Abstract
This study, for the first time, evaluates the effect of olive and juniper leaves extracts and their combination on thioacetamide (TAA)-induced nephrotoxicity in male mice. The experimental mice were divided into eight groups. Group 1 was served as control. Group 2 was exposed to TAA. Group 3 was treated with TAA and olive leaves extract. Group 4 was subjected to TAA and juniper leaves extract. Group 5 was exposed to TAA and olive and juniper leaves extracts. Groups 6, 7 and 8 were treated with olive, juniper, and olive and juniper leaves extracts respectively. In mice treated with only TAA, significant increases of blood urea nitrogen and uric acid were observed after six weeks. Moreover, levels of serum creatinine, blood urea nitrogen and uric acid were statistically increased in mice administrated with only TAA for twelve weeks. Insignificant alterations in levels of these haematobiochemical parameters were noted in other treated groups after six and twelve weeks. Histopathological evaluations of renal sections from mice treated with only TAA for twelve weeks showed severe damage of the renal corpuscles. Furthermore, the renal sections from mice treated with TAA and olive leaves extract, TAA and juniper leaves extract, TAA and olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts showed normal structures. In addition, it is conceivable therefore, that these extracts exhibit protective influences against TAA-induced nephrotoxicity, probably mediated through the antioxidative pathway roles.
Keywords: Nephrotoxicity, Thioacetamide, Olive leaves, Juniper leaves, Mice
1. Introduction
An exposure to environmental pollutants increased risks of kidney disease (Hendryx, 2009). Nephrotoxicity is one of the most common kidney problems and occurs when the body is exposed to a drug or toxin (Porter and Bennett, 1981). Toxic chemical-induced nephrotoxicity tends to be more common among certain patients and in specific clinical situations. Humans are exposed intentionally and unintentionally to a variety of diverse chemicals that harm the kidney. As drugs, natural products, industrial chemicals, and environmental pollutants that cause nephrotoxicity have increased (Prusty et al., 2012). Kidneys are highly vulnerable to damage caused by reactive oxygen species (ROSs), likely due to oxidative stress by polyunsaturated fatty acids in the composition of renal lipids (Ozbek, 2012). This damage can also be caused by a high volume of blood flowing through it, and filtering large amounts of toxins, which can concentrate in kidney lobules (Begum et al., 2011). The kidney’s response to toxicants varies by multiple morphological patterns beginning with tubular or interstitial changes to nephropathy (Silva, 2004). Various useful drugs and some environmental and industrial toxicants can cause severe renal damage through activation of these drugs to highly reactive free radicals (Olagunju et al., 2009). Most drugs found to cause nephrotoxicity exert toxic effects by one or more common pathogenic mechanisms. These include altered intraglomerular hemodynamics, tubular cell toxicity, inflammation, crystal nephropathy, rhabdomyolysis, and thrombotic microangiopathy (Zager, 1997, Schetz et al., 2005). A number of physiologic factors have been implicated in the production of the nephrotoxic response caused by chemicals. Certainly it must be recognized that the large renal blood flow (25% of the cardiac output) is an important feature. Although we recognize that response is a function of concentration, it is nonetheless true that because of the high renal blood flow, the kidney is exposed to large quantities of whatever is in that blood, including nephrotoxicants. The ability of the kidney to concentrate the tubular fluid contents is a hallmark of renal function. Any nephrotoxic or potentially nephrotoxic compound present in the tubular fluid would be concentrated in a similar manner, which could contribute to direct damage to tubular epithelial cells or at least the creation of a concentration gradient that would facilitate the movement of the compound or compounds from the tubular fluid to the blood (Berndt, 1998).
Thioacetamide (TAA) is a potent experimental hepatotoxin and hepato-carcinogenic compound; therefore, it is used often to induce fulminant hepatic failure in experimental animal models (Sarkar and Sil, 2007). Additionally, the influences of TAA are not limited to the liver as profound structural and functional changes have been described in the kidney, spleen, lung, intestine, stomach and brain (Al-Bader et al., 2000, Liu et al., 2000, Caballero et al., 2001, Avraham et al., 2009, Latha et al., 2003, Kadir et al., 2013, Gao et al., 2014). Moreover, the toxic influences of TAA depend on several factors such as its concentration, number of doses, administration period, administration methods, animal’s gender, strain, age, weight and physiological case.
Medicinal plants play an important role in pharmacology and medicine for many years. Today, it is estimated that about 80% of the world population relies on botanical preparations as medicine to meet their health needs (Ogbera et al., 2010). Apart from currently available therapeutic options, many herbal medicines have been recommended for the treatment of many diseases. Medicinal plants and herbal drugs are prescribed widely because of their effectiveness, less side effects and relatively low cost (Venkatesh et al., 2003, Tohidi et al., 2011). Therefore investigation on some active principles from traditional medicinal plants has become more important (Suba et al., 2004). Furthermore, in different countries many herbs are used in folk medicine to treat drug or toxin induced renal damage (Palani et al., 2009). The olive tree and in particular its leaves have been used for the treatment of wounds, fever, diabetes, gout, atherosclerosis and hypertension since ancient times (Jänicke et al., 2003). Olive leaves are considered a cheap raw material and a useful source of high-added value products (Briante et al., 2002, Jemai et al., 2008). Different studies have shown that olive leaves extracts and their constituents possess a wide range of pharmacologic and health promoting properties including antiarrhythmic, spasmolytic, immune-stimulant, and liver, kidney and heart protective effects. Moreover, anti-atherosclerotic, hypotensive, anti-inflammatory, antioxidant, anti-thrombic and hypoglycemic effects were reported (Visioli et al., 1998, Andrikopoulos et al., 2002, Wang et al., 2008, Wainstein et al., 2012, Kumral et al., 2015). The natural forests of Saudi Arabia spread along the Western mountainous area from North to South. Juniper trees represent more than 90% of these forests (Abo-Hassan et al., 1984). Juniperus is a unique genus from the family Cupressaceae. The genus Juniperus consists of approximately 68 species (Seca and Silva, 2006, Adams, 2011). In traditional medicine, Juniperus species are used as remedies against the common cold, urinary infections, urticaria, dysentery, hemorrhage, and rheumatic arthritis and to relieve menstrual pain worldwide (Seca and Silva, 2006). Therefore, the objective of the present study was to evaluate the possible protective influences of olive (Olea oleaster) and juniper (Juniperus procera) leaves extract against TAA-induced nephrotoxicity in male mice.
2. Materials and methods
2.1. Animals
A total of one hundred sixty male albino mice of the SWR strain, weighing 15.0–25.0 g were taken for the present study. The principles of laboratory animal care were followed throughout the duration of experiment and instruction given by King Abdulaziz University ethics committee was followed regarding experimental treatments. The mice were distributed into eight groups (twenty mice per group) and were housed in standard cages at an ambient temperature of 20 ± 1 °C with 12-h light:12-h dark cycle and humidity of 65%. The mice were fed ad libitum on normal commercial chow and had free access to water.
2.2. Preparation of olive and juniper leaves extract
The fresh leaves of olive and juniper were directly collected from the outskirts of the Albaha region of Saudi Arabia. The collected leaves were completely washed, air dried at room temperature and stored in a dry plastic container until use for extraction processes. The extract of these leaves was prepared according to the method of Al-Attar and Abu Zeid (2013). The dried leaves of olive (50 g) were powdered, added to 2 liters of cold water and mixed using an electric mixer for 20 min. Furthermore, the dried leaves of juniper (50 g) were powdered, added to 2 liters of cold water and mixed using an electric mixer for 20 min. Thereafter, the solutions of olive and juniper leaves were gently filtered. Finally, the filtrates were evaporated in an oven at 40 °C to produce dried residues (active principles). With references to the powdered samples, the yield means of olive and juniper extracts were 19.3% and 17.8% respectively.
2.3. Experimental treatments
For the experimental purpose, mice of group 1 were served as controls and intraperitoneally injected with saline solution (0.9% NaCl), twice weekly. Mice of group 2 were given 150 mg/kg body weight of TAA (Sigma–Aldrich Corp., St. Louis, MO, USA) by intraperitoneal injection, twice weekly. Mice of group 3 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with olive leaves extract at a dose of 200 mg/kg body weight/day. Mice of group 4 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with juniper leaves extract at a dose of 200 mg/kg body weight/day. Mice of group 5 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with olive leaves extract (100 mg/kg body weight/day) and juniper leaves extract (100 mg/kg body weight/day). Mice of group 6 were intraperitoneally received saline solution at the same dose given to group 1 and were orally supplemented with olive leaves extract at the same dose given to group 3. Animals of group 7 were intraperitoneally received saline solution at the same dose given to group 1 and were orally supplemented with juniper leaves extract at the same dose given to group 4. Mice of group 8 were intraperitoneally received saline solution at the same dose given to group 1 and were supplemented with olive and juniper leaves extracts at the same dose given to group 5.
2.4. Blood sampling
After six and twelve weeks, the experimental animals were fasted for 8 h, water was not restricted, and then anaesthetized with diethyl ether. Blood samples were drawn from diethyl ether anaesthetized mice via orbital venous plexus. Blood samples were centrifuged at 2500 rpm for 15 min, and the clear samples of blood serum were separated and stored at −80 °C. These serum samples were used to determine levels of creatinine, blood urea nitrogen (BUN) and uric acid. The level of serum creatinine was determined using the method of Larsen (1971). The method of Patton and Crouch (1977) was used to measure the level of serum BUN. The level of serum uric acid was estimated according to the method of Young (1990).
2.5. Histopathological examination
Kidney tissues excised from each mouse were fixed in 10% buffered formaldehyde immediately after removal from the animals. Fixed tissues were routinely processed, then embedded in paraffin, and cut into 4 μm thick sections; they were mounted on slides for hematoxylin and eosin staining. Qualitative examinations of prepared tissues and the obtaining of their photos were carried out using a light microscope (Olympus BX61- USA) connected to motorized controller unit (Olympus bx-ucb, USA) and photographed by a camera (Olympus DP72, USA).
2.6. Statistical analysis
Data were statistically analyzed using Package for Social Sciences (SPSS) for Windows version 12.0 software. All experimental data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by a two-way analysis of variance (ANOVA). The P < 0.05 level of probability was used as the criteria of significance.
3. Results
Levels of serum creatinine, BUN and uric acid in control, TAA, TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts treated mice after six and twelve weeks are shown in Table 1. After six weeks, the level of serum creatinine was significantly unchanged in all treated groups compared with the control group. In comparison with control data, levels of serum BUN (+26.3%) and uric acid (+24.0%) were significantly raised in mice treated with TAA. Insignificant changes of serum BUN and uric acid levels were noted in TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts treated mice as compared with control mice. After twelve weeks, levels of serum creatinine (+38.5%), BUN (+26.3%) and uric acid (+24.0%) were markedly elevated in mice treated with TAA compared to control mice. There were no significant changes in levels of serum creatinine, BUN and uric acid in TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts treated mice (Table 1).
Table 1.
Levels of serum creatinine, BUN and uric acid in control, TAA, TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts treated mice after six and twelve weeks.
| Treatments | Periods (weeks) | Parameters |
||
|---|---|---|---|---|
| Creatinine (μmol/L) | BUN (mmol/L) | Uric acid (μmol/L) | ||
| Control | 6 | 22.67 ± 3.27 | 6.28 ± 0.94 | 110.83 ± 16.39 |
| 12 | 25.50 ± 1.64 | 5.67 ± 0.72 | 135.33 ± 10.17 | |
| TAA | 6 | 24.33 ± 2.58 | 7.93 ± 1.95 | 137.50 ± 21.83 |
| 12 | 35.33 ± 2.25 | 7.17 ± 1.2 | 170.16 ± 15.72 | |
| TAA + olive leaves | 6 | 24.83 ± 2.40 | 6.20 ± 1.27 | 117.50 ± 15.62 |
| 12 | 23.67 ± 2.50 | 5.16 ± 0.59 | 144.00 ± 17.15 | |
| TAA + juniper leaves | 6 | 22.47 ± 4.50 | 5.65 ± 0.70 | 116.83 ± 29.05 |
| 12 | 24.83 ± 1.72 | 5.08 ± 0.62 | 132.33 ± 21.79 | |
| TAA + olive and juniper leaves | 6 | 24.50 ± 4.32 | 5.95 ± 0.51 | 111.00 ± 22.41 |
| 12 | 22.56 ± 1.94 | 5.40 ± 0.94 | 137.50 ± 18.03 | |
| Olive leaves | 6 | 23.55 ± 2.34 | 5.63 ± 1.18 | 120.77 ± 23.61 |
| 12 | 25.33 ± 3.06 | 5.93 ± 0.99 | 131.00 ± 8.08 | |
| Juniper leaves | 6 | 22.83 ± 3.87 | 6.30 ± 1.50 | 119.00 ± 17.64 |
| 12 | 23.83 ± 1.17 | 5.73 ± 0.87 | 137.06 ± 16.21 | |
| Olive and juniper leaves | 6 | 23.43 ± 3.66 | 5.73 ± 1.22 | 115.03 ± 8.51 |
| 12 | 24.50 ± 2.88 | 5.36 ± 1.04 | 136.82 ± 10.50 | |
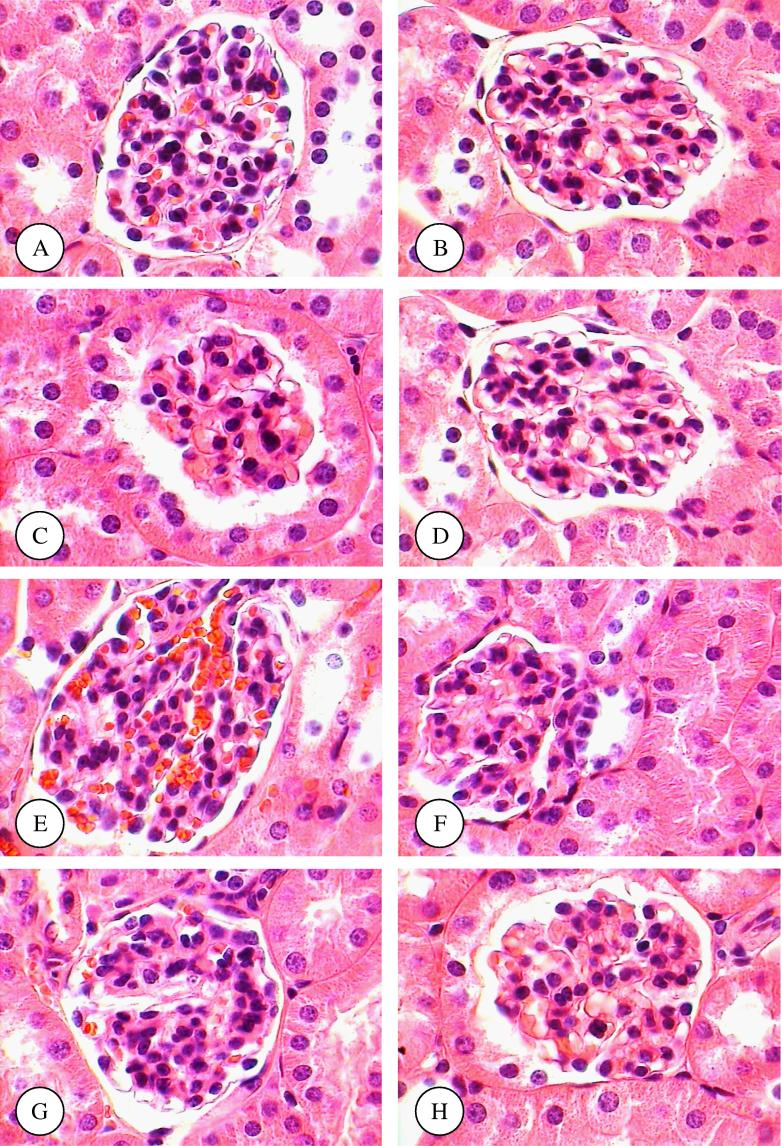
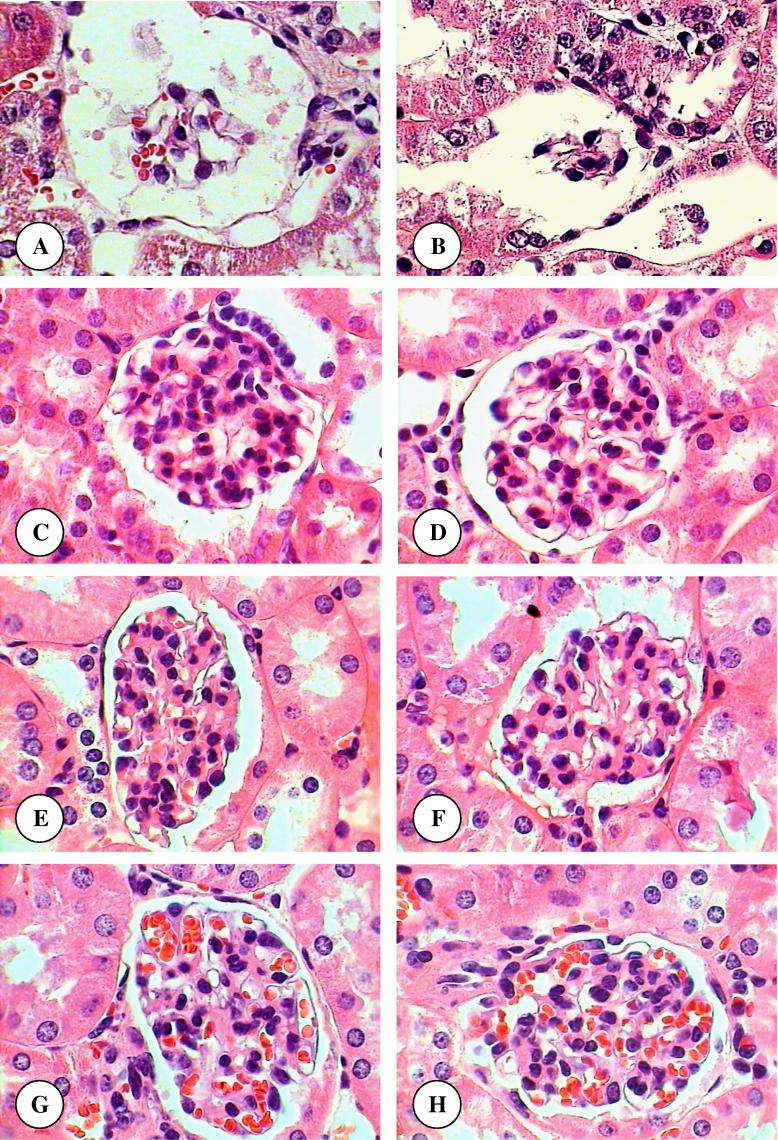
Areas of renal cortex containing renal corpuscles and associated tubules were showed more pronounced changes in treated animals compared with the control. Therefore, these areas were selected for histological examination with the light microscope. The normal renal (Malpighian) corpuscle consists of a tuft of capillaries, the glomerulus, surrounded by a double walled epithelial capsule called Bowman’s capsule. Between the two layers of the capsule is the urinary or Bowman’s space (Fig. 1A). After six weeks, the structure of renal corpuscles in mice treated with TAA, TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts showed normal appearances compared with normal mice (Fig. 1B–H). In mice treated with only TAA for twelve weeks, there were several alterations in the structure of renal corpuscles including a high degeneration and necrosis of glomeruli and Bowman’s capsules (Fig. 1A and B). In comparison with control mice, the renal sections from mice treated with TAA plus olive leaves extract, TAA plus juniper leaves extract, TAA plus olive and juniper leaves extracts, olive leaves extract, juniper leaves extract, and olive and juniper leaves extracts showed normal structures (Fig. 2C–H).
Figure 1.
(A–H) Renal corpuscle micrographs of control (A), TAA (B), TAA plus olive leaves extract (C), TAA plus juniper leaves extract (D), TAA plus olive and juniper leaves extracts (E), olive leaves extract (F), juniper leaves extract (G), and olive and juniper leaves extracts (H) treated mice for six weeks. Original magnification (×1000).
Figure 2.
(A–H) Renal corpuscle micrographs of TAA (A and B), TAA plus olive leaves extract (C), TAA plus juniper leaves extract (D), TAA plus olive and juniper leaves extracts (E), olive leaves extract (F), juniper leaves extract (G), and olive and juniper leaves extracts (H) treated mice for twelve weeks. Original magnification (×1000).
4. Discussion
The kidney is highly susceptible to toxicants for two reasons. A high volume of blood flows through it and it filters large amounts of toxins which can concentrate in the kidney tubules. It can result in systemic toxicity causing: decreased ability to excrete body wastes, inability to maintain body fluid and electrolyte balance and decreased synthesis of essential hormones (Oduola et al., 2010). Additionally, kidneys are highly vulnerable to damage caused by reactive oxygen species (ROSs), likely due to oxidative stress by polyunsaturated fatty acids (PUFs) in the composition of renal lipids (Ozbek, 2012). This damage can also be caused by a high volume of blood flowing through it, and filtering large amounts of toxins, which can concentrate in kidney lobules (Begum et al., 2011).
The present study demonstrates that mice chronically intoxicated with TAA display a pronounced impairment in kidney (renal) function which is confirmed by the enhancement of serum creatinine, BUN and uric acid levels, and histopathological alterations. Additionally, the results of the present study showed that the cortex is more affected than medulla due to long-term treatment with TAA. This could be partly due to uneven distribution of metabolites of TAA in the tissue of the kidney where about 90% of the total renal blood flow enters the cortex via the bloodstream. Accordingly, a relative high concentration of metabolites of TAA might reach the cortex via the bloodstream than that would enter the medulla. Serum levels of creatinine, BUN and uric acid are useful tools in diagnosis as they pick any disturbances to the renal system early enough to allow for projection and possible remedies. However, the present elevations of serum creatinine, BUN and uric acid are generally in accordance with the findings of several studies showing elevations of these parameters in experimental animals exposed to carbon tetrachloride (CCl4) and TAA (Sirag, 2007, Lim et al., 2011, Bakhtiary et al., 2012, Kadir et al., 2013, Al-Sayed and Abdel-Daim, 2014, Omnia et al., 2014, Fahmy et al., 2015). Histopathologically, the toxic effects of TAA on the kidney of experimental animals were investigated by several studies. These studies showed that the light microscopic examinations of renal tissues revealed severe histopathological changes including congestion of the glomeruli and focal mesengial cell proliferation, increased deposition of the collagen in the renal medulla and fibrin in the cortex, disrupted and swollen cells of convoluted tubules and lobulated atrophied glomeruli, tubular epithelial cell necrosis associated with diffuse tubular swelling, and inflammatory cell infiltration (Al-Bader et al., 1999, Mahmoud, 2006, Kadir et al., 2013).
The present study showed that the administration of olive and juniper leaves extracts and their combination in mice can prevent severe alterations of renal haematobiochemical markers and disruptions of its histological structure. Tavafi et al. (2012) studied the effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. They reported that the olive leaf extract ameliorates gentamicin nephrotoxicity via antioxidant activity, increase of renal glutathione (GSH) content, and increase of renal antioxidant enzymes activity. Zari and Al-Attar (2011) studied the possible protective effects of administration of olive leaves extract on carbendazim induced physiological and histopathological alterations in male Wistar rats. Pretreatment of carbendazim-exposed rats with olive leaves extract showed a marked improvement in both physiological and histopathological alterations. They suggested that the olive leaves extract exerts its ameliorative effect against carbendazim-induced the hematological, biochemical and histopathological alterations by preventing the decline of antioxidant defense system and direct free radical scavenging activity. Al-Attar and Abu Zeid (2013) evaluated the effect of olive leaves extract against diazinon toxicity in male mice. They indicated that the extract of olive leaves can be considered as a promising therapeutic agent against hepatotoxicity, cardiotoxicity, nephrotoxicity and metabolic disorders induced by diazinon treatment. Moreover, they suggested that the effect of olive leaves extract against diazinon was possibly due to antioxidant properties of its natural chemical constituents. Additionally, Al-Sowayan and Mousa (2014) investigated the protective effect of olive leaf extract CCl4 induced nephrotoxicity in male Wistar rats. Treatment with olive leaf extract significantly attenuated the biochemical and histopathological alterations induced by CCl4 suggesting that the olive leaf extract protected CCl4-induced nephrotoxicity through enhancement of the renal antioxidant system. Butani et al. (2003) investigated the effect of juniper oil against tacrolimus (an immunosuppressive drug) induced nephrotoxicity in rats. They reported that juniper oil displays a protective role on renal damage by exposure to tacrolimus. Additionally, Orhan et al. (2012) evaluated the antiinflammatory and antinociceptive influences of some juniper species in Swiss male albino mice. They demonstrated that the extracts and some of their constituents displayed remarkable antiinflammatory and antinociceptive activity.
In conclusion, the present findings show that oral administration of these extracts produce significant protective effects against TAA induced nephrotoxicity. However, the present findings suggest that these extracts and their chemical constituents are effective in modulation of oxidative stress induced by TAA administration. Further investigations are required to explore the mechanism of action of these extracts against TAA induced renal dysfunction and histopathological changes.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abo-Hassan A.A., El-Osta M.L.M., Sabry M.M. Scientific Research Directory, National Center for Science and Technology (King Abdulaziz City for Science and Technology); Riyadh, Saudi Arabia: 1984. Natural Forests in Kingdom of Saudi Arabia and the Possibility of Exploiting them Economically. Book No. 1, pp. 177. [Google Scholar]
- Adams R.P. third ed. Trafford Publications; Victoria, BC: 2011. The Junipers of the World: The Genus Juniperus. [Google Scholar]
- Al-Attar A.M., Abu Zeid I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed Res. Int. 2013;2013:1–6. doi: 10.1155/2013/461415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader A.A., Mathew T., Abul H., Al-Mosawi M., Dashti H.M., Kumar D., Singal P.K. Thioacetamide induced changes in trace elements and kidney damage. J. Trace Elem. Exp. Med. 1999;12:1–14. [Google Scholar]
- al-Bader A., Mathew T.C., Koursheed M., Asfar S., al-Sayer H., Dashti H.M. Thioacetamide toxicity and the spleen: histological and biochemical analysis. Anat. Histol. Embryol. 2000;29:3–8. doi: 10.1046/j.1439-0264.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Al-Sayed E., Abdel-Daim M.M. Protective role of Cupressuflavone from Cupressus macrocarpa against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Planta Med. 2014;80:1665–1671. doi: 10.1055/s-0034-1383211. [DOI] [PubMed] [Google Scholar]
- Al-Sowayan N.S., Mousa H.M. Ameliorative effect of olive leaf extract on carbon tetrachloride-induced nephrotoxicity in rats. Life Sci. J. 2014;11:238–242. [Google Scholar]
- Andrikopoulos N.K., Antonopoulou S., Kaliora A.C. Oleuropein inhibits ldl oxidation induced by cooking oil frying by-products and platelet aggregation induced by platelet-activating factor. LWT Food Sci. Technol. 2002;35:479–484. [Google Scholar]
- Avraham Y., Grigoriadis N.C., Magen I., Poutahidis T., Vorobiav L., Zolotarev O., Ilan Y., Mechoulam R., Berry E.M. Capsaicin affects brain function in a model of hepatic encephalopathy associated with fulminant hepatic failure in mice. Br. J. Pharmacol. 2009;158:896–906. doi: 10.1111/j.1476-5381.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtiary S.A., Iqbal M.M., Ibrahim Md. Hepatoprotective and nephroprotective activity of Phyllanthus amarus Schum & Thonn. seed extract. Ann. Phytomed. 2012;1:97–104. [Google Scholar]
- Begum Q., Noori S., Mahboob T. Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pak. J. Biochem. Mol. Biol. 2011;44:21–26. [Google Scholar]
- Berndt W.O. The role of transport in chemical nephrotoxicity. Toxicol. Pathol. 1998;26:52–57. doi: 10.1177/019262339802600107. [DOI] [PubMed] [Google Scholar]
- Briante R., Patumi M., Terenziani S., Bismuto E., Febbraio F., Nucci R. Olea europaea L. leaf extract and derivatives: antioxidant properties. J. Agric. Food Chem. 2002;50:4934–4940. doi: 10.1021/jf025540p. [DOI] [PubMed] [Google Scholar]
- Butani L., Afshinnik A., Johnson J., Javaheri D., Peck S., German J.B., Perez R.V. Amelioration of tacrolimus-induced nephrotoxicity in rats using juniper oil. Transplantation. 2003;76:306–311. doi: 10.1097/01.TP.0000072337.37671.39. [DOI] [PubMed] [Google Scholar]
- Caballero M.E., Berlanga J., Ramirez D., Lopez-Saura P., Gozalez R., Floyd D.N., Marchbank T., Playford R.J. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut. 2001;48:34–40. doi: 10.1136/gut.48.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy N.M., Al-Sayed E., Abdel-Daim M.M., Karonen M., Singab A.N. Protective effect of Terminalia muelleri against carbon tetrachloride-induced hepato and nephro-toxicity in mice and characterization of its bioactive constituents. Pharm. Biol. 2015;20:1–11. doi: 10.3109/13880209.2015.1035794. [DOI] [PubMed] [Google Scholar]
- Gao W., Li H.Y., Wang L.X., Hao L.J., Gao J.L., Zheng R.J., Cai C.J., Si Y.L. Protective effect of omeprazole on gastric mucosal of cirrhotic portal hypertension rats. Asian Pac. J. Trop. Med. 2014;7:402–406. doi: 10.1016/S1995-7645(14)60065-1. [DOI] [PubMed] [Google Scholar]
- Hendryx M. Mortality from heart, respiratory, and kidney disease. Int. Arch. Occup. Environ. Health. 2009;82:24–249. doi: 10.1007/s00420-008-0328-y. [DOI] [PubMed] [Google Scholar]
- Jänicke C., Grünwald J., Brendler T. Wissenschaftliche Verlagsgesellschaft; Stuttgart: 2003. Handbuch Phytotherapie. [Google Scholar]
- Jemai H., Bouaziz M., Fki I., El Feki A., Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Int. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Kadir F.A., Kassim N.M., Abdulla M.A., Yehye W.A. Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2013;13:294. doi: 10.1186/1472-6882-13-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral A., Giriş M., Soluk-Tekkeşin M., Olgaç V., Doğru-Abbasoğlu S., Türkoğlu Ü., Uysal M. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology. 2015;22:117–123. doi: 10.1016/j.pathophys.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 1971;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Latha M.S., Mirabel R.P., Pushpalatha P.K. Thioacetamide toxicity and the lung: histological analysis. Indian J. Physiol. Pharmacol. 2003;47:476–478. [PubMed] [Google Scholar]
- Lim J.H., Kim T.W., Park S.J., Song I.B., Kim M.S., Kwon H.J., Cho E.S., Son H.Y., Lee S.W., Suh J.W., Kim J.W., Yun H.I. Protective effects of Platycodon grandiflorum aqueous extract on thioacetamide-induced fulminant hepatic failure in mice. J. Toxicol. Pathol. 2011;24:223–228. doi: 10.1293/tox.24.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Han D., Ren D. Effect of intestinal endotoxemia induced by thioacetamide. Chung-HuaKan Tsang Ping Tsa Chin. 2000;8:174–179. [PubMed] [Google Scholar]
- Mahmoud N.H. Protective effect of Panax ginseng against thioacetamide cytotoxicity in liver and kidney of albino rat. J. Egypt. Soc. Toxicol. 2006;34:43–54. [Google Scholar]
- Oduola T., Bello I., Adeosun G., Abdul-Waheed A., Raheem G., Avwioro G. Hepatotoxicity and nephrotoxicity evaluation in Wistar albino rats exposed to Morinda lucida leaf extract. North Am. J. Med. Sci. 2010;2:230–233. doi: 10.4297/najms.2010.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbera A.O., Dada O., Adeyeye F., Jewo P.I. Complementary and alternative medicine use in diabetes mellitus. West Afr. J. Med. 2010;29:158–162. doi: 10.4314/wajm.v29i3.68213. [DOI] [PubMed] [Google Scholar]
- Olagunju J., Adeneye A., Fagbohunka B., Bisuga N., Ketiku A., Benebo A., Olufowobi O., Adeoye A., Alimi M., Adeleke A. Nephroprotective activities of the aqueous seed extract of Carica papaya Linn. in carbon tetrachloride induced renal injured Wistar rats: a dose-and time-dependent study. Biol. Med. 2009;1:11–19. [Google Scholar]
- Omnia M.A., Nabila M.A., Nadia R.R. Biochemical effects of propolis and bee pollen in experimentally-induced hyperammonemia in rats. Benha Vet. Med. J. 2014;27:8–24. [Google Scholar]
- Orhan N., Akkol E., Ergun F. Evaluation of antiinflammatory and antinociceptive effects of some Juniperus species growing in Turkey. Turk. J. Biol. 2012;36:719–726. [Google Scholar]
- Ozbek E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012;2012:1–9. doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani S., Raja S., Kumar R.P., Jayakumar S., Kumar B.S. Therapeutic efficacy of Pimpinella tirupatiensis (Apiaceae) on acetaminophen induced nephrotoxicity and oxidative stress in male albino rats. Int. J. PharmTech. Res. 2009;1:925–932. [Google Scholar]
- Patton G.J., Crouch S.R. Determination of urea (urease modified Berthelot reaction) Anal. Chem. 1977;49:464–469. [Google Scholar]
- Porter G.A., Bennett W.M. Nephrotoxic acute renal failure due to common drugs. Am. J. Physiol. 1981;241:F1–F8. doi: 10.1152/ajprenal.1981.241.1.F1. [DOI] [PubMed] [Google Scholar]
- Prusty K.B., Harish B., Mamatha C.H. Evaluation of nephroprotective activity of the methanolic extract of leaves of Bauhinia variegata Linn, (Family-Caesalpiniaceae) J. PharmaSciTech. 2012;2:16–19. [Google Scholar]
- Sarkar M.K., Sil P.C. Hepatocytes are protected by herb Phyllanthus niruri protein isolate against thioacetamide toxicity. Pathophysiology. 2007;14:113–120. doi: 10.1016/j.pathophys.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Schetz M., Dasta J., Goldstein S., Golper T. Drug-induced acute kidney injury. Curr. Opin. Crit. Care. 2005;11:555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- Seca A.M.L., Silva A.M.S. The chemical composition of the Juniperus genus (1970–2004) In: Govil J.N., Singh V.K., Bhardwaj R., editors. Vol. 16. Studium Press LLC; 2006. pp. 401–522. (Recent Progress in Medicinal Plants, Phytomedicines). [Google Scholar]
- Silva F.G. Chemical-induced nephropathy: a review of the renal tubulointerstitial lesions in humans. Toxicol. Pathol. 2004;32:71–84. doi: 10.1080/01926230490457530. [DOI] [PubMed] [Google Scholar]
- Sirag H.M. Biochemical studies on thioacetamide toxicity in male albino rats and the role of tomato juice as an antioxidant. Mansoura J. Forensic Med. Clin. Toxicol. 2007;XV:99–115. [Google Scholar]
- Suba V., Murugesan T., Arunachalam G., Mandal S.G., Saha B.P. Hypoglycemic potential of Barleria lupulina extract in rats. Fitoterapia. 2004;75:1–4. doi: 10.1016/s0367-326x(03)00163-1. [DOI] [PubMed] [Google Scholar]
- Tavafi M., Ahmadvand H., Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran. J. Kidney Dis. 2012;6:25–32. [PubMed] [Google Scholar]
- Tohidi M., Khayami M., Nejati V., Meftahizade H. Evaluation of antibacterial activity and wound healing of Pistacia atlantica and Pistacia khinjuk. J. Med. Plant Res. 2011;5:4310–4314. [Google Scholar]
- Venkatesh S., Reddy G.D., Reddy B.M., Ramesh M., Rao A.V. Antihyperglycemic activity of Caralluma attenuata. Fitoterapia. 2003;74:274–279. doi: 10.1016/s0367-326x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Visioli F., Bellasta S., Galli C. Oleuropein, the bitter principle of olives, enhances nitric oxide production by mouse macrophages. Life Sci. 1998;62:541–546. doi: 10.1016/s0024-3205(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Wainstein J., Ganz T., Boaz M., Bar Dayan Y., Dolev E., Kerem Z., Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food. 2012;15:605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- Wang L., Geng C., Jiang L., Gong G., Liu D., Yoshimura H., Zhong L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008;47:235–243. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]
- Young, D.S., 1990. Effects of drugs on clinical laboratory test. 3rd ed. 3, 19–25.
- Zager R.A. Pathogenetic mechanisms in nephrotoxic acute renal failure. Semin. Nephrol. 1997;17:3–14. [PubMed] [Google Scholar]
- Zari T.A., Al-Attar A.M. Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur. Rev. Med. Pharmacol. Sci. 2011;15:413–426. [PubMed] [Google Scholar]