Abstract
Introduction
Subjective cognitive decline (SCD) could help identify early stages of Alzheimer's disease. However, SCD is multidetermined and protean, and the type of cognitive complaint associated with preclinical Alzheimer's disease needs refinement.
Methods
A total of 185 nondemented elders recruited from either the community or from a memory clinic filled a questionnaire. We searched for item responses associated with medical help seeking, cognitive deficits, and β-amyloidosis.
Results
Compared with community-recruited control subjects (n = 74), help-seeking patients reported a stronger multidomain SCD that was mostly unrelated to the presence of detectable cognitive deficits. Only a few items, notably assessing temporal disorientation, distinguished help-seeking patients with (n = 78) or without (n = 33) memory deficits. Associations between SCD and β-amyloidosis were not restricted to the memory domain and varied across clinical stages.
Discussion
Detailed evaluation of SCD could provide accessible indication of the presence of β-amyloid or cognitive deficits, which might prove useful for early diagnosis and clinical trial enrichment strategies.
Keywords: Subjective cognitive decline, Memory complaint, β-Amyloid, Positron emission tomography, Preclinical, Biomarkers, Anosognosia, Prodromal Alzheimer's disease, Mild cognitive impairment, Orientation
1. Introduction
1.1. General background and previous studies
Subjective cognitive decline (SCD) has been suggested as a potential early indicator of ongoing neurodegenerative processes for decades [1], [2]. Indeed, self-reported cognitive complaint was implemented in the criteria for mild cognitive impairment (MCI) [3] that have been widely used to study the prodromal stage of Alzheimer's disease (AD). More recently, in line with the growing interest for defining and characterizing preclinical stages of AD [4], [5], researchers have assessed SCD in individuals without measurable cognitive deficits, that is, cognitively “normal” older adults. Indeed, longitudinal investigations have repeatedly shown that SCD is associated with an increased risk of subsequent cognitive decline and conversion to dementia [6]. In addition, cross-sectional studies have shown that the presence or severity of SCD is related to abnormal neuroimaging biomarkers suggestive of underlying AD pathophysiological processes [7], [8]. SCD has notably been associated with the presence of β-amyloidosis (Aβ) evidenced using positron emission tomography (PET) in several independent samples of cognitively normal elders [8], [9], [10], [11], [12].
Altogether, converging evidence indicates that SCD might be among the first clinically observable signs of AD and could potentially be used as a screening tool for enrichment strategies for clinical trials [7], [12], [13]. The development of affordable and easily accessible measures that could help predict the presence of Aβ is needed to reduce the resources, time, and costs associated with the selection of appropriate candidates for clinical trials targeting Aβ [14]. This is becoming particularly important as the field is moving toward interventions in prodromal [15] or even preclinical AD [16] populations, in which the prevalence of Aβ is at most moderate [17].
However, associations between SCD and AD biomarkers were not identified in all studies (see [8] for review), illustrating that SCD is multidetermined [18], [19] and loosely defined. Indeed, definitions or criteria used to define SCD widely vary across laboratories, hampering the direct comparison between results from different groups. For these reasons, the international SCD-initiative (SCD-I) was recently formed to stimulate standardized research and refine our understanding of SCD in the context of early AD [20], [21]. One of the main aims highlighted in the SCD-I framework is the identification of the specific features of SCD that increase the likelihood of underlying preclinical AD.
We recently showed that different approaches for defining and studying SCD led to different associations with AD biomarkers and affective symptomatology [8]. More specifically, Aβ was associated with higher levels of self-reported cognitive difficulties assessed through a questionnaire, but was not related to medical help seeking per se: asymptomatic memory clinic attendees did not have more Aβ than community-recruited individuals with similar levels of self-reported cognitive difficulties.
In addition to the recruitment setting, quantification of SCD also varies greatly across groups: members of the SCD-I systematically compared questionnaires used in 19 international studies [22] and showed little overlap among measures (item phrasing, number of items, response options, and so forth). Authors encouraged researchers to identify specific and relevant items, notably by assessing their relationships to AD biomarkers.
1.2. Overview and aims of the present study
Following these recommendations, we aimed at better characterizing the relevance of different types of self-reported cognitive difficulties to identify early AD stages in nondemented older adults. We studied three groups of individuals: (1) healthy aged subjects recruited from the community (HAS), (2) patients who sought help at a memory clinic because of concerns about their memory but whose clinical and neuropsychological examination did not show any deficit (SCDclinic), and (3) patients who sought help at a memory clinic because of concerns about their memory and who actually fulfilled criteria for amnestic MCI (aMCI).
All participants filled out a standardized SCD questionnaire, the Cognitive Difficulties Scale (CDS [23]), which covers multiple cognitive domains. First, the questionnaire was analyzed to identify patterns of responses that were specifically associated (1) with medical help seeking in cognitively normal individuals (SCDclinic > HAS) or (2) with the presence of detectable episodic memory deficits (aMCI > SCDclinic). Second, we searched for associations between patterns of responses and the presence of Aβ deposition, assessed using Florbetapir-PET.
For the sake of completeness, the CDS was analyzed in two complementary ways. Analyses were first performed for each item separately using nonparametric tests. Then, exploratory factor analysis (EFA) was conducted to reveal latent variables and to obtain more reliable estimates of different aspects of SCD by grouping highly correlated items.
2. Methods
2.1. Participants
The participants included in the current article were drawn from two academic studies conducted by the same investigators: the multimodal neuroimaging study of early AD (Imagerie Multimodale de la maladie d'Alzheimer à un stade Précoce, IMAP+) study [8], [24], [25], [26], [27] and an earlier study of patients with aMCI [28]. All participants were aged 55 years or older.
The control group (HAS) included volunteers to our academic study on aging and AD and were recruited through advertising in local media and word of mouth. Only those volunteers who had never consulted a memory clinic and showed normal neuropsychological examination (i.e., scores within the normal range) were included.
Patients with SCDclinic and aMCI were recruited from local memory clinics they had visited because of memory concerns. During the screening interview, the clinician ensured that the complaint was not related to current medication, major psychiatric, or neurologic conditions (including major depressive disorder), or other medical conditions. For this specific study, we only selected nondemented individuals and classified them as SCDclinic or aMCI depending on the results of their cognitive assessment. SCDclinic had no “objective” evidence for impaired cognition (i.e., they scored within the normal range on all tests), whereas aMCI patients had detectable episodic memory deficits, that is, at least one score less than the fifth percentile using adapted norms on the Free and Cued Selective Reminding Test (FCSRT) [29].
The two studies were approved by local ethical committees, and all participants gave written consent before undergoing further investigation including quantification of SCD, detailed neuropsychological assessment, and neuroimaging magnetic resonance imaging (MRI) and PET scans. Demographics are provided in Table 1.
Table 1.
Group description
| Measure | HAS (n = 74) | SCDclinic (n = 33) | aMCI (n = 78) | F and P values | Effect size | Pairwise comparisons |
|---|---|---|---|---|---|---|
| Age (y) | 69 ± 7.2 | 68 ± 7.3 | 73 ± 7.2 | F (2, 182) = 7.36 | η2 = 0.07 | aMCI > HAS∗∗ |
| [63, 68.5, 73] | [63, 68, 72] | [68, 73, 78] | P < .001 | aMCI > SCDclinic∗∗ | ||
| Female: n | 40 (54%) | 14 (42%) | 38 (49%) | Fisher's exact test | ||
| P = .53 | ||||||
| Education (y) | 12 ± 3.9 | 13 ± 3.5 | 11 ± 3.6 | F (2, 179) = 5.65 | η2 = 0.06 | HAS > aMCI∗ |
| [9, 12, 15] | [10, 14, 15] | [7, 10, 14] | P = .004 | SCDclinic > aMCI∗∗ | ||
| MMSE (/30)† | 29 ± 1.2 | 29 ± 1.1 | 27 ± 1.7 | F (2, 180) = 42.85 | η2 = 0.32 | HAS > aMCI∗∗∗ |
| [28, 29, 30] | [28, 29, 30] | [26, 27, 28] | P < .001 | SCDclinic > aMCI∗∗∗ | ||
| FCSRT (/48)† | 30 ± 5.2 | 31 ± 5.9 | 17 ± 6.4 | F (2, 176) = 117.08 | η2 = 0.57 | HAS > aMCI∗∗∗ |
| Sum of 3 free recalls | [26, 30, 33] | [27, 29, 36] | [11, 17, 21] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| FCSRT (/48)† | 46 ± 2.1 | 47 ± 1.8 | 36 ± 7.8 | F (2, 176) = 81.70 | η2 = 0.48 | HAS > aMCI∗∗∗ |
| Sum of 3 total recalls | [45, 47, 48] | [45, 47, 48] | [30, 37, 43] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| FCSRT (/16)† | 12 ± 2.3 | 12 ± 2.2 | 5 ± 3.6 | F (2, 179) = 120.89 | η2 = 0.57 | HAS > aMCI∗∗∗ |
| Delayed free recall | [10, 12, 14] | [10, 12, 13] | [2, 6, 8] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| FCSRT (/16)† | 15.7 ± 0.6 | 15.7 ± 0.5 | 12 ± 3.6 | F (2, 176) = 61.01 | η2 = 0.41 | HAS > aMCI∗∗∗ |
| Delayed total recall | [16, 16, 16] | [15, 16, 16] | [10, 12.5, 15] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| FCSRT (/16)† | 15.9 ± 0.3 | 15.9 ± 0.4 | 14.8 ± 1.6 | F (2, 173) = 24.85 | η2 = 0.22 | HAS > aMCI∗∗∗ |
| Recognition | [16, 16, 16] | [16, 16, 16] | [14, 15, 16] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| Verbal abilities | 0.32 ± 0.89 | 0.39 ± 0.75 | −0.65 ± 0.94 | F (2, 155) = 24.72 | η2 = 0.28 | HAS > aMCI∗∗∗ |
| Composite score | [−0.21, 0.32, 0.80] | [−0.24, 0.24, 0.94] | [−1.42, −0.49, 0.01] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| Executive function | 0.28 ± 0.76 | 0.47 ± 0.83 | −0.59 ± 1.07 | F (2, 161) = 22.10 | η2 = 0.22 | HAS > aMCI∗∗∗ |
| Composite score | [−0.08, 0.35, 0.76] | [0.05, 0.39, 0.95] | [−1.20, −0.70, 0.04] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| Episodic memory | 0.57 ± 0.69 | 0.37 ± 0.81 | −0.90 ± 0.73 | F (2, 161) = 77.58 | η2 = 0.49 | HAS > aMCI∗∗∗ |
| Composite score | [0.13, 0.64, 1.06] | [−0.08, 0.31, 0.94] | [−1.44, −1.00, −0.46] | P < .001 | SCDclinic > aMCI∗∗∗ | |
| Depression† | 0.8 ± 1.9 | 3.4 ± 3.0 | 3.8 ± 5.4 | F (2, 153) = 12.47 | η2 = 0.14 | SCDclinic > HAS∗∗∗ |
| MADRS total score | [0, 0, 1] | [1.5, 2.5, 5] | [0, 2, 5] | P < .001 | aMCI > HAS∗∗∗ | |
| SCD | 40 ± 17 | 57 ± 22 | 60 ± 20 | F (2, 182) = 21.08 | η2 = 0.19 | SCDclinic > HAS∗∗∗ |
| CDS total score | [29, 39.5, 50] | [41, 55, 70] | [45, 57, 73] | P < .001 | aMCI > HAS∗∗∗ | |
| APOE ε4 carriers: n (%) | 17 (24%) | 4 (15%) | 25 (50%) | Fisher's exact test | HAS > aMCI∗∗ | |
| P = .002 | SCDclinic > aMCI∗∗ |
Abbreviations: aMCI, amnestic mild cognitive impairment; CDS, Cognitive Difficulties Scale; FCSRT, Free and Cued Selective Reminding Test; HAS, healthy aged subjects; MADRS, Montgomery-Asberg Depression Rating Scale; MMSE, Mini Mental State Examination; SCD, subjective cognitive decline.
NOTE. For numerical variables, we indicated the mean ± standard deviation [first quartile, median, and third quartile]. Fisher's LSD test was used as a post hoc test: ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. For the sake of readability, patients values are given in bold when different from the HAS group (P < .05). Group comparisons were also assessed using Kruskal-Wallis and Mann-Whitney tests because some variables (recognition, depression) had strongly skewed distributions; results were unchanged. Percentages of APOE ε4 carriers are calculated based on the number of available genotypes within each group (n = 72 for HAS, n = 26 for SCDclinic, n = 50 for aMCI).
Scores that were used in the inclusion battery.
2.2. Neuropsychological evaluation
2.2.1. Self-reported cognitive difficulties
Self-reported cognitive difficulties were assessed using the CDS [23], a 39-item questionnaire that requires participants to rate how often they currently experience cognitive difficulties in everyday life using a 5-point scale (from “never” = 0 to “very often” = 4). The 39 items, detailed in Table 2, cover a large span of domains (retrospective and prospective memory, attention, language, orientation, praxis, and so forth), and previous independent studies have confirmed the multidimensionality of the scale [30], [31]. The questionnaire was not part of the screening process and was acquired once participants were already classified into one of the three clinical groups. It should also be highlighted that the questionnaire was filled by the participants alone (without any intervention from the experimenter) and that none of the participants knew their apolipoprotein E (APOE) genotype or Florbetapir status when enrolling in the study.
Table 2.
List of the 39 items included in the Cognitive Difficulties Scale
| Item order | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| 1. I have trouble recalling frequently used phone numbers | −0.037 | 0.547 | −0.04 |
| 2. I put down things (glasses, keys, wallet, papers) and have trouble finding them | 0.104 | 0.473 | −0.056 |
| 3. When interrupted while reading, I have trouble finding my place again | 0.526 | 0.176 | −0.095 |
| 4. I need a written list when I do errands to avoid forgetting things | 0.09 | 0.607 | −0.208 |
| 5. I forget appointments, dates or classes | −0.008 | 0.679 | 0.022 |
| 6. I forget to return phone calls | −0.055 | 0.48 | 0.281 |
| 7. I have trouble getting my keys into a lock | n/i | n/i | n/i |
| 8. I forget errands I planned to do on my way | 0.215 | 0.474 | 0.007 |
| 9. I have trouble recalling names of people I know | 0.398 | 0.375 | −0.121 |
| 10. I find it hard to keep my mind on a task or a job | 0.6 | −0.02 | 0.111 |
| 11. I have trouble describing a program I just watched on television | 0.69 | 0.129 | −0.152 |
| 12. I do not say quite what I mean | 0.475 | 0.055 | 0.134 |
| 13. I fail to recognize people I know | 0.393 | 0.037 | 0.145 |
| 14. I have trouble getting out information that is at the tip of my tongue | 0.539 | 0.017 | −0.005 |
| 15. I have trouble finding the name of objects | 0.449 | −0.071 | 0.369 |
| 16. I find it hard to understand what I read | 0.758 | −0.335 | 0.204 |
| 17. I miss the point of what other people are saying | 0.573 | 0.033 | 0.117 |
| 18. I forget names of people soon after being introduced | 0.372 | 0.319 | −0.014 |
| 19. I lose my train of thought when I listen to somebody else | 0.718 | 0.272 | −0.253 |
| 20. I forget steps in recipes I know well and have to look them up | 0.182 | 0.108 | 0.439 |
| 21. I forget what day of the week it is | −0.082 | 0.672 | 0.102 |
| 22. I forget to button or zip my clothing | n/i | n/i | n/i |
| 23. I need to check or double check whether I locked the door, turned off the stove, and so forth | 0.009 | 0.412 | 0.147 |
| 24. I make mistakes in writing, typing, or operating a calculator | 0.168 | 0.143 | 0.322 |
| 25. I cannot keep my mind on one thing | 0.484 | −0.11 | 0.311 |
| 26. I need to have instructions repeated several times | 0.129 | 0.439 | 0.192 |
| 27. I leave out ingredients when I cook | 0.119 | −0.031 | 0.64 |
| 28. I have trouble manipulating buttons, fasteners, scissors, or bottle caps | 0.073 | −0.105 | 0.719 |
| 29. I misplace my clothing | −0.046 | 0.049 | 0.602 |
| 30. I have trouble sewing or mending | −0.097 | −0.084 | 0.76 |
| 31. I find it hard to keep my mind on what I am reading | 0.667 | 0.004 | 0.031 |
| 32. I forget right away what people say to me | 0.422 | 0.414 | −0.063 |
| 33. When walking or riding, I forget how I had gotten from one place to another | n/i | n/i | n/i |
| 34. I have trouble deciding if I have received the correct change | n/i | n/i | n/i |
| 35. I forget to pay bills, record checks, or mail letters | −0.152 | 0.3 | 0.491 |
| 36. I have to do things very slowly to be sure I am doing them right | 0.017 | 0.251 | 0.411 |
| 37. My mind goes blank at times | 0.194 | 0.456 | 0.047 |
| 38. I forget the date of the month | −0.283 | 0.943 | 0 |
| 39. I have trouble using tools (hammers, pliers, and so forth) for minor household repairs | −0.097 | −0.013 | 0.844 |
NOTE. The three columns on the right show the loading scores resulting from the exploratory factor analysis (see Section 2 for further information). Factor loadings greater than 0.4 are in bold. Four items were not included (n/i) in the factor analysis because of insufficient variability but were still included in item-by-item analyses.
2.2.2. Standardized measures of cognition
Participants underwent an extensive neuropsychological evaluation. To obtain robust proxies of cognitive abilities, composite scores were created for executive functions, verbal abilities, and episodic memory. The latter was calculated without using FCSRT scores to avoid circularity (this test was used as an inclusion criteria and was by definition normal in all HAS and SCDclinic but low in aMCI, see Table 1). For all composite scores, only scores showing no ceiling or floor effects were used and higher values indicate better performances (see Supplementary Material for further detail). Depressive symptomatology was assessed using the Montgomery-Asberg Depression Rating Scale.
2.3. Neuroimaging measures
A subset of 151 participants (68 HAS, 33 SCDclinic, 50 aMCI) underwent both structural MRI and PET with Florbetapir. Details on image acquisition and preprocessing are available in previous publications [8] and in the Supplementary Methods. Briefly, PET images were preprocessed using MRI data for partial volume effect correction and extraction of individual uptake values in a predetermined neocortical mask [25], [31]. Participants were classified as Aβ-positive or Aβ-negative based on Florbetapir-PET data acquired in a group of 41 healthy adults aged less than 40 years [8], [26], [32].
2.4. Statistical analyses
2.4.1. Item-by-item analyses
Analyses were first conducted item-by-item to identify the responses showing a significant clinical group difference; nonparametric tests were applied because of the ordinal nature of the dependent variables [33]. Kruskal-Wallis tests were first used to identify the effect of clinical group, a stringent Bonferroni correction (α < 0.001282 = 0.05/39) was used when significant, and Mann-Whitney tests were used for pairwise comparisons. To limit multiple testing, post hoc tests were limited to the two contrasts of interest, that is, to identify items that were related to medical help seeking (HAS vs. SCDclinic) or cognitive impairment (SCDclinic vs. aMCI). Bonferroni correction was applied at this step as well to define statistical significance (α < 0.025 = 0.05/2). In a second set of analyses, we tested for associations between item endorsement and Aβ status using Mann-Whitney tests. Finally, we assessed correlations between item endorsement and cognitive or affective measures using nonparametric Spearman correlation coefficients.
2.4.2. Exploratory factor analysis
EFA was conducted using the freely available FACTOR package (http://psico.fcep.urv.es/utilitats/factor/). This choice was motivated by the optimal implementation of both polychoric correlations and parallel analysis. Resulting SCD factors were rotated using the oblique promin method, allowing factors to be intercorrelated. FACTOR methods are described in detail elsewhere [34] and in the Supplementary Methods. Factor scores were extracted for each participant to be used in subsequent analyses. These SCD factor scores, which have a continuous distribution, were analyzed using parametric statistics, enabling to test for both main effects and interactions with between (clinical group, Aβ status) and within subject (SCD factor) factors.
3. Results
3.1. Description of clinical groups
Group description is available in Table 1. On average, patients with aMCI were slightly older, less educated, and more likely to carry the APOE ε4 allele than the other two groups. Per inclusion criteria, patients with aMCI had lower FCSRT scores than HAS and SCDclinic, and this difference was observed for all FCSRT subscores. In contrast, the SCDclinic group did not show any significant difference on any FCSRT subscore compared with the HAS group. The same pattern was observed with the three independent composite scores, with aMCI performing lower than the other two groups, whereas HAS and SCDclinic had very comparable distributions (see Supplementary Fig. 1). In contrast, both the SCDclinic and aMCI groups had increased levels of SCD endorsement (measures on the total CDS score) and subclinical depressive symptomatology compared with HAS.
3.2. Factorial structure of the CDS
Analysis of the polychoric correlation matrix confirmed the suitability of the data for factor analysis: the Kaiser-Meyer-Olkin index value was 0.898 and the Bartlett sphericity test was highly significant (χ2 = 2935.3, df = 595, P < .001). Parallel analysis recommended the extraction of three factors accounting for 46.4% of total variance, and resulting model showed a good fit of data (goodness of fit index = 0.98, root mean square of residual = 0.0505). The rotated factor loading matrix is presented in Table 2. SCD factor 1 (eigenvalue = 11.70, 33.4% of variance) had strong factor loadings on items related to attention and language, SCD factor 2 (eigenvalue = 2.45, 7.0% of variance) was driven by items on orientation and memory (including both prospective and retrospective memory), and SCD factor 3 (eigenvalue = 2.07, 5.9% of variance) corresponded to praxis and domestic activities.
Demographic variables were not associated with these SCD factor scores in any group (see Supplementary Table 1) and were not included in the statistical models.
3.3. Patterns of SCD in clinical groups
3.3.1. Item-by-item analysis
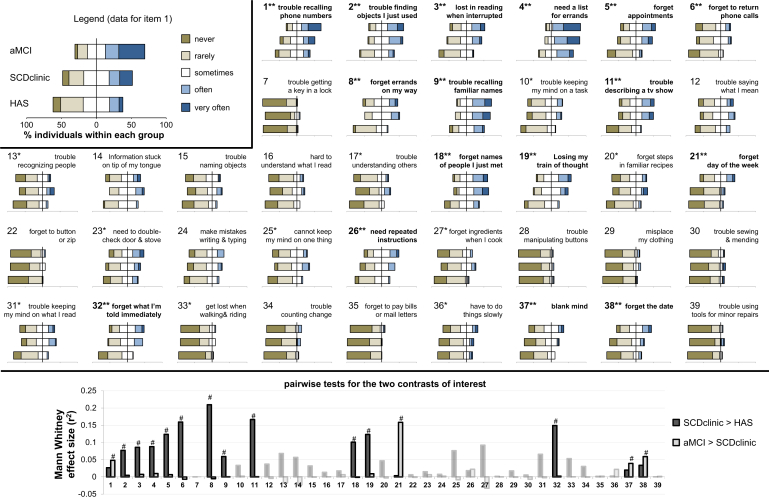
Item endorsement is visually presented in Fig. 1, Supplementary Fig. 2, and further described in the Supplementary Table 2. Of the 39 items included in the questionnaire, 16 (41%) showed significant between-group differences when applying a stringent Bonferroni correction (α = 0.001282), nine (23%) additional items were only significant at an uncorrected α = 0.05, whereas 14 (36%) did not show a significant effect of clinical group using Kruskal-Wallis test (see Supplementary Table 3 for further statistical details). Post hoc tests were conducted in the 16 significant items; most (11) of them were significant in the medical help-seeking contrast (HAS < SCDclinic). These items mainly related to retrospective (e.g., 2, 9, 11) and prospective (e.g., 4, 6, 8) aspects of memory, as well as attentional processes (e.g., 3, 19). In contrast, only four items were associated with the presence of memory deficits in patients consulting at a memory clinic (SCDclinic < aMCI). Two of these items assessed temporal disorientation (items 21 and 38, “I forget what day of the week/month it is”), one assessed semantic memory (item 1 “I have trouble recalling frequently used phone numbers”), and the last one referred to a feeling of a “blank mind” (item 37). It is to note that no item showed an unexpected gradient (higher endorsement in SCDclinic compared with HAS, or in aMCI compared with SCDclinic).
Fig. 1.
Item-by-item responses and group comparisons. The top panel shows the distribution of responses for each item within each clinical group (an alternative version of the figure is available in Supplementary Fig. 2 and numerical data are available in Supplementary Table 2). The top left box illustrates data presentation using diverging stacked bar plots, which enable precise visualization of the responses for each item within each group while showing global between-group trends (stronger endorsement in one group shifts the corresponding bar to the right). Note that the axis scale and increment were kept identical for all items to allow visual comparison. The full phrasing of all items is available in Table 2 but keywords are indicated in the present figure for the sake of simplicity. Kruskal-Wallis test was first used to identify items that showed group differences: ∗indicates an uncorrected P < .05, whereas ∗∗highlights items surviving stringent Bonferroni correction (P < .001282 = .05/39). The two contrasts of interest were tested using Mann-Whitney tests; bottom panel shows effect sizes (r2 = Z2/n) for each contrast (plain color for items surviving Bonferroni correction on the Kruskal-Wallis test and transparent color for others); #indicates significant group difference surviving Bonferroni correction for post hoc tests (P < .025 = .05/2). Numerical data are described in Supplementary Table 2 and details of the statistical tests can be found in Supplementary Table 3. Abbreviations: aMCI, amnestic mild cognitive impairment; HAS, healthy aged subjects; SCD, subjective cognitive decline.
Overall, these analyses suggested that item endorsement was strongly associated to medical help seeking and that a more subtle pattern was related to the presence of detectable memory deficits.
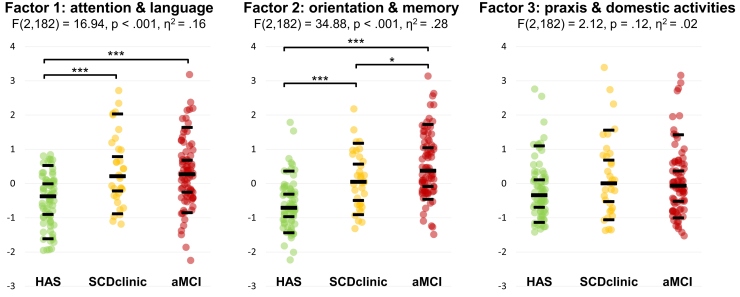
3.3.2. SCD factor scores
Analysis of SCD factor scores confirmed this pattern: the clinical group*SCD factor interaction was highly significant (F (4, 364) = 11.33, P < .001), indicating that group differences were not identical for the three SCD factors. Post hoc testing (see Fig. 2 and Supplementary Table 4) showed that SCD factor 1 was elevated in both memory clinic groups (SCDclinic and MCI) compared with control subjects but was very comparable in the two patient groups (Cohen's d: 0.09, IC95% [−0.27, 0.31]). SCD factor 2 showed a different pattern with an incremental increase from HAS to aMCI, all between-group differences being statistically significant. Finally, SCD factor 3 did not significantly differ between groups.
Fig. 2.
SCD factor scores across clinical groups. After the group*factor interaction was significant (F (4, 364) = 11.33, P < .001), between-group differences were assessed for each factor. Fisher's Least Significant Difference (LSD) was used as a post hoc test: ∗P < .05, and ∗∗∗P < .001. Scatterplot shows individual values, as well as 10th, 30th, 50th, 70th, and 90th percentiles within each group (black lines). Numerical data are described in Supplementary Table 4. Abbreviation: SCD, subjective cognitive decline.
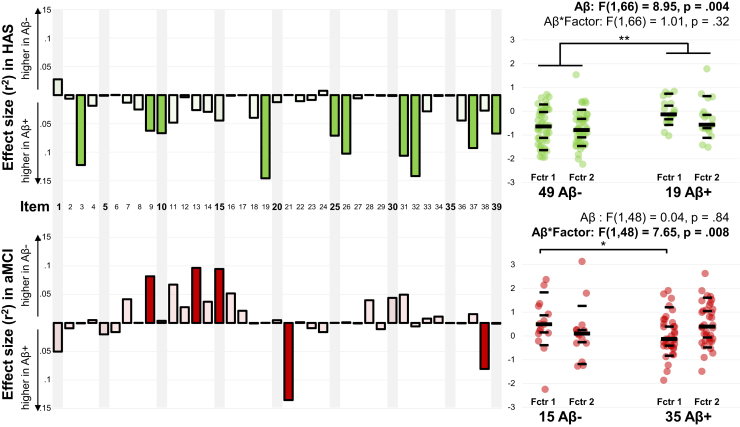
3.4. Association with Aβ status
Because of the small sample size in the SCDclinic group (nine Aβ-positive (28%), 18 Aβ-negative) compared with the other two groups (HAS: 19 Aβ-positive (33%), 49 Aβ-negative; aMCI: 35 Aβ-positive (70%), 15 Aβ-negative), the analyses were restricted to HAS and aMCI groups to avoid unbalanced statistical power.
3.4.1. Item-by-item analyses
In HAS, we observed a general trend for stronger item endorsement on almost all items in individuals harboring Aβ deposits compared with Aβ-negative participants (see Fig. 3, top left panel, and Supplementary Table 5). Although no difference was significant using a stringent Bonferroni correction, 10 items had uncorrected P values <.05. These items spanned a broad range of cognitive domains including memory (e.g., item 9 “I have trouble recalling names of people I know,” item 32 “I forget right away what people say to me,” and item 26 “I need to have instructions repeated several times”) and attention (e.g., item 10 “I find it hard to keep my mind on a task or a job,” item 19 “I lose my train of thought when I listen to somebody else,” and item 25 “I cannot keep my mind on one thing”).
Fig. 3.
Association between SCD and Aβ status in HAS and patients with aMCI. Left panel: item-by-item analyses. Mann-Whitney tests were used to compare individuals with and without evidence of Aβ using Florbetapir-PET. The figure illustrates the effect size (r2 = Z2/n) of the comparison. Filled bars indicate that the group difference reached an uncorrected threshold of α = 0.05 (but none was significant when using Bonferroni procedure to correct 39 tests). Further description of item scores and statistical comparison is available in Supplementary Table 5 (for HAS) and Supplementary Table 6 (for patients with aMCI). Right panel: factor score analysis. After the (clinical group*factor*Aβ status) interaction was significant (F (1, 114) = 9.00, P = .003), a repeated model analysis of variance was conducted within each clinical group. ∗P < .05, ∗∗P < .01. Scatterplot shows individual values, as well as 10th, 30th, 50th, 70th, and 90th percentiles within each group (black lines). Abbreviations: Aβ, β-amyloidosis; aMCI, amnestic mild cognitive impairment; HAS, healthy aged subjects; PET, positron emission tomography; SCD, subjective cognitive decline.
In aMCI, five items showed association with Aβ status, although not surviving Bonferroni correction. Interestingly, except for item 9, there was no overlap with the significant items identified in HAS; also, the direction of the association with Aβ was variable (see Fig. 3, bottom left panel, and Supplementary Table 6). On the one hand, three items related to semantic memory or language were more endorsed by Aβ-negative patients than Aβ-positive patients (item 9 “I have trouble recalling names of people I know,” item 13 “I fail to recognize people I know,” and item 15 “I have trouble finding the name of objects”). The opposite (higher endorsement in Aβ-positive patients) was only found for the two temporal orientation items (items 21 and 38 “I forget what day of the week/month it is”).
3.4.2. SCD factor scores
Further analyses were performed using SCD factor scores to confirm that the presence of Aβ modified the SCD pattern in a clinical group-dependent manner, as suggested by the item-by-item investigations. These analyses only included SCD factors 1 and 2 (as factor 3 did not differ between clinical groups and was of little relevance, being mainly driven by praxis-related items).
Indeed, in a full statistical model, the triple (clinical group*Aβ status*factor) interaction was significant (F (1, 114) = 9.00, P = .003), confirming that Aβ was not associated with the same SCD pattern in the two groups. Subsequent analyses in the HAS group showed a main effect of Aβ status (Aβ-positive individuals showing increased SCD factor scores) but no Aβ status*factor interaction (see Fig. 3, top right panel). In contrast, only the Aβ status*factor interaction was significant in the aMCI group, with post hoc tests showing that Aβ-positive aMCI patients had lower scores than their Aβ-negative counterparts on the SCD factor 1 (see Fig. 3, bottom right panel).
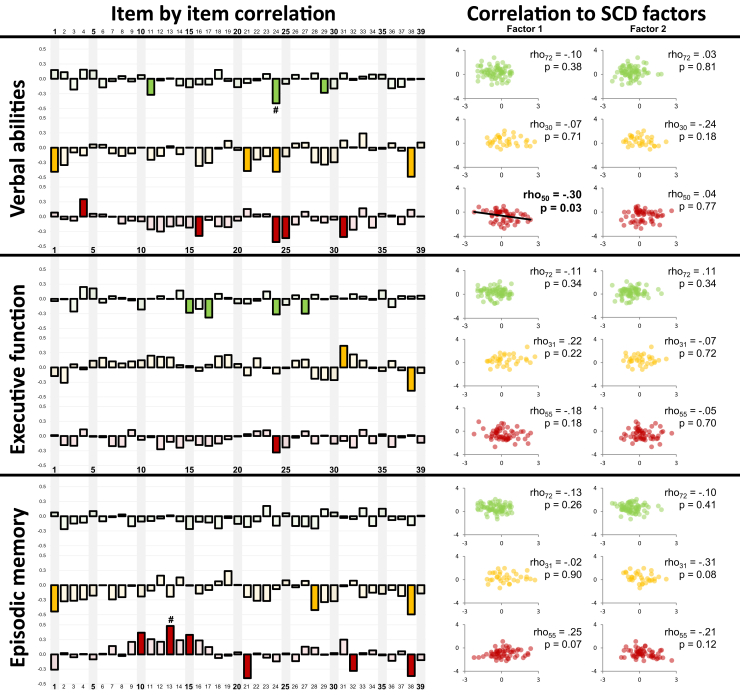
3.5. Association with cognitive measures
For each clinical group, correlations were assessed between responses on each item and each composite cognitive score (Fig. 4, left panel and Supplementary Table 7). Globally, results show that significant correlations with cognitive scores were very sparse and were found in both positive and negative directions. Analysis conducted with SCD factor scores 1 and 2 confirmed this pattern (Fig. 4, right panel): SCD was poorly correlated to cognitive scores, except for a mild association between SCD factor 1 and verbal abilities restricted to aMCI.
Fig. 4.
Association between SCD and cognitive scores. Left panel: item-by-item correlations between item endorsement and cognitive scores within each group (green, HAS; yellow, SCDclinic; red, aMCI). Correlations were assessed using nonparametric Spearman's coefficient; filled bars indicate that the correlation reached an uncorrected threshold of α = 0.05 whereas #indicates items that survived stringent Bonferroni correction (P < .001282 = .05/39). Positive coefficients indicate that higher levels of self-reported cognitive difficulties are associated with better cognitive performances. Right panel: correlation between SCD factor scores and cognitive scores within each group. Correlations were assessed using nonparametric Spearman's coefficient. Positive coefficients indicate that higher levels of self-reported cognitive difficulties are associated with better cognitive performances. Abbreviations: aMCI, amnestic mild cognitive impairment; HAS, healthy aged subjects; SCD, subjective cognitive decline.
4. Discussion
Consistent with the growing interest of studying SCD in early stages of AD and in line with the framework defined by the SCD-I, the present study aimed at refining our knowledge on SCD in nondemented elders. More precisely, we searched for specific self-reported cognitive difficulties associated with clinical features and Aβ imaging. To address these questions, we benefited from (1) using a questionnaire spanning multiple relevant cognitive domains and (2) including three groups of nondemented elders. This design allowed to distinguish correlates of medical help seeking (community-recruited HAS vs. SCDclinic) and detectable memory impairment (SCDclinic vs. aMCI).
4.1. Patterns of SCD in clinical groups
Clinical group comparisons revealed a pattern that was consistent when considering items separately or using SCD factor scores. Globally, responses of the SCDclinic group were closer to aMCI than HAS, suggesting that item endorsement was more influenced by the recruitment setting than by the presence of detectable memory impairment. Previous reports have also shown that medical help seeking is associated with a marked and generalized SCD [35], [36], [37], with a large overlap of global scores between asymptomatic and impaired patients [38], [39]. In addition, a few studies have used a more qualitative and quantitative approach, assessing different SCD items in aMCI and SCDclinic groups. In these studies, questionnaires were centered on subjective memory difficulties and showed no [40] or subtle [41] group differences. Our findings are consistent, but also expand these previous works: three of four items that differed between aMCI and SCDclinic groups were not memory related. Thus, self-reports of temporal disorientation and blank mind were specifically significantly elevated in aMCI. The subjective temporal disorientation is a robust finding (both items survived stringent multiple testing correction) and echoes clinical studies suggesting that objectively measured disorientation correlates with episodic memory deficits and indicates a higher risk for subsequent cognitive decline [42]. Finally, both pathology [43] and neuroimaging [42], [44] investigations showed that, in AD, temporal disorientation was related to neurodegeneration in the hippocampal regions and posterior association cortices. Although memory complaint itself is multidetermined and poorly specific to early AD, the co-occurrence of self-reported temporal disorientation and memory difficulties may provide converging evidence that the AD-sensitive hippocampoparietal network [27], [45] is dysfunctional, increasing the likelihood of underlying AD etiology.
4.2. SCD and Aβ
Associations between Aβ and SCD varied across domains and clinical stages. In community-recruited control subjects, we replicated previous findings [9], [10], [11], [12] showing higher SCD in Aβ-positive individuals. Interestingly, this relationship was not specific to self-reported memory difficulties; it was also found for language and attention items (see items 19 and 31, as well as SCD factor 1). Although most questionnaires currently in use are centered on memory complaints [22], our finding reinforces the importance of considering subjective cognition at large rather than focusing only on memory complaints, as suggested by the SCD-I [20], [22].
In aMCI, a reverse association was found (less SCD in Aβ-positive patients), especially on language items and factor scores. This might reflect the fact that Aβ-positive aMCI could actually have less language impairment than their Aβ-negative counterparts, but previous independent studies have not found such differences [46], [47]. Alternatively, the lower language SCD in Aβ-positive patients might be because of anosognosia. This would be consistent with the previous finding that an AD profile of cerebrospinal fluid biomarkers was associated with an underestimation of language difficulties in aMCI [48]. Future analyses of the neuropsychological and neuroimaging profiles of our patients with aMCI will help address these hypotheses. Interestingly, Aβ was associated with higher endorsement in both temporal orientation items. However, these differences did not survive Bonferroni correction and were not reflected in the factor analyses, because orientation items were grouped with memory items.
4.3. Limitations, conclusions, and perspectives
Our study has limitations. First, our questionnaire assessed current cognitive difficulties rather than feeling of decline over time, which might be more sensitive to detect AD-related SCD. Moreover, other potentially important features of SCD highlighted by the SCD-I (corroboration by informant, associated concern, and so forth) were not assessed here and should be investigated. In addition, patients were recruited from our regional university hospital, which might constitute a selection bias and prevent generalization to all medical help-seeking patients. Indeed, patients' cognitive complaints were likely considered serious enough by their primary care physicians to be referred to our center. Also it should be noted that additional complementary statistical approaches could be used to identify the most relevant items or combination of items, using logistic regression [49] or item response theory [50], [51]. Although interesting, the latter was not adapted to our goal (assessing different aspects of SCD) as it relies on the assumption that the questionnaire is unidimensional. Finally, it should be noted that, although we used advanced EFA methods on the CDS questionnaire, our sample size (n = 185) is limited and likely underpowered to optimally analyze the factorial structure of the CDS; replication in larger sample is therefore warranted to confirm and expand these results.
Overall, this report confirms the interest of studying SCD in nondemented elders and points to the relevance of assessing SCD beyond the memory domain. Subtle temporal disorientation seems particularly interesting in a clinical setting: corresponding items were associated with the presence of both detectable memory deficits and amyloidosis.
Future studies are needed to replicate and expand our findings, for example with additional AD biomarkers or through a longitudinal design, and with larger samples, especially in the SCDclinic group.
Research in Context.
-
1.
Systematic review. The authors recently reviewed the literature on subjective cognitive decline (SCD) and Alzheimer's disease (AD) neuroimaging biomarkers (http://dx.doi.org/10.1016/j.jalz.2016.08.011). The present work falls within the framework defined by the SCD-initiative (http://dx.doi.org/10.1016/j.jalz.2014.01.001) and addresses questions raised in a recent overview of questionnaires currently used to assess SCD in preclinical AD (http://dx.doi.org/10.3233/JAD-150154).
-
2.
Interpretation. SCD endorsement was strongly related to medical help seeking, whereas more subtle nuances notably related to self-reported temporal disorientation were specifically associated with objectively detectable memory deficits. The presence of Aβ was associated with different patterns of SCD beyond memory complaint in both healthy control subjects and patients with MCI.
-
3.
Future directions. Our data reinforce the need to consider subjective cognition at large rather than a more specific memory complaint to detect early AD. Pending replication, these results could help improve enrichment strategies to screen for relevant candidates for antiamyloid trials conducted in preclinical and prodromal stages.
Acknowledgments
The authors thank J. Gonneaud, R. De Flores, J. Mutlu, B. Landeau, G. Poisnel, M. Leblond, T. Anquetil, K. Mevel, N. Villain, M. Fouquet, A. Quillard, C. Schupp, J. Dayan, A. Chocat, L. Barre, A. Manrique, and the Cyceron MRI-PET staff members for their help with data acquisition. We are grateful to Adrienne Visani, Jacob Vogel, Alexandre Bejanin, and Eider Arenaza-Urquijo for their insightful comments and to the participants of the IMAP + study.
Conflicts of interest: Dr Perrotin now works for Piramal Imaging Ltd. None of the other authors report any conflict of interest.
Sources of funding and support: The study was supported by Fondation Plan Alzheimer (Alzheimer Plan 2008–2012); Programme Hospitalier de Recherche Clinique (PHRCN 2011-A01493-38 and PHRCN 2012 12-006-0347); Agence Nationale de la Recherche (LONGVIE 2007); Région Basse-Normandie; Association France Alzheimer et maladies apparentées AAP 2013. Funding sources were not involved in the study design, data acquisition, data analysis, or manuscript writing.
The first author had full access to the data and takes responsibility for the integrity of data analysis.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.12.005.
Supplementary data
References
- 1.Reisberg B., Ferris S.H., de Leon M.J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 2.McGlone J., Gupta S., Humphrey D., Oppenheimer S., Mirsen T., Evans D.R. Screening for early dementia using memory complaints from patients and relatives. Arch Neurol. 1990;47:1189–1193. doi: 10.1001/archneur.1990.00530110043015. [DOI] [PubMed] [Google Scholar]
- 3.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 7.Lista S., Molinuevo J.L., Cavedo E., Rami L., Amouyel P., Teipel S.J. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis. 2015;48:S171–S191. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- 8.Perrotin A., La Joie R., De La Sayette V., Barré L., Mézenge F., Mutlu J. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrotin A., Mormino E.C., Madison C.M., Hayenga A.O., Jagust W.J. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielke M.M., Wiste H.J., Weigand S.D., Knopman D.S., Lowe V.J., Roberts R.O. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;9:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley R.F., Villemagne V.L., Masters C.L., Ellis K.A., Rowe C.C., Johnson K. A conceptualization of the utility of subjective cognitive decline in clinical trials of preclinical Alzheimer's disease. J Mol Neurosci. 2016;60:354–361. doi: 10.1007/s12031-016-0810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel P.S., Palmqvist S., Mackin R.S., Nosheny R.L., Hansson O., Weiner M.W. Assessing risk for preclinical β-amyloid pathology with APOE, cognitive, and demographic information. Alzheimers Dement (Amst) 2016;4:76–84. doi: 10.1016/j.dadm.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sevigny J., Suhy J., Chiao P., Chen T., Klein G., Purcell D. Amyloid PET screening for enrichment of early-stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1–7. doi: 10.1097/WAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 16.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R.J. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn D.J., Wakefield S., Shanks M.F., Harkness K., Reuber M., Venneri A. Memory difficulties are not always a sign of incipient dementia: a review of the possible causes of loss of memory efficiency. Br Med Bull. 2014;112:71–81. doi: 10.1093/bmb/ldu029. [DOI] [PubMed] [Google Scholar]
- 19.Comijs H.C., Deeg D.J., Dik M.G., Twisk J.W., Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 20.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinuevo J.L., Rabin L.A., Amariglio R., Buckley R., Dubois B., Ellis K.A. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabin L.A., Smart C.M., Crane P.K., Amariglio R.E., Berman L.M., Boada M. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 International Research Studies. J Alzheimers Dis. 2015;48:S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNair D.M., Kahn R.J. Mark Powley Associates; New Canaan: 1983. Self-assessment of cognitive deficits. Assessment in geriatric psychopharmacology. [Google Scholar]
- 24.Perrotin A., Desgranges B., Landeau B., Mézenge F., La Joie R., Egret S. Anosognosia in Alzheimer disease: disconnection between memory and self-related brain networks. Ann Neurol. 2015;78:477–486. doi: 10.1002/ana.24462. [DOI] [PubMed] [Google Scholar]
- 25.Perrotin A., de Flores R., Lamberton F., Poisnel G., La Joie R., de la Sayette V. Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. J Alzheimers Dis. 2015;48:S141–S150. doi: 10.3233/JAD-150087. [DOI] [PubMed] [Google Scholar]
- 26.Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mézenge F., Egret S. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J Neurosci. 2015;35:10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Joie R., Landeau B., Perrotin A., Bejanin A., Egret S., Pélerin A. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014;81:1417–1428. doi: 10.1016/j.neuron.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Fouquet M., Desgranges B., La Joie R., Rivière D., Mangin J.-F., Landeau B. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic mild cognitive impairment. Neuroimage. 2012;59:3309–3315. doi: 10.1016/j.neuroimage.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Grober E., Sanders A.E., Hall C., Lipton R.B. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24:284–290. doi: 10.1097/WAD.0b013e3181cfc78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gass C.S., Apple C. Cognitive complaints in closed-head injury: relationship to memory test performance and emotional disturbance. J Clin Exp Neuropsychol. 1997;19:290–299. doi: 10.1080/01688639708403858. [DOI] [PubMed] [Google Scholar]
- 31.Derouesné C., Dealberto M.J., Boyer P., Lubin S., Sauron B., Piette F. Empirical evaluation of the “Cognitive Difficulties Scale” for assessment of memory complaints in general practice: a study of 1628 cognitively normal subjects aged 45–75 years. Int J Geriatr Psychiatry. 1993;8:599–607. [Google Scholar]
- 32.Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Winter J.C.F., Dodou D. Five-point likert items: T test versus Mann-Whitney-Wilcoxon. Pract Assess Res Eval. 2010;15:1–16. [Google Scholar]
- 34.Baglin J. Improving your exploratory factor analysis for ordinal data: a demonstration using FACTOR. Pract Assess Res Eval. 2014;19:1–14. [Google Scholar]
- 35.Pires C., Silva D., Maroco J., Ginó S., Mendes T., Schmand B.A. Memory complaints associated with seeking clinical care. Int J Alzheimers Dis. 2012;2012:725329. doi: 10.1155/2012/725329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckerström M., Skoogh J., Rolstad S., Göthlin M., Steineck G., Johansson B. Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire (SASCI-Q)—a research tool discriminating between subjectively cognitively impaired patients and healthy controls. Int Psychogeriatr. 2013;25:420–430. doi: 10.1017/S1041610212001846. [DOI] [PubMed] [Google Scholar]
- 37.Buelow M.T., Tremont G., Frakey L.L., Grace J., Ott B.R. Utility of the cognitive difficulties scale and association with objective test performance. Am J Alzheimers Dis Other Demen. 2014;29:755–761. doi: 10.1177/1533317514539032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salem L.C., Vogel A., Ebstrup J., Linneberg A., Waldemar G. Subjective cognitive complaints included in diagnostic evaluation of dementia helps accurate diagnosis in a mixed memory clinic cohort. Int J Geriatr Psychiatry. 2015;30:1177–1185. doi: 10.1002/gps.4272. [DOI] [PubMed] [Google Scholar]
- 39.Lehrner J., Moser D., Klug S., Gleiß A., Auff E., Dal-Bianco P. Subjective memory complaints, depressive symptoms and cognition in patients attending a memory outpatient clinic. Int Psychogeriatr. 2014;26:463–473. doi: 10.1017/S1041610213002263. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed S., Mitchell J., Arnold R., Dawson K., Nestor P.J., Hodges J.R. Memory complaints in mild cognitive impairment, worried well, and semantic dementia patients. Alzheimer Dis Assoc Disord. 2008;22:227–235. doi: 10.1097/WAD.0b013e31816bbd27. [DOI] [PubMed] [Google Scholar]
- 41.Ryu S.Y., Lee S.B., Kim T.W., Lee T.J. Memory complaints in subjective cognitive impairment, amnestic mild cognitive impairment and mild Alzheimer's disease. Acta Neurol Belg. 2016;116:535–541. doi: 10.1007/s13760-016-0604-7. [DOI] [PubMed] [Google Scholar]
- 42.Sousa A., Gomar J.J., Goldberg T.E. Neural and behavioral substrates of disorientation in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement (N Y) 2015;1:37–45. doi: 10.1016/j.trci.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannakopoulos P., Gold G., Duc M., Michel J.P., Hof P.R., Bouras C. Neural substrates of spatial and temporal disorientation in Alzheimer's disease. Acta Neuropathol (Berl) 2000;100:189–195. doi: 10.1007/s004019900166. [DOI] [PubMed] [Google Scholar]
- 44.Hirono N., Mori E., Ishii K., Ikejiri Y., Imamura T., Shimomura T. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;64:552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 46.Hanseeuw B., Dricot L., Lhommel R., Quenon L., Ivanoiu A. Patients with amyloid-negative mild cognitive impairment have cortical hypometabolism but the hippocampus is preserved. J Alzheimers Dis. 2016;53:651–660. doi: 10.3233/JAD-160204. [DOI] [PubMed] [Google Scholar]
- 47.Ye B.S., Seo S.W., Kim C.H., Jeon S., Kim G.H., Noh Y. Hippocampal and cortical atrophy in amyloid-negative mild cognitive impairments: comparison with amyloid-positive mild cognitive impairment. Neurobiol Aging. 2014;35:291–300. doi: 10.1016/j.neurobiolaging.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Edmonds E.C., Delano-Wood L., Galasko D.R., Salmon D.P., Bondi M.W. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J Int Neuropsychol Soc. 2014;20:836–847. doi: 10.1017/S135561771400068X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall G.A., Zoller A.S., Kelly K.E., Amariglio R.E., Locascio J.J., Johnson K.A. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2014;11:853–861. doi: 10.2174/1567205011666141001120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gifford K.A., Liu D., Romano R.R., Jones R.N., Jefferson A.L. Development of a subjective cognitive decline questionnaire using item response theory: a pilot study. Alzheimers Dement (Amst) 2015;1:429–439. doi: 10.1016/j.dadm.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snitz B.E., Yu L., Crane P.K., Chang C.C., Hughes T.F., Ganguli M. Subjective cognitive complaints of older adults at the population level: an item response theory analysis. Alzheimer Dis Assoc Disord. 2012;26:344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.