Abstract
Cerebrovascular pathologies (CVPs) are common pathologies associated with age-related cognitive decline along with Alzheimer disease pathologies. The impact of CVP on the prevalence of dementia is increasingly being recognized. The goal of this review is to improve our understanding of the pathophysiological underpinnings and the multimodal magnetic resonance imaging and positron emission tomography imaging changes that are associated with the hallmarks of CVP. This knowledge will facilitate the development of early detection, intervention, and prevention strategies that may contribute to lowering the risk of dementia. In this review, we will first discuss currently known risk factors of CVPs including cardiovascular, lifestyle, genetic, sex differences, and head injury. Next, we will focus on the pathophysiology of CVPs and their impact on neurodegeneration and downstream cognitive impairment. Specifically, we will discuss three of the most common cerebrovascular lesions seen on MRI: white-matter hyperintensity, microbleeds, and infarcts. Finally, we will discuss the unanswered open questions in this field.
Keywords: Cerebrovascular, Pathophysiology, Imaging, Aging
1. Introduction
Cerebrovascular pathologies (CVPs) are one of the most prevalent pathologies in older adults [1], [2]. There is a monotonic age-related increase in the prevalence of cases with CVP, and it has been noted that there is detectable pathological evidence of CVP in 75%–90% of persons over age of 90 years [3]. The three main vessel disorders frequently underlying CVP are atherosclerosis (degenerative disorder of large- and medium-sized arteries), cerebral small-vessel disease, and cerebral amyloid angiopathy (CAA) [4]. All three disorders are related to cerebral infarction and hemorrhage. The pathological hallmarks of CVP are the presence of microvascular changes (white-matter hyperintensities [WMHs], microbleeds, and microinfarcts) and macrovascular changes (subcortical and cortical macroinfarcts) in the brain. With the advent of sophisticated magnetic resonance and PET imaging methodologies, many previously invisible cerebrovascular changes can now be detected using multimodal imaging techniques.
Recently, there has been renewed interest in the field of CVP research because it contributes significantly to the risk of dementia by lowering the threshold of dementia detection [5] and is one of the more preventable pathologies associated with cognitive impairment [6]. Increased interdisciplinary research efforts are currently being undertaken to improve our understanding of vascular contributions to cognitive impairment and mechanisms through which CVP can be targeted and prevented. In this review, we describe currently known risk factors and pathophysiology of CVPs and their impact on neurodegeneration and downstream cognitive impairment. Specifically, we will discuss three of the most common cerebrovascular lesions seen on MRI: WMH, microbleeds, and infarcts.
2. Risk factors for cerebrovascular pathologies
Based on neuropathology studies, about 30% of nondemented elderly subjects have CVP [7], [8], [9]. Although the strongest risk factor for the occurrence of CVP is age [10], [11], [12], [13], [14], [15], there are several other risk factors discussed in the following sections that are associated with increased risk of CVP.
2.1. Cardiovascular and lifestyle risk factors
Common cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, atrial fibrillation, and smoking are associated with greater WMH burden [16], infarcts [17], and microbleeds [11], particularly when they occur in midlife [18]. It has been shown that subjects with metabolic syndrome have twice the probability of presenting with WMH [19]. These common risk factors contribute to cerebrovascular changes through build-up of atherosclerotic plaque, lipohyalinosis, arteriolosclerosis, and fibrinoid necrosis [20], [21]. Healthy lifestyle behaviors specifically healthy diets and physical activity that are associated with lower cardiovascular disease have been associated with lower risk of CVP [22], [23].
2.2. Genetic risk
Studies investigating genetic risk factors for CVP aim to identify genes that effect risk of disease, influence outcome after a cerebrovascular event, and interact with various therapeutics [24]. Genome-wide association studies (GWASs) have yielded possible genetic associations with different cerebrovascular lesions. The odds of having ischemic stroke are increased patients with genetic variations in HDAC9 (related to large-vessel disease) and PITX2 and ZFHX3 (related to cardioembolism) [25]. The most common genetic risk factors for lobar microbleeds are APOE ε2 [26] and APOE ε4 [10] allele carrier status [13], [27]. Mutations associated with familial conditions including cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy [28], Dutch-type or Iowa-type CAA and APP and presenilin mutations in familial AD are also associated with microbleeds [29]. WMH is likely associated with many different genes as it has heterogeneous etiologies [24]. APOE genotype is a well-known genetic indicator of risk for Alzheimer disease and is also shown to be associated with cardiovascular risk factors [30]. Many other gene candidates have presented themselves as links to CVP through GWAS investigations of cerebrovascular lesions as endophenotypes. However, the diversity of pathologies contributing to these various imaging hallmarks of CVP makes it difficult to firmly identify genetic underpinnings to pathology [17].
2.3. Sex differences
Risks and effects of cardiovascular disease differ greatly between males and females. Premenopausal women appear to have fewer strokes than age-matched men; however, rate and severity of strokes in postmenopausal women surpasses those of age-matched men [28]. There is evidence that the regional distribution and contributing risk factors to WMH differs among older men and women and that WMH is more common in women [31]. The impact of sex on risks and effects of cardiovascular disease might be attributed to sex-specific conditions, for example, pregnancy or menopause; a disproportionate effect of a disease or condition on one sex; and distinct causes, manifestations, outcomes, or treatments that are sex dependent [32].
2.4. Head injury
Acute and chronic traumatic encephalopathies (CTEs) as a result of one or more incidents of traumatic brain injury are gaining public and scientific notice as significant contributors to cognitive impairment and dementia [33]. Following an acute insult, there can be severe vascular pathology including contusions, intracerebral hemorrhage, and vasoconstriction in response to blood products. Even in “closed head” injuries in which the skull remains intact or mild head trauma without readily apparent brain damage, there is injury to the vasculature and meninges due to the strain exerted on them by the impact [33]. The study of pathologies ensuing from head injuries is evolving; current and future work will attempt to characterize the epidemiology, in vivo biomarkers, and pathologies of various types of traumatic brain injuries [33], [34]. Although criteria for staging of CTE are still in progress, CTE pathology is being recognized largely as accumulation of tau pathology at the depths of the sulci and perivascularly, suggesting a connection between insult to vasculature and subsequent tauopathy [35]. Work has also begun to investigate possible therapeutic targets in the secondary immune reactions following vascular injury to ameliorate symptoms and progression of pathology [36].
3. Imaging hallmarks of cerebrovascular pathologies
Alzheimer disease has imaging biomarkers that are available for assessing the accumulation of amyloid β and tau pathologies; similarly several different cerebrovascular changes can be observed on imaging (Fig. 1). Although CT has been most commonly used clinically for assessing stroke, MRI is valuable in noncritical clinical assessments and research studies because it provides a more sensitive and detailed visualization of CVP damage to the brain. Fluid Attenuated Inversion Recovery (FLAIR) MRI and T2* MRI are the most common imaging sequences used to visualize ischemic cerebrovascular disease. In this section, we will describe the imaging hallmarks of CVP that are observed on MRI.
Fig. 1.
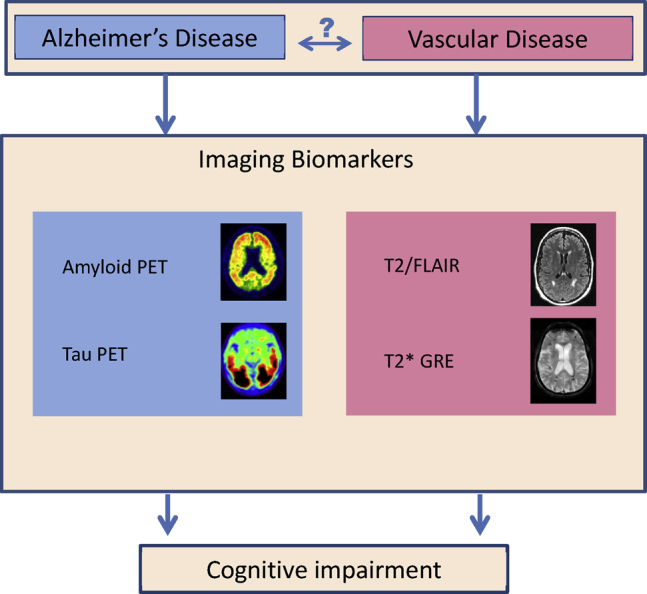
Alzheimer disease has positron emission tomorgraphy imaging biomarkers (left) that are available for assessing the accumulation of amyloid β and tau pathologies. Only surrogates of cerebrovascular pathology using imaging are possible. T2/FLAIR and T2* GRE magnetic resonancy imaging (right) provide surrogates of ischemic pathology and microhemorrhages, respectively. The relationship between Alzheimer disease and vascular disease in their contributions to cognitive impairment is still unknown. Abbreviations: FLAIR, Fluid Attenuated Inversion Recovery; GRE, gradient recalled echo.
3.1. White-matter hyperintensities
3.1.1. Imaging
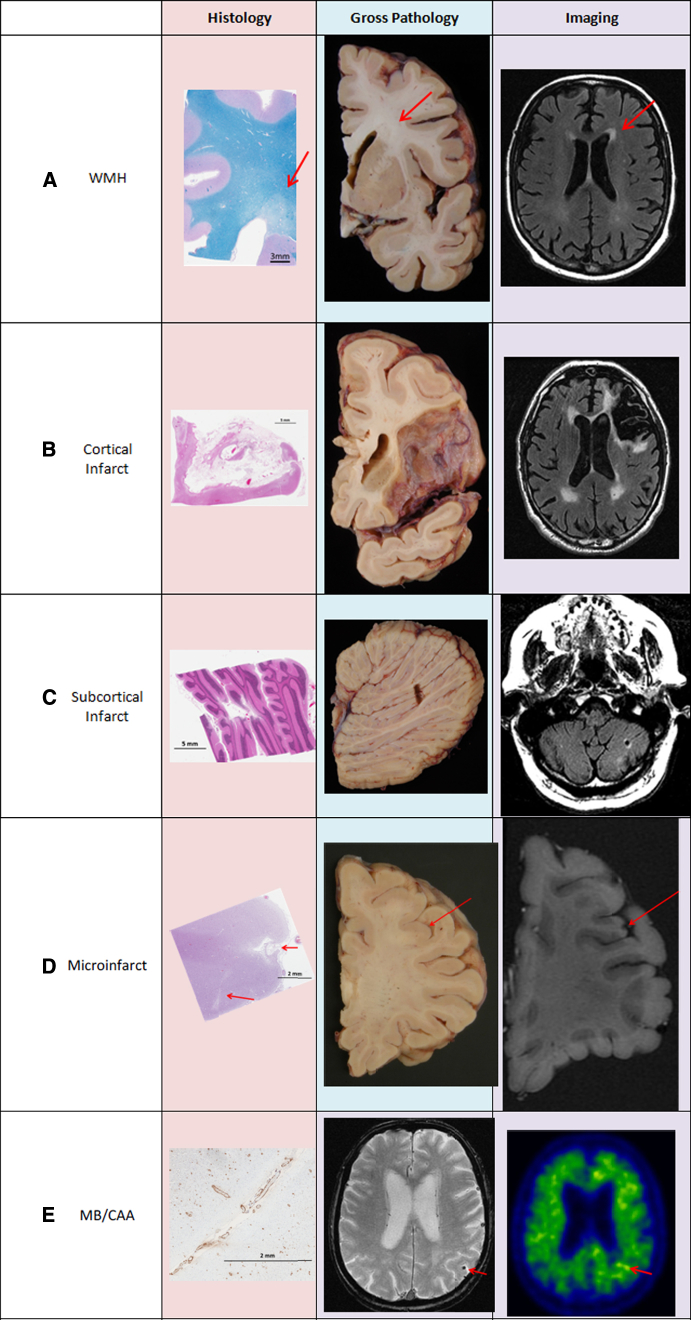
WMHs, also termed leukoaraiosis, white-matter lesions, or leukoencephalopathy, are areas of bilateral hyperintense signal in brain white matter on T2-weighted or FLAIR MRI (Fig. 2A). They occur in subcortical and periventricular white matter. These lesions are associated with pathologies in multiple disease contexts including vascular disease, multiple sclerosis, and leukodystrophies [21]. In addition to having heterogeneous contributing pathologies, studies show that, similar to heterogeneity in make-up of a tumor, WMH and its surrounding tissue can represent varying degrees of white-matter injury within an individual [37], [38]. Here, we discuss WMH as an imaging feature of underlying vascular pathology.
Fig. 2.
Cerebrovascular disease pathologies on histology, gross pathology, and imaging. (A) White-matter hyperintensities: rarefied tissue (indicated by arrows) on histology, discolored white matter on gross pathology, and hyperintensity on FLAIR MRI. (B) Large cortical infarct: tissue loss and gliosis on H&E stained histology slide, apparent tissue loss on gross pathology, tissue loss with surrounding hyperintensity. (C) Subcortical cerebellar infarct: tissue loss and gliosis on H&E stained histology slide, apparent tissue loss on gross pathology, tissue loss with surrounding hyperintensity. (D) Cortical microinfarct (indicated by arrows): tissue loss and gliosis on H&E stained histology slide, not apparent on gross pathology, microinfarcts >1 mm seen on high-resolution MRI. (E) Lobar microbleeds/CAA: amyloid uptake in vessel walls seen on parietal lobe histology slide with amyloid stain, not apparent on gross pathology, microbleed visible in parietal lobe (shown by arrow), and increased focal uptake seen on amyloid PET scan (shown by arrow). Abbreviations: CAA, cerebral amyloid angiopathy; FLAIR, Fluid Attenuated Inversion Recovery; GRE, gradient recalled echo; WMH, white-matter hyperintensity.
3.1.2. Underlying pathology
Even within the context of vascular disease, WMHs have been shown to be correlated with multiple pathologies. Autopsy studies correlating histology findings with WMH on MRI indicate that WMHs are associated with small-vessel ischemic disease [39], [40], [41], [42], [43], [44], [45], [46]. Hyperintense WM on antemortem FLAIR appears to have vacuolation and decreased myelin and small-vessel density when assessed microscopically at pathology [47]. There is also evidence that periventricular and subcortical WMHs have a different underlying pathophysiology [44]. Hyperintense periventricular WM has decreased myelin and oligodendrocyte density with astrogliosis and activated microglia, whereas hyperintense subcortical WM has loss of myelin that is not accompanied by oligodendrocyte loss. Pathologic white matter also occurs with affected vasculature including “string vessels,” which are capillaries that only have their basement membrane and tortuous arterioles, which occur commonly in patients with hypertension or other cardiovascular diseases [48], [49]. The presence of WMH is not specific but can also occur in neurodegenerative disorders.
There are several biomarker studies that investigated the possible effects of WMH on neurodegenerative changes in the brain. Higher WMHs are associated with reduced cerebral perfusion on arterial spin labeling [50], gray-matter volume on structural MRI, and glucose metabolism on fluorodeoxyglucose-PET [51].
3.1.3. Clinical effects
It has been extensively shown that WMH is associated with and can be predictive of increased global cognitive impairment in cognitively normal adults and individuals with various neurodegenerative diseases or neurological or psychiatric disorders. The effects are seen most often as impairment in attention and executive function [52], [53], [54]. Acceleration in the accumulation of WMH burden over time is associated with progression to mild cognitive impairment [55]. WMHs also contribute to disruption of normal gait, balance, and postural stability [52]. Particularly in older adults, WMH is associated with late-life depression [56]. However, WMH can also be seen as a part of normal aging [57]; the differentiation of disease-related WMH from normal age-related WMH is an ongoing area of investigation.
3.2. Brain infarcts
Infarctions occur when there is disruption of the neurovascular unit—a term describing the intimate relationship between cerebral vasculature and brain tissue. When cerebral blood flow is disrupted, the high energy needs of neurons are no longer met, eventually leading to ischemic infarctions. The process is usually initiated with occlusion of a vessel by thrombi or emboli or systemic pathologies that significantly reduce focal blood flow. This leads to a cascade of events beginning with failure of ionic pumps maintaining ionic gradients, followed by inflammatory responses, release of excitotoxins, and generation of free radicals [58]. This manifests as cytotoxic edema, seen as restricted diffusion on diffusion-weighted MRI, that is later followed by increased diffusion after breakdown of the cell membrane, which ultimately results in cell death [59], [60].
Cortical macroinfarcts are usually the result of cerebral emboli from a myocardial infarction or atrial fibrillation. Plaque deposition in atherosclerosis is the most common cause of large-vessel thrombosis leading to large infarcts. Subcortical macroinfarcts or lacunar infarcts occur in the subcortical WM or deep gray-matter structures. They are usually caused by the effects of small-vessel disease, which result in occlusion of the small penetrating arteries that perfuse subcortical gray and white matter [61]. Pathologic changes that decrease the vessel lumen include tortuous vessels seen in hypertensive patients, lipohyalinosis which makes the vessel walls glass-like, or atherosclerosis which deposits plaques and decreases cerebral blood flow [49]. Reduced cerebral perfusion not caused by vessel occlusion can also lead to infarcts. For instance, disrupted autoregulation in elderly with compromised vascular systems or overly aggressive treatment of hypertension might cause hypoperfusion that leads to brain ischemia [62], [63].
Microinfarcts occur very commonly in older adults [1], [7], [64] and are also very numerous—there may be up to hundreds or thousands distributed throughout the brain of even one subject [65]. Microinfarcts cannot be detected on gross pathologic examination unless numerous and associated with cortical granular atrophy [66], [67]. Granular atrophy manifests as pitting of the cortical surface due to multiple cortical microinfarcts and is most often found in the watershed distributions between the middle and anterior cerebral arteries [68]. Evidence suggests that this atrophy can be seen as macroscopic loss of GM volume over time in the cortical watershed zones on antemortem MRI of autopsied patients with microinfarcts [69]. This atrophy was independent of Braak and Braak stage of neurofibrillary tangles, indicating that the contribution of this CVP to neuronal loss was additional to those of AD pathology.
3.2.1. Imaging
3.2.1.1. Acute infarction
The process of acute infarction can be tracked using diffusion-weighted imaging (DWI) within hours after the onset of stroke. During this time frame, restricted diffusion (decreased apparent diffusion coefficient or hyperintensity on DWI) represents cytotoxic edema occurring in response to cell membrane breakdown [59], [60]. A chronic ischemic lesion is seen as increased diffusivity due to clearance of dead neurons and replacement of the infarcted tissue by CSF. In this way, DWI can be used to distinguish acute from chronic ischemic lesions [70], [71].
3.2.1.2. Chronic macroinfarcts
Chronic macroinfarcts are best seen on FLAIR and T1-weighted MRI; they appear as areas of tissue loss that have been replaced by CSF and on FLAIR are surrounded by a hyperintense rim-reflecting gliosis (Fig. 2B). Cortical macroinfarcts are best seen on FLAIR MRI as cortical lesions with high signal intensity that extend to the cortical surface [72]. Infarcts smaller than 5 mm may not be diagnosed with high confidence on conventional MRI as they may be reflecting a pulsation artifact caused by CSF in the subarachnoid space or by blood flow in the leptomeningeal blood vessels [73]. Subcortical macroinfarcts (Fig. 2C) are also seen on FLAIR MRI but occur in subcortical WM or deep gray-matter structures and are lesions that are surrounded by a hyperintense rim. Subcortical infarcts <3 mm may be difficult to distinguish from dilatation of perivascular spaces [39].
3.2.1.3. Microinfarcts
Microinfarcts (Fig. 2D) are ischemic lesions that occur in both cortical and subcortical regions. They are described pathologically as infarcts that are not visible on gross tissue but are found microscopically. Microinfarcts are typically below the resolution of conventional clinical MRI. Fig. 2D shows a histology slide with 2 chronic cortical microinfarcts. Neither lesion is visible on the gross pathology image in the middle panel, but the larger microinfarct (∼1.3 mm) is visible on high-resolution ex vivo MRI in the third panel. The definition of microinfarct size varies greatly across different groups. The definitions range from the smallest criteria at 50–400 μm and the largest criteria at ≤5 mm [74]. Recently, imaging studies have begun investigating the feasibility of imaging large cortical microinfarcts (1–3 mm) on high-resolution in vivo structural and diffusion-weighted MRI [75], [76], [77], [78]. These studies aimed to detect the microscopic lesions in vivo because clinical-pathologic studies have shown an effect of microinfarcts on cognition [40], [79], [80]. Pathologically, microinfarcts have a mean diameter of ∼0.2 mm [80], [81]. The large (>1 mm) lesions that these studies were able to capture on MRI represent ∼0.5% of pathologically identified microinfarcts, so we are still only able to see a small fraction of the overall burden [78].
3.2.2. Clinical effects
Clinical presentations of macroinfarcts are dependent on their location. For instance, so-called “strategic” infarcts in the striatum or other subcortical structures can lead to vascular parkinsonism [82]. A pathologic study showed that cortical microinfarcts and lacunes in the white matter, basal ganglia, and thalamus were all significantly associated with lower CDR scores and explained much of the clinical variability when compared against age and amyloid burden [83]. Subcortical macroinfarcts are often clinically silent but are associated with increased risk of stroke and gait impairment [84], [85]. Silent infarcts particularly those in the thalamus may increase the risk of cognitive impairment and dementia [84], [85], [86]. Cortical microinfarcts are associated with cognitive impairment and have been shown to increase the risk of dementia [79], [80], [87]. Although a relationship has been shown between the volume of infarcts and cognitive impairment, regional associations between infarcts and cognitive outcomes are not clearly defined [20].
3.3. Cerebral amyloid angiopathy and microbleeds
3.3.1. Imaging
Microbleeds or microhemorrhages are microscopic (∼200 μm) areas of blood leakage from weakened vessels. After the leakage of blood from a damaged vessel, hemosiderin is deposited in macrophages, and these deposits are visible as blooming hypointense lesions <10 mm in size on T2* gradient recalled echo or susceptibility weighted MR images. Therefore, these imaging findings are considered markers of cerebral microangiopathy [88], [89].
3.3.2. Pathophysiology and clinical effects
Microbleeds can occur in both subcortical and lobar cortical brain regions. Subcortical microbleeds are associated with small-vessel disease pathologies associated with vascular risk factors [17]. Lobar cortical microbleeds (Fig. 2E), however, are associated with CAA [29]. In CAA, amyloid deposits are found in the vessel wall, which leads to weakening and rupture of the vessel and subsequent blood leakage. In patients with microbleeds that are associated with CAA, increased focal uptake of Pittsburgh compound B is seen on positron emission tomography, indicating presence of amyloid pathology in the area of the microbleed [90]. Microbleeds and increased amyloid burden seen together on imaging is considered a marker for presence of cerebral amyloid angiopathy [90]. In patients with CAA, microbleeds seem to occur most often in posterior brain regions [10], [91]. Microbleeds are also seen as a side effect in patients receiving AD immunotherapy which is typically accompanied by vasogenic edema [92], [93]. An association has also been established between microhemorrhages and greater WMH burden [29], [45], [91], [94], [95]. Additionally, a higher number of baseline microbleeds is associated with a faster accumulation of microbleeds [10], [95], [96], [97].
Microbleeds occur more frequently in subjects with MCI and AD but are also seen in cognitively normal individuals [29]. There is some evidence that subcortical microbleeds may also be associated with gait changes [98]. The association between microbleeds and clinical impact is an area of active investigation.
4. Open questions and future directions
Although much has been established in the imaging, pathophysiology, and clinical effects of CVP, there are still some unanswered questions that are still being investigated:
-
1)What levels of CVP are abnormal?
- Although we are able to detect imaging findings during life associated with CVPs, criteria are still being evaluated to understand the levels of CVP that are abnormal and contribute to cognitive impairment. In the case of WMH, a heterogeneous marker with numerous contributing pathologies, clinical utility will have to be determined due to possible lack of specificity. The goal will be to develop in vivo staging criteria to mirror existing histopathologic staging criteria [99].
-
2)Can midlife interventions for vascular risk factors be used to reduce risk of dementia?
- Epidemiological studies have found that presence of vascular and metabolic risk factors during midlife is most strongly associated with risk of cognitive impairment and dementia, suggesting that this would be the opportune time for preventive measures and treatment of vascular and metabolic disease [18], [100]. Studies have found that antihypertensive treatment can have a beneficial effect in the risk of dementia, particularly when treated over a long period [20]. Additionally, the interaction between age and sex indicates that sex should also be considered for age-specific preventive measures. There need to be longitudinal imaging studies designed to study the evolution of CVP in subjects at higher risk of cardiovascular disease starting at midlife. This will enable us to design better early CVP prevention trials which may have significant impact on the number of dementia cases.
-
3)What is the relationship between AD and CVP?
- There is some evidence that AD may contribute to increased risk of CVP and CVP in turn may increase the risk of AD [91], [101], [102], [103], [104], [105], [106]. However, there are also studies in the field that have found that AD and CVP are independent pathologies, and CVP mainly lowers the threshold of dementia detection in subjects with comorbid AD pathology thus increasing the likelihood that the AD pathology will produce clinical symptoms [107]. There need to be more mechanistic studies to resolve this debate by studying the interplay between AD and CVP. Autopsy data show that the most common pathologic make-up in the oldest old is AD pathology mixed with vascular pathology, with each having independent and similarly severe impact on cognition [108]. Recent evidence suggests that vascular care can reduce the risk of non-AD dementias (possibly by reducing the risk of CVP). Both AD and CVP have been shown to contribute to the loss of brain structure and function which is the proximal surrogate of cognitive impairment. Understanding the interplay between vascular risk factors and imaging surrogates of AD and CVP pathologies (illustrated in Fig. 1) will aid in the development of effective intervention strategies.
-
4)Can we detect small infarcts? What is their impact on cognition?
- Many autopsy studies have defined microinfarcts as infarcts that are not visible on gross pathology. However, given the evidence that they are the most widespread CVP at autopsy and have been correlated with cognitive impairment, there is a push to assess them in vivo [79], [87]. With the availability of high-resolution MRI imaging, large microinfarcts can be detected using in vivo imaging [75]. There is a need to establish consensus criteria on the definitions of micro and macroinfarcts.
-
5)Can we use advanced methodologies to detect early changes due to vascular dysfunction in the brain?
- Diffusion tensor imaging (DTI): DTI measures the diffusion properties of water molecules in the brain and therefore is useful in visualizing the white-matter tracts and microstructural changes due to hypoxic-ischemic injury [109]. It has been shown that DTI changes underlying WMH captures severity of vascular disease including extent of WMH [110] and baseline DTI measures on normal appearing WM may be an early predictor of subsequent WMH incidence on the subsequent FLAIR scans [111]. DTI may prove to be a powerful biomarker for CVP.
- Blood-brain barrier (BBB) imaging: There is pathologic evidence that the BBB is disrupted during the course of vascular disease [17]. A recent study used dynamic contrast-enhanced MRI to capture high-resolution MR images and measure BBB permeability disruption in the brains of cognitively normal and MCI subjects [112]. Methods such as these that are sensitive to changes in permeability of the BBB before the occurrence of overt lesions can be useful as a biomarker for early detection and treatment of vascular changes in the brain.
Current and future work will investigate unknown mechanisms of vascular injury to the brain, new imaging methods and other biomarkers for effective diagnosis, and better understand which treatments are beneficial in maintaining cognitive function.
Research in Context.
-
1.
Systematic review: The authors reviewed the existing literature on cerebrovascular pathologies using standard database search engines for biomedical research journal articles. Cerebrovascular pathologies (CVPs) are one of the most common pathologies among older adults. The understanding of the pathophysiology of CVP is important to guide intervention strategies and understand the relationship with common comorbidities in the elderly population, such as Alzheimer disease. The literature describing CVP has been appropriately cited.
-
2.
Interpretation: Our study describes the current understanding of common CVP in the elderly population, including descriptions of clinical phenotypes, imaging biomarkers, and underlying pathologies identified histologically.
-
3.
Future directions: The gaps in our current knowledge to be addressed by future studies of cerebrovascular pathologies are (1) What levels of CVP are abnormal? (2) Can midlife interventions for vascular risk factors be used to reduce risk of dementia? (3) What is the relationship between AD and CVP? (4) Can we detect small infarcts? What is their impact on cognition? (5) Can we use advanced methodologies to detect early changes due to vascular dysfunction in the brain?
References
- 1.Schneider J.A., Aggarwal N.T., Barnes L., Boyle P., Bennett D.A. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinger K.A. Understanding the pathology of vascular cognitive impairment. J Neurol Sci. 2005;229-230:57–63. doi: 10.1016/j.jns.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Nelson P.T., Head E., Schmitt F.A., Davis P.R., Neltner J.H., Jicha G.A. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jellinger K.A. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vemuri P., Knopman D.S. The role of cerebrovascular disease when there is concomitant Alzheimer disease. Biochim Biophys Acta. 2016;1862:952–956. doi: 10.1016/j.bbadis.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder H.M., Corriveau R.A., Craft S., Faber J.E., Greenberg S.M., Knopman D. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longstreth W.T., Jr., Sonnen J.A., Koepsell T.D., Kukull W.A., Larson E.B., Montine T.J. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovitch H., Ross G.W., Steinhorn S.C., Abbott R.D., Markesbery W., Davis D. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 9.Schneider J.A., Wilson R.S., Cochran E.J., Bienias J.L., Arnold S.E., Evans D.A. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci K., Gunter J.L., Tosakulwong N., Weigand S.D., Senjem M.S., Petersen R.C. Focal hemosiderin deposits and beta-amyloid load in the ADNI cohort. Alzheimers Dement. 2013;9:S116–S123. doi: 10.1016/j.jalz.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernooij M.W., van der Lugt A., Ikram M.A., Wielopolski P.A., Niessen W.J., Hofman A. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 12.Jeerakathil T., Wolf P.A., Beiser A., Hald J.K., Au R., Kase C.S. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 13.Poels M.M., Vernooij M.W., Ikram M.A., Hofman A., Krestin G.P., van der Lugt A. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 14.Sveinbjornsdottir S., Sigurdsson S., Aspelund T., Kjartansson O., Eiriksdottir G., Valtysdottir B. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- 16.Pantoni L., Garcia J.H. The significance of cerebral white matter abnormalities 100 years after Binswanger's report. A review. Stroke. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloppenborg R.P., van den Berg E., Kappelle L.J., Biessels G.J. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585:97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Portet F., Brickman A.M., Stern Y., Scarmeas N., Muraskin J., Provenzano F.A. Metabolic syndrome and localization of white matter hyperintensities in the elderly population. Alzheimers Dement. 2012;8:S88–S95. doi: 10.1016/j.jalz.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson S.C., Akesson A., Wolk A. Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 2014;83:1699–1704. doi: 10.1212/WNL.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaffe K., Hoang T.D., Byers A.L., Barnes D.E., Friedl K.E. Lifestyle and health-related risk factors and risk of cognitive aging among older veterans. Alzheimers Dement. 2014;10:S111–S121. doi: 10.1016/j.jalz.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren A. Stroke genetics: a review and update. J Stroke. 2014;16:114–123. doi: 10.5853/jos.2014.16.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traylor M., Farrall M., Holliday E.G., Sudlow C., Hopewell J.C., Cheng Y.C. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarron M.O., Nicoll J.A. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann N Y Acad Sci. 2000;903:176–179. doi: 10.1111/j.1749-6632.2000.tb06366.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim M., Bae H.J., Lee J., Kang L., Lee S., Kim S. APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient-echo MRI. Neurology. 2005;65:1474–1475. doi: 10.1212/01.wnl.0000183311.48144.7f. [DOI] [PubMed] [Google Scholar]
- 28.Haast R.A., Gustafson D.R., Kiliaan A.J. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32:2100–2107. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yates P.A., Villemagne V.L., Ellis K.A., Desmond P.M., Masters C.L., Rowe C.C. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. doi: 10.3389/fneur.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichner J.E., Dunn S.T., Perveen G., Thompson D.M., Stewart K.E., Stroehla B.C. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 31.Sachdev P.S., Parslow R., Wen W., Anstey K.J., Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Miller V.M., Garovic V.D., Kantarci K., Barnes J.N., Jayachandran M., Mielke M.M. Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ. 2013;4:6. doi: 10.1186/2042-6410-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeKosky S.T., Blennow K., Ikonomovic M.D., Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roozenbeek B., Maas A.I., Menon D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 35.McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth T.L., Nayak D., Atanasijevic T., Koretsky A.P., Latour L.L., McGavern D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maillard P., Fletcher E., Lockhart S.N., Roach A.E., Reed B., Mungas D. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindemer E.R., Salat D.H., Smith E.E., Nguyen K., Fischl B., Greve D.N. White matter signal abnormality quality differentiates mild cognitive impairment that converts to Alzheimer's disease from nonconverters. Neurobiol Aging. 2015;36:2447–2457. doi: 10.1016/j.neurobiolaging.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantarci K., Petersen R.C., Przybelski S.A., Weigand S.D., Shiung M.M., Whitwell J.L. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65:1621–1628. doi: 10.1001/archneur.65.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Groot J.C., De Leeuw F.E., Oudkerk M., Van Gijn J., Hofman A., Jolles J. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 41.Silbert L.C., Nelson C., Howieson D.B., Moore M.M., Kaye J.A. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maillard P., Carmichael O., Fletcher E., Reed B., Mungas D., DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagust W.J., Zheng L., Harvey D.J., Mack W.J., Vinters H.V., Weiner M.W. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray M.E., Vemuri P., Preboske G.M., Murphy M.C., Schweitzer K.J., Parisi J.E. A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. J Neuropathol Exp Neurol. 2012;71:1113–1122. doi: 10.1097/NEN.0b013e318277387e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erten-Lyons D., Woltjer R., Kaye J., Mattek N., Dodge H.H., Green S. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81:977–983. doi: 10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmichael O., Mungas D., Beckett L., Harvey D., Tomaszewski Farias S., Reed B. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33:83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fazekas F., Kleinert R., Offenbacher H., Schmidt R., Kleinert G., Payer F. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 48.Challa V.R., Thore C.R., Moody D.M., Brown W.R., Anstrom J.A. A three-dimensional study of brain string vessels using celloidin sections stained with anti-collagen antibodies. J Neurol Sci. 2002;203-204:165–167. doi: 10.1016/s0022-510x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 49.Brown W.R., Thore C.R. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alosco M.L., Brickman A.M., Spitznagel M.B., Garcia S.L., Narkhede A., Griffith E.Y. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest Heart Fail. 2013;19:E29–E34. doi: 10.1111/chf.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeCarli C., Murphy D.G., Tranh M., Grady C.L., Haxby J.V., Gillette J.A. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 52.Murray M.E., Senjem M.L., Petersen R.C., Hollman J.H., Preboske G.M., Weigand S.D. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ylikoski R., Ylikoski A., Erkinjuntti T., Sulkava R., Raininko R., Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- 54.Kloppenborg R.P., Nederkoorn P.J., Geerlings M.I., van den Berg E. Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology. 2014;82:2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 55.Silbert L.C., Dodge H.H., Perkins L.G., Sherbakov L., Lahna D., Erten-Lyons D. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79:741–747. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aizenstein H.J., Baskys A., Boldrini M., Butters M.A., Diniz B.S., Jaiswal M.K. Vascular depression consensus report - a critical update. BMC Med. 2016;14:161. doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vannorsdall T.D., Waldstein S.R., Kraut M., Pearlson G.D., Schretlen D.J. White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol. 2009;24:209–217. doi: 10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brott T., Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 59.Li T.Q., Chen Z.G., Hindmarsh T. Diffusion-weighted MR imaging of acute cerebral ischemia. Acta Radiol. 1998;39:460–473. doi: 10.1080/02841859809172209. [DOI] [PubMed] [Google Scholar]
- 60.van Everdingen K.J., van der Grond J., Kappelle L.J., Ramos L.M., Mali W.P. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998;29:1783–1790. doi: 10.1161/01.str.29.9.1783. [DOI] [PubMed] [Google Scholar]
- 61.Moody D.M., Bell M.A., Challa V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 62.van Beek A.H., Claassen J.A., Rikkert M.G., Jansen R.W. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 63.Birns J., Markus H., Kalra L. Blood pressure reduction for vascular risk: is there a price to be paid? Stroke. 2005;36:1308–1313. doi: 10.1161/01.STR.0000165901.38039.5f. [DOI] [PubMed] [Google Scholar]
- 64.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 65.Westover M.B., Bianchi M.T., Yang C., Schneider J.A., Greenberg S.M. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013;80:1365–1369. doi: 10.1212/WNL.0b013e31828c2f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jellinger K.A. The pathology of ischemic-vascular dementia: an update. J Neurol Sci. 2002;203-204:153–157. doi: 10.1016/s0022-510x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 67.Dickson D.W. Neuropathology of Alzheimer's disease and other dementias. Clin Geriatr Med. 2001;17:209–228. doi: 10.1016/s0749-0690(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 68.Murray M.E., Knopman D.S., Dickson D.W. Vascular dementia: clinical, neuroradiologic and neuropathologic aspects. Panminerva Med. 2007;49:197–207. [PubMed] [Google Scholar]
- 69.Raman M.R., Preboske G.M., Przybelski S.A., Gunter J.L., Senjem M.L., Vemuri P. Antemortem MRI findings associated with microinfarcts at autopsy. Neurology. 2014;82:1951–1958. doi: 10.1212/WNL.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lutsep H.L., Albers G.W., DeCrespigny A., Kamat G.N., Marks M.P., Moseley M.E. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol. 1997;41:574–580. doi: 10.1002/ana.410410505. [DOI] [PubMed] [Google Scholar]
- 71.Albers G.W. Diffusion-weighted MRI for evaluation of acute stroke. Neurology. 1998;51:S47–S49. doi: 10.1212/wnl.51.3_suppl_3.s47. [DOI] [PubMed] [Google Scholar]
- 72.Knopman D.S., Griswold M.E., Lirette S.T., Gottesman R.F., Kantarci K., Sharrett A.R. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herlihy A.H., Hajnal J.V., Curati W.L., Virji N., Oatridge A., Puri B.K. Reduction of CSF and blood flow artifacts on FLAIR images of the brain with k-space reordered by inversion time at each slice position (KRISP) AJNR Am J Neuroradiol. 2001;22:896–904. [PMC free article] [PubMed] [Google Scholar]
- 74.Brundel M., de Bresser J., van Dillen J.J., Kappelle L.J., Biessels G.J. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Veluw S.J., Zwanenburg J.J., Engelen-Lee J., Spliet W.G., Hendrikse J., Luijten P.R. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auriel E., Edlow B.L., Reijmer Y.D., Fotiadis P., Ramirez-Martinez S., Ni J. Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis. Neurology. 2014;83:182–188. doi: 10.1212/WNL.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Dalen J.W., Scuric E.E., van Veluw S.J., Caan M.W., Nederveen A.J., Biessels G.J. Cortical microinfarcts detected in vivo on 3 Tesla MRI: clinical and radiological correlates. Stroke. 2015;46:255–257. doi: 10.1161/STROKEAHA.114.007568. [DOI] [PubMed] [Google Scholar]
- 78.Auriel E., Westover M.B., Bianchi M.T., Reijmer Y., Martinez-Ramirez S., Ni J. Estimating Total Cerebral Microinfarct Burden From Diffusion-Weighted Imaging. Stroke. 2015;46:2129–2135. doi: 10.1161/STROKEAHA.115.009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonnen J.A., Larson E.B., Crane P.K., Haneuse S., Li G., Schellenberg G.D. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 80.Arvanitakis Z., Leurgans S.E., Barnes L.L., Bennett D.A., Schneider J.A. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okamoto Y., Ihara M., Fujita Y., Ito H., Takahashi R., Tomimoto H. Cortical microinfarcts in Alzheimer's disease and subcortical vascular dementia. Neuroreport. 2009;20:990–996. doi: 10.1097/WNR.0b013e32832d2e6a. [DOI] [PubMed] [Google Scholar]
- 82.Benamer H.T., Grosset D.G. Vascular parkinsonism: a clinical review. Eur Neurol. 2009;61:11–15. doi: 10.1159/000165343. [DOI] [PubMed] [Google Scholar]
- 83.Gold G., Kovari E., Herrmann F.R., Canuto A., Hof P.R., Michel J.P. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 84.Choi P., Ren M., Phan T.G., Callisaya M., Ly J.V., Beare R. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke. 2012;43:1505–1510. doi: 10.1161/STROKEAHA.111.647271. [DOI] [PubMed] [Google Scholar]
- 85.Snowdon D.A., Greiner L.H., Mortimer J.A., Riley K.P., Greiner P.A., Markesbery W.R. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 86.Vermeer S.E., Longstreth W.T., Jr., Koudstaal P.J. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 87.White L., Petrovitch H., Hardman J., Nelson J., Davis D.G., Ross G.W. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 88.Fazekas F., Kleinert R., Roob G., Kleinert G., Kapeller P., Schmidt R. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 89.Vernooij M.W., Ikram M.A., Hofman A., Krestin G.P., Breteler M.M., van der Lugt A. Superficial siderosis in the general population. Neurology. 2009;73:202–205. doi: 10.1212/WNL.0b013e3181ae7c5e. [DOI] [PubMed] [Google Scholar]
- 90.Greenberg S.M., Grabowski T., Gurol M.E., Skehan M.E., Nandigam R.N., Becker J.A. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol. 2008;64:587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettersen J.A., Sathiyamoorthy G., Gao F.Q., Szilagyi G., Nadkarni N.K., St George-Hyslop P. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 92.Rinne J.O., Brooks D.J., Rossor M.N., Fox N.C., Bullock R., Klunk W.E. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 93.Sperling R., Salloway S., Brooks D.J., Tampieri D., Barakos J., Fox N.C. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yakushiji Y., Yokota C., Yamada N., Kuroda Y., Minematsu K. Clinical characteristics by topographical distribution of brain microbleeds, with a particular emphasis on diffuse microbleeds. J Stroke Cerebrovasc Dis. 2011;20:214–221. doi: 10.1016/j.jstrokecerebrovasdis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Poels M.M., Ikram M.A., van der Lugt A., Hofman A., Krestin G.P., Breteler M.M. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 96.Greenberg S.M., Eng J.A., Ning M., Smith E.E., Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y.W., Gurol M.E., Rosand J., Viswanathan A., Rakich S.M., Groover T.R. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Laat K.F., van den Berg H.A., van Norden A.G., Gons R.A., Olde Rikkert M.G., de Leeuw F.E. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42:494–497. doi: 10.1161/STROKEAHA.110.596122. [DOI] [PubMed] [Google Scholar]
- 99.Deramecourt V., Slade J.Y., Oakley A.E., Perry R.H., Ince P.G., Maurage C.A. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts R.O., Knopman D.S., Przybelski S.A., Mielke M.M., Kantarci K., Preboske G.M. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–1141. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakata-Kudo Y., Mizuno T., Yamada K., Shiga K., Yoshikawa K., Mori S. Microbleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dement Geriatr Cogn Disord. 2006;22:8–14. doi: 10.1159/000092958. [DOI] [PubMed] [Google Scholar]
- 102.Haller S., Bartsch A., Nguyen D., Rodriguez C., Emch J., Gold G. Cerebral microhemorrhage and iron deposition in mild cognitive impairment: susceptibility-weighted MR imaging assessment. Radiology. 2010;257:764–773. doi: 10.1148/radiol.10100612. [DOI] [PubMed] [Google Scholar]
- 103.Gao T., Wang Y., Zhang Z. Silent cerebral microbleeds on susceptibility-weighted imaging of patients with ischemic stroke and leukoaraiosis. Neurol Res. 2008;30:272–276. doi: 10.1179/016164107X251556. [DOI] [PubMed] [Google Scholar]
- 104.Goos J.D., Henneman W.J., Sluimer J.D., Vrenken H., Sluimer I.C., Barkhof F. Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population. Neurology. 2010;74:1954–1960. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- 105.Kimberly W.T., Gilson A., Rost N.S., Rosand J., Viswanathan A., Smith E.E. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fiehler J. Cerebral microbleeds: old leaks and new haemorrhages. Int J Stroke. 2006;1:122–130. doi: 10.1111/j.1747-4949.2006.00042.x. [DOI] [PubMed] [Google Scholar]
- 107.Chui H.C., Zheng L., Reed B.R., Vinters H.V., Mack W.J. Vascular risk factors and Alzheimer's disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res Ther. 2012;4:1. doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawas C.H., Kim R.C., Sonnen J.A., Bullain S.S., Trieu T., Corrada M.M. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Papma J.M., de Groot M., de Koning I., Mattace-Raso F.U., van der Lugt A., Vernooij M.W. Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum Brain Mapp. 2014;35:2836–2851. doi: 10.1002/hbm.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maniega S.M., Valdes Hernandez M.C., Clayden J.D., Royle N.A., Murray C., Morris Z. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maillard P., Carmichael O., Harvey D., Fletcher E., Reed B., Mungas D. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013;34:54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]