to controls (p < 0.001 for all).
CAD group also had significantly lower BAFMD values in comparison to subjects with normal coronaries (p < 0.001).
3.1.1. Correlations between serum total testosterone levels, CAD risk factors, Gensini score and BAFMD
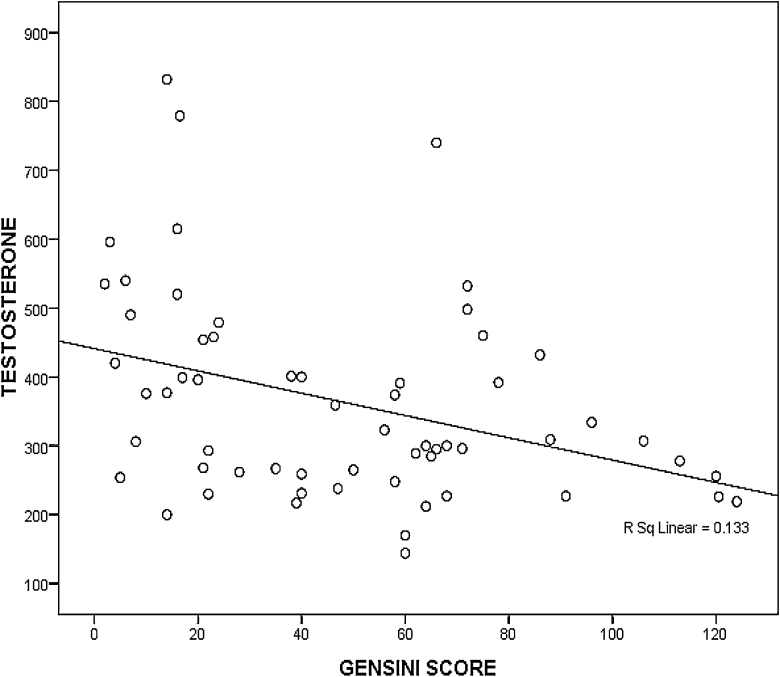
There was a significant negative correlation between serum total testosterone levels and Gensini scores (r = −0.481, p < 0.001) (Fig. 1 – scatter plot). The total testosterone concentrations also correlated negatively with the traditional risk factors for CAD like age, hypertension, BMI and serum cholesterol levels, but this correlation was statistically significant only for serum triglycerides (r = −0.224, p = 0.032) and diabetes – FBS (r = −0.303, p = 0.003) and PPBS (r = −0.284, p = 0.006) (Table 3). There was a significant positive correlation between BAFMD and serum total testosterone levels (r = 0.572, p < 0.001).
Fig. 1.
Scatter diagram showing a negative correlation between serum total testosterone levels and Gensini scores (r = −0.481, p < 0.001).
Table 3.
Association of serum total testosterone with CAD risk factors, Gensini score and brachial artery flow mediated dilation.
| Variables | Correlation coefficient | p value |
|---|---|---|
| Age | −0.088 | 0.405 |
| BMI (kg/m2) | −0.077 | 0.464 |
| Fasting blood sugar (mg/dl) | −0.303 | 0.003 |
| Post-prandial blood sugar (mg/dl) | −0.284 | 0.006 |
| Systolic B.P. (mmHg) | −0.042 | 0.691 |
| Disatolic B.P. (mmHg) | −0.143 | 0.174 |
| Total cholesterol (TC) (mg/dl) | −0.181 | 0.083 |
| Triglyceride (TG) (mg/dl) | −0.224 | 0.032 |
| HDL (mg/dl) | 0.074 | 0.481 |
| LDL (mg/dl) | −0.140 | 0.183 |
| Brachial artery flow mediated dilation (%) | 0.572 | 0.001 |
| Gensini score | −0.481 | 0.001 |
p value is statistically significant as mentioned under Section 2.4.1.
Similarly, serum free testosterone levels also showed statistically significant negative correlation with Gensini score (r = −0.480, p < 0.001) and statistically significant positive correlation with BAFMD (r = +0.525, p < 0.001). Bioavailable testosterone levels also correlated negatively with Gensini score (r = −0.524, p < 0.001) and positively with BAFMD (r = 0.547, p < 0.001).
3.1.2. Predictors of CAD severity
The influence of serum testosterone levels on CAD was assessed after adjustment for age, BMI, smoking history, hypertension, diabetes mellitus, dyslipidemia and history and treatment of IHD in a multivariate regression model. Age, smoking status and serum testosterone levels were found to be independent predictors for higher Gensini score or severity of CAD (Table 4).
Table 4.
Multiple linear regression analysis with Gensini score as the dependent variable.
| Variables | β coefficient | Lower limit | Upper limit | p value |
|---|---|---|---|---|
| Age (years) | 0.099 | 1.011 | 1.205 | 0.028 |
| BMI (kg/m2) | 0.097 | 0.777 | 1.059 | 0.218 |
| Diabetes | 0.943 | 0.785 | 8.387 | 0.119 |
| Dyslipidemia | 0.040 | 0.323 | 3.349 | 0.947 |
| Hypertension | 0.005 | 0.299 | 3.310 | 0.993 |
| Smoker | 1.166 | 0.951 | 10.824 | 0.050 |
| Serum total testosterone (ng/dl) | −0.007 | 0.989 | 0.997 | <0.001 |
p value is statistically significant as mentioned under Section 2.4.1.
4. Discussion
The present study, a cross sectional observational study, demonstrated a statistically significant negative correlation between severity of CAD and serum total, free and bioavailable testosterone levels in middle aged Indian men. This study also showed that levels of total, free and bioavailable testosterone correlated positively with BAFMD, an indicator of endothelial function. This association remained significant even after adjustment for well-established traditional cardiovascular risk factors. To the best of our knowledge, no study has been done till date to assess the relationship of serum testosterone levels with CAD severity and impairment of endothelial function in middle aged Indian men.
The findings are similar to previous studies done by Hu et al.19 and Li et al.26 that showed an inverse association between serum testosterone and Gensini score. However, some prior studies have failed to show a definite association between serum testosterone levels and CAD.20, 27, 28 Yet another study suggested a nonlinear association of testosterone levels with CAD: lower levels have a preventive effect on CAD, whereas higher values increase the risk of CAD.29 Some studies that showed a significant correlation between androgens and CAD severity included patients with acute coronary syndrome.16 Inclusion of these patients could confound the results since hormone levels might be affected by acute myocardial infarction. Hence, our study included only patients with stable CAD. The heterogeneous findings concerning association of testosterone and CAD likely reflect complexity of pathogenesis of CAD. Given this backdrop of contradictory evidences, findings of our study lend a stronger support toward a relevant impact of testosterone on CAD and endothelial function in males.
The levels of total, free and bioavailable testosterone were significantly lower in the CAD group as compared to that in patients with normal coronaries, on univariate analysis. Some baseline characteristics such as age, diabetes mellitus and BMI may affect testosterone levels.30, 31 In our study groups, BMI was similar. But there were significant differences in the mean age and prevalence of diabetes, and therefore we analyzed the association between serum testosterone and CAD in a multivariate regression model, after correcting for traditional risk factors. Results showed low serum testosterone to be an independent predictor for Gensini score or severity of CAD. The current study findings thus provide evidence for a role of testosterone in the pathogenesis of CAD along with traditional coronary risk factors. The study excluded patients with acute and chronic illnesses, and hence it is unlikely that these factors contributed to lower levels of testosterone in our study.
In the present study, the absolute value of BAFMD was significantly higher in the controls than in cases, which suggests that endothelial function was better inpatients with higher testosterone levels. Since all the traditional risk factors for CAD affect endothelial function adversely, the relationship between testosterone levels and BAFMD was also assessed after adjusting for all coronary risk factors using a regression model. The testosterone levels were associated with BAFMD %, independent of all confounding risk factors.
In our study total, free and bioavailable testosterone correlated negatively with age, but the correlation was significant only for bioavailable testosterone. A significant negative correlation was observed between total, free and bioavailable testosterone levels and blood sugars. Studies done by Stellato et al.,32 Selvin et al.,33 Haring et al.,34 and Oh et al.35 have shown a negative correlation between testosterone and diabetes or components of the metabolic syndrome. Our study also showed negative correlation between total, free and bioavailable testosterone levels with BMI, total cholesterol and low density lipoprotein (LDL) cholesterol and triglyceride levels (statistically insignificant). Earlier studies have found that testosterone levels correlate inversely with obesity and cholesterol levels.9, 36 Testosterone replacement therapy has been shown to improve BMI in clinical studies.37, 38 The testosterone levels correlated positively with HDL levels in our study, but it was also not statistically significant.
Exact mechanisms linking low testosterone and CAD have not been fully elucidated, and are likely to involve complex inter-connected processes including accelerated atherosclerosis, abnormal activation of inflammatory response, impaired vasomotion and endothelial dysfunction. Additional investigations are required to further clarify the relationship between low testosterone levels and CAD.
5. Limitations
The study had a few limitations. Firstly, cross-sectional design of this study made it impossible to determine whether CAD preceded or followed the decline in serum testosterone level. Hence, a causal relationship between CAD and testosterone could not be deduced. Since measurement of testosterone was single sample, fluctuations in serum testosterone values over time could not be assessed. As study was performed in a single center and of a relatively smaller sample size, a selection bias cannot be ruled out. There were significant differences pertaining to age, diabetes and history of IHD between the two groups which could have affected our final results. However, these differences do exist between CAD subjects and the general population and furthermore, a multivariate analysis performed after adjusting for these potential confounders showed independent association between CAD and testosterone levels. Even though testosterone levels were correlated with CAD severity, our patients were not followed up to monitor the final outcomes in terms of mortality. Longitudinal studies might provide valuable additional information regarding role of testosterone in the pathogenesis and outcomes of CAD.
6. Conclusion
Our study suggests low testosterone levels is an independent risk predictor for CAD and endothelial dysfunction. A randomized controlled trial of testosterone replacement in hypogonadal males and evaluation of its effects on endothelial function, risk factors for cardiovascular disease, CAD progression and outcomes, is needed to decide the role of testosterone as a modifiable risk factor. The present study is the first study done on an Indian population and it corroborates with studies done in other population groups. The exact mechanism underlying our results and its clinical relevance require further elucidation by pathogenetic and long-term clinical studies.
Conflicts of interest
The authors have none to declare.
Acknowledgement
This work was completed by a grant from ‘Fluid Research Grant Project’ of Christian Medical College, Vellore.
References
- 1.Murray C.J., Lopez A.D. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.James P.T., Leach R., Kalamara E. The worldwide obesity epidemic. Obes Res. 2001;9(Suppl 4) doi: 10.1038/oby.2001.123. 228S–233S. [DOI] [PubMed] [Google Scholar]
- 3.Kearney P.M., Whelton M., Reynolds K. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R., Joshi P., Mohan V. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 5.Balagopal P.B., de Ferranti S.D., Cook S. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 6.English K.M., Mandour O., Steeds R.P. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 7.Shores M.M., Matsumoto A.M., Sloan K.L., Kivlahan D.R. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 8.Heufelder A.E., Saad F., Bunck M.C. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 9.Traish A.M., Haider A., Doros G. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English K.M., Steeds R.P., Jones T.H. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 11.Phillips G.B., Pinkernell B.H., Jing T.Y. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- 12.Yeap B.B., Alfonso H., Chubb S.A. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–E18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 13.Dobrzycki S., Serwatka W., Nadlewski S. An assessment of correlations between endogenous sex hormone levels and the extensiveness of coronary heart disease and the ejection fraction of the left ventricle in males. J Med Investig. 2003;50:162–169. [PubMed] [Google Scholar]
- 14.Zhao S.P., Li X.P. The association of low plasma testosterone level with coronary artery disease in Chinese men. Int J Cardiol. 1998;63:161–164. doi: 10.1016/s0167-5273(97)00295-7. [DOI] [PubMed] [Google Scholar]
- 15.Akishita M., Hashimoto M., Ohike Y. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–236. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Alkamel A., Shafiee A., Jalali A. The association between premature coronary artery disease and level of testosterone in young adult males. Arch Iran Med. 2014;17:545–550. [PubMed] [Google Scholar]
- 17.Rosano G.M., Sheiban I., Massaro R. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–182. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 18.Morgentaler A. Testosterone deficiency and cardiovascular mortality. Asian J Androl. 2015;17:26–31. doi: 10.4103/1008-682X.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X., Rui L., Zhu T. Low testosterone level in middle-aged male patients with coronary artery disease. Eur J Intern Med. 2011;22:e133–e136. doi: 10.1016/j.ejim.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Kabakci G., Yildirir A., Can I. Relationship between endogenous sex hormone levels, lipoproteins and coronary atherosclerosis in men undergoing coronary angiography. Cardiology. 1999;92:221–225. doi: 10.1159/000006977. [DOI] [PubMed] [Google Scholar]
- 21.Durukan A.B., Gurbuz H.A., Tanalp A.C. The association between serum testosterone levels and coronary artery disease in middle-aged men. Kardiochirurgia i Torakochirurgia Polska. 2013;10:211–215. [Google Scholar]
- 22.Empen K., Lorbeer R., Dörr M. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012;32:481–486. doi: 10.1161/ATVBAHA.111.232876. [DOI] [PubMed] [Google Scholar]
- 23.Akishita M., Hashimoto M., Ohike Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–1034. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 24.Ringqvist I., Fisher L.D., Mock M. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Investig. 1983;71:1854–1866. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gensini G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 26.Li L., Guo C.Y., Jia E.Z. Testosterone is negatively associated with the severity of coronary atherosclerosis in men. Asian J Androl. 2012;14:875–878. doi: 10.1038/aja.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett-Connor E., Khaw K.T. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 28.Arnlöv J., Pencina M.J., Amin S. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;145:176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 29.Fallah N., Mohammad K., Nourijelyani K. Nonlinear association between serum testosterone levels and coronary artery disease in Iranian men. Eur J Epidemiol. 2009;24:297–306. doi: 10.1007/s10654-009-9336-9. [DOI] [PubMed] [Google Scholar]
- 30.Al Hayek A.A., Khader Y.S., Jafal S. Prevalence of low testosterone levels in men with type 2 diabetes mellitus: a cross-sectional study. J Family Community Med. 2013;20:179–186. doi: 10.4103/2230-8229.122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlsson C., Barrett-Connor E., Bhasin S. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58:1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Stellato R.K., Feldman H.A., Hamdy O. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 33.Selvin E., Feinleib M., Zhang L. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 34.Haring R., Völzke H., Felix S.B. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–2031. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh J.Y., Barrett-Connor E., Wedick N.M. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 36.Haffner S.M., Mykkänen L., Valdez R.A. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77:1610–1615. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- 37.Mårin P., Holmäng S., Gustafsson C. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–251. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 38.Snyder P.J., Peachey H., Hannoush P. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]