Abstract
Introduction
We aimed to investigate if thinner cortex of the Alzheimer's disease (AD)-signature region was related to clinical progression in patients with subjective cognitive decline (SCD).
Methods
We included 302 SCD patients with clinical follow-up (≥1 year) and three-dimensional T1 magnetic resonance imaging. We estimated AD-signature cortical thickness, consisting of nine frontal, parietal, and temporal gyri and hippocampal volume. We used Cox proportional hazard models (hazard ratios and 95% confidence intervals) to evaluate cortical thickness in relation to clinical progression to mild cognitive impairment (MCI) or dementia.
Results
After a follow-up of the mean (standard deviation) 3 (2) years, 49 patients (16%) showed clinical progression to MCI (n = 32), AD (n = 9), or non-AD dementia (n = 8). Hippocampal volumes, thinner cortex of the AD-signature (hazard ratio [95% confidence interval], 5 [2–17]) and various AD-signature subcomponents were associated with increased risk of clinical progression. Stratified analyses showed that thinner AD-signature cortex was specifically predictive for clinical progression to dementia but not to MCI.
Discussion
In SCD patients, thinner regional cortex is associated with clinical progression to dementia.
Keywords: Subjective cognitive decline, Cortical thickness, MRI, Cognitively normal, Cognitive complaint, Dementia, MCI, Alzheimer's disease cortical signature
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by behavioral changes and gradual decline of cognitive function and daily functioning [1]. Atrophy, particularly of the medial temporal lobe, is the magnetic resonance imaging (MRI) hallmark of dementia due to AD [2], [3]. It has been suggested that a specific pattern of regional atrophy in the temporal, parietal, and frontal gyri, which has been coined as the cortical AD-signature, shows changes very early in the disease process [4], [5]. Moreover, cortical thinning of the AD-signature region in cognitively normal elderly has been suggested to predict AD dementia due to as early as a decade before diagnosis [6].
Cognitively normal memory clinic patients who perceive subjective cognitive decline (SCD), are at a threefold increased risk of clinical progression to dementia [7], [8], [9], [10], [11]. In an effort to explore the earliest signs of AD, a framework has been proposed that provides a common concept and terminology for studying subjective experience of cognitive decline [12].
Memory clinic–based studies have demonstrated decreased gray matter volumes [13], [14], [15] and cortical thinning [16] in medial temporal regions in SCD compared with healthy controls. It is still unclear, however, whether such smaller brain structures are related to clinical progression over time in SCD patients. Therefore, we investigated whether baseline cortical thickness is related to incident clinical progression in patients with SCD in a tertiary referral center. More specifically, if thinner cortex of the AD-signature in cognitively intact patients with self-perceived memory complaints is associated with increased risk of mild cognitive impairment (MCI) or dementia over time.
2. Methods
2.1. Study population
We included 302 SCD patients from the Amsterdam Dementia Cohort [17], [18] according to the following inclusion criteria: availability of brain MRI and clinical follow-up (≥1 year). Patients were referred by a general practitioner or local hospital (according to Dutch healthcare system regulations) and subsequently visited our memory clinic between 2000 and 2012. At baseline, all patients underwent a standardized dementia screening, including extensive neuropsychological assessment, physical, and neurologic examination as well as laboratory tests and brain MRI. Patients were labeled as having SCD when they presented with cognitive complaints, and results of clinical assessments were within normal range. Patients were excluded if criteria for MCI, dementia, or any other neurologic or major psychiatric (e.g., major depression) disorders known to cause cognitive complaints were met (at baseline) during a multidisciplinary consensus meeting according to international research consensus criteria [12]. In addition, we offered patients a lumbar puncture for research purposes. We determined β-amyloid1–42 and total tau in cerebrospinal fluid (CSF) using sandwich enzyme-linked immunoassays (Innogenetics, Belgium) [19], [20].
Follow-up took place by annual visits to our memory clinic in which medical history, neuropsychological assessment, and general physical and neurologic examination were repeated. The primary outcome in this study was clinical progression, which was defined as progression to MCI, AD, or another type of dementia as diagnosed at follow-up by an interdisciplinary consensus meeting based on international diagnostic or research consensus criteria [21], [22], [23], [24], [25], [26]. In this study, for subjects who progressed to MCI, we further determined the MCI subtype (amnestic, multidomain, or nonamnestic) based on the neuropsychological evaluation at the time of clinical progression. Our neuropsychological test battery included tests that measured cognitive functioning in the domains of memory, attention, executive functioning, and language [17]. For the memory domain, we used the Dutch version of the Rey Auditory Verbal Learning Test (RAVLT) direct and delayed recall and visual association task A. For attention we used digit span forward, Trialmaking Test-A (TMT-A), and Stroop 1 and 2. For the executive function domain, we used Trialmaking Test-B (TMT-B), digit span backward, and Stroop color-word test. For the language domain, we used category fluency animals and visual association task naming. The medical ethics committee of the VU University Medical Center approved the study. All patients provided written informed consent for their clinical data to be used for research purposes.
2.2. MRI acquisition
Structural MRI was performed at the first visit to the memory clinic using 1.0 T (n = 182) Siemens Magnetom Impact (Siemens, Erlangen, Germany) and 3.0 T (n = 120) Signa HDxt (General Electric, Milwaukee, WI) scanners.
For cortical thickness estimations, three-dimensional (3D) T1-weighted images were acquired using the following sequences: magnetization-prepared rapid acquisition gradient-echo (MPRAGE) on 1.0 T (168 slices, matrix = 256 × 256, voxel size = 1 × 1 × 1.5 mm3, echo time = 7 ms, repetition time = 15 ms, inversion time = 300 ms, and flip angle, 15°) and Fast Spoiled Grass Sequence with Inversion Recovery-Prepared (IR-FSPGR) on 3.0 T (176 slices, matrix = 256 × 256, voxel size = 1 × 0.9 × 0.9 mm3, echo time = 3 ms, repetition time = 7.8 ms, inversion time = 450 ms, and flip angle 12°). In addition, the scan protocol included T2-weighted images and fluid attenuated inversion recovery. A standard circular head coil was used. Motion was restricted using expandable foam cushions. Scans with movement or any other image (reconstruction) artifacts were excluded (1.0 T, n = 5; 3.0 T, n = 7). T1-weighted images acquired on 3.0 T were corrected for gradient nonlinearity in all three directions.
2.3. Image analysis
Freesurfer (v5.3, Harvard, MA) was used to analyze cortical thickness (https://surfer.nmr.mgh.harvard.edu) in 3D T1-weighted images. Briefly, Freesurfer constructs models of the boundaries between gray and white matter as well as pial surface to estimate cortical thickness. The distance between these surfaces gives the vertex-wise cortical thickness of cortical areas (i.e., the perpendicular thickness at each location). Automated cortical parcellations were run using a default script template (recon-all) [27], [28], providing 34 cortical estimations per hemisphere (Killiany/Desikan atlas based) [28]. The output was visually inspected for segmentation errors. We evaluated whole-brain cortical thickness and cortical thickness in the AD-signature region, which consists of nine bilateral regions: angular, precuneus, supramarginal, superior frontal, superior parietal, temporal pole, inferior temporal, medial temporal, and inferior frontal cortex [4], [5].
Subsequently, we performed vertex-wise analyses. First, preprocessing using default settings (mris_preproc) was performed. Optimized Gaussian smoothing kernel was used for detecting focal abnormalities [29]. Next, group main effects were investigated in the context of a general linear model using a default design (Different Offset, Different Slope [DODS]), for visualization purposes it was thresholded on sig greater than 2.00 uncorrected. Analyses were adjusted for age, gender, scanner type (Nclasses = 2, Nvariables = 3). Significant brain region positions were described according to Right Anterior Superior (RAS) coordinate system with corresponding significance on log-scale.
In addition, hippocampal volumes were estimated using FMRIB Integrated Registration and Segmentation Tool (FIRST) (Functional Magnetic Resonance Imaging of the Brain [FMRIB] software library v5, Oxford, UK). The FIRST algorithm consists of a two-stage linear registration to achieve a robust and accurate alignment of subcortical volumes. First, it applies a registration between the 3D T1 images and the Montreal Neurological Institute 152 template. Second, using a subcortical mask, segmentation was done based on shape models and voxel intensities to obtain hippocampal volumes (normalized for head size using Structural Image Evaluation using Normalization of Atrophy cross-sectional (SIENAX)) for further statistical analyses. All registrations were visually inspected for errors [30].
2.4. Statistical analyses
Statistical analyses were performed with SPSS version 20.0.0 (IBM Corp, Armonk, NY). Comparisons between stable versus progressive patients of baseline demographic and clinical characteristics were analyzed with analyses of variance (ANOVA) for continuous variables and χ2 tests for discrete variables. Between group regional cortical thickness estimations were analyzed with ANOVA (covariates: age, sex, and scanner) with Bonferroni-adjustment for multiple comparisons. Cox proportional hazard models adjusted for age, gender, baseline mini-mental state examination (MMSE) score, and scanner type were used to investigate the risk of incident clinical progression (outcome measure) associated with baseline cortical thickness (in millimeters) and hippocampal volumes (in millimeters [3]). Estimations were inverted; thinner cortex represents therefore increased risk of clinical progression. Separate Cox proportional hazard models were performed for each cortical variable (continuous) as predictor (i.e., whole-brain cortical thickness, cortical thickness of the AD-signature, the nine AD-signature components, and hippocampal volumes). Subsequently, we evaluated the respective contributions of the nine individual AD-signature regions and hippocampal volumes using a forward stepwise model. In addition, we performed an additional exploratory analysis, stratified for clinical outcome (i.e., MCI, AD, other dementia).
Cortical thickness estimations are represented as the mean (standard deviation), whereas results on Cox proportional hazard models are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). For visualization purposes, we constructed Kaplan-Meier curves based on tertiles (low/medium/high cortical thickness).
3. Results
Demographic and clinical characteristics are presented in Table 1. There were 136 (54%) male SCD patients, with a mean (standard deviation) age of 62 (10) years, and MMSE of 28 (2). Forty-nine patients (16%) showed incident clinical progression to MCI (n = 32, 65%), dementia due to AD (n = 9, 18%), or other dementia subtypes (vascular dementia n = 4 [8%], behavioral variant frontotemporal dementia n = 3 [6%], and primary progressive aphasia n = 1 [2%]). Of the patients who progressed to MCI, n = 25 (79%) had an amnestic cognitive profile, n = 5 (15%) multidomain, and n = 2 (6%) nonamnestic. SCD patients with clinical progression were older, and more often apolipoprotein E (APOE) ε4 positive than stable patients. MMSE baseline scores were comparable between patients with and without clinical progression. Duration of follow-up, sex, education level, cardiovascular risk factors, systolic and diastolic blood pressure, family history of AD, and cerebrovascular disease were comparable between groups. Among those with available CSF data (68%), patients with clinical progression had lower CSF β-amyloid1–42, and higher tau than those who remained stable.
Table 1.
Demographic and clinical data
| Demographic and clinical variables | SCD stable (n = 253) | SCD progression (n = 49) | P value |
|---|---|---|---|
| Male/female (%male) | 136/117 (54%) | 27/22 (55%) | .64 |
| Age (y) | 61 (9) | 69 (6) | <.01 |
| Education (range 1–7)∗ | 5 (1) | 5 (1) | .32 |
| Scanner system (1 T/3T) | 151/102 | 31/18 | .84 |
| Clinical | |||
| Cardiovascular risk factors | |||
| Smoking, current (n (%) yes) | 30 (12%) | 5 (9%) | .81 |
| Diabetes (n (%) yes) | 23 (9%) | 3 (6%) | .41 |
| Hypertension (n (%) yes) | 52 (20%) | 15 (30%) | .13 |
| Blood pressure (systolic/diastolic mm Hg) | 139/84 | 147/84 | .37/.87 |
| Family history cardiovascular disease (n (%) yes) | 81 (32%) | 15 (31%) | .38 |
| Family history dementia (n (%) yes) | 100 (39%) | 22 (45%) | .06 |
| MMSE | 28 (2) | 28.0 (2) | .10 |
| APOE ε4 positive† | 80 (37%) | 22 (53%) | .05 |
| Follow-up time | 3 (2) | 4 (3) | .12 |
| Diagnosis at progression | NA | aMCI n = 25 (51%) | |
| mMCI n = 5 (10%) | |||
| naMCI n = 2 (4%) | |||
| Probable AD n = 9 (18%) | |||
| bvFTD n = 3 (6%) | |||
| VaD n = 4 (8%) | |||
| PPA n = 1 (2%) | |||
| CSF β-amyloid1-42‡ | 857 (237) | 705 (303) | <.01 |
| CSF total tau‡ | 268 (146) | 448 (258) | <.001 |
| Preclinical AD§ | |||
| Stage 0 (%) | n = 114 (64%) | n = 8 (29%) | <.001 |
| Stage I (%) | n = 20 (12%) | n = 4 (14%) | .44 |
| Stage II (%) | n = 12 (7%) | n = 10 (36%) | <.01 |
| SNAP (%) | n = 31 (18%) | n = 6 (21%) | .34 |
Abbreviations: AD, Alzheimer's disease; aMCI, amnestic MCI; APOE, apolipoprotein E; bvFTD, behavioral FTD; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; mMCI, multidomain MCI; MMSE, mini-mental state examination; NA, not applicable; naMCI, nonamnestic MCI; PPA, primary progressive aphasia, SCD, subjective cognitive decline; SD, standard deviation; SNAP, suspected non-Alzheimer's pathophysiology; VaD, vascular dementia.
NOTE. Data are presented as n (%) or mean (SD). Comparisons between groups were made with analyses of variance for continuous variables and χ2 tests for discrete variables. An AD biomarker profile was defined based on the following cutoffs Aβ42: 640 and tau: 375 ng/L.
According to the Verhage classification.
Fourteen percent missing data.
Thirty-two percent missing data.
According to the National Institute on Aging-Alzheimer's Association preclinical AD stages (2012).
Cortical thickness estimations are presented in Table 2. ANOVAs showed that SCD patients who showed clinical progression had a thinner medial temporal lobe and smaller hippocampus than those who remained stable.
Table 2.
Cortical thickness of SCD patients with and without clinical progression
| Cortical thickness (mm) | SCD stable (n = 253) | SCD progression to MCI/dementia (n = 49) | SCD progression to MCI (n = 32) | SCD progression to AD (n = 9) | SCD progression to non-AD (n = 8) |
|---|---|---|---|---|---|
| Whole-brain | 2.17 (0.31) | 2.04 (0.37) | 2.15 (0.30)∗ | 1.84 (0.27) | 1.80 (0.48)† |
| AD-signature | 2.39 (0.34) | 2.21 (0.39) | 2.34 (0.35) | 1.96 (0.31) | 2.05 (0.41) |
| Angular gyrus | 2.06 (0.35) | 1.93 (0.37) | 2.04 (0.34) | 2.04 (0.36) | 1.72 (0.37) |
| Precuneus | 1.95 (0.36) | 1.81 (0.39) | 1.98 (0.35)∗ | 1.89 (0.30) | 1.70 (0.39)† |
| Supramarginal | 2.22 (0.32) | 2.10 (0.35) | 2.19 (0.33) | 1.83 (0.33) | 1.93 (0.35) |
| Frontal superior | 2.34 (0.37) | 2.20 (0.40) | 2.30 (0.37) | 1.94 (0.36) | 2.00 (0.40) |
| Parietal superior | 1.80 (0.32) | 1.71 (0.40) | 1.85 (0.30)∗ | 1.71 (0.21) | 1.60 (0.40)† |
| Temporal pole | 3.38 (0.60) | 3.06 (0.65) | 3.24 (0.59) | 2.44 (0.59) | 2.97 (0.65) |
| Temporal inferior | 2.62 (0.32) | 2.44 (0.35) | 2.54 (0.30) | 2.13 (0.36) | 2.29 (0.35) |
| Medial temporal | 2.85 (0.42) | 2.56 (0.47)† | 2.69 (0.44) | 2.16 (0.46) | 2.44 (0.47) |
| Frontal inferior | 2.34 (0.29) | 2.21 (0.32) | 2.28 (0.30) | 1.98 (0.32) | 2.07 (0.32) |
| Hippocampus‡ | 7.18 (0.91) | 6.41 (0.92)† | 6.73 (0.97) | 5.99 (0.92) | 6.84 (0.85) |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
NOTE. Data are presented as the mean (standard deviation). Analyses of variance were used to investigate cortical thickness between groups (SCD stable, MCI, AD, and non-AD) with Bonferroni post hoc tests.
Significantly different between SCD progression to MCI and SCD progression to non-AD.
P < .05 significantly different between SCD progression to MCI, AD, or non-AD compared with SCD stable.
In cubic millimeters estimated with FIRST (FMRIB software library v5).
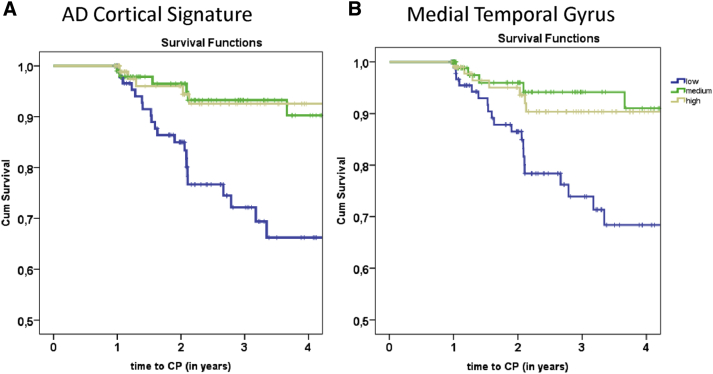
Cox proportional hazard analyses showed that adjusted for age, gender, baseline MMSE score, and scanner type, thinner cortical thickness of the AD-signature (HR [95% CI]: 5 [2–17]) and whole-brain (HR [95% CI]: 5 [2–15]) were associated with an increased risk of clinical progression (Table 3; Fig. 1A). If analyses were repeated with SCD patients older than age 60 years (n = 175, including n = 44 with clinical progression), results did not change essentially (data not shown). When we analyzed the individual cortical regions in separate models, thinner angular, supramarginal, superior parietal, precuneus, superior frontal, temporal poles, medial temporal gyri, and hippocampal volumes were associated with increased risk of clinical progression (Fig. 1B). When we additionally adjusted for APOE ε4 carrier status, results did not change essentially (data not shown). Subsequently, we ran a forward stepwise model, which identified that thinner medial temporal cortex (HR [95% CI]: 5 [2–11]) was most strongly associated with increased risk of clinical progression (Supplementary Table 1).
Table 3.
Cortical thickness hazard ratios of SCD patients with progression to MCI, AD, or non-AD dementia or progression to MCI/dementia
| AD-signature components | SCD progression to MCI/dementia (n = 49) | SCD progression to MCI (n = 32) | SCD progression to AD (n = 9) | SCD progression to non-AD (n = 8) |
|---|---|---|---|---|
| Whole-brain thickness | 5 (2–15)∗ | 1 (0–8) | 8 (0–88) | 14 (2–126)∗ |
| AD-signature | 5 (2–17)∗ | 2 (0–9) | 18 (1–284)∗ | 25 (1–433)∗ |
| Angular | 6 (2–20)† | 2 (0–9) | 15 (1–303) | 58 (2–2062)∗ |
| Supramarginal | 4 (1–14)∗ | 1 (0–7) | 6 (1–278)∗ | 29 (1–746)∗ |
| Parietal superior | 7 (1–38)∗ | 1 (0–6) | 126 (1–14,926)∗ | 6225 (6–67,700)∗ |
| Precuneus | 7 (2–25)† | 2 (0–12) | 10 (1–170) | 212 (4–11,636)∗ |
| Frontal inferior | 2 (0–5) | 1 (0–6) | 5 (0–61) | 14 (1–231) |
| Frontal superior | 3 (1–9)∗ | 1 (0–6) | 4 (0–44) | 16 (1–213)∗ |
| Temporal poles | 2 (1–4)∗ | 1 (1–3) | 6 (1–25)∗ | 2 (1–9) |
| Temporal inferior | 4 (1–12) | 2 (0–6) | 12 (1–133)∗ | 14 (1–206) |
| Temporal medial | 5 (2–11)∗ | 3 (1–8) | 14 (2–109)∗ | 11 (1–96)∗ |
| Hippocampus | 2 (1–2)∗ | 2 (1–3)∗ | 2 (1–4)∗ | 1 (0–3) |
Abbreviations: AD, dementia due to Alzheimer's disease; MCI, mild cognitive impairment; non-AD, dementia due to other dementia; SCD, subjective cognitive decline.
NOTE. Data are presented as hazard ratios with accompanying 95% confidence intervals in brackets.
P < .05.
P < .01.
Fig. 1.
Kaplan-Meier curves of the AD-signature cortical thickness (A) and medial temporal cortical thickness (B) in relation to clinical progression to MCI or dementia. Abbreviations: AD, Alzheimer's disease; CP, clinical progression.
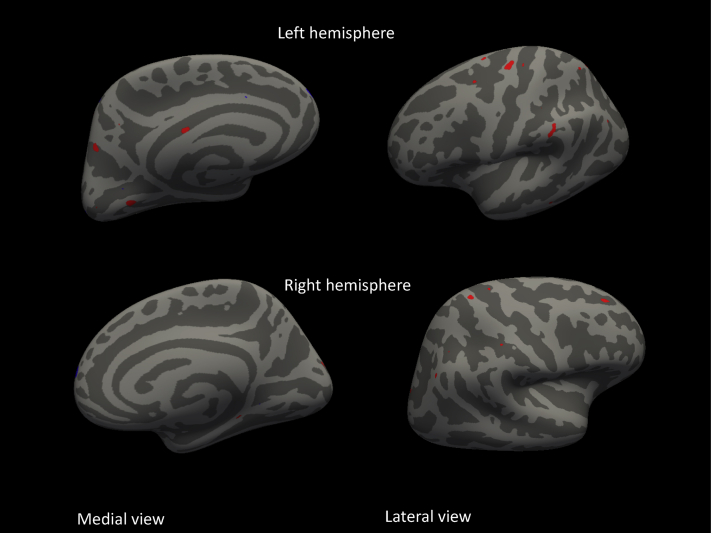
Subsequently, we explored the pattern of reduced cortical thickness using vertex-wise analyses. SCD patients who showed clinical progression had a thinner left anterior lingual cortex (coordinates: 21/−68/−28, sig = 3), left supramarginal gyrus (−63/−35/34, sig = 2), superior parietal (−16/−69/61, sig = 3), precuneus (27/−76/28, sig = 2), right parahippocampus (18/−32/−13, sig = 2) and cuneus (−23/96/3, sig = 2), middle frontal (−6/71/34, sig = 2), and postcentral (−12/27/70, sig = 2) (Fig. 2). In the right superior frontal cortex (12/68/14, sig = 4), a small area seemed to be thicker in SCD patients who showed clinical progression in comparison to stable patients.
Fig. 2.
Vertex-wise analyses of cortical thickness between SCD patients with and without clinical progression to MCI or dementia superimposed on an average pail surface. Red reflects thinner cortex, whereas blue reflects thicker cortex at baseline in SCD patients with clinical progression compared with patients without clinical progression.
In an additional set of exploratory analyses, we stratified for clinical outcome (i.e., progression to MCI, AD, or non-AD type of dementia). ANOVAs showed that SCD patients who progressed to non-AD dementia had thinner precuneus and superior parietal cortex compared with patients with stable SCD and SCD patients who progressed to MCI (Table 2). Cortical thickness or hippocampal volume of SCD patients who progressed to MCI or AD dementia did not differ significantly from stable SCD patients.
Cox proportional hazard models showed that for patients progressing to MCI, smaller hippocampal volumes were associated with clinical progression, whereas thinner medial temporal cortex was associated at trend level (P = .06) with clinical progression (Table 3). None of the other AD-signature regions were associated with progression to MCI. For clinical progression to AD (n = 9) and non-AD (n = 8) thinner cortex of the AD-signature was predictive for clinical progression. Within the regions constituting the AD-signature the inferior temporal cortex and temporal poles were more predictive for progression to AD, whereas thickness of the angular gyrus and precuneus were more predictive for progression to non-AD (Table 3).
4. Discussion
The main finding of the present study was that in patients with SCD, thinner temporal and parietal cortex is associated with increased risk of future clinical progression to dementia.
In the present study, we have investigated cognitively normal patients with SCD. Self-perceived decline could be a reflection of underlying neurodegeneration, but could also be related to many other factors, such as sleep disturbances, mental illness, substance use/abuse, personality traits (e.g., neuroticism), or normal aging. A former study has shown that cognitive complaints together with excessive worrying are associated with an increased risk of dementia [7]. This illustrates that although most SCD patients do not have AD, a small proportion of people may be at the earliest stages of AD, and these individuals might benefit from future preventive strategies or disease modifying therapies [12], [31]. Compared with community-dwelling elderly with complaints, SCD patients are a unique group to study AD because they actively seek help by visiting a memory clinic, but still have intact cognitive function.
AD develops gradually and the earliest brain changes occur years probably decades before clinical onset of the disease. Previous studies have suggested that a specific pattern of cortical thinning, which has been coined as the cortical AD-signature, could be present up to a decade before AD diagnosis [4], [5], [6]. In general, this proposed AD-signature was associated with an increased risk of clinical progression in SCD patients. When we further stratified analyses based on clinical outcome, we found that this association was driven by those patients progressing to dementia, but not by patients progressing to MCI. The predictive value seemed to be nonspecific for progression to AD or non-AD. The strongest predictor of progression to AD dementia was cortical thinning in the temporal and parietal gyri, whereas other regions, particularly the frontal lobe, were not associated with increased risk of progression to AD dementia.
We observed that thinner cortex of the AD-signature was also associated with progression to non-AD. Although it needs to be interpreted with caution, cortical patterns associated with increased risk to non-AD were somewhat more widespread compared with SCD patients with progression to AD dementia and seemed to cover multiple frontotemporoparietal regions within the AD-signature. Other studies on cortical thickness found pronounced temporoparietal and global atrophy in AD compared with frontotemporal dementia (FTD) [32], [33] and vascular dementia [34]. An explanation could be that the cortical AD-signature has been developed based on results from individuals that were on average 10 years older [4], [5], thus reflecting a more general neurodegenerative process with concomitant cerebral vascular disease [35], [36]. In addition, other studies have consistently demonstrated that cortical thinning during normal aging is predominantly found in the prefrontal cortex [36], [37], [38], suggesting that frontal gyri of the AD-signature are likely influenced by age.
In our study, the AD-signature was not associated with progression to MCI, despite most MCI patients were classified with predominant amnestic cognitive deficits. A former study demonstrated that thinner cortex of the AD-signature predicts progression to AD dementia in MCI [39], whereas in our study we extend the predictive value of the AD-signature in SCD patients with progression to AD. Our results illustrate that amnestic MCI is possibly a heterogeneous syndrome, which could be due to underlying AD, but likely also induced by other factors such as subclinical mental illness.
It could be that presymptomatic neural changes were already more extensive at the first visit in SCD patients with incident clinical progression to dementia. Therefore, future studies should compare the temporal evolution of cortical atrophy patterns across several presymptomatic dementia syndromes to identify unique disease signatures. Also, but beyond the scope of the present study, we have not taken into account the role of cognitive brain reserve or compensatory mechanisms. In a former study, it was demonstrated that education or cognitive activity could mediate associations between cortical thickness and time to AD progression [40], [41]. Effects of cognitive reserve were demonstrated for posterior cingulate, medial, and lateral temporal regions, but it remains to be clarified if cognitive reserve plays a role in the AD-signature.
Partly in line with the literature, we found thinner temporoparietal cortex in patients that were going to progress on average 3 years later [6]. Within the regions constituting the AD-signature region, the medial temporal lobe entailed the highest risk of future progression to MCI and AD, which is in line with former neuroimaging studies predicting progression to AD-dementia in patients with MCI [42], [43], [44], [45]. To date only a few neuroimaging studies have investigated brain structure in SCD patients and found decreased medial temporal volumes in patients with complaints compared with without complaints [13], [14], [16]. These former studies were all cross-sectional in nature; hence, they were not able to relate imaging abnormalities to incident clinical progression. Compared with other studies we found slightly thinner medial temporal cortex in our patients [6], [16], [46], but we furthermore showed that thinner medial temporal cortex had a fivefold increased risk of clinical progression. The predictive values of hippocampal volumes and medial temporal cortex were quite comparable, although the predictive value of the medial temporal lobe was slightly stronger. Moreover, hippocampal volume did not add any predictive value over the effect of cortical thickness of the medial temporal lobe [39], [47]. Of note, hippocampus volume was the only brain region associated with clinical progression to MCI, thus likely an early marker for AD. In contrast to previous SCD studies [13], [14], [15], [16], our exploratory vertex-wise analyses did not indicate parahippocampal or entorhinal abnormalities in patients who progressed, but strongest effects were found in the adjacent anterior lingual cortex, which is also located in the medial temporal lobe. Lingual gyri are related to explicit memory encoding, topographical orientation, and exhibit lesions in early AD neuropathologic stages [43], [48], [49], [50]. Using a more lenient threshold, vertex-wise analyses revealed thinner supramarginal, superior parietal, and parahippocampal gyri, which are all parts of the AD-signature, in the SCD progressive group.
The strength of this study was our thorough standardized workup. All patients underwent extensive investigations and showed no signs of (mild) cognitive impairment at baseline. In addition, subjects with a psychiatric diagnosis (e.g., major depression and schizophrenia) were not included. Several potential limitations merit attention. First, we investigated SCD in a memory clinic; hence, it is unknown to what extent our results can be extrapolated to community-dwelling elderly with subjective cognitive complaints. On the other side, because subjects visited a hospital, our results are clinically relevant as these patients do seek help. Second, MRI scans were acquired on two different systems, which might influence our results. Nonetheless, we deliberately controlled for scanner type in all statistical analyses, and our results robustly identified thinning of regional cortex in SCD patients at the risk of clinical progression. Third, although we had an average follow-up time of 3 years, which is rather long compared with other studies, it is likely that more patients will progress at later times because the earliest brain changes leading to AD probably occur as early as 20 years before clinical manifestation of the disease. Our current progression rates to dementia were slightly lower than the rates reported by a former study on patients with complaints and worries [7], but comparable to incidence rates in community-dwelling elderly [51], [52], [53]. Notwithstanding, we investigated a relatively young patient group, and our results illustrate that cognitively intact patients with cognitive complaints and thinner cortex in memory clinics might have an increased risk of AD. Finally, a limited number of SCD patients progressed to AD and non-AD dementia during the time of study. Nevertheless, we studied the AD-signature in a relatively large patient population, and our effect sizes were comparable to other single-center studies investigating cognitively intact elderly [5], [6].
5. Conclusions
Our findings suggest that reduced cortical thickness, especially of the temporal and parietal cortex, was associated with clinical progression to dementia in patients presenting with SCD at a memory clinic. The strongest predictor of clinical progression was cortical thinning in the medial temporal lobe, which is in line with neuropathologic Alzheimer-related disease staging models. Future studies using repeated neuropsychological assessment should investigate associations between regional cortical thickness and specific cognitive changes in the trajectory to MCI and dementia due to AD.
Research in Context.
-
1.
Systematic review: It has been suggested that atrophy of specific cortical areas, that is, a cortical Alzheimer's disease (AD)-signature, occurs up to 10 years before the onset of dementia in healthy elderly [6]. Memory clinic–based studies have demonstrated decreased gray matter and cortical thinning in medial temporal regions in subjects with cognitive complaints compared with healthy controls [16]. It is still unclear, however, whether these smaller brain structures in subjective cognitive decline patients are related to clinical progression over time.
-
2.
Interpretation: Our findings indicate that thinner cortex of the AD-signature was associated with an increased risk of incident clinical progression to dementia but not to mild cognitive impairment. Furthermore, we demonstrate that thinner temporal and parietal thickness was associated with progression to dementia.
-
3.
Future directions: Further research should investigate how cortical thinning spreads through the cortex in the trajectory to mild cognitive impairment and dementia due to AD and to what extent it is correlated with cognitive function.
Acknowledgments
Research of the VUmc Alzheimer center is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The VUmc Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc Fonds. The clinical database structure was developed with funding from Stichting Dioraphte. S.V. is supported by a research grant from Gieske-Strijbis Fonds. F.B. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Author contributions: F.B. contributed toward analysis and interpretation and critical revision of the manuscript for important intellectual content. M.R.B. and F.H. B. contributed toward acquisition of data, critical revision of the manuscript for important intellectual content. W.M.F. contributed toward study concept and design, analysis and interpretation, critical revision of the manuscript, and study supervision. P.S contributed toward study supervision and critical revision of the manuscript for important intellectual content. B.T. contributed toward analysis and interpretation and critical interpretation of the manuscript for important intellectual content. S.C.J.V. contributed toward acquisition of data, analysis and interpretation, and study concept and design. A.V. contributed toward analysis and interpretation. H.V contributed toward study concept and design, analysis and interpretation, and critical revision of the manuscript for important intellectual content.
Footnotes
All authors are affiliated with the Neuroscience Campus Amsterdam and VU University Medical Center, Amsterdam, The Netherlands.
Disclosures: S.C.J. Verfaillie, M.R. Benedictus, F.H. Bouwman, B. Tijms, and A. Versteeg report no disclosures. Dr F. Barkhof serves on the editorial boards of Brain, European Radiology, Radiology, Multiple Sclerosis, Neuroradiology, and Neurology and serves as a consultant for Bayer-Schering Pharma, Sanofi-Aventis, Biogen-Idec, EVA, Synthon BV, Merck-Serono, Jansen Alzheimer Immunotherapy, Novartis, Genzyme, and Roche. Dr Barkhof receives research support from the Dutch MS Society (EU-FP7). Dr Barkhof has received consulting fees or honoraria for the consultancy mentioned previously. Dr W. M. van der Flier has received research funding and speaker honorarium from Boehringer Ingelheim; all funds were paid to her institution. Dr P. Scheltens receives grant support (for the institution; the VUmc Alzheimer center) from GE Healthcare, Danone Research, and MERCK. In the past 2 years, he has received speaker's fees (paid to the institution) from Lilly, GE Healthcare, Lundbeck, Danone, and Jansen AI-Pfizer. Dr H. Vrenken has received funding for research projects from Pfizer, Merck-Serono, Novartis, and Teva and speaker honoraria from Novartis. All funds are paid directly to his institution.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.10.007.
Supplementary data
References
- 1.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet (London, England) 2016;6736:1–13. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack C.R., Jr., Petersen R.C., Xu Y.C., Waring S.C., O'Brien P.C., Tangalos E.G. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerson B.C., Bakkour A., Salat D.H. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakkour A., Morris J.C., Dickerson B.C. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson B.C., Stoub T.R., Shah R.C., Sperling R.A., Killiany R.J., Albert M.S. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessen F., Wiese B., Bachmann C., Eifflaender-Gorfer S., Haller F., Kölsch H. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 8.Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kölsch H. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 9.Schultz S.A., Oh J.M., Koscik R.L., Dowling N.M., Gallagher C.L., Carlsson C.M. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement. 2015;1:33–40. doi: 10.1016/j.dadm.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmand B., Jonker C., Hooijer C., Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 11.Van Harten A.C., Visser P.J., Pijnenburg Y.A. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Flier W.M., van Buchem M.A., Weverling-Rijnsburger A.W., Mutsaers E.R., Bollen E.L., Admiraal-Behloul F. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251:671–675. doi: 10.1007/s00415-004-0390-7. [DOI] [PubMed] [Google Scholar]
- 14.Hafkemeijer A1., Altmann-Schneider I., Oleksik A.M., van de Wiel L., Middelkoop H.A., van Buchem M.A. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3:353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saykin A.J., Wishart H.A., Rabin L.A., Santulli R.B., Flashman L.A., West J.D. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meiberth D., Scheef L., Wolfsgruber S., Boecker H., Block W., Träber F. Cortical thinning in individuals with subjective memory impairment. J Alzheimers Dis. 2015;45:139–146. doi: 10.3233/JAD-142322. [DOI] [PubMed] [Google Scholar]
- 17.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 18.Benedictus M.R., van Harten A.C., Leeuwis A.E., Koene T., Scheltens P., Barkhof F. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46:2661–2664. doi: 10.1161/STROKEAHA.115.009475. [DOI] [PubMed] [Google Scholar]
- 19.Mulder C., Verwey N.A., van der Flier W.M., Bouwman F.H., Kok A., van Elk E.J. Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem. 2010;56:248–253. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- 20.Zwan M., van Harten A., Ossenkoppele R., Bouwman F., Teunissen C., Adriaanse S. Concordance between cerebrospinal fluid biomarkers and [C]PIB PET in a memory clinic cohort. J Alzheimers Dis. 2014;41:801–807. doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 23.Román G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 24.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B., Van Der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D.H. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 28.Desikan R.S.1., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Lerch J.P., Evans A.C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 30.Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M.A. Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling R. Can we detect Alzheimer's disease 10 years before dementia and why would we want to? Alzheimers Dement. 2011;7:S805. [Google Scholar]
- 32.Du A.T., Schuff N., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130(Pt 4):1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartikainen P., Räsänen J., Julkunen V., Niskanen E., Hallikainen M., Kivipelto M. Cortical thickness in frontotemporal dementia, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis. 2012;29:1–18. doi: 10.3233/JAD-2012-112060. [DOI] [PubMed] [Google Scholar]
- 34.Kim C.H., Seo S.W., Kim G.H., Shin J.S., Cho H., Noh Y., Kim S.H. Cortical thinning in subcortical vascular dementia with negative 11C-PiB PET. J Alzheimers Dis. 2012;31:315–323. doi: 10.3233/JAD-2012-111832. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt R., Ropele S., Enzinger C., Petrovic K., Smith S., Schmidt H. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 36.Bakkour A., Morris J.C., Wolk D.A., Dickerson B.C. The effects of aging and Alzheimer's disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 38.Hurtz S., Woo E., Kebets V., Green A.E., Zoumalan C., Wang B. Age effects on cortical thickness in cognitively normal elderly individuals. Dement Geriatr Cogn Dis Extra. 2014;4:221–227. doi: 10.1159/000362872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickerson B.C., Wolk D.A. Biomarker-based prediction of progression in MCI: comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau. Front Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Querbes O., Aubry F., Pariente J., Lotterie J.A., Démonet J.F., Duret V. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132(Pt 8):2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins R., Joanette Y., Monchi O. The implications of age-related neurofunctional compensatory mechanisms in executive function and language processing including the new temporal hypothesis for compensation. Front Hum Neurosci. 2015;9:221. doi: 10.3389/fnhum.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spulber G., Niskanen E., MacDonald S., Kivipelto M., Padilla D.F., Julkunen V. Evolution of global and local grey matter atrophy on serial MRI scans during the progression from MCI to AD. Curr Alzheimer Res. 2012;9:516–524. doi: 10.2174/156720512800492486. [DOI] [PubMed] [Google Scholar]
- 43.Chételat G., Landeau B., Eustache F., Mézenge F., Viader F., de la Sayette V. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Risacher S.L., Saykin A.J., West J.D., Shen L., Firpi H.A., McDonald B.C. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.den Heijer T., van der Lijn F., Koudstaal P.J., Hofman A., van der Lugt A., Krestin G.P. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010;133(Pt 4):1163–1172. doi: 10.1093/brain/awq048. [DOI] [PubMed] [Google Scholar]
- 46.Möller C., Hafkemeijer A., Pijnenburg Y.A., Rombouts S.A., van der Grond J., Dopper E. Different patterns of cortical gray matter loss over time in behavioral variant FTD and AD. Neurobiol Aging. 2015;38:21–31. doi: 10.1016/j.neurobiolaging.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Hwang J., Kim C.M., Jeon S., Lee J.M., Hong Y.J., Roh J.H. Prediction of Alzheimer's disease pathophysiology based on cortical thickness patterns. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2015;2:58–67. doi: 10.1016/j.dadm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golby A., Silverberg G., Race E., Gabrieli S., O'Shea J., Knierim K. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128(Pt 4):773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi N., Kawamura M. Pure topographical disorientation—the anatomical basis of landmark agnosia. Cortex. 2002;38:717–725. doi: 10.1016/s0010-9452(08)70039-x. [DOI] [PubMed] [Google Scholar]
- 51.Roberts R., Knopman D.S. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ott A., Breteler M.M., van Harskamp F., Stijnen T., Hofman A. Incidence and risk of dementia. the Rotterdam Study. Am J Epidemiol. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 53.Fratiglioni L., De Ronchi D., Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.