Abstract
Coronary artery disease (CAD) is one of the important causes of cardiovascular morbidity and mortality globally, giving rise to more than 7 million deaths annually. An increasing burden of CAD in India is a major cause of concern with angina being the leading manifestation. Stable coronary artery disease (SCAD) is characterised by episodes of transient central chest pain (angina pectoris), often triggered by exercise, emotion or other forms of stress, generally triggered by a reversible mismatch between myocardial oxygen demand and supply resulting in myocardial ischemia or hypoxia. A stabilised, frequently asymptomatic phase following an acute coronary syndrome (ACS) is also classified as SCAD. This definition of SCAD also encompasses vasospastic and microvascular angina under the common umbrella.
Keywords: Management standards, Stable CAD, Treatment algorithms, Guidelines, Developing countries, Practice guidelines
1. Definition, prevalence and pathophysiology
Coronary artery disease (CAD) is one of the important causes of cardiovascular morbidity and mortality globally, giving rise to more than 7 million deaths annually.1 An increasing burden of CAD in India is a major cause of concern with angina being the leading manifestation. Stable coronary artery disease (SCAD) is characterised by episodes of transient central chest pain (angina pectoris), often triggered by exercise, emotion or other forms of stress, generally triggered by a reversible mismatch between myocardial oxygen demand and supply resulting in myocardial ischaemia or hypoxia.2 A stabilised, frequently asymptomatic phase following an acute coronary syndrome (ACS) is also classified as SCAD. This definition of SCAD also encompasses vasospastic and microvascular angina under the common umbrella.
The common clinical presentation of SCAD is chronic stable angina. The underlying mechanisms may include atherogenesis and plaque formation in epicardial arteries, spasm of normal or plaque containing arteries, micro-vascular, or left ventricular dysfunction due to prior acute myocardial necrosis or ischaemic cardiomyopathy.2 In addition to chest discomfort, dyspnoea, palpitations, syncope or fatigue may also be present and sometimes may be the only symptom. Classical angina and micro-vascular angina are difficult to differentiate owing to the fact that both are exercise induced. Vasospastic angina, on the other hand, occurs at rest and has preserved effort tolerance. As opposed to ACS, SCAD lesions are more fibrotic, have a small necrotic core and little or no overlying thrombus.3 Besides, they do not display erosion or rupture of endothelial lining.4 Vasospasm is mostly due to various vasoconstrictor stimuli acting on hyper-reactive vascular smooth muscle cells, like cellular rho-kinase activity, abnormalities in adenosine triphosphate (ATP) sensitive potassium channels and/or membrane Na+-H+ counter-current transport.5 The diffuse distal spastic reaction usually underlies micro-vascular angina, while focal spasm is classically observed in variant angina.
In the last three decades, the prevalence of CAD has increased from 1.1% to about 7.5% in the urban population and from 2.1% to 3.7% in the rural population.6 Coronary artery disease tends to occur at a younger age in Indians with 50% of cardiovascular mortality occurring in individuals aged <50 years.7, 8 The younger CAD patients have more extensive angiographic involvement contributed by genetic, metabolic, conventional and nonconventional risk factors. Although several risk factors have been suggested, smoking, dyslipidemia and hypertension remain the major risk factors in the younger CAD patients.6
A need for developing practice standards for the management of stable CAD was perceived by experts across India. This document aims to assist physicians, particularly cardiologists, in clinical decision-making by delineating a gamut of commonly acceptable modalities for the diagnosis, management, and prevention of stable angina. The current management standards have defined practices that meet the needs of most patients in the Indian context.7 A modified GRADE system was used to derive quality of evidence and grades of recommendations.
2. Diagnosis
The initial and important step in the management of stable angina is the diagnosis. A detailed clinical history of angina includes assessing magnitude, location, severity, duration and precipitating factors of angina. Presence of typical nature of the discomfort, precipitating factors and relieving factors should be suggestive angina. Taking the medical history of a patient is the foremost and most vital process. Indeed in most cases a clear diagnosis can be made based on the history alone of a patient.9 Physical examination is required to corroborate and strengthen the diagnosis.10
Besides history and physical examination, diagnosis of stable angina needs the supporting evidence from non-invasive investigations to confirm it and sometimes provide additional prognostic information. It has been demonstrated that there is an association between low haemoglobin concentration and increased mortality in patients with stable angina.11, 12 Similarly, fasting blood glucose has a prognostic importance in patients with stable angina.13, 14 Furthermore, a full lipid profile, renal, liver and thyroid function tests, a 12-lead electrocardiography (ECG), resting echocardiography and chest X-ray can be helpful in evaluating stable angina.2 An elevated resting heart rate is also associated with poor prognosis in patients with SCAD.15 Patients with resting heart rate ≥70 bpm (beats per minute) exhibit a higher risk of vascular events compared to patients with a heart rate <70 bpm.16 Therefore, it is prudent to include heart rate determination as a part of the routine cardiac assessment.
Fractional flow reserve (FFR) is an evidence-based diagnostic test gradually gaining prognostic value.17 In a meta-analysis non-invasive FFR demonstrated high diagnostic efficacy comparable with invasively measured FFR for the detection of ischaemia in stable patients with suspected or known CAD.18 Therefore, in select patients FFR testing can be recommended if resources permit.
The diagnosis of microvascular angina can be done by a combination of various modalities. It can be diagnosed by normal coronary angiogram and the absence of epicardial coronary spasm at rest or during acetylcholine provocation. A coronary flow reserve <2.5 on adenosine-induced hyperemia,19 data from positron emission tomography20 and cardiac magnetic resonance21 have been also used in diagnosis of micro-vascular angina. Similarly, vasospastic angina can be diagnosed by ECG and coronary arteriography showing epicardial coronary spasm at rest or during acetylcholine provocation.2 Further, exercise stress test and Holter monitoring can be used to detect silent ischaemia.22
Recommendations on diagnosis.
-
•
Patient's history and physical examination should be considered to identify all the symptoms and signs of cardiovascular diseases (CVDs), medical history, CV risk factors, and other cardiac etiologies. [Grade A, Evidence 3]9, 10
-
•
The basic first-line testing in patients with suspected SCAD includes standard laboratory biochemical testing (including haemoglobin, HbA1C (glycated haemoglobin), lipid profile, liver, renal and thyroid function tests), a resting ECG, resting echocardiography and, in selected patients, a chest X-ray. [Grade A, Evidence 3]2, 23, 24, 25, 26, 27
-
•
It is recommended to include assessment of resting heart rate in SCAD patients as a routine clinical practice. [Grade A, Evidence 2]15, 28, 29
-
•
Exercise ECG testing, if possible, should be preferred in patients with a pre-test probability (based on character of symptom, age and sex) of 15–65% as it is more relevant to their activities than pharmacological testing. [Grade A, Evidence 2]30, 31
-
•
In patients who cannot exercise to an adequate workload, pharmacological testing with adenosine-induced vasodilator perfusion imaging or dobutamine echocardiography should be considered. [Grade A, Evidence 4]2
-
•
An invasive coronary angiogram is indicated in significantly symptomatic patients, patients with high risk features on non-invasive testing.
-
•
Certain specific types of angina (microvascular, vasospastic and silent angina) should be diagnosed by a combination of available diagnostic techniques and should be individualised. [Grade A, Evidence 4]2
3. Management
Diverse risk factor distribution, across various geographical locations in India mandates implementation of aggressive strategies for risk factor control and prevention. An effective usage of primary and secondary prevention strategies is another important aspect of CVD prevention and control. Developing the clinical practice algorithms translates best evidence into best practice and promotes quality by reducing healthcare variations, refining diagnostic accuracy, promoting appropriate therapy, and preventing the use of ineffective or potentially harmful interventions.
The treatment of stable angina includes anti-anginal medication, medication to modify atherosclerosis and aggressive treatment of causative risk factors. All patients with SCAD require life-long supervised treatment. Overall, management strategy includes, lifestyle changes, pharmacological management and prevention of cardiovascular events (Fig. 1), various revascularisation techniques and management considerations for special groups such as women, elderly, renal dysfunction and diabetic patients.
Fig. 1.
An algorithm for pharmacological management and event prevention. (A) Pharmacological management and event prevention. (B) Medical management options. Abbreviations: ACEIs: angiotensin-converting-enzyme inhibitors; ARB: angiotensin receptor blockers; ASA: acetyl salicylic acid (aspirin); BBs: beta-blockers; BP: blood pressure; CABG: coronary artery bypass grafting; CCBs: calcium channel blockers; CT: computed tomographic; DHP: dihydropyridine; DVD: double vessel disease; ECG: electrocardiogram; EF: ejection fraction; HR: heart rate; LAD: left anterior descending; LV: left ventricular; PCI: percutaneous coronary intervention; SCAD: stable coronary artery disease; SVD: single vessel disease; TVD: triple vessel disease.
4. Lifestyle management and control of risk factors
4.1. Smoking
Tobacco smoking is one of the frequent causes of mortality; indeed smoking can result in at least 2.8–3.1 fold increase in the risk of CAD.32 Passive smoking or second-hand smoke has been demonstrated to exhibit a positive association with CAD (RR 1.27; 95% CI 1.10–1.48) and has displayed a crucial impact on the prognosis of CVDs.33 It is known that about 35–40% of tobacco consumption in India is in smokeless form, mostly chewing.34 Similar to smoking, smokeless tobacco also has a strong association with myocardial infarction (MI) (RR 1.13 (95% confidence interval (CI) 1.06–1.21)) and stroke 1.40 (95% CI 1.28–1.54).35 Abstaining from all forms smoking (direct and second-hand or passive smoking) is an important factor in the primary and secondary prevention of cardiac events and has become an essential part of lifestyle management. Nicotine replacement and sustained release bupropion (bupropion SR) are the potential treatments for smoking. Nicotine patches have been examined extensively in patients with SCAD and shown to be safe.36
4.2. Diet
Several studies show that specific and conscious modification of diet is an essential step towards attaining healthy diet, which could decrease diet-induced CV risk. Indeed, in a prospective cohort study, 31,546 high-risk individuals from 40 countries, higher-quality diet decreased risk of recurrent CVD events among people ≥55 years of age with CVD or diabetes mellitus.37 A Mediterranean diet, Dietary Approach to Stop Hypertension (DASH) diet, rich in fruits, vegetables, and low-fat dairy foods and with reduced saturated and total fat has demonstrated a reduction in blood pressure and is effective as a nutritional strategy for preventing CVD.38 In the Indian context, a diet rich in fruits and vegetable has a positive association with the heart diseases. Indeed, a couple of studies have observed a significant protective effect of vegetable intake against CAD risk.39 The effects of a low-carbohydrate diet have also been investigated in a meta-analysis and was found correlative of modest but significant weight loss.40 Further, drinking plenty of water,41 and reducing the consumption of sweetened beverages and juices42 are advisable, as they can reduce the excessive body weight considerably. Consumption of alcoholic beverages has not been found to be beneficial in Indian patients and if at all should be limited to 2 glasses per day (20 g/day of alcohol) for men and 1 glass per day (10 g/day of alcohol) for non-pregnant women. Moreover, Omega-3 polyunsaturated fatty acids has a promising role in primary but particularly in the secondary prevention of CVD.43, 44
4.3. Physical activity
Regular physical activity and aerobic exercises have demonstrated decreased risk of fatal and non-fatal coronary events.45 Moreover, a sedentary lifestyle is one of the key risk factors for CVD.46 Therefore, occupational and leisure time physical activity can be recommended for the prevention of CVD. In this context, a meta-analysis of observational data demonstrated that high level occupational physical activity protected against ischaemic stroke compared with both moderate (RR = 0.77, 95% CI 0.60–0.98) and inactive occupational levels (RR = 0.57, 95% CI 0.43–0.77).47
4.4. Weight management
Overweight and obesity are the well-recognised risk factors for CAD.48 The metabolic consequences of obesity such as dyslipidemia (particularly hyper-triglyceridemia, low high density lipoprotein [HDL] and increased numbers of small dense low-density lipoprotein [LDL] particles) and dysglycaemia (insulin resistance and type 2 diabetes), are apparent even at a lower absolute levels of total body fat in South Asians than in whites.49 Taking it into consideration, the Asian Indian consensus defines body mass index (BMI) cut off: normal BMI, 18.0–22.9 kg/m2; overweight, 23.0–24.9 kg/m2; obesity: >25 kg/m2.50 Besides BMI, waist circumference (WC) has emerged as an important indicator for the prognosis of CVDs.50, 51 For Asian Indians, the WC cut-offs at action level 1 are: men, 78 cm; women, 72 cm; and the corresponding values at action level 2 are 90 cm and 80 cm for men and women, respectively. Any person attaining action level 1 should avoid gaining weight and maintain physical activity to avoid developing any of the cardiovascular risk factors. Similarly, action level 2 indicates need for medical advice.50
4.5. Lipid management
Dyslipidemia is one of the critical risk factors for CVD.52 Dyslipidemia should be managed according to lipid management guidelines with pharmacological and lifestyle interventions.53 Treatment objectives include LDL-cholesterol (LDL-C) below 1.8 mmol/L (<70 mg/dL) or >50% (LDL-C) reduction when an objective level cannot be reached. Patients having CAD are considered as highly vulnerable subjects for cardiovascular events and statin treatment should be considered as early as possible, regardless of LDL-C concentrations. Indeed, a meta-analysis demonstrated that statin pre-treatment in patients with stable angina reduces procedural myocardial necrosis and overall major adverse cardiac event.54 Other interventions like fibrates, nicotinic acid, ezetimibe have also been examined for reducing cardiovascular events.
4.6. Diabetes mellitus
Comprehensive risk reduction is essential for the management of diabetic patients with CAD. General measures should comprise diet, physical activity, complete cessation of smoking, and weight and lipid profile management. Furthermore, pharmacological management for glycaemic control has its important role to achieve precise glycaemic control (objective HbA1C < 7.0%).2
4.7. Arterial hypertension
Arterial hypertension is one of the risk factors for SCAD and other CVDs.55 It is advisable to lower the systolic blood pressure (SBP) to <140 mmHg and diastolic blood pressure (DBP) to <90 mmHg in SCAD patients with hypertension. Moreover, diabetes patients are advised to achieve a goal of at least lower than 140/85 mmHg BP.44
Recommendations on lifestyle management and risk factor modifications.
-
•
It is recommended to stop all forms of tobacco (smoking and smokeless) for the prevention and control of cardiovascular risk. [Grade A, Evidence 1]33, 35, 56, 57
-
•
Patients with previous acute MI, coronary artery bypass graft (CABG), percutaneous coronary interventions (PCI), stable angina pectoris, or stable chronic heart failure should undergo moderate-to-vigorous intensity aerobic exercise training ≥3 times a week and 30 min per session. Sedentary patients should be strongly encouraged to start light-intensity exercise programmes after adequate exercise-related risk stratification. [Grade A, Evidence 3]44
-
•
Weight reduction in overweight and obese people is recommended to have favourable effects on blood pressure and dyslipidaemia, which may lead to less CVD. [Grade A, Evidence 1]. More precisely, it is recommended to attain BMI <22.9 kg/m2and WC (men: 90 cm; women: 80 cm) to minimise the cardiovascular risk. [Grade A, Evidence 1]50, 58, 59, 60, 61
-
•
All the SCAD patients should be treated with statins to achieve optimal LDL-C goal <70 mg/dl. [Grade A, Evidence 2]54, 62, 63, 64
-
•
All the SCAD patients with hypertension should be recommended to attain SBP/DBP goal of 140/90 mmHg with medical management. [Grade A, Evidence 2]65, 66, 67, 68, 69
-
•
HbA1C of <7.0% should be the objective while treating SCAD patients with diabetes. [Grade A, Evidence 2]70, 71, 72
5. Pharmacological management
The main objectives of the pharmacological management of SCAD include relief of anginal symptoms and to avert cardiovascular anginal episodes or cardiovascular event prevention.
5.1. Relief of anginal symptoms
Besides anti-ischaemic agents, other initiatives such as lifestyle changes, including regular physical exercise, and revascularisation may play a crucial role in symptom amelioration. In addition, patient education is vital to improving adherence to the measures of primary and secondary prevention in the course of disease management over the long tenure.
5.1.1. Long-term relief of symptoms
5.1.1.1. Beta-blockers
Beta-blockers improve oxygen supply and demand balance by reducing heart rate, myocardial contractility and after-load. Reduction in heart rate allows greater diastolic time with increased coronary perfusion that improves myocardial oxygen supply.
Though there is no direct evidence from placebo-controlled trials that demonstrate the benefits of beta-blockers in stable CAD patients in absence of prior MI, a meta-analysis has demonstrated that cardio-selective beta-blockers may improve mortality when compared with placebo.73 The use of beta-blockers has certainly been found beneficial in relieving angina in the randomised trials enrolling post-MI or heart failure patients. Beta-blockers are also found to be effective in regulating exercise-induced angina, increasing exercise capacity and controlling both symptomatic as well as asymptomatic ischaemic attacks.
5.1.1.2. Calcium channel blockers
Calcium channel blockers (CCBs) noncompetitively inhibit calcium influx through voltage-dependent L-type calcium channels, resulting in negative inotropic effects, chronotropic effects, slowing conduction, and smooth muscle relaxation. CCBs are chemically of two types: dihydropyridine (DHP) CCBs and non-DHP CCBs. The non-DHP CCBs (verapamil and diltiazem), due to their sinus node inhibiting activity, reduce the heart rate and act as anti-anginal drugs. A meta-analysis revealed that when compared with placebo, CCBs resulted in a 28% reduction in the risk of heart failure (95% CI 0.73–0.92) in patients with CAD.74 This meta-analysis also demonstrated that long-acting CCBs (either DHPs or non-DHPs) were associated with a decrease in the risk of stroke, angina pectoris and heart failure with similar outcomes for other cardiovascular events as the comparison.74
5.1.1.3. Beta-blockers vs. calcium channel blockers
Beta-blockers have been compared to CCBs in numerous trials for the management of stable angina. With regard to the treatment efficacy against angina, beta-blockers and CCBs are found to be similar.75, 76, 77 Indeed, a couple of meta-analyses revealed that the CCBs and beta-blockers exert similar anti-anginal effects; nevertheless, beta-blockers were found to be associated with fewer adverse events than CCBs.73, 78
5.1.1.4. Beta-blockers plus calcium channel blockers
Beta-blockers in combination with DHP-CCBs were found to be more effective in relieving angina. A meta-analysis based on effects on exercise testing concluded that the combination of a calcium antagonist and a beta-blocker was statistically more effective than either monotherapy.79
The choice of the first-line agent should be individualised based on the desired pharmacological actions, co-morbidities and side effects. However, the use of beta-blockers in combination with diltiazem and verapamil should be avoided due to the probable risk of bradycardia.2, 80
5.1.1.5. Trimetazidine
Trimetazidine is a novel metabolic modulator. It inhibits reduction of intracellular ATP levels via conservation of cellular metabolism in ischaemic regions and stimulates myocardial glucose consumption through inhibition of fatty acid metabolism.81 Eventually, it leads to the formation of ATP while consuming less oxygen (which is inadequate in ischaemia) without affecting heart rate, blood pressure and myocardial contractility.82, 83
The Trimetazidine European Multicenter Study compared the efficacy of trimetazidine with beta-blocker propranolol and found no difference between the two groups with regards to anginal attack rate per week, exercise duration or time to 1 mm ST-segment depression.84 A Cochrane systematic review (n = 23), revealed that in comparison with placebo, trimetazidine alone or combined with conventional anti-anginal agents, decreased the number of weekly angina attacks (mean difference −1.44, 95% CI −2.10 to −0.79; p < 0.0001), reduced weekly nitroglycerin intake (95% CI −1.47 to −2.20, −0.73; p < 0.0001) and improved exercise time to 1 mm ST segment depression (p = 0.0002).85 In a retrospective analysis assessing the independent effect of anti-anginal drugs on subsequent mortality risk in patients with stable angina, trimetazidine treatment was associated with significantly reduced risk of death after surviving an acute myocardial infarction (AMI).86 Trimetazidine has been evaluated in diverse patient populations ranging from stable angina, post-MI, pre, post PCI and heart failure and was found to have excellent safety and tolerability profile without any known drug interactions. It was found to be safe when added to ongoing therapy with beta-blockers, CCBs, and nitrates, without unfavourable drug interactions in SCAD.87 In fact, it has been shown that combining a metabolic agent with a haemodynamic agent may offer a better synergistic efficacy when compared to a combination of two haemodynamic agents.88 In a recent systematic review and meta-analysis of AMI patients, adjunctive trimetazidine treatment was associated with a significant reduction of major adverse cardiac events (MACE) (OR = 0.33, 95% CI = 0.15–0.74; p = 0.007).89 The usual dose of trimetazidine is 20 mg three times daily or sustained release 35 mg twice daily with meals.90 The European Society of Cardiology (ESC) guidelines recommend the use of trimetazidine as a second-line treatment for the treatment of angina on the basis of current evidence.2 However, use of trimetazidine in patients with Parkinson's disease and motion disorders such as restless leg syndrome, tremor muscle rigidity and walking disorders, is contraindicated.2
5.1.1.6. Ranolazine
Ranolazine is an anti-anginal agent acting via inhibition of the late sodium influx in the myocardium, in voltage and frequency dependent manner.91
A systematic review demonstrated that in comparison to placebo, alone or in combination with other anti-anginal drugs (amlodipine and atenolol), ranolazine reduced anginal symptoms among patients with symptomatic chronic stable angina pectoris on traditional anti-anginal medications.92 Ranolazine has been studied in several trials such as Monotherapy Assessment of Ranolazine In Stable Angina (MARISA) study,93 the Efficacy of Ranolazine in Chronic Angina (ERICA) trial94 and the Combination Assessment of Ranolazine In Stable Angina (CARISA) study.95 In the Metabolic Efficiency with Ranolazine for Less Ischaemia in Non–ST-Elevation Acute Coronary Syndromes (MERLIN-TIMI) trial involving 6560 patients with NSTE-ACS, ranolazine did not reduce the primary composite endpoint of cardiovascular death, MI or recurrent ischaemia.96 In a more recent RIVER-PCI trial enrolling 2651 chronic angina patients with incomplete revascularisation post PCI, ranolazine had no effect on primary endpoint consisting of ischaemia driven revascularisation and hospitalisation without revascularisation (HR 0.95, 95% CI 0.82–1.10; p = 0.48).97
Co-administration of ranolazine with cytochrome P3A (CYP3A) inhibitors like verapamil, diltiazem, macrolide antibiotics and grapefruit juice, should be monitored as these drugs may cause an increase of ranolazine levels in plasma.91 Ranolazine clearance is reduced in renal and hepatic impairment. Ranolazine should be used cautiously with QT-prolonging drugs as it may increase QTc.91 Ranolazine may be recommended as an adjunctive therapy in patients with chronic stable angina who are symptomatic despite the use of conventional treatment. The current ESC guidelines recommend ranolazine as a second-line treatment as it is devoid of any effects on heart rate, blood pressure and tolerance.2 Ranolazine is well tolerated; the major adverse effects reported with ranolazine include constipation, nausea, dizziness, and headache. The effective recommended dose of ranolazine is 500 mg to 1000 mg twice daily.
5.1.1.7. Ivabradine
Ivabradine blocks the f channel in the sino-atrial node and inhibits the If current, causing a specific heart rate reduction without affecting the force of contraction. This exclusive heart rate reduction helps in prolonging diastole and thereby improving myocardial oxygen balance. Further, ivabradine is devoid of any effect on blood pressure or myocardial contractility or conduction time.
Ivabradine or ivabradine plus beta-blocker, when compared to placebo alone, produced dose-dependent improvements in exercise tolerance and time to development of ischaemia during exercise.98, 99 When added to beta-blocker (atenolol), in the Morbidity-Mortality Evaluation of the If inhibitor ivabradine in patients with coronary artery disease and left ventricular dysfunction (BEAUTIFUL) trial, ivabradine decreased the admission to hospital for fatal and non-fatal myocardial infarction and coronary revascularisation. It was noteworthy that the effects were more pronounced in patients with heart rate ≥70 bpm.16 Ivabradine was found to be as effective as beta-blocker (atenolol) in increasing total exercise duration and reducing the number of anginal attacks.100 Moreover, combining ivabradine with low dose beta-blocker (bisoprolol) vs. uptitration of beta-blocker in stable angina patients with left ventricular (LV) systolic dysfunction produced additional anti-anginal and anti-ischaemic benefits and improved chronotropic reserve and exercise tolerance.101
Further, ivabradine was found to be as effective as CCB (amlodipine) in improving exercise tolerance and demonstrated superior reduction of myocardial oxygen consumption with similar safety.102 Ivabradine has also displayed a good tolerability and anti-anginal efficacy in patients with chronic stable angina treated with concomitant anti-anginal medications (anti-thrombotic agents, lipid-lowering agents, long-acting nitrates and dihydropyridine CCBs).103
Ivabradine is well tolerated and recommended at a dose of 5 and 7.5 mg twice daily for chronic stable angina and the maintenance dose should not exceed beyond 7.5 mg twice daily. Concomitant use of verapamil and diltiazem with ivabradine is contraindicated.104 The current ESC-2013 guidelines recommend ivabradine as a second line therapy for symptom relief in stable coronary artery disease patients based on the available evidence.2
5.1.1.8. Nicorandil
Nicorandil functions via activating ATP–sensitive potassium channels and promoting systemic venous and coronary vasodilatation, eventually it causes a rise in coronary blood flow, a decrease in after-load, preload and oxidative injury.105 The efficacy of nicorandil in patients with stable angina has been compared to placebo, alone or in combination with standard anti-anginal medications in randomised trials, including the Impact Of Nicorandil in Angina (IONA) randomised study,106 the Study of Women's health Across the Nation (SWAN) group study,107 and some Asian studies.108, 109, 110 A meta-analysis evaluating the short-term efficacy of nicorandil compared with anti-anginal drugs for stable angina found that nicorandil did not show a significant reduction of weekly angina frequency. Further, there were no significant differences in total exercise duration, time to 1-mm ST-segment depression, and time to onset of pain.111 Chronic use of nicorandil could stabilise coronary plaques in patients with stable angina.112 Use of nicorandil may be associated with the side-effects such as oral, intestinal and peri-anal ulceration as well as frequent headaches. Concomitant use of nicorandil with sulphonylureas was an important exclusion criterion in the IONA trial since nicorandil and sulphonylureas compete and have opposite effects at potassium channels.106 Presently, ESC-2013 guidelines recommend nicorandil as a second line therapy for symptom relief in stable coronary artery disease patients.2
5.1.1.9. Nitrates
Nitrates can be effective in combating all forms of angina. Nitrates cause vascular smooth muscle relaxation resulting in both the coronary arteriolar and venous dilatation together leading to reduced preload. A meta-analysis assessed the effect of nitrates for stable angina and reported that long-term administration of nitrates was beneficial for angina prophylaxis and improved exercise performance. With continuous regimen, low-dose nitrates were more effective than high-dose for improving exercise performance. On the contrary, with the intermittent regimen, high-dose nitrates were more effective.113
5.1.2. Long-acting nitrates for angina prophylaxis
Long-acting nitrates are recommended for treating angina when additional therapy to control angina is necessary or if the initial therapy with a beta-blocker or non-DHP CCB is contraindicated or poorly tolerated. Tolerance to nitrates could develop if long-acting nitrates are regularly administered over a prolonged period (around 8–10 h). Moreover, exacerbation of endothelial dysfunction is a possible complication of long-acting nitrates.
A meta-analysis of trials comparing beta-blockers, CCBs, and nitrates for stable angina, found no significant differences in weekly anginal episodes, time to ST-segment depression, total exercise time, or sublingual nitroglycerin use when long-acting nitrates were compared to either beta-blocker or CCBs.78 However, it is important to note that only a few studies compared long-acting nitrates with beta-blockers or CCBs. In agreement with these results, the current NICE guidelines also find poor quality of evidence for the use of long-acting nitrates in combination with beta-blockers in patients with stable angina.114
Often oral administration of isosorbide dinitrate can be used for angina prophylaxis. In a placebo-controlled clinical study, duration of exercise increased substantially for around 6–8 h upon a single dose (15–120 mg) of oral isosorbide dinitrate; but the effect was sustained for only 2 h when similar doses were administered repeatedly for 4 times daily.115 Similarly, extended-release formulation of isosorbide dinitrate was also found to be non-superior to placebo in a large multicenter clinical study. Therefore, presently the use of isosorbide dinitrate is not evidence-based. Moreover, mono-nitrates also exhibit similar complications.116, 117
5.1.3. Short-acting nitrates for acute angina
Fast acting formulations of nitroglycerin can show immediate relief from angina. They can be employed in cases where the anginal episode has just started or when the symptoms are likely to appear (such as activity after a meal, emotional stress, sexual activity and in colder weather). During the course of anginal attacks, the patient should be advised to attain sitting position and self-administer sublingual nitroglycerin (0.3–0.6 mg) for every 5 min until the pain is relieved or a maximum of 1.2 mg is used (within 15 min).2
In summary, the level of evidence for emerging therapies such as trimetazidine, ranolazine, nicorandil and ivabradine is similar to the older drugs like beta-blockers and CCBs. The only difference is the widespread availability of the older drugs. However, considering similar efficacy in prognosis and symptom relief, it is recommended to individualise patient treatment according to their characteristics, co-morbidities, and contraindications.118, 119 Emerging therapies are certainly effective 2nd line agents when angina is not relieved by conventional drugs.
Recommendations on relief of angina symptoms.
-
•
Short-acting nitrates are indicated for the immediate relief of anginal symptoms. [Grade A, Evidence 2]2, 120, 121
-
•
Beta-blockers and/or CCBs are the initial agents for long-term symptoms management and heart rate control based on co-morbidities, contraindications and patient preference. [Grade A, Evidence 1]2, 79, 122
-
•
The combination of non-DHP CCB with beta-blocker should be avoided in patients with anticipated risk of atrio-ventricular block or severe bradycardia. [Grade A, Evidence: 4]123, 124
-
•
The addition of long-acting nitrates, trimetazidine, ivabradine, ranolazine or nicorandil is proposed in case of intolerance or contraindications or failure in achieving control by beta-blockers and/or CCBs. The choice of the drug should be made on the basis of blood pressure, heart rate and tolerance. [Grade A, Evidence 2]98, 100, 106, 114, 115, 117, 125, 126
-
•
Ivabradine may be considered in symptomatic patients who do not tolerate beta-blockers or in whom the resting heart rate remains above 70 bpm, despite administration of the full tolerable dose of beta-blockers. [Grade A, Evidence 2]16, 100, 101
-
•
When two haemodynamically acting drugs fail to achieve the desired results in reducing angina, preference may be given to cardio-metabolic agents like trimetazidine or ranolazine as they have a different mode of action and may offer better efficacy in combination with a haemodynamic agent. [Grade A, Evidence 2]87, 88
5.1.4. Event prevention
The measures to stop myocardial ischaemia and CVD related deaths, chiefly focus on decreasing the occurrence of acute thrombotic events and preventing ventricular dysfunction. These objectives can be attained by pharmacological or lifestyle approaches which may modify the natural history by diminishing progression or regression of plaques, stabilising plaques by decreasing inflammation and thus preventing plaque rupture and thrombosis.
5.1.4.1. Anti-platelet agents
Anti-platelet drugs reduce platelet accumulation and prevent the development of thrombus. Among anti-platelet drugs, low-dose of aspirin or clopidogrel are the most common agents used. While considering effectiveness, safety and cost issues, low-dose aspirin is the choice of anti-platelet drug in many cases.
Low-dose of aspirin: Aspirin is an important drug for prevention of arterial thrombosis. It functions by inhibiting platelet cyclooxygenase-1 (COX-1) irreversibly and eventual thromboxane production. Considering effectiveness and safety, aspirin 75 mg daily dose is recommended for all patients with stable angina.
A meta-analysis reviewed the usage of low-dose of aspirin in patients with SCAD and found that aspirin reduces all-cause mortality, adverse cardiovascular events, nonfatal MI, and nonfatal stroke. However, aspirin increases the risk of major bleeding. Moreover, no additional beneficial effects were seen with a higher dose of aspirin (300 mg vs. 50–100 mg/d).127 The beneficial effects of aspirin are attained within the dose range of about 75–150 mg/day; more precisely the ideal anti-thrombotic activity of aspirin was found to be at 75–80 mg/day. Aspirin, when used in larger doses can precipitate gastrointestinal side-effects.128, 129, 130, 131
Clopidogrel: Clopidogrel inhibits platelet accumulation by selective and irreversible inhibition of adenosine diphosphate P2Y12 receptor. Clopidogrel 75 mg daily can be recommended in patients who are intolerant to aspirin. This is backed by Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study. The study compared clopidogrel (75 mg) with aspirin (325 mg) in subjects with previous MI, stroke, or symptomatic peripheral artery disease (PAD).132 The findings of CAPRIE study demonstrated the superiority of clopidogrel over aspirin in reducing the combined risk of ischaemic stroke, MI, or vascular death; however, the extent of difference was small. Overall safety profile of clopidogrel was at least as good as that of medium-dose aspirin.132 Clopidogrel is mainly metabolised by cytochrome P450, CYP2C19 variant. Other drugs, such as proton pump inhibitors (PPIs) and statins that are metabolised by CYP2C19 can competitively inhibit the enzyme and impair metabolism of clopidogrel.133 Additionally, a study compared clopidogrel with aspirin plus esomeprazole (PPI) for the prevention of recurrent bleeding from ulcers in high-risk patients and found that the combination of aspirin plus esomeprazole was superior to clopidogrel.134 Therefore, in patients who have gastrointestinal bleeding while on aspirin, adding a PPI could be more effective than using clopidogrel instead of aspirin.134
Prasugrel and ticagrelor are newer P2Y12 inhibitors that demonstrate better platelet inhibition, when compared to clopidogrel.135, 136 Both prasugrel and ticagrelor have exhibited a marked decrease of cardiovascular outcomes when compared to clopidogrel in patients with ACS. However, the clinical benefits of these drugs have not been demonstrated in SCAD patients and therefore cannot be currently recommended for use in these patients.137, 138
5.1.4.2. Combination of anti-platelet agents
Combinations of anti-platelet agents are currently not routinely recommended for SCAD. In a Cochrane systematic review, the use of clopidogrel plus aspirin, compared with placebo plus aspirin, was associated with a lower risk of cardiovascular events (OR: 0.87, 95% CI 0.81–0.94; p < 0.01) and a higher risk of major bleeding (OR 1.34, 95% CI 1.14–1.57; p < 0.01). The review concluded that dual anti-platelet therapy is not advisable for routine management of SCAD, except in patients after stenting, where benefits outweigh harm.139
Furthermore, adding an antagonist of protease activated receptor type 1 (PAR-1), vorapaxar to standard anti-platelet therapy in patients with stable atherosclerosis displayed a significant decrease in cardiovascular death, MI or stroke. The beneficial effects were more prominent in the post-MI group of patients.140 However, vorapaxar caused an increase in the risk of moderate or severe bleeding.140, 141 In summary, combined anti-platelet therapy may be advised only in select SCAD patients at high risk of ischaemic events.
5.1.4.3. Renin–angiotensin–aldosterone system modulators
The renin–angiotensin–aldosterone system plays a vital role in maintaining haemodynamic stability through regulation of arterial blood pressure, water and electrolyte balance.142 Angiotensin-converting-enzyme inhibitors (ACEIs) prevent the conversion of angiotensin I to angiotensin II and that eventually prevents the effects of angiotensin II, such as narrowing of blood vessels and raising blood pressure. Furthermore, ACE inhibition eventually prevents the degradation of bradykinin, hence promotes vasodilatation and corrects endothelial dysfunction. Moreover, it is important to note that angiotensin II receptor blockers (ARBs) are devoid of such bradykinin related actions.143
The beneficial effect of ACEI, ramipril at a target dose of 10 mg daily at night was examined in the HOPE (Heart Outcomes Prevention Evaluation) study that enrolled the patients with high risk for cardiovascular events and devoid of LV dysfunction and heart failure. The study demonstrated that ramipril compared to placebo, considerably (p < 0.001) reduced the primary composite endpoints of cardiovascular death (ramipril [6.1%] vs. placebo [8.1%]), AMI (9.9% vs. 12.3%), and stroke (3.4% vs. 4.9%).144 In contrast to the HOPE study conducted in high-risk patients, the EUROPA (EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease) study done in documented CAD patients, who were optimally protected with aspirin, beta-blockers and statins, confirmed the benefits of ACE inhibitor perindopril in all CAD patients; whether low, medium or high risk CAD patients.144, 145 Together, the HOPE and EUROPA studies provide strong evidence that patients with SCAD, vascular disease, and diabetes (with one further high-risk factor), regardless of LV function should be considered for treatment with either of these two ACE inhibitors.146
Further, in a meta-analysis of ACEI therapy compared with placebo in patients (n = 31,555) with CAD and preserved LV systolic function, ACEI therapy demonstrated a reduction in cardiovascular mortality (RR 0.83, 95% CI 0.72–0.96, p = 0.01), non-fatal MI (RR 0.84, 95% CI 0.75–0.94, p = 0.003), all-cause mortality (RR 0.87, 95% CI 0.81–0.94, p = 0.0003) and revascularisation rates (RR 0.93, 95% CI 0.87–1.00, p = 0.04).147
In the EUROPA-CCB post hoc analysis, perindopril given on top of amlodipine significantly reduced total mortality by 46% (p < 0.01) and primary end point (composite of cardiovascular-mortality, non-fatal MI and resuscitated cardiac arrest) by 35% (p < 0.05) and provided a significant supplementary impact.148
The Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) compared the effectiveness of amlodipine (5–10 mg) plus perindopril (4–8 mg) with atenolol (50–100 mg) plus bendroflumethiazide (1.25–2.5 mg), in patients with hypertension. The study revealed that compared with the atenolol-based regimen, though not significant, fewer individuals on the perindopril-based regimen had a primary endpoint (429 vs. 474; unadjusted HR 0.90, 95% CI 0.79–1.02, p = 0.1052), fatal and non-fatal stroke (327 vs. 422; 0.77, 0.66–0.89, p = 0.0003), total cardiovascular events and procedures (1362 vs. 1602; 0.84, 0.78–0.90, p <0 0001), and all-cause mortality (738 vs. 820; 0.89, 0.81–0.99, p = 0.025).149 Furthermore, the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial compared the effectiveness of benazepril plus amlodipine with benazepril plus hydrochlorothiazide. In this study, combination therapy with benazepril and amlodipine exhibited an excellent blood-pressure control and a clear benefit with respect to cardiovascular outcomes.150 In view of these findings, the combination of amlodipine and an ACE inhibitor seems to be a better alternative in SCAD patients with hypertension.
5.1.5. Combination of angiotensin II receptor blocker (ARB) with angiotensin-converting-enzyme inhibitors (ACEI) vs. ARB alone
The ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) – study examined the effect of ACE inhibitor ramipril, ARB telmisartan and their combination in patients with vascular disease or high-risk diabetes. The results demonstrated non-inferiority of telmisartan with primary outcome (death from cardiovascular causes, MI, stroke, or hospitalisation for heart failure) occurring in 16.5% and 16.7% patients of ramipril group and telmisartan group, respectively (relative risk (RR), 1.01; 95% CI 0.94–1.09). In the combination therapy group, the primary outcome occurred in 16.3% patients (RR, 0.99; 95% CI 0.92–1.07) and as compared to the ramipril group, there was an increased risk of hypotension (4.8% vs. 1.7%, p < 0.001), syncope (0.3% vs. 0.2%, p = 0.03), and renal dysfunction (13.5% vs. 10.2%, p < 0.001). It is noteworthy that the findings of this study could not be generalised to say ACEIs and ARBs are similar.151 A previous meta-analysis inferred that ARBs are indeed inferior to ACEIs with respect to major cardiovascular events like MI and cardiovascular death.152 Further, the meta-analysis stated that when clinicians have to choose either an ACEI or an ARB in high-risk patients, they should be cognizant of the unique differences between each class of medications, particularly with respect to MI and cardiovascular death.152 ACEIs should be the first choice across the spectrum of cardio-metabolic risk reduction. A combination of ACEI and ARB therapy should be avoided. ARBs can be considered as an alternative therapy for patients with SCAD when ACE inhibitors are not tolerated.151, 152, 153
5.1.5.1. Statins
Several studies have demonstrated the beneficial effects of statins in preventing cardiovascular events. An initial network meta-analysis (n = 65,000 patients) showed a significant reduction in cardiovascular deaths (RR: 0.89, 95% CI 0.81–0.98, p = 0.01); moreover the analysis concluded that statins have a clear role in primary prevention of CVD mortality and major cardiovascular events.154 A prospective meta-analysis of data from 14 randomised (n = 90,056) trials of statins revealed that statin therapy can safely reduce the 5-year incidence of major coronary events (MI or coronary death [0.77, 0.74–0.80; p < 0.0001]), coronary revascularisation (0.76, 0.73–0.80; p < 0.0001), and stroke (0.83, 0.78–0.88; p < 0.0001) by about one fifth per mmol/L reduction in LDL cholesterol.62 Further, a recent Cochrane meta-analysis reviewed harms and benefits of statins in people with no history of CVD. The analysis found reduction in all-cause mortality (OR 0.86, 95% CI 0.79–0.94) as well as in revascularisation rates (RR 0.62, 95% CI 0.54–0.72).155 Moreover, meta-analysis of cardiovascular outcomes trials comparing intensive vs. moderate statin therapy, reported a significant 16% reduction in coronary death or MI (p < 0.00001), as well as a significant 16% reduction of coronary death or any cardiovascular event (p < 0.00001).156 Hence, statin in high intensity (or at least moderate intensity) should be prescribed in all patients with SCAD irrespective of lipid levels.
5.1.5.2. Beta-blockers
There are no confirmatory trials of the beneficial effects of beta-blockers in SCAD patients. Indeed, the anticipated beneficial effects of beta-blockers in SCAD patients are eventual extrapolations from the studies in the patients with MI and patients with new-onset of CHD.157 However, in the international REACH registry, beta-blockers use couldn’t lower event rate of cardiovascular events at 44-month follow-up, even among patients with prior history of MI.158 Moreover, a meta-analysis of randomised studies involving MI patients concluded that β-blockers have no mortality benefit but reduce recurrent MI and angina (short-term).159 In summary, at present, studies are warranted for the use of β-blockers in event prevention in SCAD patients.
5.1.5.3. Other anti-anginal agents and event reduction
A few other drugs such as trimetazidine, nicorandil, ivabradine and ranolazine also have been examined for prevention of cardiovascular events in patients with stable angina. The Impact Of Nicorandil in Angina (IONA) study in patients with stable angina found a significant difference in achieving primary endpoint (coronary heart disease death, non-fatal MI, or unplanned hospital admission for cardiac chest pain, HR 0.83, 95% CI 0.72–0.97; p = 0.014) by nicorandil therapy. However, it could not attain the secondary endpoint (combined outcome of coronary heart disease death or non-fatal MI, HR 0.79, 95% CI 0.61–1.02; p = 0.068).106 In addition, the Metabolic Efficiency with Ranolazine for Less Ischaemia in Non-ST-Elevation Acute Coronary Syndromes (MERLIN)-TIMI 36 trial observed that addition of ranolazine to standard treatment for ACS was not effective in reducing major cardiovascular events. However, ranolazine did not adversely affect the risk of all-cause death or symptomatic documented arrhythmia.160 The morBidity-mortality EvAlUation of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction (BEAUTIFUL) study, found no effect of ivabradine on the primary composite endpoint (cardiovascular death, admission to hospital due to AMI, and admission to hospital for new onset or worsening heart failure) (HR 1.00, 95% CI 0.91–1.1, p = 0.94).161 However, it reduced secondary endpoints: admission to hospital for fatal and non-fatal MI (0.64, 95% CI 0.49–0.84, p = 0.001) and coronary revascularisation (0.70, 95% CI 0.52–0.93, p = 0.016) in a pre-specified subgroup of patients with heart rate of 70 bpm or greater. Based on this, ivabradine could be used in a subgroup of patients who have elevated heart rate (≥70 bpm). Similarly, the Study assessInG the morbidity–mortality beNefits of the If Inhibitor ivabradine in patients with coronary arterY disease (SIGNIFY) found that ivabradine, added to guideline-recommended medical therapy, did not improve the outcome in patients who had SCAD without clinical heart failure.162 However, BEAUTIFUL and SIGNIFY varied in terms of patient population and dose of ivabradine, which might have led to different clinical outcomes.162 A preliminary study, METRO (ManagEment of angina: a reTRospectivecOhort) found that inclusion of trimetazidine in the anti-anginal treatment of stable angina is independently associated with a significant reduction in mortality after surviving an MI.86
5.1.5.4. Chelation therapy
Chelation therapy includes a series of intravenous infusions of disodium ethylene diamine tetraacetic acid (EDTA) in combination with other substances. It has been suggested that EDTA chelation therapy can result in relief of angina in patients with stable ischaemic heart disease.163 A few earlier clinical studies could not confirm positive effects of chelation therapy. However, recently the Trial to Assess Chelation Therapy (TACT) was conducted in subjects with CAD and found reduction in the risk of cardiovascular events by 18% (HR = 0.82; 95% CI 0.69–0.99; p = 0.035).164 Nevertheless, additional studies are needed to confirm the utility of chelation therapy.
Recommendations on event prevention.
-
•
Indefinite daily low-dose aspirin is recommended in all SCAD patients if not contraindicated. [Grade A, Evidence 1]127, 129, 130
-
•
Clopidogrel is recommended in patients with aspirin intolerance. [Grade A, Evidence 2]132, 165, 166, 167
-
•
In view of absence of any trial showing the benefit of prasugrel or ticagrelor in stable angina patients and also considering their cost in this sub-set of patients, they may be avoided pending results of the trials addressing this issue. [Grade A, Evidence 4]
-
•
High intensity statin should be prescribed in all patients with SCAD irrespective of lipid levels. [Grade A, Evidence Level 2]156
-
•
All stable angina patients with diabetes, hypertension, heart failure or chronic kidney disease should be recommended to receive ACEIs if not contraindicated. [Grade A, Evidence 1]144, 145, 168, 169
-
•
Rest of the patients with SCAD also be recommended to receive ACEIs [Grade A, Evidence 2]144, 145, 147
-
•
A combination of ACEI and amlodipine may be considered in hypertensive CAD patients for improving cardiovascular outcomes. [Grade A, Evidence 2]148, 149, 150
-
•
ARB treatment may be used as an alternative therapy for patients who are intolerant to ACEIs. [Grade A, Evidence 2]151, 153
-
•
At present chelation therapy cannot be routinely recommended for event prevention in stable angina patients. [Grade A, Evidence 3]163, 164
6. Treatment of certain forms of stable CAD
6.1. Silent myocardial ischaemia
Silent myocardial ischaemia is defined as objective evidence of myocardial ischaemia in the absence of chest discomfort or other anginal equivalents. Myocardial ischaemia either silent or symptomatic, generally results from increased oxygen demand or reduced blood supply and/or the combination of both. Therefore, the treatment modalities should address both supply and demand.
Studies have examined whether pharmacological interventions could ameliorate silent ischaemia. Medical therapy has been also compared with revascularisation in silent ischemia patients.
Silent myocardial ischemia should be treated like asymptomatic CAD. Mangement of silent ischaemia should include aspirin, statin, combined with beta-blockers, nitrates, CCBs. The use of metabolic modulators mainly trimetazidine and ranolazine have also been examined. In a study, administration of trimetazidine to diabetic patients receiving standard anti-anginal therapy exhibited a significant reduction of silent myocardial ischaemic episodes and the total silent ischaemic burden compared with placebo.170 Furthermore, trimetazidine (70 mg/day and 140 mg/day) in symptomatic and asymptomatic patients with chronic ischaemia receiving background beta-blockers, was found to ameliorate effort-induced myocardial ischaemia and functional capacity.171 In addition, in a study comparing the efficacy of lacidipine, bisoprolol and trimetazidine, only trimetazidine was found to reduce the late afternoon peak transient ischaemic episodes.172
Further in a study, in CAD patients with diabetes mellitus, trimetazidine treatment was found to show significant improvement (p = 0.009) in silent myocardial ischaemia.173 Similarly, another study compared the efficacy of diltiazem and trimetazidine, in patients suffering from silent myocardial ischaemia, after CABG surgery. Trimetazidine and diltiazem were effective in reducing silent myocardial ischaemia following CABG. Moreover, trimetazidine found to be superior to diltiazem at 6 months on 24-hr ambulatory ECG monitoring and myocardial scintigraphy.174
Recently a phase II study revealed that lowering of heart rate with ivabradine can result in reduction or resolution of silent ambulatory myocardial ischaemia (SAMI) recorded as episodes of ST-segment depression in ambulatory monitoring of the ECG. However, a larger study is required to confirm these results and to demonstrate the effect of SAMI reduction on long-term mortality of chronic ischaemic heart disease.175
Another study examined the efficacy of ranolazine in patients of atrial fibrillation (AF) with CAD176, 177; (a major proportion of AF episodes were asymptomatic in nature). Ranolazine demonstrated shorter total AF duration (81.56 ± 45.24 h vs. 68.71 ± 34.84 h, p = 0.002), decreased AF burden (1.89 ± 1.05% vs. 1.59 ± 0.81%, p = 0.002), and shortened mean AF duration (1.15 ± 0.41 h vs. 0.92 ± 0.35 h, p = 0.01).176, 177 However, specific studies are needed address the efficacy of ranolazine in silent ischaemia.178, 179
Recommendations for management of silent angina.
-
•
Silent myocardial ischaemia should be managed like asymptomatic CAD and may need administration of anti-ischaemic therapy and revascularisation as required. [Grade A, Evidence 4]2
-
•
Use of optimal medical therapies such as lipid-lowering agents, beta-blockers and metabolic modulators such as trimetazidine or ranolazine can be prescribed after careful examination of the patient on individual case by case basis. [Grade A, Evidence 3]170, 172, 173, 174
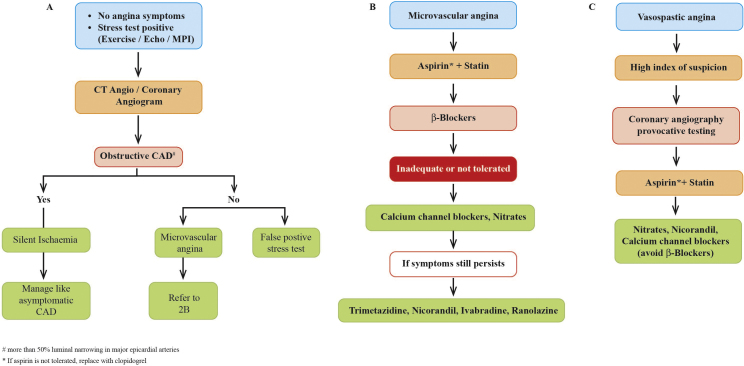
6.2. Microvascular angina
Microvascular angina can be described as angina pectoris caused by anomalies of small coronary arteries. The main objective of medical therapy of micro-vascular angina is to control the symptoms and improve the quality of life because there is inadequate information about its causes and risk factors.
Initial therapy can be started with the classical anti-ischaemic agents.180 For symptomatic relief of angina, short-acting nitrates can be prescribed. Use of beta-blockers is an appropriate choice, especially in the patients with increased activity of the adrenergic system. Propranolol, as well as atenolol, demonstrated a decrease in anginal attacks and ischaemic burden. Indeed, atenolol exhibited more efficiency than nitrates and CCBs.181, 182 Further, nebivolol was found to increase nitric oxide release from vascular endothelium and significantly improve coronary flow.183 However, the activity of beta-blockers has been disparate and fluctuated between 19% and 60%; requiring further therapeutic approaches.184 Calcium channel blockers and nitrates were also associated with variable effects. Nisoldipine and sublingual nifedipine reduced ischaemic burden and improved anginal symptoms while diltiazem and verapamil failed to show any beneficial effects.180, 185 These agents can be added to beta-blockers in case of inadequate control of symptoms.186, 187
Further, the metabolic modulator, trimetazidine has been examined in two studies in patients with exercise-induced ischaemia and normal coronary arteries. These studies demonstrated that trimetazidine augmented exercise capacity and reduced angina frequency.188, 189 Similarly, ivabradine has been examined for micro-vascular angina. In a recent study, a four-week treatment with ivabradine (5 mg twice daily) or ranolazine (375 mg twice daily) in addition to the standard anti-anginal therapy improved symptoms in patient with micro-vascular angina without affecting coronary flow reserve and endothelial function.190 In another study, a 4-week treatment of ranolazine exhibited improvement in anginal symptoms and myocardial ischaemia in women with micro-vascular angina.191
Recommendations on management of microvascular angina.
-
•
Micro-vascular angina patients can be initially treated with beta-blockers in addition to secondary preventive agents including aspirin and statins. [Grade A, Evidence 3]180, 181, 182
-
•
Calcium channel blockers can be prescribed if beta-blockers are inadequate or intolerable. [Grade A, Evidence 3]185, 186, 187
-
•
Novel agents like trimetazidine, ranolazine and ivabradine may be effective. [Grade A, Evidence 3]188, 189, 190
6.3. Vasospastic angina
Vasospastic angina is a form of angina resulting from coronary artery spasm, which comprises of a sudden obstructive vasoconstriction of a part of an epicardial artery, manifesting into a decrease in coronary blood flow.192 The clinical feature is dominated by rest angina, preserved effort tolerance and ST segment elevation in the ECG. In general, vasospastic angina responds to CCBs.193, 194 A meta-analysis evaluating the efficacy of CCBs (amlodipine, nifedipine, benidipine and diltiazem) on vasospastic angina revealed that benidipine showed a statistically significant reduction in major cardiovascular events than amlodipine, nifedipine or diltiazem.194 Further, long-acting nitrates can also improve the efficacy of treatment and should be scheduled to cover the period of the day in which ischaemic episodes most frequently occur, to prevent nitrate tolerance.193 Beta-blockers should be avoided, to prevent probable spasm.193 Furthermore, in refractive attacks of vasospastic angina, anti-alpha-adrenergic drugs, such as guanethidine or clonidine, may be helpful.195 (The management of silent, micro-vascular and vasospastic angina is outlined in Fig. 2).
Recommendations for management of vasospastic angina.
-
•
The treatment of vasospastic angina should be individualised according to the diagnosis of each case. [Grade A, Evidence 4]2
-
•
Calcium channel blockers can be used for effective prevention of vasospastic angina. [Grade A, Evidence 3]2, 196, 197
-
•
In patients who continue to be symptomatic, agents like trimetazidine, nicorandil, ranolazine and ivabradine may be effective. [Grade A, Evidence 3]188, 189, 190
Fig. 2.
Management of special type of angina. (A) Management of silent ischaemia. (B) Management of microvascular angina. (C) Management of vasospastic angina. Abbreviations: CAD: coronary artery disease; CT: computed tomographic; MPI: myocardial perfusion imaging.176, 177
7. Revascularisation
Coronary revascularisation is a process of restoring perfusion to ischaemic myocardium. Revascularisation in patients with SCAD is mandated whenever medical treatment does not improve prognosis or relieve symptoms.2 The need for revascularisation in a SCAD patient is based on the presence of significant obstructive coronary artery stenosis, the degree of related ischaemia and the expected advantage to prognosis and/or symptoms. The dual purpose of revascularisation being: to improve survival and relieve symptoms. Further, the decision to revascularise is also based not only on symptom status but also the individual risk of procedure in the given patient. Revascularisation can be opted early when high-risk features are recognised in non-invasive test findings.10
In view of unreliable findings on non-invasive examination revascularisation should not be considered without invasive findings of critical or multi-vessel disease.198 Cardiac computed tomography angiography is sometimes used before proceeding to invasive angiography; however this practice remains controversial.10 Further, invasive angiography is suitable and a necessary requirement for selecting the best revascularisation modality, even with the concomitant optimisation of medical therapy.
There could be several factors such as clinical, anatomical and associated co-morbidities to be taken into account while considering revascularisation for a patient. Based on combination of clinical factors it may be difficult to arrive at a decision in a given patient. In such situations, a clinical decision should be made after consulting and discussing with an expert heart team in the clinical setting.
Several meta-analyses reveal that revascularisation may alleviate myocardial ischaemia more efficiently than medical treatment alone.199, 200 Coronary revascularisation involves two modalities, one is the PCI and the other is CABG.
7.1. Percutaneous coronary intervention
The clinical efficiency of PCI in combination with medical therapy in SCAD patients has been examined in several clinical studies201, 202 and reviewed in several meta-analyses.203, 204, 205, 206, 207 A recent meta-analysis reviewed PCI vs. optimal medical therapy in patients with SCAD. The analysis found that compared with medical therapy, PCI did not show significant improvement in all-cause mortality [RR 0.85; 95% CI 0.71–1.01], cardiac death (RR 0.71; 95% CI 0.47–1.06), MI (RR 0.93; 95% CI 0.70–1.24), or repeat revascularisation (RR 0.93; 95% CI 0.76–1.14) during short or long-term follow-up. However, PCI demonstrated a better angina relief compared to medical therapy alone.208 Another meta-analysis compared the effect of PCI and medical therapy with medical therapy alone in patients with SCAD and objectively documented myocardial ischaemia; the review found that there were no differences between PCI and medical treatment in terms of death, MI, unplanned revascularisation or angina during a median follow-up of five years.199
Further, PCI with drug-eluting stents (DES) compared with bare-metal stents (BMS) was compared in few studies. A meta-analysis revealed that risks of mortality associated with drug-eluting and bare-metal stents are comparable. Sirolimus-eluting stents were found to be clinically better than bare-metal and paclitaxel-eluting stents.209 A recent meta-analysis found that DES are highly effective in decreasing the risk of target-vessel revascularisation without an increase in any safety outcomes, including stent thrombosis. However, among the DES generations there were significant differences, such that everolimus-eluting stent (EES), sirolimus-eluting stent (SES), and zotarolimus-eluting stent-Resolute (ZES)-R were the most efficacious and EES was the safest stent.210, 211
7.1.1. Coronary artery bypass grafting
Usage of CABG for specific subsets of SCAD patients was compared with medical therapy in a meta-analysis. The analysis showed that The CABG group had significantly lower mortality than the medical treatment group at 5 years (10.2 vs. 15.8%; OR 0.61 [95% Cl 0.48–0.77], p = 0.0001), 7 years (15.8 vs. 21.7%; 0.68 [0.56–0.83], p < 0.001), and 10 years (26.4 vs. 30.5%; 0.83 [0.70–0.98]; p = 0.03). Benefits were more in those patients with severe symptoms, early positive exercise tests, and depressed LV function.212 Similar findings were seen in other meta-analyses too.200, 204
Further, the Medicine, Angioplasty, or Surgery Study (MASS II) compared effects of PCI, CABG and medical treatment in patients with multi-vessel CAD, stable angina, and preserved ventricular function. In this study, compared with CABG, medical therapy demonstrated a significantly higher incidence of subsequent MI, a higher rate of additional revascularisation, a higher incidence of cardiac death, and consequently, a 2.29-fold increased risk of combined events. Similarly, PCI exhibited a recurring need for further revascularisation, a higher incidence of MI, and a 1.46-fold increased risk of combined events compared with CABG. Furthermore, CABG was better than medical therapy at eliminating anginal symptoms.202
Moreover, several studies compared PCI and CABG and found that neither of them could demonstrate effectiveness for the full spectrum of SCAD patients who require revascularisation.213 Nevertheless, CABG demonstrated a more complete revascularisation than PCI. Revascularisation is in itself a very broad topic for which separate guidelines are already available. The detailed evaluation of PCI or CABG is out of scope; thus in this document only the recommendations on indications for revascularisation have been given.
Recommendations on revascularisation.
-
•
The decision of considering revascularisation in patient with SCAD should be individualised. Revascularisation can be opted early when patients symptoms are uncontrolled by medical therapy alone and/or have high-risk features. [Grade A, Evidence 4]2
-
•
While selecting whether PCI or CABG for revascularisation, the decision should be purely individualised and consensus based. [Grade A, Evidence 4]2
8. Treatment of special groups of population
8.1. Women
CAD is one of the leading causes of death in women as well. Women with CAD exhibit symptoms that are different from those in men.2 Similarly, women have unique risk factors for CAD, including those related to pregnancy and autoimmune diseases. Coronary artery disease starts appearing 5–10 years later in women as compared to men.2 Women are more prone to CAD than men; however, its detection could be hindered by coronary artery positive remodelling. Indeed, the intravascular ultrasound sub-study within the Women's Ischemia Syndrome Evaluation (WISE) demonstrated that, in a sample of 100 women with normal coronary arteries, ≈80% had definite coronary atherosclerosis that was concealed by positive remodelling.214 These findings emphasise the need for better diagnostic and treatment modalities for women with concealed CAD. Until adequate evidence is presented, women with chest pain should be screened for CVD risk factors and treated accordingly.44
8.2. Patients with diabetes
Mortality due to CVD is amplified three-fold in diabetic men and two to five-fold in diabetic women, compared with the age and sex-matched non-diabetic persons.2 The treatment of CAD in diabetic patients presents a challenge. Probable approaches for ideal care of patients with CAD and diabetes include the stringent and constant regulation of blood glucose and control of other risk factors such as dyslipidemia, renal disease, overweight and smoking.2
The classical anti-anginal interventions such as nitrates, beta-blockers, CCBs, statins, anti-platelet drugs and coronary revascularisation procedure have similar indications in diabetic and non-diabetic patients. Furthermore, in patients with coronary or other vascular disease or with diabetes, ACEIs were found to reduce rates of death from cardiovascular causes or AMI when compared with placebo. Therefore, ACE inhibitors can be the ideal choice in case of stable angina patients who also suffer from diabetes.146, 215 Moreover, emerging therapies such as trimetazidine85 and nicorandil216 have shown beneficial effects in stable angina patients with diabetes. In diabetic patients with documented multi-vessel CAD and cardiomyopathy, trimetazidine as compared to control group significantly improved systolic wall thickening (1.7 ± 0.9 vs. 2.3 ± 0.9; p < 0.05), ejection fraction (43 ± 6% vs. 38 ± 6%; p = 0.007) and exercise duration (440 ± 140 s to 530 ± 145 s; p < 0.05).85 Additionally, achieving a goal of HbA1C < 7.0% and blood pressure < 140/85 mmHg can be beneficial for the prevention of diseases progression in patients with diabetes and SCAD.2, 217 In diabetic hypertensive's, PreterAx and DiamicroN Controlled Evaluation (ADVANCE) trial blood pressure-lowering arm revealed that a fixed combination of perindopril + indapamide significantly reduced total mortality by 14%, risk of cardiovascular death by 18% along with a significant 14% reduction in total coronary events and a 21% reduction in renal event.218, 219 In fact, ADVANCE-ON is the first study ever to demonstrate sustained post-trial benefits of blood pressure lowering therapy on cardiovascular and total mortality in patients with type 2 diabetes.220
There is now greater awareness regarding drug treatment strategy to achieve optimal glycemic goal but with cardiovascular safety, in type 2 diabetes. Metformin remains an ideal 1st line agent and amongst the sulphonylureas (SUs), the relative safety of a gliclazide makes it a preferred SU.221, 222, 223 Latest Braunwald's text book of cardiology refers to the cardiovascular safety of gliclazide.224 In addition, empagliflozin, an SGLT2 inhibitor has been examined in EMPA-REG OUTCOME trial; the study found that as compared with placebo, empagliflozin had a lower rate of the primary composite cardiovascular outcome and of death from any cause on the top of standard care. Therefore, empagliflozin can be useful in patients with type 2 diabetes who are at high risk for cardiovascular events.225
Coronary artery revascularisation in diabetes remains a potential challenge. A study by Frye and co-workers revealed that there was no significant difference in the rates of death and major cardiovascular events among patients undergoing prompt revascularisation and those undergoing medical therapy in patients with diabetes and CAD.226 Moreover, the choice of either PCI or CABG for revascularisation must be based on both anatomical and clinical factors.2 A meta-analysis observed survival advantage for CABG over PCI in diabetic patients.203 Another meta-analysis confirmed that revascularisation of patients with diabetes and multi-vessel disease by CABG decreased long-term mortality by about one-third compared with PCI using either BMS or DES.227 A recent patient-level pooled analysis of three clinical studies found that CABG plus optimal medical therapy decreased the primary endpoint (composite of death, myocardial infarction (MI), or stroke) during long-term follow-up in patients with type 2 diabetes and stable CAD, making this as the ideal treatment strategy.228
Recommendations on management of SCAD patients with diabetes.
-
•
An objective for HbA1C of <7.0% and blood pressure <140/85 mmHg is recommended for the prevention of micro-vascular disease. [Grade A, Evidence 2]65, 66, 70, 71, 72
-
•
All SCAD patients with should be recommended to receive an aspirin, high intensity statin and ACE inhibitor or a combination of ACE inhibitor with diuretic if not contraindicated. [Grade A, Evidence 1]44, 215, 228
-
•
For symptomatic treatment of SCAD patients with diabetes, classical anti-anginal agents are 1st line agents but long-acting nitrates, trimetazidine, ivabradine, ranolazine or nicorandil may be considered as an alternate especially when β-blockers are contraindicated. [Grade A, Evidence 3]85, 216
-
•
Trimetazidine is particularly beneficial in diabetic multi-vessel coronary artery disease patients who may also have diffuse disease and LV dysfunction. [Grade A, Evidence 3]85
-
•
SCAD patients with diabetes should be treated with Oral Anti-diabetics (OADs) which have shown cardiovascular safety/benefits such as metformin, gliclazide, gliptins and SGLT2 inhibitors. [Grade A, Evidence 2]220, 222, 223, 225
-
•
Revascularisation is recommended in diabetic patients, with persistent symptoms despite optimal medical therapy or high risk features on non-invasive testing. [Grade A, Evidence 4]2, 229
-
•
Coronary artery bypass grafting can be recommended in diabetic patients with multi-vessel disease, left main coronary artery disease or in the presence of LV dysfunction. [Grade A, Evidence 4]2, 229
-
•
PCI may be considered in single vessel disease and select cases of multi-vessel disease in consultation with heart team. [Grade A, Evidence 4]2
8.3. Patients with chronic kidney disease
Renal dysfunction is an independent predictor of CAD.25, 230 Patients with chronic kidney disease (CKD) should be closely observed for symptoms suggestive of CAD. Management of a suspected CAD in a symptomatic patient with renal disease can be similar to patients with normal renal function with careful monitoring of patients on drugs like ACE inhibitors. Likewise, treatment for patients with CAD and renal dysfunction is also similar to patients with normal renal function. However, consideration for revascularisation should be done with utmost caution in view of problems of contrast-induced nephropathy and generally worse outcome of CKD patients following CABG.
Recommendations on management of SCAD patients with chronic kidney disease.
-
•
All stable angina patients with chronic kidney disease should receive optimal medical therapy. ACE inhibitors can be used if not contraindicated with careful monitoring of serum creatinine and potassium levels. [Grade A, Evidence Level 4]231
8.4. Elderly patients
Data based on Randomized Controlled Trials (RCTs) to guide therapy in elderly patients are relatively scarce because of the common omission of elderly patients from most clinical trials. In elderly patients, diagnosis of angina would be difficult due to atypical symptoms and difficulties in performing stress testing.232, 233 Elderly SCAD patients may generally suffer from other serious co-morbidities. Thus personalised discussion about goals of treatments, risk and benefit of therapeutic approaches, especially revascularisation of obstructive CAD by either PCI or CABG surgery, is important. Patient's choice can differ as a function of age. In elderly patients, the medical therapy is as effective as in younger patients, in terms of benefit and risk ratio.234, 235 However, adverse effects, intolerance of anti-anginal agents are common.236 Selection of anti-anginal drugs for elderly patients should be customised according to co-morbidities, contra-indications, adverse effects, costs, and personal choice.237 Moreover, annual influenza vaccination can have beneficial effects in elderly SCAD patients.238, 239, 240
The decision of opting for revascularisation is more challenging in elderly patients. Age may influence greatly the choice of revascularisation, especially in the patients with multi-vessel disease and/or left main stenosis. The TIME (Trial of Invasive versus Medical therapy in Elderly patients) study, which randomised patients with SCAD despite standard therapy, to an invasive vs. standard medical therapy, revealed that patients aged ≥75 years benefited from revascularisation over standard medical therapy in terms of symptom relief and quality of life (QOL).241 Moreover, the invasive treatment was associated with a small early intervention risk, while medical treatment was associated with nearly 50% chance for later hospitalisations and revascularisations for increasing or refractory symptoms. Although 1-year outcomes in terms of mortality, symptom status and QOL were similar in both the groups, after 4 years, non-fatal events happened more often in standard medical therapy patients and survival was better for patients who were revascularised within the first year.236, 242 Elderly women differed from men in disease presentation, perception and outcome. Despite similar angina at baseline and lower disease severity they had a lower QOL and worse survival.243 Therefore, the choice of modality for managing SCAD in elderly patients should be individualised based on all the prognostic factors.
Recommendations on management of elderly SCAD patients.
8.5. The patients after revascularisation
Secondary prevention and cardiac rehabilitation are essential parts of chronic management after revascularisation because such procedures decrease future morbidity and mortality.244, 245 Compliance to lifestyle and risk factor adjustment requires personalised behavioural education and can be implemented during exercise-based cardiac rehabilitation. Education should be interactive, with full participation of patient care providers, providing an explanation for each intervention, while early mobilisation and physical conditioning should vary according to individual clinical status.244, 245 Further, follow-up approaches should focus on the assessment of the patient's symptoms, functional status and secondary prevention, and not rely only on the detection of re-stenosis or graft occlusion.
8.6. Repeat revascularisation of the patient with prior coronary artery bypass graft revascularisation
Patients with prior revascularisation may present with recurence of anginal symptoms (as SCAD). Therapeutic option of repeat revascularisation is to be considered if symptoms persist despite optimal medical management.246, 247, 248 The factors to be considered while deciding modality of the repeat revascularisation include the age of patients, co-morbidities and diffuseness of coronary disease, as well as the potential for damage to patent grafts, intra-luminal embolisation in saphenous vein grafts, lack of suitable arterial and venous conduits and instability of a graft-independent circulation. Generally, PCI is ideal modality for the patients with distinct lesions in grafts and preserved LV function or accessible native vessel disease. Repetition of bypass surgery may be required when the target vessels are inappropriate for PCI and when there are good distal vessel targets for bypass graft placement. The use of distal embolic protection devices is recommended in saphenous vein graft interventions. Any type of revascularisation modality should be accompanied with medical therapy with anti-anginal drugs and decreasing risk factors.2
8.7. Chronic total occlusions
Chronic total occlusions (CTO) are found in 15–30% of all the patients recommended for coronary angiography.249, 250, 251 Chronic total occlusions denote the most common mode of failure of PCI.252 Indeed, the comparison of surgical vs. PCI registries shows that presence of a chronic occlusion of one or more arteries in the PCI group as one of the most influential predictors of worse outcome, versus complete surgical revascularisation.253, 254 Percutaneous coronary intervention of CTOs is technically difficult and needs acquaintance with advanced techniques and specialised equipment. The complexity of percutaneous treatment of CTOs is demonstrated by the comparatively low procedural success rates observed in CTOs (60–85%) compared with sub-total stenosis (>98%).255, 256 Further, the success of percutaneous CTO re-canalization has been enhanced by the introduction of DES, which has radically decreased re-stenosis rates, compared with BMS.257, 258, 259, 260 Moreover, a recent systematic review demonstrated that DES use in CTO recanalization is showed lower target vessel revascularisation.261 In addition, a surgical modality with the implantation of a distal bypass graft is an effective alternative, particularly with a left internal mammary artery on the left anterior descending and is technically simpler.204, 262
8.8. Refractory angina
Refractory angina is a clinical condition where angina cannot be controlled by medical therapy, angioplasty or revascularisation. To address refractory angina a number of new pharmacological and non-pharmacological modalities has been developed. Enhanced external counter pulsation (EECP) therapy263, 264, 265, 266 and neurostimulatory techniques [trans-cutaneous electrical nerve stimulation (TENS)]267, 268; spinal cord stimuation (SCS)269, 270 have shown that they can ameliorate symptoms and improve the quality of life. With regard to EECP, a meta-analysis demonstrated that approximately 86% of chronic stable angina patients who underwent EECP treatment improved by at least one CCS (Canadian Cardiovascular Society) class at the end of therapy. Further, it is suggested that EECP therapy should be considered for patients with stable angina who are refractory to or not suitable for invasive therapy and/or medical management.271 However, transmyocardial or percutaneous myocardial revascularisations have been restricted as they are found ineffective.272
8.9. Heart failure
Management of co-morbidities such as stable angina is a crucial component of the holistic care of heart failure (HF) patients. As already outlined, β-blockers are the preferred treatment to relieve angina in heart failure patients. Ivabradine is an effective alternative to β-blockers in patients with reduced ejection fraction and HR ≥70 bpm. As an add-on to beta-blockers, trimetazidine has been shown to exert some beneficial effect in patients with HF and angina.170, 171, 273 It improves NYHA functional capacity, exercise duration and LV function in patients with reduced ejection fraction. The other alternatives in patients with reduced ejection fraction include CCBs (amlodipine), and nitrates. While the recent ESC 2016 HF guidelines have regarded the trimetazidine evidence as level ‘A’, Ranolazine and nicorandil have been given weak recommendations due to their uncertain safety profile.274
The treatment algorithms were published in the November–December 2016 Issue of Indian Heart Journal as Executive Summary. Same are available in the next section.275
Conflicts of interest
The authors have none to declare.
References
- 1.The World Health Organization . 2012. The Top Ten Causes of Death Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index2.html Accessed 02.07.16. [Google Scholar]
- 2.Montalescot G., Sechtem U., Achenbach S. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 3.Depre C., Wijns W., Robert A.M. Pathology of unstable plaque: correlation with the clinical severity of acute coronary syndromes. J Am Coll Cardiol. 1997;30:694–702. doi: 10.1016/s0735-1097(97)00213-1. [DOI] [PubMed] [Google Scholar]
- 4.Crea F., Andreotti F. The unstable plaque: a broken balance. Eur Heart J. 2009;30:1821–1823. doi: 10.1093/eurheartj/ehp266. [DOI] [PubMed] [Google Scholar]
- 5.Lanza G.A., Careri G., Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R., Bhairappa S., Prasad S. Clinical characteristics, angiographic profile and in hospital mortality in acute coronary syndrome patients in south Indian population. Heart India. 2014;2:65–69. [Google Scholar]
- 7.Nag T., Ghosh A. Cardiovascular disease risk factors in Asian Indian population: a systematic review. J Cardiovasc Dis Res. 2013;4:222–228. doi: 10.1016/j.jcdr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalal J., Hiremath M.S., Das M.K. Vascular disease in young Indians (20–40 years): role of ischemic heart disease. J Clin Diagn Res. 2016;10:OE08–OE12. doi: 10.7860/JCDR/2016/20206.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pryor D.B., Shaw L., McCants C.B. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mancini G.B., Gosselin G., Chow B. Canadian Cardiovascular Society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30:837–849. doi: 10.1016/j.cjca.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Shah A.D., Nicholas O., Timmis A.D. Threshold haemoglobin levels and the prognosis of stable coronary disease: two new cohorts and a systematic review and meta-analysis. PLoS Med. 2011;8:e1000439. doi: 10.1371/journal.pmed.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebnord E.W., Pedersen E.R., Strand E. Glycated hemoglobin and long-term prognosis in patients with suspected stable angina pectoris without diabetes mellitus: a prospective cohort study. Atherosclerosis. 2015;240:115–120. doi: 10.1016/j.atherosclerosis.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Held C., Bjorkander I., Forslund L. The impact of diabetes or elevated fasting blood glucose on cardiovascular prognosis in patients with stable angina pectoris. Diabet Med. 2005;22:1326–1333. doi: 10.1111/j.1464-5491.2005.01643.x. [DOI] [PubMed] [Google Scholar]
- 14.Karetnikova V., Gruzdeva O., Uchasova E. Glucose levels as a prognostic marker in patients with ST-segment elevation myocardial infarction: a case–control study. BMC Endocr Disord. 2016;16:31. doi: 10.1186/s12902-016-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz Ortiz M., Romo E., Mesa D. Prognostic value of resting heart rate in a broad population of patients with stable coronary artery disease: prospective single-center Cohort study. Revista Española de Cardiología (English Edition) 2010;63:1270–1280. doi: 10.1016/s1885-5857(10)70252-8. [DOI] [PubMed] [Google Scholar]
- 16.Fox K., Ford I., Steg P.G. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 17.Johnson N.P., Tóth G.G., Lai D. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 18.Deng S.B., Jing X.D., Wang J. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2015;184:703–709. doi: 10.1016/j.ijcard.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Mittal S.R. Diagnosis of coronary microvascular dysfunction – present status. Indian Heart J. 2015;67:552–560. doi: 10.1016/j.ihj.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler T.H., Schelbert H.R., Quercioli A., Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Arai A.E. The cardiac magnetic resonance (CMR) approach to assessing myocardial viability. J Nucl Cardiol. 2011;18:1095–1102. doi: 10.1007/s12350-011-9441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A.H., Shankar K., Eftekhari H. Silent myocardial ischemia: current perspectives and future directions. Exp Clin Cardiol. 2007;12:189–196. [PMC free article] [PubMed] [Google Scholar]
- 23.da Silveira A.D., Ribeiro R.A., Rossini A.P. Association of anemia with clinical outcomes in stable coronary artery disease. Coron Artery Dis. 2008;19:21–26. doi: 10.1097/MCA.0b013e3282f27c0a. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz P.E., Li J., Lindstrom J. Tools for predicting the risk of type 2 diabetes in daily practice. Horm Metab Res. 2009;41:86–97. doi: 10.1055/s-0028-1087203. [DOI] [PubMed] [Google Scholar]
- 25.Di Angelantonio E., Danesh J., Eiriksdottir G. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med. 2007;4:1497–1507. doi: 10.1371/journal.pmed.0040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rydén L., Grant P.J., Anker S.D. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 27.Bartnik M., Ryden L., Malmberg K. Euro Heart Survey I. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007;93:72–77. doi: 10.1136/hrt.2005.086975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz A., Bourassa M.G., Guertin M.C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 29.Palatini P. Heart rate: a strong predictor of mortality in subjects with coronary artery disease. Eur Heart J. 2005;26:943–945. doi: 10.1093/eurheartj/ehi235. [DOI] [PubMed] [Google Scholar]
- 30.Gianrossi R., Detrano R., Mulvihill D. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80:87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 31.Shaw L.J., Mieres J.H., Hendel R.H. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011;124:1239–1249. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnan S., Bhatt K., Dubey A.K. Acute electrocardiographic changes during smoking: an observational study. BMJ Open. 2013;3:e002486. doi: 10.1136/bmjopen-2012-002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer F., Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. doi: 10.1186/s12889-015-2489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobe M., Sinha D.N., Rahman K. Smokeless tobacco use and its implications in WHO South East Asia Region. Indian J Public Health. 2006;50:70–75. [PubMed] [Google Scholar]
- 35.Boffetta P., Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009;339:b3060. doi: 10.1136/bmj.b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McRobbie H., Thornley S. The importance of treating tobacco dependence. Rev Esp Cardiol. 2008;61:620–628. [PubMed] [Google Scholar]
- 37.Dehghan M., Mente A., Teo K.K. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation. 2012;126:2705–2712. doi: 10.1161/CIRCULATIONAHA.112.103234. [DOI] [PubMed] [Google Scholar]
- 38.Siervo M., Lara J., Chowdhury S. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 39.Shridhar K., Dhillon P.K., Bowen L. The association between a vegetarian diet and cardiovascular disease (CVD) risk factors in India: the Indian Migration Study. PLOS ONE. 2014;9:e110586. doi: 10.1371/journal.pone.0110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sackner-Bernstein J., Kanter D., Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. A meta-analysis. PLOS ONE. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vij V.A., Joshi A.S. Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants. J Nat Sci Biol Med. 2014;5:340–344. doi: 10.4103/0976-9668.136180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik V.S., Schulze M.B., Hu F.B. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavie C.J., Milani R.V., Mehra M.R. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 44.Perk J., De Backer G., Gohlke H. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 45.Lollgen H., Bockenhoff A., Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. Int J Sports Med. 2009;30:213–224. doi: 10.1055/s-0028-1128150. [DOI] [PubMed] [Google Scholar]
- 46.Warren T.Y., Barry V., Hooker S.P. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42:879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wendel-Vos G.C., Schuit A.J., Feskens E.J. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z.J., Zhou Y.J., Galper B.Z. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015;101:1631–1638. doi: 10.1136/heartjnl-2014-307119. [DOI] [PubMed] [Google Scholar]
- 49.Sniderman A.D., Bhopal R., Prabhakaran D. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–225. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 50.Misra A., Chowbey P., Makkar B.M. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170. [PubMed] [Google Scholar]
- 51.Pradeepa R., Anjana R.M., Joshi S.R. Prevalence of generalized & abdominal obesity in urban & rural India – the ICMR-INDIAB Study (Phase-I) [ICMR-NDIAB-3] Indian J Med Res. 2015;142:139–150. doi: 10.4103/0971-5916.164234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catapano A.L., Reiner Z., De Backer G. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:S1–S44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Enas E.A., Kuruvila A., Khanna P. Benefits & risks of statin therapy for primary prevention of cardiovascular disease in Asian Indians – a population with the highest risk of premature coronary artery disease & diabetes. Indian J Med Res. 2013;138:461–491. [PMC free article] [PubMed] [Google Scholar]
- 55.Zengin E., Bickel C., Schnabel R.B. Risk factors of coronary artery disease in secondary prevention – results from the AtheroGene – study. PLOS ONE. 2015;10:e0131434. doi: 10.1371/journal.pone.0131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He J., Vupputuri S., Allen K. Passive smoking and the risk of coronary heart disease – a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340:920–926. doi: 10.1056/NEJM199903253401204. [DOI] [PubMed] [Google Scholar]
- 57.Gupta R., Gupta N., Khedar R.S. Smokeless tobacco and cardiovascular disease in low and middle income countries. Indian Heart J. 2013;65:369–377. doi: 10.1016/j.ihj.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudeja V., Misra A., Pandey R.M. BMI does not accurately predict overweight in Asian Indians in northern India. Br J Nutr. 2001;86:105–112. doi: 10.1079/bjn2001382. [DOI] [PubMed] [Google Scholar]
- 59.Misra A., Pandey R.M., Sinha S. Receiver operating characteristics curve analysis of body fat & body mass index in dyslipidaemic Asian Indians. Indian J Med Res. 2003;117:170–179. [PubMed] [Google Scholar]
- 60.Vikram N.K., Misra A., Pandey R.M. Anthropometry and body composition in northern Asian Indian patients with type 2 diabetes: Receiver Operating Characteristics (ROC) Curve analysis of body mass index with percentage body fat as standard. Diab Nutr Metab. 2003;16:32–40. [PubMed] [Google Scholar]
- 61.Singh K.D., Dhillon J.K., Arora A., Gill B.S. Receiver operating characteristic curve analysis of BMI and percentage body fat in type 2 diabetics of Punjab. Indian J Physiol Pharmacol. 2004;48:73–80. [PubMed] [Google Scholar]
- 62.Baigent C., Keech A., Kearney P.M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 63.Ebrahimi R., Saleh J., Toggart E. Effect of preprocedural statin use on procedural myocardial infarction and major cardiac adverse events in percutaneous coronary intervention: a meta-analysis. J Invasive Cardiol. 2008;20:292–295. [PubMed] [Google Scholar]
- 64.Dorresteijn J.A., Boekholdt S.M., van der Graaf Y. High-dose statin therapy in patients with stable coronary artery disease: treating the right patients based on individualized prediction of treatment effect. Circulation. 2013;127:2485–2493. doi: 10.1161/CIRCULATIONAHA.112.000712. [DOI] [PubMed] [Google Scholar]
- 65.Hansson L., Zanchetti A., Carruthers S.G. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 66.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 67.Zanchetti A., Grassi G., Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens. 2009;27:923–934. doi: 10.1097/HJH.0b013e32832aa6b5. [DOI] [PubMed] [Google Scholar]
- 68.Liu L., Zhang Y., Liu G. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23:2157–2172. doi: 10.1097/01.hjh.0000194120.42722.ac. [DOI] [PubMed] [Google Scholar]
- 69.Weber M.A., Julius S., Kjeldsen S.E. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 70.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 71.Patel A., MacMahon S., Chalmers J. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 72.Piepoli M.F., Hoes A.W., Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang H.L., Fox K.A. The impact of β-blockers on mortality in stable angina: a meta-analysis. Scott Med J. 2012;57:69–75. doi: 10.1258/smj.2011.011274. [DOI] [PubMed] [Google Scholar]
- 74.Bangalore S., Parkar S., Messerli F.H. Long-acting calcium antagonists in patients with coronary artery disease: a meta-analysis. Am J Med. 2009;122:356–365. doi: 10.1016/j.amjmed.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 75.Wallace W.A., Wellington K.L., Chess M.A. Comparison of nifedipine gastrointestinal therapeutic system and atenolol on antianginal efficacies and exercise hemodynamic responses in stable angina pectoris. Am J Cardiol. 1994;73:23–28. doi: 10.1016/0002-9149(94)90721-8. [DOI] [PubMed] [Google Scholar]
- 76.de Vries R.J., van den Heuvel A.F. Nifedipine gastrointestinal therapeutic system versus atenolol in stable angina pectoris. The Netherlands Working Group on Cardiovascular Research (WCN) Int J Cardiol. 1996;57:143–150. doi: 10.1016/s0167-5273(96)02806-9. [DOI] [PubMed] [Google Scholar]
- 77.Fox K.M., Mulcahy D., Findlay I., Ford I., Dargie H.J. The Total Ischemic Burden European Trial (TIBET). Effects of atenolol, nifedipine SR and their combination on the exercise test and the total ischemic burden in 608 patients with stable angina. The TIBET Study Group. Eur Heart J. 1996;17:96–103. doi: 10.1093/oxfordjournals.eurheartj.a014699. [DOI] [PubMed] [Google Scholar]
- 78.Heidenreich P.A., McDonald K.M., Hastie T. Meta-analysis of trials comparing β-blockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281:1927–1936. doi: 10.1001/jama.281.20.1927. [DOI] [PubMed] [Google Scholar]
- 79.Klein W.W., Jackson G., Tavazzi L. Efficacy of monotherapy compared with combined antianginal drugs in the treatment of chronic stable angina pectoris: a meta-analysis. Coron Artery Dis. 2002;13:427–436. doi: 10.1097/00019501-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Koskinas K.C., Lillis L., Ziakas A. Diltiazem: a reversible cause of atrioventricular block – until proven otherwise. Open Cardiovasc Med J. 2013;7:46. doi: 10.2174/1874192401307010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neubauer S. The failing heart – an engine out of fuel. N Engl J Med. 2007;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 82.Barsness G.W., Holmes D.R., editors. Coronary Artery Disease: New Approaches Without Traditional Revascularisation. Springer Science & Business Media; 2011. [Google Scholar]
- 83.Dalal J.J. Trimetazidine effects on cardiac biomarkers in acute coronary syndrome. Heart Metab. 2015;67:26–29. [Google Scholar]
- 84.Detry J.M., Sellier P., Pennaforte S. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Trimetazidine European Multicenter Study Group. Br J Clin Pharmacol. 1994;37:279–288. doi: 10.1111/j.1365-2125.1994.tb04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belardinelli R., Cianci G., Gigli M. Effects of trimetazidine on myocardial perfusion and left ventricular systolic function in type 2 diabetic patients with ischemic cardiomyopathy. J Cardiovasc Pharmacol. 2008;51:611–615. doi: 10.1097/FJC.0b013e31817bdd66. [DOI] [PubMed] [Google Scholar]
- 86.Iyengar S.S., Rosano G.M. Effect of antianginal drugs in stable angina on predicted mortality risk after surviving a myocardial infarction: a preliminary study (METRO) Am J Cardiovasc Drugs. 2009;9:293–297. doi: 10.2165/11316840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Chazov E., Lepakchin V., Zharova E. Trimetazidine in Angina Combination Therapy-the TACT study: trimetazidine versus conventional treatment in patients with stable angina pectoris in a randomized, placebo-controlled, multicenter study. Am J Ther. 2005;12:35–42. doi: 10.1097/00045391-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Jackson G. Combination therapy in angina: a review of combination haemodynamic treatment and the role of combined haemodynamic and cardio-metabolic agents. Int J Clin Pract. 2001;55:256–261. [PubMed] [Google Scholar]
- 89.Li Y., Wang D., Hu C. Efficacy and safety of adjunctive trimetazidine therapy for acute myocardial infarction: a systematic review and meta-analysis. Cardiology. 2016;135:188–195. doi: 10.1159/000446640. [DOI] [PubMed] [Google Scholar]
- 90.Seth S. Textbook of pharmacology. Indian J Pharmacol. 1998;30:58. [Google Scholar]
- 91.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45:469–491. doi: 10.2165/00003088-200645050-00003. [DOI] [PubMed] [Google Scholar]
- 92.Banon D., Filion K.B., Budlovsky T., Franck C., Eisenberg M.J. The usefulness of ranolazine for the treatment of refractory chronic stable angina pectoris as determined from a systematic review of randomized controlled trials. Am J Cardiol. 2014;113:1075–1082. doi: 10.1016/j.amjcard.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 93.Chaitman B.R., Skettino S.L., Parker J.O. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43(8):1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 94.Stone P.H., Gratsiansky N.A., Blokhin A. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 95.Chaitman B.R., Pepine C.J., Parker J.O. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 96.Scirica B.M., Morrow D.A., Budaj A. Ischemia detected on continuous electrocardiography after acute coronary syndrome: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol. 2009;53:1411–1421. doi: 10.1016/j.jacc.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 97.Weisz G., Généreux P., Iñiguez A. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER-PCI): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:136–145. doi: 10.1016/S0140-6736(15)00459-6. [DOI] [PubMed] [Google Scholar]
- 98.Tardif J.C., Ponikowski P., Kahan T. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving β-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–548. doi: 10.1093/eurheartj/ehn571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borer J.S., Fox K., Jaillon P. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 100.Tardif J.C., Ford I., Tendera M. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 101.Amosova E., Andrejev E., Zaderey I. Efficacy of ivabradine in combination with β-blocker versus uptitration of β-blocker in patients with stable angina. Cardiovasc Drugs Ther. 2011;25:531–537. doi: 10.1007/s10557-011-6327-3. [DOI] [PubMed] [Google Scholar]
- 102.Ruzyllo W., Tendera M., Ford I. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs. 2007;67:393–405. doi: 10.2165/00003495-200767030-00005. [DOI] [PubMed] [Google Scholar]
- 103.Lopez-Bescos L., Filipova S., Martos R. Long-term safety and efficacy of ivabradine in patients with chronic stable angina. Cardiology. 2007;108:387–396. doi: 10.1159/000108387. [DOI] [PubMed] [Google Scholar]
- 104.Agency EM. Procoralan Film-coated Tablets: Summary of Product Characteristics. Available from: http://www.ema.europa.eu; Accessed 19.09.16.
- 105.Markham A., Plosker G.L., Goa K.L. Nicorandil. An updated review of its use in ischemic heart disease with emphasis on its cardioprotective effects. Drugs. 2000;60:955–974. doi: 10.2165/00003495-200060040-00007. [DOI] [PubMed] [Google Scholar]
- 106.IONA Study Group Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269–1275. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- 107.Swan Study Group Comparison of the antiischemic and antianginal effects of nicorandil and amlodipine in patients with symptomatic stable angina pectoris: the SWAN study. J Clin Basic Cardiol. 1999;2:213–217. [Google Scholar]
- 108.Hughes L.O., Rose E.L., Lahiri A. Comparison of nicorandil and atenolol in stable angina pectoris. Am J Cardiol. 1990;66:679–682. doi: 10.1016/0002-9149(90)91129-t. [DOI] [PubMed] [Google Scholar]
- 109.Zhu W.L., Shan Y.D., Guo J.X. Double-blind, multicenter, active-controlled, randomized clinical trial to assess the safety and efficacy of orally administered nicorandil in patients with stable angina pectoris in China. Circ J. 2007;71:826–833. doi: 10.1253/circj.71.826. [DOI] [PubMed] [Google Scholar]
- 110.Udaykumar P., Adhikari P., Periera P. Safety and efficacy of nicorandil in chronic stable angina – a double blind comparative randomised trial with isosorbide mononitrate. J Indian Acad Clin Med. 2003;4:205–209. [Google Scholar]
- 111.Hanai Y., Mita M., Hishinuma S. Systematic review on the short-term efficacy and safety of nicorandil for stable angina pectoris in comparison with those of β-blockers, nitrates and calcium antagonists. Yakugaku Zasshi. 2010;130:1549–1563. doi: 10.1248/yakushi.130.1549. [DOI] [PubMed] [Google Scholar]
- 112.Izumiya Y., Kojima S., Araki S. Long-term use of oral nicorandil stabilizes coronary plaque in patients with stable angina pectoris. Atherosclerosis. 2011;214:415–421. doi: 10.1016/j.atherosclerosis.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 113.Wei J., Wu T., Yang Q. Nitrates for stable angina: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol. 2011;146:4–12. doi: 10.1016/j.ijcard.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 114.NICE Guidelines-Stable Angina, 2011. Available from: https://www.nice.org.uk/guidance/cg126/evidence/full-guideline-183176605; Accessed 07.11.16.
- 115.Thadani U., Fung H.L., Darke A.C. Oral isosorbide dinitrate in angina pectoris: comparison of duration of action an dose-response relation during acute and sustained therapy. Am J Cardiol. 1982;49:411–419. doi: 10.1016/0002-9149(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 116.Parker J.O. Eccentric dosing with isosorbide-5-mononitrate in angina pectoris. Am J Cardiol. 1993;72:871–876. doi: 10.1016/0002-9149(93)91098-3. [DOI] [PubMed] [Google Scholar]
- 117.Chrysant S.G., Glasser S.P., Bittar N. Efficacy and safety of extended-release isosorbide mononitrate for stable effort angina pectoris. Am J Cardiol. 1993;72:1249–1256. doi: 10.1016/0002-9149(93)90292-k. [DOI] [PubMed] [Google Scholar]
- 118.Ohman E.M. Clinical practice. Chronic stable angina. N Engl J Med. 2016;374:1167–1176. doi: 10.1056/NEJMcp1502240. [DOI] [PubMed] [Google Scholar]
- 119.Manolis A.J., Poulimenos L.E., Ambrosio G. Medical treatment of stable angina: a tailored therapeutic approach. Int J Cardiol. 2016;220:445–453. doi: 10.1016/j.ijcard.2016.06.150. [DOI] [PubMed] [Google Scholar]
- 120.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 121.Savonitto S., Motolese M., Agabiti-Rosei E. Antianginal effect of transdermal nitroglycerin and oral nitrates given for 24 h a day in 2,456 patients with stable angina pectoris. The Italian Multicenter Study. Int J Clin Pharmacol Ther. 1995;33:194–203. [PubMed] [Google Scholar]
- 122.Davies R.F., Habibi H., Klinke W.P. Effect of amlodipine, atenolol and their combination on myocardial ischemia during treadmill exercise and ambulatory monitoring. Canadian Amlodipine/Atenolol in Silent Ischemia Study (CASIS) Investigators. J Am Coll Cardiol. 1995;25:619–625. doi: 10.1016/0735-1097(94)00436-t. [DOI] [PubMed] [Google Scholar]
- 123.Mills T.A., Kawji M.M., Cataldo V.D. Profound sinus bradycardia due to diltiazem, verapamil, and/or beta-adrenergic blocking drugs. JLa State Med Soc. 2004;156:327–331. [PubMed] [Google Scholar]
- 124.Zeltser D., Justo D., Halkin A. Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol. 2004;44:105–108. doi: 10.1016/j.jacc.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 125.de Vries R.J., Dunselman P.H., van Veldhuisen D.J. Comparison between felodipine and isosorbide mononitrate as adjunct to beta blockade in patients > 65 years of age with angina pectoris. Am J Cardiol. 1994;74:1201–1206. doi: 10.1016/0002-9149(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 126.Wilson S.R., Scirica B.M., Braunwald E. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebocontrolled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol. 2009;53:1510–1516. doi: 10.1016/j.jacc.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 127.Berger J.S., Brown D.L., Becker R.C. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121:43–49. doi: 10.1016/j.amjmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Trialists A Collaborative overview of randomised trials of antiplatelet therapy – I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 129.Trialists’ Collaboration A Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Juul-Moller S., Edvardsson N., Jahnmatz B. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet. 1992;340:1421–1425. doi: 10.1016/0140-6736(92)92619-q. [DOI] [PubMed] [Google Scholar]
- 131.Ridker P.M., Manson J.E., Gaziano J.M., Buring J.E., Hennekens C.H. Low-dose aspirin therapy for chronic stable angina. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1991;114:835–839. doi: 10.7326/0003-4819-114-10-835. [DOI] [PubMed] [Google Scholar]
- 132.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 133.Fihn S.D., Gardin J.M., Abrams J. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery. Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–3137. doi: 10.1161/CIR.0b013e3182776f83. [DOI] [PubMed] [Google Scholar]
- 134.Chan F.K., Ching J.Y., Hung L.C. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352:238–244. doi: 10.1056/NEJMoa042087. [DOI] [PubMed] [Google Scholar]
- 135.Gurbel P.A., Bliden K.P., Butler K. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 136.Jernberg T., Payne C.D., Winters K.J. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166–1173. doi: 10.1093/eurheartj/ehi877. [DOI] [PubMed] [Google Scholar]
- 137.Wiviott S.D., Antman E.M., Gibson C.M. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) Am Heart J. 2006;152:627–635. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 138.Cannon C.P., Harrington R.A., James S. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–293. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 139.Keller T.T., Squizzato A., Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2007;(3) doi: 10.1002/14651858.CD005158.pub2. Cd005158. [DOI] [PubMed] [Google Scholar]
- 140.Morrow D.A., Braunwald E., Bonaca M.P. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 141.Scirica B.M., Bonaca M.P., Braunwald E. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet. 2012;380:1317–1324. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 142.Skeggs L.T., Dorer F.E., Kahn J.R. The biochemistry of the renin–angiotensin system and its role in hypertension. Am J Med. 1976;60:737–748. doi: 10.1016/0002-9343(76)90888-3. [DOI] [PubMed] [Google Scholar]
- 143.Landmesser U., Drexler H. Effect of angiotensin II type 1 receptor antagonism on endothelial function: role of bradykinin and nitric oxide. J Hypertens Suppl. 2006;24:S39–S43. doi: 10.1097/01.hjh.0000220405.38622.23. [DOI] [PubMed] [Google Scholar]
- 144.Yusuf S., Sleight P., Pogue J. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 145.Fox K.M. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 146.Boos C.J. Cardiovascular protection with ace inhibitors – more HOPE for EUROPA? Med Sci Monit. 2004;10:SR23–SR28. [PubMed] [Google Scholar]
- 147.Al-Mallah M.H., Tleyjeh I.M., Abdel-Latif A.A. Angiotensin-converting enzyme inhibitors in coronary artery disease and preserved left ventricular systolic function: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2006;47:1576–1583. doi: 10.1016/j.jacc.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 148.Bertrand M.E., Ferrari R., Remme W.J. Clinical synergy of perindopril and calcium-channel blocker in the prevention of cardiac events and mortality in patients with coronary artery disease. Post hoc analysis of the EUROPA study. Am Heart J. 2010;159:795–802. doi: 10.1016/j.ahj.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 149.Dahlof B., Sever P.S., Poulter N.R. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 150.Jamerson K., Weber M.A., Bakris G.L. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 151.Yusuf S., Teo K.K., Pogue J. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 152.Strauss M.H., Hall A.S. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 153.Mann J.F., Schmieder R.E., McQueen M. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 154.Mills E.J., Rachlis B., Wu P. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–1781. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 155.Taylor F., Huffman M.D., Macedo A.F. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:Cd004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cannon C.P., Steinberg B.A., Murphy S.A. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 157.Andersson C., Shilane D., Go A.S. beta-Blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247–252. doi: 10.1016/j.jacc.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 158.Bangalore S., Steg G., Deedwania P. beta-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 159.Bangalore S., Makani H., Radford M. Clinical outcomes with beta-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014;127:939–953. doi: 10.1016/j.amjmed.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 160.Morrow D.A., Scirica B.M., Karwatowska-Prokopczuk E. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 161.Fox K., Ford I., Steg P.G. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 162.Fox K., Ford I., Steg P.G. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 163.Fihn S.D., Blankenship J.C., Alexander K.P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery. Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2015;149:e5–e23. doi: 10.1016/j.jtcvs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 164.Lamas G.A., Boineau R., Goertz C. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: the factorial group results of the Trial to Assess Chelation Therapy. Am Heart J. 2014;168 doi: 10.1016/j.ahj.2014.02.012. 37–44.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhatt D.L., Chew D.P., Hirsch A.T. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001;103:363–368. doi: 10.1161/01.cir.103.3.363. [DOI] [PubMed] [Google Scholar]
- 166.Ringleb P.A., Bhatt D.L., Hirsch A.T. Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke. 2004;35:528–532. doi: 10.1161/01.STR.0000110221.54366.49. [DOI] [PubMed] [Google Scholar]
- 167.Schiano P., Steg P.G., Barbou F. A strategy for addressing aspirin hypersensitivity in patients requiring urgent PCI. Eur Heart J Acute Cardiovasc Care. 2012;1:75–78. doi: 10.1177/2048872612441580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 169.Pfeffer M.A., Braunwald E., Moye L.A. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 170.Marazzi G., Wajngarten M., Vitale C. Effect of free fatty acid inhibition on silent and symptomatic myocardial ischemia in diabetic patients with coronary artery disease. Int J Cardiol. 2007;120:79–84. doi: 10.1016/j.ijcard.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 171.Vitale C., Spoletini I., Malorni W. Efficacy of trimetazidine on functional capacity in symptomatic patients with stable exertional angina – the VASCO-angina study. Int J Cardiol. 2013;168:1078–1081. doi: 10.1016/j.ijcard.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 172.Kikalishvili T.T., Chumburidze V.B., Kurashvili R.B. P-459: exercise-induced and silent ischemia: different effects of monotherapy. Am J Hypertens. 2001;14:185A. [Google Scholar]
- 173.Xu X., Zhang W., Zhou Y. Effect of trimetazidine on recurrent angina pectoris and left ventricular structure in elderly multivessel coronary heart disease patients with diabetes mellitus after drug-eluting stent implantation: a single-centre, prospective, randomized, double-blind study at 2-year follow-up. Clin Drug Investig. 2014;34:251–258. doi: 10.1007/s40261-014-0170-9. [DOI] [PubMed] [Google Scholar]
- 174.Kuralay E., Demirkiliç U., Özal E. Myocardial ischemia after coronary bypass: comparison of Trimetazidine and Diltiazem. Asian Cardiovasc Thorac Ann. 1999;7:84–89. [Google Scholar]
- 175.Kadro W. 2D.09: the effect of ivabradine on silent ambulatory myocardial ischemia. J Hypertens. 2015;33:e30. [Google Scholar]
- 176.Leftheriotis D., Flevari P., Theodorakis G. The effects of ranolazine on paroxysmal atrial fibrillation in patients with coronary artery disease: a preliminary observational study. JAFIB: J Atrial Fibril. 2014;6:25–31. doi: 10.4022/jafib.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leftheriotis D., Flevari P., Theodorakis G. The effects of ranolazine on paroxysmal atrial fibrillation in patients with coronary artery disease. Eur Heart J. 2013;34:P4113. doi: 10.4022/jafib.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thadani U. Should ranolazine be used for all patients with ischemic heart disease or only for symptomatic patients with stable angina or for those with refractory angina pectoris? A critical appraisal. Expert Opin Pharmacother. 2012;13:2555–2563. doi: 10.1517/14656566.2012.740458. [DOI] [PubMed] [Google Scholar]
- 179.Conti C.R., Bavry A.A., Petersen J.W. Silent ischemia: clinical relevance. J Am Coll Cardiol. 2012;59:435–441. doi: 10.1016/j.jacc.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 180.Marinescu M.A., Loffler A.I., Ouellette M. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–220. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lanza G.A., Colonna G., Pasceri V. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–856. doi: 10.1016/s0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 182.Bugiardini R., Borghi A., Biagetti L. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63:286–290. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 183.Togni M., Vigorito F., Windecker S. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. 2007;21:99–108. doi: 10.1007/s10557-006-0494-7. [DOI] [PubMed] [Google Scholar]
- 184.Kaski J.C., Valenzuela Garcia L.F. Therapeutic options for the management of patients with cardiac syndrome X. Eur Heart J. 2001;22:283–293. doi: 10.1053/euhj.2000.2152. [DOI] [PubMed] [Google Scholar]
- 185.Ozcelik F., Altun A., Ozbay G. Antianginal and anti-ischemic effects of nisoldipine and ramipril in patients with syndrome X. Clin Cardiol. 1999;22:361–365. doi: 10.1002/clc.4960220513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cannon R.O., 3rd, Watson R.M., Rosing D.R. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–246. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 187.Wang L., Cheng Z., Gu Y. Short-term effects of verapamil and diltiazem in the treatment of no reflow phenomenon: a meta-analysis of randomized controlled trials. Biomed Res Int. 2015;2015:382086. doi: 10.1155/2015/382086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rogacka D., Guzik P., Wykretowicz A. Effects of trimetazidine on clinical symptoms and tolerance of exercise of patients with syndrome X: a preliminary study. Coron Artery Dis. 2000;11:171–177. doi: 10.1097/00019501-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 189.Nalbantgil S., Altintiğ A., Yilmaz H. The effect of trimetazidine in the treatment of microvascular angina. Int J Angiol. 1999;8:40–43. doi: 10.1007/BF01616842. [DOI] [PubMed] [Google Scholar]
- 190.Villano A., Di Franco A., Nerla R. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 191.Mehta P.K., Goykhman P., Thomson L.E. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–522. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaski J.C., Maseri A., Vejar M. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989;14:1456–1463. doi: 10.1016/0735-1097(89)90382-3. [DOI] [PubMed] [Google Scholar]
- 193.Harris J.R., Hale G.M., Dasari T.W. Pharmacotherapy of vasospastic angina. J Cardiovasc Pharmacol Ther. 2016;21:439–451. doi: 10.1177/1074248416640161. [DOI] [PubMed] [Google Scholar]
- 194.Minatoguchi S. Vasospastic angina and Ca channel blockers. Curr Hypertens Rev. 2013;9:219–223. doi: 10.2174/1573402110666140131161338. [DOI] [PubMed] [Google Scholar]
- 195.Frenneaux M., Kaski J.C., Brown M. Refractory variant angina relieved by guanethidine and clonidine. Am J Cardiol. 1988;62:832–833. doi: 10.1016/0002-9149(88)91238-6. [DOI] [PubMed] [Google Scholar]
- 196.Sueda S., Kohno H., Fukuda H. Limitations of medical therapy in patients with pure coronary spastic angina. Chest. 2003;123:380–386. doi: 10.1378/chest.123.2.380. [DOI] [PubMed] [Google Scholar]
- 197.Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745–1762. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 198.Gibbons R.J., Abrams J., Chatterjee K. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina – summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107(1):149–158. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 199.Stergiopoulos K., Boden W.E., Hartigan P. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med. 2014;174:232–240. doi: 10.1001/jamainternmed.2013.12855. [DOI] [PubMed] [Google Scholar]
- 200.Windecker S., Stortecky S., Stefanini G.G. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859. doi: 10.1136/bmj.g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Boden W.E., O’Rourke R.A., Teo K.K. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 202.Hueb W., Lopes N., Gersh B.J. Ten-year follow-up survival of the medicine. Angioplasty, or surgery study (MASS II). A randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–957. doi: 10.1161/CIRCULATIONAHA.109.911669. [DOI] [PubMed] [Google Scholar]
- 203.Hlatky M.A., Boothroyd D.B., Bravata D.M. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 204.Jeremias A., Kaul S., Rosengart T.K. The impact of revascularisation on mortality in patients with nonacute coronary artery disease. Am J Med. 2009;122:152–161. doi: 10.1016/j.amjmed.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 205.Katritsis D.G., Ioannidis J.P. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111:2906–2912. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 206.Schomig A., Mehilli J., de Waha A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:894–904. doi: 10.1016/j.jacc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 207.Trikalinos T.A., Alsheikh-Ali A.A., Tatsioni A. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373:911–918. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pursnani S., Korley F., Gopaul R. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv. 2012;5:476–490. doi: 10.1161/CIRCINTERVENTIONS.112.970954. [DOI] [PubMed] [Google Scholar]
- 209.Stettler C., Wandel S., Allemann S. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 210.Bangalore S., Kumar S., Fusaro M. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 211.Mishra S. Are all stents equal-Need for scoring system to evaluate stents. Indian Heart J. 2016;68(5):589–591. doi: 10.1016/j.ihj.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yusuf S., Zucker D., Peduzzi P. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 213.Mishra S. Does modern medicine increase life-expectancy: quest for the Moon Rabbit? Indian Heart J. 2016;68:19–27. doi: 10.1016/j.ihj.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Khuddus M.A., Pepine C.J., Handberg E.M. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart. Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 216.Shehata M. Cardioprotective effects of oral nicorandil use in diabetic patients undergoing elective percutaneous coronary intervention. J Interv Cardiol. 2014;27:472–481. doi: 10.1111/joic.12142. [DOI] [PubMed] [Google Scholar]
- 217.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 218.Patel A., ADVANCE Collaborative Group, MacMahon S. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 219.Poulter N.R. Major findings from ADVANCE: blood pressure lowering arm. Medicographia. 2009;31:223–231. [Google Scholar]
- 220.Zoungas S., Chalmers J., Neal B. ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 221.Lavrenko A.V., Kutsenko L.A., Solokhina I.L. Efficacy of metformin as initial therapy in patients with coronary artery disease and diabetes type 2. Lik Sprava. 2011;(1–2):89–95. [PubMed] [Google Scholar]
- 222.Hong J., Zhang Y., Lai S. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–1311. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schramm T.K., Gislason G.H., Vaag A. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900–1908. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 224.Braunwald E., Mann D.L., Zipes D.P. Elsevier Saunders; 2014. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. [Google Scholar]
- 225.Zinman B., Lachin J.M., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016;374:1094. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 226.Frye R.L., August P., Brooks M.M. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Verma S., Farkouh M.E., Yanagawa B. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2013;1(4):317–328. doi: 10.1016/S2213-8587(13)70089-5. [DOI] [PubMed] [Google Scholar]
- 228.Mancini G.B., Farkouh M.E., Brooks M.M. Medical treatment and revascularisation options in patients with type 2 diabetes and coronary disease. J Am Coll Cardiol. 2016;68:985–995. doi: 10.1016/j.jacc.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 229.Singh M., Arora R., Kodumuri V. Coronary revascularisation in diabetic patients: current state of evidence. Exp Clin Cardiol. 2011;16:16–22. [PMC free article] [PubMed] [Google Scholar]
- 230.Manjunath G., Tighiouart H., Ibrahim H. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 231.Bicket D.P. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461–468. [PubMed] [Google Scholar]
- 232.Jeger R.V., Zellweger M.J., Kaiser C. Prognostic value of stress testing in patients over 75 years of age with chronic angina. Chest. 2004;125:1124–1131. doi: 10.1378/chest.125.3.1124. [DOI] [PubMed] [Google Scholar]
- 233.Kasser I.S., Bruce R.A. Comparative effects of aging and coronary heart disease on submaximal and maximal exercise. Circulation. 1969;39(6):759–774. doi: 10.1161/01.cir.39.6.759. [DOI] [PubMed] [Google Scholar]
- 234.Maron D.J., Spertus J.A., Mancini G.B. Impact of an initial strategy of medical therapy without percutaneous coronary intervention in high-risk patients from the Clinical Outcomes Utilizing Revascularisation and Aggressive DruG Evaluation (COURAGE) trial. Am J Cardiol. 2009;104:1055–1062. doi: 10.1016/j.amjcard.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 235.Pfisterer M., Buser P., Osswald S. Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one-year results of the randomized TIME trial. JAMA. 2003;289:1117–1123. doi: 10.1001/jama.289.9.1117. [DOI] [PubMed] [Google Scholar]
- 236.Montamat S.C., Cusack B.J., Vestal R.E. Management of drug therapy in the elderly. N Engl J Med. 1989;321:303–309. doi: 10.1056/NEJM198908033210507. [DOI] [PubMed] [Google Scholar]
- 237.Dai X., Busby-Whitehead J., Forman D.E. Stable ischemic heart disease in the older adults. J Geriatr Cardiol. 2016;13:109–114. doi: 10.11909/j.issn.1671-5411.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nichol K.L., Nordin J., Mullooly J. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 239.Ciszewski A., Bilinska Z.T., Brydak L.B. Influenza vaccination in secondary prevention from coronary ischemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–1358. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 240.Chen C.I., Kao P.F., Wu M.Y. Influenza vaccination is associated with lower risk of acute coronary syndrome in elderly patients with chronic kidney disease. Medicine (Baltimore) 2016;95:e2588. doi: 10.1097/MD.0000000000002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary-artery disease (TIME): a randomised trial. Lancet. 2001;358:951–957. doi: 10.1016/S0140-6736(01)06100-1. [DOI] [PubMed] [Google Scholar]
- 242.Pfisterer M. Long-term outcome in elderly patients with chronic angina managed invasively versus by optimized medical therapy: four-year follow-up of the randomized Trial of Invasive versus Medical therapy in Elderly patients (TIME) Circulation. 2004;110:1213–1218. doi: 10.1161/01.CIR.0000140983.69571.BA. [DOI] [PubMed] [Google Scholar]
- 243.Kuster G.M., Buser P., Osswald S. Comparison of presentation, perception, and six-month outcome between women and men > or =75 years of age with angina pectoris. Am J Cardiol. 2003;91:436–469. doi: 10.1016/s0002-9149(02)03240-x. [DOI] [PubMed] [Google Scholar]
- 244.Wijns W., Kolh P., Danchin N. Guidelines on myocardial revascularisation. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 245.Piepoli M.F., Corra U., Benzer W. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17:1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 246.Goldman S., Zadina K., Moritz T. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 247.Fitzgibbon G.M., Kafka H.P., Leach A.J. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 248.Riley R.F., Don C.W., Powell W. Trends in coronary revascularisation in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4:193–197. doi: 10.1161/CIRCOUTCOMES.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Christofferson R.D., Lehmann K.G., Martin G.V., Every N., Caldwell J.H., Kapadia S.R. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005;95:1088–1091. doi: 10.1016/j.amjcard.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 250.Kahn J.K. Angiographic suitability for catheter revascularisation of total coronary occlusions in patients from a community hospital setting. Am Heart J. 1993;126:561–564. doi: 10.1016/0002-8703(93)90404-w. [DOI] [PubMed] [Google Scholar]
- 251.Fefer P., Knudtson M.L., Cheema A.N. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991–997. doi: 10.1016/j.jacc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 252.Ge J.B. Current status of percutaneous coronary intervention of chronic total occlusion. J Zhejiang Univ Sci B. 2012;13:589–602. doi: 10.1631/jzus.B1201009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hannan E.L., Racz M.J., Walford G. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–2183. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 254.Hannan E.L., Racz M., Holmes D.R. Impact of completeness of percutaneous coronary intervention revascularisation on long-term outcomes in the stent era. Circulation. 2006;113:2406–2412. doi: 10.1161/CIRCULATIONAHA.106.612267. [DOI] [PubMed] [Google Scholar]
- 255.Stone G.W., Rutherford B.D., McConahay D.R. Procedural outcome of angioplasty for total coronary artery occlusion: an analysis of 971 lesions in 905 patients. J Am Coll Cardiol. 1990;15:849–856. doi: 10.1016/0735-1097(90)90285-w. [DOI] [PubMed] [Google Scholar]
- 256.Rathore S., Matsuo H., Terashima M. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: impact of novel guidewire techniques. JACC Cardiovasc Interv. 2009;2:489–497. doi: 10.1016/j.jcin.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 257.Patel M.R., Marso S.P., Dai D. Comparative effectiveness of drug-eluting versus bare-metal stents in elderly patients undergoing revascularisation of chronic total coronary occlusions: results from the National Cardiovascular Data Registry, 2005–2008. JACC Cardiovasc Interv. 2012;5:1054–1061. doi: 10.1016/j.jcin.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 258.Rubartelli P., Petronio A.S., Guiducci V. Comparison of sirolimus-eluting and bare metal stent for treatment of patients with total coronary occlusions: results of the GISSOC II-GISE multicentre randomized trial. Eur Heart J. 2010;31:2014–2020. doi: 10.1093/eurheartj/ehq199. [DOI] [PubMed] [Google Scholar]
- 259.Van den Branden B.J., Rahel B.M., Laarman G.J. Five-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II study) EuroIntervention. 2012;7:1189–1196. doi: 10.4244/EIJV7I10A190. [DOI] [PubMed] [Google Scholar]
- 260.Reifart N., Hauptmann K.E., Rabe A. Short and long term comparison (24 months) of an alternative sirolimus-coated stent with bioabsorbable polymer and a bare metal stent of similar design in chronic coronary occlusions: the CORACTO trial. EuroIntervention. 2010;6:356–360. doi: 10.4244/EIJV6I3A59. [DOI] [PubMed] [Google Scholar]
- 261.Colmenarez H.J., Escaned J., Fernandez C. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1854–1866. doi: 10.1016/j.jacc.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 262.Aziz O., Rao C., Panesar S.S. Meta-analysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ. 2007;334:617. doi: 10.1136/bmj.39106.476215.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Arora R.R., Chou T.M., Jain D. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 264.Soran O., Kennard E.D., Bart B.A. Impact of external counterpulsation treatment on emergency department visits and hospitalizations in refractory angina patients with left ventricular dysfunction. Congest Heart Fail. 2007;13:36–40. doi: 10.1111/j.1527-5299.2007.05989.x. [DOI] [PubMed] [Google Scholar]
- 265.Soran O., Kennard E.D., Kfoury A.G. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from The International EECP Patient Registry) Am J Cardiol. 2006;97:17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 266.Loh P.H., Cleland J.G., Louis A.A. Enhanced external counterpulsation in the treatment of chronic refractory angina: a long-term follow-up outcome from the International Enhanced External Counterpulsation Patient Registry. Clin Cardiol. 2008;31:159–164. doi: 10.1002/clc.20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.West P.D., Colquhoun D.M. TENS in refractory angina pectoris. Three case reports. Med J Aust. 1993;158:488–489. doi: 10.5694/j.1326-5377.1993.tb137584.x. [DOI] [PubMed] [Google Scholar]
- 268.Irurita M., Culebras C., Gopar S. Transcutaneous electrical nerve stimulation in refractory angina. J Am Coll Cardiol. 2014;63 [Google Scholar]
- 269.Taylor R.S., De Vries J., Buchser E. Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials. BMC Cardiovasc Disord. 2009;9:13. doi: 10.1186/1471-2261-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mannheimer C., Eliasson T., Augustinsson L.E. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY study. Circulation. 1998;97:1157–1163. doi: 10.1161/01.cir.97.12.1157. [DOI] [PubMed] [Google Scholar]
- 271.Shah S.A., Shapiro R.J., Mehta R. Impact of enhanced external counterpulsation on Canadian Cardiovascular Society angina class in patients with chronic stable angina: a meta-analysis. Pharmacotherapy. 2010;30:639–645. doi: 10.1592/phco.30.7.639. [DOI] [PubMed] [Google Scholar]
- 272.Schofield P.M., McNab D. NICE evaluation of transmyocardial laser revascularisation and percutaneous laser revascularisation for refractory angina. Heart. 2010;96:312–313. doi: 10.1136/hrt.2009.185769. [DOI] [PubMed] [Google Scholar]
- 273.Gao D., Ning N., Niu X., Hao G., Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 274.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 275.Mishra S., Ray S., Dalal J.J. Management of stable coronary artery disease in India: executive summary. Indian Heart J. 2016;68:868–873. doi: 10.1016/j.ihj.2016.11.318. [DOI] [PMC free article] [PubMed] [Google Scholar]