Abstract
Background
The assessment of the IVC diameter is self explanatory for evaluation of the individuals’ volume status. Studies regarding IVC diameter estimation in normal individuals are scarce.
Aim
The present study aimed to define normal criteria of size and dynamics of the inferior vena cava (IVC) by M-mode echocardiography in normal individuals.
Methods
This was a prospective, single-center, observational study carried out at Sri Jayadeva Institute of Cardiovascular Sciences and Research between December 2011 and April 2014. A total of 4126 consecutive individuals were enrolled. Normal IVC diameter was measured both during inspiration and expiration by M-mode echocardiography in subcostal view.
Results
The IVC diameter varied from 0.46 to 2.26 cm in the study individuals. The IVC diameter ranged from 0.97 to 2.26 cm during expiration and from 0.46 to 1.54 cm during inspiration. A strong correlation was observed between IVC diameter and height, weight and BMI of the individuals, calculated using Pearson correlation. The correlation coefficients for expiratory and inspiratory IVC diameters as a function of BMI were 0.686 and 0.7, respectively.
Conclusions
Our findings corroborate the correlations between height, weight and BMI with IVC diameter. Future studies could be focused to bring about a steadfast formula for calculating IVC diameter based on demographic parameters of an individual.
Keywords: Body mass index, Echocardiography, Expiration, Inferior vena cava, Inspiration
1. Introduction
Echocardiography assists complete estimation of cardiac function. Through echocardiography, inferior vena cava (IVC) can easily be depicted by a transthoracic, subcostal view.1
Assessment of IVC diameter in different phases of respiratory cycle is a consistent guide for assessment of volume status in the haemodynamically stable individuals. In a spontaneously breathing, healthy subject, cyclic variations in pleural pressure can cause variation of inferior vein cava diameter. The echocardiography can be used as a reliable tool for this purpose.2 The measurements and indices that could be useful are IVC diameter and IVC collapsibility index.
The IVC is a major collapsible vein; its diameter directly relates to right side cardiac functions.3 Literature suggests that IVC and collapsibility index of patients correlate with their intravascular volume.4 Moreover, it reproduces volume status more precisely than other parameters based on arterial system such as blood pressure, pulse rate, diameter of aorta, and others.5
Studies in the past have utilized IVC diameter in monitoring volume status in patients undergoing haemodialysis, in patients under mechanical ventilation in intensive care units,5, 6 in patients with severe sepsis, severe preeclampsia, acute circulatory failure, sub-arachnoid hemorrhage,7 and heart failure.8 However, the IVC diameter in normal population has not been quantified. As the use of the degree of collapsibility of the IVC in conjunction with IVC diameter offers more accurate non-invasive information regarding right atrial pressure.9, 10, 11 Its assessment in normal individuals poses a great importance in clinical assessment of the individuals. Thus we conducted this study to define normal criteria of size and dynamics of the IVC based on age, height, weight and body mass index (BMI), by M-mode echocardiography in normal individuals.
2. Methods
2.1. Study design and patient population
This was a prospective, single-center, observational study in which 4126 consecutive individuals were enrolled at Sri Jayadeva Institute of Cardiovascular Sciences and Research between December 2011 and April 2014. The study complies with Declaration of Helsinki and was approved by the institutional ethics committee.
Individuals eligible for inclusion were healthy, above the age of 18 years and had structurally normal heart with normal left ventricular ejection fraction (LVEF). In all the individuals’ clinical examination, ECG and chest X-ray were normal. The study population did not have any other clinical, electrocardiographic, or echocardiographic evidence of increased right atrial pressure (RAP). Right atrium and right ventricle (RV) were normal in size and RV contractility was normal. There was no regional wall motion abnormality in any of the individuals. Subjects were excluded if they had ischemic heart disease (IHD), IHD combined with LV dysfunction or decompensated heart failure, rheumatic heart disease, mitral regurgitation, mitral stenosis, aortic stenosis, aortic regurgitation, chronic lung disease, constrictive cardiomyopathy, restrictive cardiomyopathy and dilated cardiomyopathy.
2.2. Study methods
Conventional echocardiography (HD7xe, Koninklijke Philips N.V., Amsterdam, Netherlands) was performed in all the individuals. The IVC diameter was visualized, with individuals in supine position, using subcostal 4 chamber view (midline, inferior to the xyphoid, angling to the right). The technique was performed using 2–4 MHz transducer. The transducer was placed immediately below the xiphisternum and 1–2 cm to the right of the midline, in such a way that the marker dot points toward the sternal notch. The cross-section image of IVC was visualized at the right atrial/hepatic vein/IVC junction and rotated counter-clockwise so that long axis view of IVC merging into right atrium was obtained. Once the 2-dimensional image of IVC entering into right atrium was acquired, then the M-mode line was placed through IVC, 1 cm caudal from its junction with hepatic vein, and M-mode tracing was attained. This ensured that we did not measure intrathoracic IVC during any part of the respiratory cycle. Individuals were asked to take several short sniffs during M-mode recording. Since, normal inspiration may not elicit the inspiratory response; brief sniffs are often required, as this leads to a rapid and distinct decrease in IVC size.12 In each of the individual, IVC diameter was recorded both during inspiration and expiration. The M-mode image was freezed, using calipers measurements of maximum and minimum diameter of IVC were made. All individuals were subsequently grouped as per their inspiratory IVC diameter and expiratory IVC diameter. Then the grouped individuals were correlated with their corresponding age, height, weight, and BMI.
2.3. Statistical analysis
The continuous variables were presented as mean, standard deviation and ranges. The correlation between IVC diameter and collapsibility index as a function of demographic parameters was calculated using Pearson correlation coefficient. A p-value of ≤0.05 was considered statistically significant. Scatter plots of expiratory and inspiratory IVC diameter of individuals against BMI and all data were analyzed using the Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) program, version 15.
3. Results
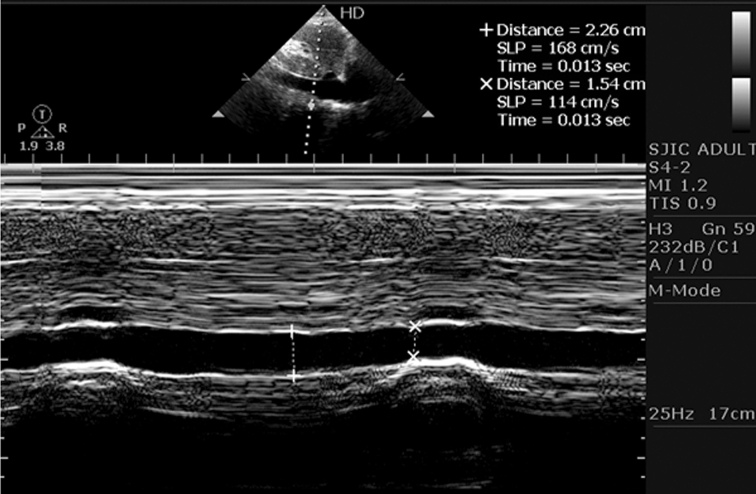
Out of 4126 individuals enrolled for the study, 2336 (56.62%) were male. During inspiration, the IVC caliber decreased in every individual. The decrease was quite variable and dependent on individual characteristics. Expiration led to increase in the IVC diameter in all individuals. Fig. 1 demonstrates echocardiography image of changes in IVC diameter of an individual during expiration and inspiration. During inspiration, IVC diameter value ranged from 0.46 to 1.54 cm, and during expiration it ranged between 0.97 and 2.26 cm. The demographics of individuals are outlined in Table 1. The mean age of individuals was 38.16 years. The average BMI was 22.90 kg/m2, which ranged between 14.19 and 36.84 kg/m2.
Fig. 1.
Echocardiography image showing changes in IVC diameter during expiration and inspiration in an individual.
Table 1.
Demographics of the individuals.
|
N = 4126 |
Female (n = 1790) |
Male (n = 2336) |
||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| IVC diameter (expiratory) (cm) | 1.69 ± 0.37 | 0.97–2.26 | 1.64 ± 0.38 | 0.97–2.23 | 1.74 ± 0.35 | 1.06–2.26 |
| IVC diameter (inspiratory) (cm) | 1.04 ± 0.22 | 0.46–1.54 | 1.00 ± 0.23 | 0.46–1.50 | 1.08 ± 0.21 | 0.52–1.54 |
| Age (years) | 38.16 ± 5.50 | 28–68 | 38.19 ± 5.38 | 28–65 | 38.13 ± 5.58 | 28–68 |
| Height (m) | 1.64 ± 0.08 | 1.32–1.87 | 1.61 ± 0.08 | 1.38–1.76 | 1.65 ± 0.08 | 1.32–1.87 |
| Weight (kg) | 62.58 ± 13.13 | 31.07–97.59 | 59.30 ± 12.99 | 33.64–88.40 | 65.10 ± 12.67 | 31.07–97.59 |
| BMI (kg/m2) | 22.90 ± 3.64 | 14.19–36.84 | 22.32 ± 3.75 | 14.19–36.84 | 23.34 ± 3.49 | 14.55–35.42 |
IVC, inferior vena cava; BMI, body mass index.
The individuals, both males and females, were distributed as per expiratory IVC diameter into four groups: ≤1.20 cm, 1.21–1.50 cm, 1.51–2.00 cm, and ≥2.01 cm. The demographic characteristics of individuals as per expiratory diameter are detailed in Table 2. Likewise, the individuals were grouped as per inspiratory IVC diameter: ≤0.80 cm, 0.81–1.00 cm, 1.01–1.20 cm, and ≥1.21 cm. Table 3 depicts demographic characteristics of individuals as per inspiratory diameter.
Table 2.
Demographic characteristics of individuals as per expiratory IVC diameter.
| Expiratory IVC diameter (cm) | N = 4126 | IVC diameter (mean ± SD, cm) | Age (mean ± SD, years) | Height (mean ± SD, m) | Weight (mean ± SD, kg) | BMI (mean ± SD, kg/m2) |
|---|---|---|---|---|---|---|
| Female | ||||||
| ≤1.20 | 328 | 1.11 ± 0.05 | 38.48 ± 5.36 | 1.48 ± 0.04 | 39.05 ± 2.12 | 18.34 ± 2.60 |
| 1.21–1.50 | 472 | 1.36 ± 0.06 | 38.21 ± 5.66 | 1.59 ± 0.05 | 52.79 ± 4.37 | 20.81 ± 2.98 |
| 1.51–2.00 | 541 | 1.81 ± 0.09 | 38.17 ± 5.44 | 1.64 ± 0.03 | 64.32 ± 3.66 | 23.38 ± 1.92 |
| ≥2.01 | 449 | 2.13 ± 0.04 | 38.00 ± 5.03 | 1.66 ± 0.03 | 74.90 ± 3.92 | 25.55 ± 3.41 |
| Male | ||||||
| ≤1.20 | 201 | 1.10 ± 0.04 | 38.29 ± 5.41 | 1.53 ± 0.08 | 42.34 ± 4.62 | 19.23 ± 3.06 |
| 1.21–1.50 | 574 | 1.35 ± 0.05 | 38.07 ± 5.38 | 1.61 ± 0.05 | 54.65 ± 5.70 | 21.02 ± 2.88 |
| 1.51–2.00 | 869 | 1.82 ± 0.08 | 38.04 ± 5.55 | 1.66 ± 0.04 | 66.49 ± 4.48 | 23.04 ± 2.18 |
| ≥2.01 | 692 | 2.13 ± 0.03 | 38.25 ± 5.84 | 1.70 ± 0.08 | 78.62 ± 7.56 | 26.84 ± 1.96 |
IVC, inferior vena cava; BMI, body mass index.
Table 3.
Demographic characteristics of individuals as per inspiratory IVC diameter.
| Inspiratory IVC diameter (cm) | N = 4126 | IVC diameter (mean ± SD, cm) | Age (mean ± SD, years) | Height (mean ± SD, m) | Weight (mean ± SD, kg) | BMI (mean ± SD, kg/m2) |
|---|---|---|---|---|---|---|
| Female | ||||||
| ≤0.80 | 344 | 0.65 ± 0.05 | 38.51 ± 5.36 | 1.48 ± 0.04 | 39.04 ± 2.09 | 18.31 ± 2.54 |
| 0.81–1.00 | 487 | 0.89 ± 0.04 | 38.23 ± 5.70 | 1.60 ± 0.04 | 53.30 ± 3.55 | 20.92 ± 2.88 |
| 1.01–1.20 | 524 | 1.09 ± 0.04 | 38.11 ± 5.44 | 1.65 ± 0.03 | 64.99 ± 2.51 | 23.53 ± 1.84 |
| ≥1.21 | 435 | 1.29 ± 0.05 | 38.00 ± 4.96 | 1.67 ± 0.03 | 75.21 ± 3.57 | 25.61 ± 3.45 |
| Male | ||||||
| ≤0.80 | 237 | 0.65 ± 0.05 | 38.33 ± 5.31 | 1.53 ± 0.08 | 41.12 ± 4.54 | 18.95 ± 2.93 |
| 0.81–1.00 | 586 | 0.93 ± 0.04 | 38.19 ± 5.58 | 1.62 ± 0.04 | 55.46 ± 4.51 | 21.19 ± 2.76 |
| 1.01–1.20 | 842 | 1.12 ± 0.03 | 37.91 ± 5.44 | 1.67 ± 0.04 | 67.20 ± 3.29 | 23.17 ± 2.11 |
| ≥1.21 | 671 | 1.30 ± 0.06 | 38.30 ± 5.86 | 1.70 ± 0.08 | 78.99 ± 7.35 | 26.99 ± 1.71 |
IVC, inferior vena cava; BMI, body mass index.
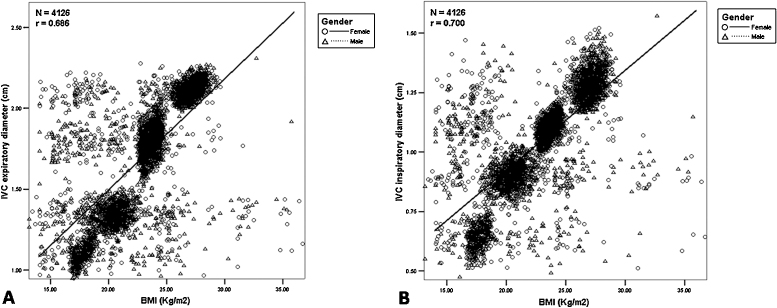
As expiratory and inspiratory IVC diameters were grouped in increasing order, so was the raise in height, weight and thus BMI. This indicated a probable correlation between all these characteristics and IVC diameter. Thus, Pearson's correlation was performed. Table 4 shows correlation of inspiratory IVC diameter with demographic characteristics. IVC diameter was found to be unrelated to age, but strongly related to height, weight and BMI, in both males and females. Fig. 2(A) illustrates scatter plot of expiratory IVC diameter of individuals against BMI. A strong correlation (r = 0.686) was observed. Similarly, Fig. 2(B) illustrates scatter plot of inspiratory IVC diameter of individuals against BMI, indicating a strong correlation (r = 0.7).
Table 4.
Correlation of inspiratory IVC diameter with demographics of the individuals.
| Female |
Male |
|||
|---|---|---|---|---|
| Pearson's r-value | p-value | Pearson's r-value | p-value | |
| Age (years) | −0.032 | 0.172 | −0.001 | 0.952 |
| Height (m) | 0.814* | <0.001 | 0.623* | <0.001 |
| Weight (kg) | 0.952* | <0.001 | 0.886* | <0.001 |
| BMI (kg/m2) | 0.674* | <0.001 | 0.710* | <0.001 |
IVC, inferior vena cava; BMI, body mass index.
Indicates correlation is significant at 0.01 level (2-tailed).
Fig. 2.
(A) Scatter plot of the expiratory diameter of the inferior vena cava (IVC) against body mass index (BMI) of all individuals; a strong correlation (r = 0.686) was observed; (B) Scatter plot of the inspiratory diameter of the inferior vena cava (IVC) against body mass index (BMI) of all individuals; a strong correlation (r = 0.700) was observed.
The average collapsibility index was 0.38. It was computed by the formula [(maximum IVC diameter − minimum IVC diameter)/maximum IVC diameter]. There was no correlation (r = 0.0) between collapsibility index and BMI of the individuals.
4. Discussion
In the present study, IVC diameter of all the individuals, during inspiration as well as expiration, was categorized as per height, weight and BMI of individuals. A strong correlation was obtained between the IVC diameter and height, weight and BMI; such that an increased height, weight and BMI presented with increased IVC diameter, both during expiratory and inspiratory phases. The average expiratory and inspiratory diameters were 1.69 cm and 1.04 cm, respectively. Average height, weight and BMI of the individuals were 1.64 m, 62.58 kg, and 22.90 kg/m2, respectively. Maximum number of individuals (N = 1410) had expiratory IVC diameter between 1.51 and 2.00 cm; with average height of 1.66 m, weight of 65.66 kg and BMI of 23.17 kg/m2, simulating demographics of normal Indian population.
A previous study reported that >10% alteration in the IVC collapsibility index was observed with 2 kg weight decrease after haemodialysis. In such setting, collapsibility index was superior to dry-weight assessments in predicting adverse outcomes related with haemodialysis.13 This depicted the probability of correlation of IVC dimensions with demographics of individuals indicating a decrease in IVC size with decrease in weight. Another study by Taniguchi et al.14 demonstrated correlation between IVC diameter and body surface area (BSA) in patients undergoing right-heart catheterization or central venous catheter insertion. It reported that IVC diameter cutoff was smaller in smaller patients (low BSA) and larger in larger patients (high BSA); men had higher IVC diameter than women; IVC diameter was not related to age and collapsibility index was not related with BSA. The results of our study are similar to this study, manifesting the higher IVC diameter for individuals with higher BMI and vice versa. Similarly, our study also evinced no significant relation of age and collapsibility with BMI. On the contrary, a study has reported a decrease in IVC diameter with increasing age (r = −0.221); weak positive correlation with BMI (r = 0.332) and BSA (r = 0.238).15 Various studies have been reported that include a control group along with the patient group, i.e., as case–control study.16, 17 However, no study is available that involves only healthy individuals to find the IVC diameter normal ranges on the basis of baseline demographics.
The IVC caliber alters on change of volume status of an individual. In case of hypervolemia, the IVC dilates and becomes less elastic due to alterations in venous return with inspiration.18 Assuming that changes in systemic venous return are more marked in hypovolemic than in normovolemic conditions, the deviation in vena cava diameter could also be useful in recognizing patients who may be benefited from a volume load.19 On the other side, there have been various studies which demonstrate that changes in IVC do not strongly predict the fluid responsiveness of patients.20, 21 Nevertheless, measuring the IVC right away presents clinicians with useful information, specifically in the emergency department when evaluating patients with dyspnea of an unknown cause.22 Moreover, regular measurements of IVC diameter and collapsibility with respiration have been used in patients with shock to consistently guide fluid management decisions.23 Thus, evaluating IVC diameter in normal individuals becomes important to manage some emergency conditions relating to fluid status and RAP. Once the standard values of IVC diameter are known, the deviation in the IVC caliber will be of immense utility for timely diagnosis of the hemodynamic changes and their causes. A recent study has investigated IVC diameter in normovolemic, healthy children of up to 22 years of age. It demonstrated a positive correlation between age, height, weight, BMI and IVC diameter of the children.24 But a similar study for healthy adults has not been performed till date. To the best of our knowledge, this is the first study to investigate the relationship between IVC diameter variability and demographic characteristics in normal Indian adult population.
4.1. Study limitations
The patients were assumed to be euvolemic based on their clinical signs and presentation, yet there might have been chances of selection bias during enrolment.
5. Conclusion
In this study we found that the diameter of IVC ranged from 0.97 to 2.26 cm during expiration and from 0.46 to 1.54 cm during inspiration in normal Indian population. Any values outside this range should be considered abnormal, and needs further investigation and treatment accordingly. Our findings substantiate the explicit correlations between height, weight and body mass index with IVC diameter. Future studies could be focused to bring about a steadfast formula for calculating IVC diameter based on demographic parameters of an individual.
Conflicts of interest
The authors have none to declare.
References
- 1.Barbier C., Loubières Y., Schmit C. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensiv Care Med. 2004;30(9):1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 2.De Lorenzo R.A., Morris M.J., Williams J.B. Does a simple bedside sonographic measurement of the inferior vena cava correlate to central venous pressure? J Emerg Med. 2012;42(4):429–436. doi: 10.1016/j.jemermed.2011.05.082. [DOI] [PubMed] [Google Scholar]
- 3.Moreno F.L., Hagan A.D., Holmen J.R. Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol. 1984;53(4):579–585. doi: 10.1016/0002-9149(84)90034-1. [DOI] [PubMed] [Google Scholar]
- 4.Yanagawa Y., Nishi K., Sakamoto T. Early diagnosis of hypovolemic shock by sonographic measurement of inferior vena cava in trauma patients. J Trauma Acute Care Surg. 2005;58(4):825–829. doi: 10.1097/01.ta.0000145085.42116.a7. [DOI] [PubMed] [Google Scholar]
- 5.Dipti A., Soucy Z., Surana A. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am J Emerg Med. 2012;30(8) doi: 10.1016/j.ajem.2011.10.017. 1414–9.e1. [DOI] [PubMed] [Google Scholar]
- 6.Mandelbaum A., Ritz E. Vena cava diameter measurement for estimation of dry weight in haemodialysis patients. Nephrol Dial Transplant. 1996;11(suppl 2):24–27. doi: 10.1093/ndt/11.supp2.24. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Xu X., Ye S. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853. doi: 10.1016/j.ultrasmedbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Miller J.B., Sen A., Strote S.R. Inferior vena cava assessment in the bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;30(5):778–783. doi: 10.1016/j.ajem.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Pudil R., Tichy M., Praus R. NT-proBNP and echocardiographic parameters in patients with acute heart failure. Acta Med. 2007;50(1):51. [PubMed] [Google Scholar]
- 10.Stein J.H., Neumann A., Marcus R.H. Comparison of estimates of right atrial pressure by physical examination and echocardiography in patients with congestive heart failure and reasons for discrepancies. Am J Cardiol. 1997;80(12):1615–1618. doi: 10.1016/s0002-9149(97)00776-5. [DOI] [PubMed] [Google Scholar]
- 11.Kimura B.J., Shaw D.J., Agan D.L. Value of a cardiovascular limited ultrasound examination using a hand-carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol. 2007;100(2):321–325. doi: 10.1016/j.amjcard.2007.02.104. [DOI] [PubMed] [Google Scholar]
- 12.Grant E., Rendano F., Sevinc E. Normal inferior vena cava: caliber changes observed by dynamic ultrasound. Am J Roentgenol. 1980;135(2):335–338. doi: 10.2214/ajr.135.2.335. [DOI] [PubMed] [Google Scholar]
- 13.Brennan J.M., Ronan A., Goonewardena S. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1(4):749–753. doi: 10.2215/CJN.00310106. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi T., Ohtani T., Nakatani S. Impact of body size on inferior vena cava parameters for estimating right atrial pressure: a need for standardization? J Am Soc Echocardiogr. 2015;28(12):1420–1427. doi: 10.1016/j.echo.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Masugata H., Senda S., Okuyama H. Age-related decrease in inferior vena cava diameter measured with echocardiography. Tohoku J Exp Med. 2010;222(2):141–147. doi: 10.1620/tjem.222.141. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R., Bouldin J.M., Light R.P. Inferior vena cava diameter and left atrial diameter measure volume but not dry weight. Clin J Am Soc Nephrol. 2011;6(5):1066–1072. doi: 10.2215/CJN.09321010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zengin S., Al B., Genc S. Role of inferior vena cava and right ventricular diameter in assessment of volume status: a comparative study: ultrasound and hypovolemia. Am J Emerg Med. 2013;31(5):763–767. doi: 10.1016/j.ajem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Jardin F., Vieillard-Baron A. Springer; 2009. Ultrasonographic Examination of the Venae Cavae. Applied Physiology in Intensive Care Medicine; pp. 181–184. [DOI] [PubMed] [Google Scholar]
- 19.Feissel M., Michard F., Faller J.-P. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensiv Care Med. 2004;30(9):1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 20.Airapetian N., Maizel J., Alyamani O. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19(1):1–8. doi: 10.1186/s13054-015-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhl-Olsen P., Vistisen S.T., Christiansen L.K. Ultrasound of the inferior vena cava does not predict hemodynamic response to early hemorrhage. J Emerg Med. 2013;45(4):592–597. doi: 10.1016/j.jemermed.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Jang T., Aubin C., Naunheim R. Ultrasonography of the internal jugular vein in patients with dyspnea without jugular venous distention on physical examination. Ann Emerg Med. 2004;44(2):160–168. doi: 10.1016/j.annemergmed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Lanspa M.J., Grissom C.K., Hirshberg E.L. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock (Augusta, GA) 2013;39(2):155. doi: 10.1097/SHK.0b013e31827f1c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kathuria N., Ng L., Saul T. The baseline diameter of the inferior vena cava measured by sonography increases with age in normovolemic children. J Ultrasound Med. 2015;34(6):1091–1096. doi: 10.7863/ultra.34.6.1091. [DOI] [PubMed] [Google Scholar]