ABSTRACT
Sex assignment in newborns depends on the anatomy of the external genitalia, despite this stage being the final in embryogenesis. According to the current view, the genital tubercle is the embryonic precursor of penis and clitoris. It originates from mesenchymal tissue, but mesenchymal cells are arranged across the embryonal body and do not have specific androgen receptors. The nature of the signal that initiates early derivation of the indifferent genital tubercle is unknown at present. The aims of this article are to improve surgical management of intersex disorders and investigate the development of the genital tubercle. Clinical examination of 114 females with various forms of DSD revealed ambiguous (bisexual) external genitalia in 73 patients, and 51 of them underwent feminizing surgery. Intersexuality (ambiguity) in 46,XY patients results from disruptors in the pathways of sex steroid hormones or receptors; in 46,XX females arises from excessive levels of androgens. Systematization of intersex disorders distinguishes the karyotype, gonadal morphology, and genital anatomy to provide a differential diagnosis and guide appropriate surgical management. Modified feminizing clitoroplasty with preservation of the dorsal and ventral neurovascular bundles to retain erogenous sensitivity was performed in females with severe virilization (Prader degree III-V). The outgrowth of the genital tubercle and the fusion of the urethral fold proceed in an ordered fashion; but in some cases of ambiguity, there was discordance due to different pathways. Speculation about the derivation of the genital tubercle have discussed with a literature review. The genital tubercle derives from the following 3 layers: the ectodermal glans of the tubercle, the mesodermal corpora cavernosa and the endodermal urogenital groove. According to the new hypothesis, during the indifferent stages, the 5 sacral somites have to recede from their segmentation and disintegrate: the sclerotomes form the pelvic bones, the fused myotomes follow with their genuine neurotomes and the angiotomes join to the corpora cavernosa of the genital tubercle. Sexual differentiation of external genitalia is final in gender embryogenesis, but surprisingly derivation of the indifferent genital tubercle from 5 somites occurs before gonadal and internal organs development.
KEYWORDS: ambiguity, cliitororeduction, disorders of sex development, embryogenesis of external genitalia, erogenous sensitivity, feminizing plasty, genital tubercle, intersexuality
INTRODUCTION
Human organogenesis begins in the third week of gestation when the paraxial mesoderm organizes into segments, known as somitomeres (somites). During that period, the 3 germ layers, consisting of the ectoderm, the mesoderm, and the endoderm, give rise to a number of specific tissues and organs.1
The first somites appear in the cephalic region of the embryo at approximately the 20th day of development, and their formation proceeds cephalo-caudally at a rate of approximately 3 pairs per day. At the end of the fifth week, the embryo has 42-44 pairs of somites; these consist of 4 occipital, 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 8 to 10 coccygeal pairs.1
Each somitomere consists of mesodermal cells arranged in concentric whorls around the center of the unit. Somites give rise to the following 5 components: the myotome (segmental muscle component), the sclerotome (cartilage and bone component), and the dermatome (forms the skin of the back). All of these tissues are supporting tissues of the body; each myotome and dermatome retains its own innervation and vascularization from its originating segments, and these form the neurotome and angiotome, respectively.1,2
The vertebral column and ribs retain segmental structure. They develop from the sclerotome compartments of the somites, and they join in the ventral body wall. Each segment includes all of the following 5 components: a vertebra connected to a rib (sclerotome), with intercostal muscles (myotome) beneath, and its own nerve (neurotome) and vessels (angiotome), covered in skin (dermatome). The first occipital and the last 5 to 7 coccygeal somites later disappear, while the remaining somites form the axial skeleton.1
Initially, the genital system consists of (1) gonads or primitive sex glands, (2) genital ducts and (3) indifferent external genitalia. All three components go through indifferent stages, after which they develop following male or female pathways. For a review, see refs. 1-5.
The organogenesis of the external genitalia proceeds from the third to the 12th weeks of embryonic development. In the third week of development, mesenchymal cells originating around the primitive streak migrate to the cloacal membrane to form a pair of slightly elevated cloacal folds.
Cranially of the cloacal membrane, the folds unite to form the genital tubercle. Caudally, the folds are subdivided into urethral (anterior) and anal (posterior). Meanwhile, another pair of elevations, the genital swellings, becomes visible on each side of the urethral folds. These swellings later form the scrotal buds (in males) and the labia majora (in females).
The external genitalia in both sexes develop from the genital tubercle, genital swellings, and genital folds. At the end of the sixth week, the genital tubercle is indistinguishable between male and female.1
The indifferent duct system and external genitalia develop under the influence of hormones.
In males, the SRY gene on the Y chromosome produces testes-determining factor and regulates male sexual development. Testosterone produced by Leydig cells in the testes stimulates development of the mesonephric ducts into the vas deferens and epididymis. Dihydrotestosterone stimulates development of the male genitalia (virilization), consisting of the penis, scrotum, and prostate. The genital swellings become the scrotum, and the genital tubercle elongates to form the penis (phallus) and the penile urethra, which terminates in the glans penis. The prostate forms in the walls of the urogenital sinus. For a review, see refs. 1-5.
In the absence or inactivity of androgens, the fetus remains in the indifferent stages and becomes phenotypically female. Estrogens stimulate development of the external genitalia in females. In females, the genital tubercle becomes the clitoris, the genital swellings become the labia majora, and the genital folds become the labia minora. The genital tubercle elongates slightly and forms the clitoris, which is larger than that in the male during the early stages of development. The urethral fold does not fuse, as in the male embryo, and develops into the labia minora. Genital swellings enlarge and form the labia majora. The urogenital groove is open and forms the vestibulum vaginae. For a review, see refs. 1-6.
Abnormal androgen effects during embryogenesis may cause persistence on between developmental stages, which may result in the fetus clinically presenting as intersexual (disorders of sex development), or it could result in the fetus developing and being assigned female sex at birth. For a review, see refs. 7-16.
Disorders of sex development (DSD) or intersexuality (ambiguity) are present in 0.018% (1.8 per 10,000 live births) of newborns, and the incidence of 46,XY DSD is estimated at 1 in 20,000 live male births. More than 90% of 46,XX patients (congenital adrenal hyperplasia) and 46,XY DSD patients (androgen insensitivity syndrome, testicle dysgenesis) are assigned as females and require for feminizing plasty.7,8
Feminizing genital surgery is considered in cases of severe virilization (Prader degree of III-V) and should be performed with introitoplasty of the common urogenital sinus. For a review, see refs. 9-18.
Surgical procedures should be anatomically based to preserve the innervation of the clitoris, which possesses erogenous sensation and orgasmic function.17
Management of intersex disorders has been addressed in clinical guidelines, but appropriate surgical corrections have not been established in a standardized fashion for gynecological practice. For a review, see refs. 9-14.
MATERIALS AND METHODS
Clinical examination of 114 females with various forms of DSD (Table 1) revealed ambi-guous (bisexual) external genitalia in 73 patients.
TABLE 1.
The systematization of 114 females with various disorders of sex development (DSD).
| Systematization of Intersex Disorders | ||||||
|---|---|---|---|---|---|---|
| Nosology | Karyotype | Gonadal morphology | Internal genital anatomy | External genital anatomy | Surgical treatment | N |
| Congenital adrenal hyperplasia | 46,XX | ovary | uterus and vagina | ambiguous genitalia | feminizing plasty: clitororeduction, introitoplasty | 29 |
| Turner syndrome (45,X) | 45,X | fibrous streak gonad | PMDS | female | colpopoesis | 7 |
| Turner syndrome (45,X/46,XX) | 45,X/46,XX | ovarian dysgenesis | uterus and vagina normal | female | laparoscopy gonadal biopsy | 8 |
| Turner syndrome (45,X/46,XY) | 45,X/46,XY | fibrous streak, testicular dysgenesis, ovotestis | PMDS | ambiguous genitalia | laparoscopy gonadectomy; feminizing plasty: clitororeduction, introitoplasty; colpopoesis | 5 |
| 46,XY gonadal dysgenesis, incomplete form | 46,XY | testicular dysgenesis | PMDS, hypoplastic uterus | ambiguous genitalia | laparoscopy gonadectomy; feminizing plasty: clitororeduction, introitoplasty; colpopoesis | 19 |
| 46,XY gonadal dysgenesis, complete form | 46,XY | testicular dysgenesis | uterus and vagina (normal or hypoplastic) | female | laparoscopy gonadectomy | 12 |
| Androgen insensitivity syndrome, complete form | 46,XY | testicles | PMDS | female | laparoscopy gonadectomy, colpopoesis | 14 |
| Androgen insensitivity syndrome, incomplete form | 46,XY | testicles | PMDS | ambiguous genitalia | laparoscopy gonadectomy; feminizing plasty: clitororeduction, introitoplasty; colpopoesis | 14 |
| Ovotesticular DSD | 46,XY 46,XX/46,XY | ovotestis | PMDS | ambiguous genitalia | laparoscopy gonadectomy; feminizing plasty: clitororeduction, introitoplasty;colpopoesis | 6 |
In total, 51 females (5 to 25 y of age) with ambiguous external genitalia (Prader degree III-V) underwent of feminizing surgery between 2007 and 2016 in the Federal State Scientific Center of Obstetrics, Gynecology and Perinatology in Moscow, Russia (Table 1).
All individuals received an assignment of female gender after clinical examination, karyotyping, ultrasonography, MRI, laparoscopy, studies of gonadal morphology, and hormonal measurement.
Surgical correction was performed according to the European Consensus (2006, 2009) and the UK Guidance (2011) on management of Disorders of Sexual Development (DSD). For a review, see refs. 9-14.
Adnexectomy recommended in SRY-positive female patients with androgen insensitivity syndrome (AIS); 46,XY gonadal dysgenesis, and ovotesticular DSD, to prevent the development of malignancy in adulthood. Gonadal biopsy in SRY negative patients allow for verification of ovarian dysgenesis (45,X/46,XX) or streak-gonads with X-monosomy and mosaicism. Feminizing surgery includes clitororeduction and introitoplasty, which are intended to provide sexual function, retain erogenous sensitivity and achieve a satisfactory cosmetic result. For a review, see refs. 9-14.
The investigation of gonadal morphology among patients with DSD was published in “Studies of gonadal sex differentiation.”19
Uterovaginal anomalies were researched in the “New theory of uterovaginal embryogenesis.”20
The degree of external genitalia virilization was estimated by Prader classification stages I-V (Table 2).15,16
TABLE 2.
Classification of ambiguous external genitalia by Prader stages.
| Prader stages | Clitoromegally | Introitus | Operation | n |
|---|---|---|---|---|
| 0 stage | Female phenotype, normal clitoris | Normal vestibulum vaginae and labia minora | 25 | |
| I stage | Slightly enlarged clitoris | Normal vaginal orifice | 22 | |
| II stage | Mild enlarged cliitoris | Slightly reduced vaginal orifice and posterior labial fusion. The vagina and urethra open into a funnel-shaped urogenital sinus. | Introitoplasty | 16 |
| III stage | Clitoromegaly | Incomplete posterior fusion of the labia minora. The vagina and urethra share a single opening in the urogenital sinus. | Clitororeduction, introitoplasy | 31 |
| IV stage | Clitoromegaly appears as male phallus | Complete posterior fusion of the labia minora. The urogenital sinus opens near of the base clitoris. | Clitororeduction, introitoplasy | 17 |
| V stage | Male phenotype due to penile transformation (male phallus) | Complete fusion of the labial folds. The urogenital sinus transforming to penile urethra, has single orifice at the glans penis. The normally formed scrotum empty. | Clitororeduction, introitoplasy | 3 |
| Total | 114 |
Clitoreduction was performed for patients with severe virilization (Prader degrees III–V). For a review, see refs. 9-14.
RESULTS
Differential diagnosis of patients with DSD involves the investigation of karyotype, gonadal morphology, internal genital anatomy and degree of external genital virilization, which guides surgical treatment.
Prader's classification of ambiguous external genitalia includes the following (Table 2):
Prader stage 0 corresponds to females with normal external genitalia. This includes females with 45,X and 45,X/46,XX Turner syndrome and those with a complete form of 46,XY gonadal dysgenesis and androgen insensitivity syndromes (AIS) who present with feminine external genitalia (Prader stage 0).
Prader stage I is characterized by a slightly enlarged clitoris. Prader I may be regarded as a common condition present in the general female population.
Prader stage II distinguishes a mild degree of virilization and does not require surgical correction.
Prader stage III-V is recognized as ambiguous genitalia or intersex DSD. Patients with ambiguous genitalia (Prader degree III-V) undergo feminizing genitoplasty according to the European Consensus recommendations.
Prader VI stage indicates a normal male presentation with typical external genitalia and normal testes in the scrotum.
First-line surgery is performed for patients with 46,XY gonadal dysgenesis, testicular feminization syndrome and ovotesticular DSD: laparoscopic adnexectomy to remove the testicular tissue or rudimentary gonads.
Feminizing surgery of the external genitalia was performed in 51 cases (Prader degree III-V), and 3 patients of them had V degree of masculinization.
Feminizing surgery includes clitororeduction and introitoplasty, which are intended to retain erogenous sensitivity and achieve a satisfactory cosmetic result.
The clitoris is innervated by sensory nerves that pass within the nervus dorsalis clitoridis and the nervus pudendalis originating from S2-S5 segments. Surgical incision of the clitoral glans and corpora cavernosa may risk damaging the innervation, which disturbs the erogenous sensation essential for orgasm. Problems related with clitoroplasty are decreased sexual sensitivity, loss of clitoral tissue, and cosmetic issues. The surgical procedure of clitororeduction relies anatomically on the precise incision of the corpora cavernosa and preservation of innervation of the glans clitoris.
The modified clitororeduction, as opposed to clitorectomia, is performed by resection of the corpora cavernosa and preservation of the glans clitoridis on the dorsal and ventral neurovascular bundles (Figs. 1 and 2).
FIGURE 1.
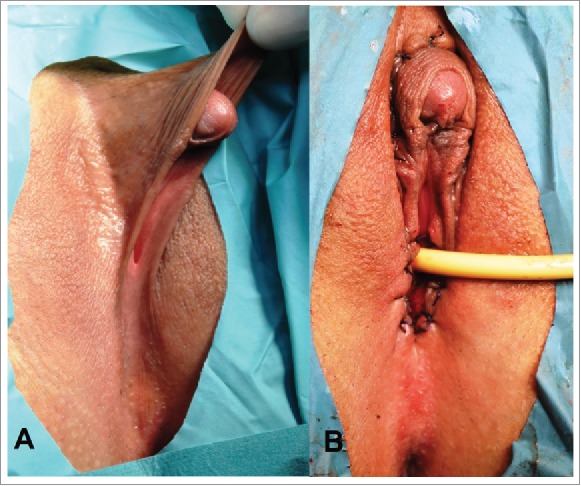
Congenital adrenal hyperplasia. Prader stage III. (A) External genitalia before operation. Clitoromegaly and sinus urogenitalis resulted of incomplete posterior labial fusion. (B) External genitalia after clitororeduction and introitoplasty.
FIGURE 2.
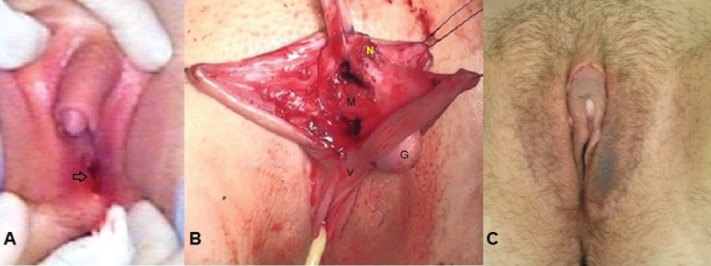
46,XY gonadal (testicle) dysgenesis, incomplete form. (A) External genital view before operation. Clitoromegaly (appr. 5-6 sm), corresponds to Prader stage III-IV. The vagina introitus normal corresponds to Prader 0 (arrow).(B) The stage of clitororeduction: separated glans clitoris connected with dorsal (N) and ventral (V) neurovascular bundles; corpora cavernosa clitoridis (V). (C) The external genitalia after clitororeduction by recection of corpora cavernosa, in 3 week after operation.
The surgical operation stages proceed as follows. A semilunar incision is made around the glans clitoridis at a distance of 5-7 mm. The isolated clitoral shaft (body) is completely separated before the pubic bone. On the sides of the clitoris shaft, longitudinally lateral cuts of Buck's fascia and the tunica albuginea are made. The corpora cavernosa is sharply mobilized and separated from the glans clitoris until bifurcation of the crura clitoridis, and it is cut off just above of symphysis (pubic bone). The isolated dorsal and ventral neurovascular bundle is mobilized and preserved, and the connection to the glans clitoridis is maintained. The glans clitoris on the dorsal neurovascular bundle is connected and sutured with the cult of the resected corpora cavernosa. The ventral sheaf is underlined on the vestibulum vaginae. The capuche is formed above the glans clitoris and around the pudendal labia minora from prepuceal skin.
In patients with a common urogenital sinus, was performed the Y-shape introitoplasty. Briefly, the surgery began with vertical cutting of the urogenital orificium (opening) down to the perineum. Then, the internal mucosa was turned outwards, circumcised along the sides and sewed edge-to-edge with the skin incision. The widely shaped vaginal introitus was formed (Fig. 4A, B).
FIGURE 4.
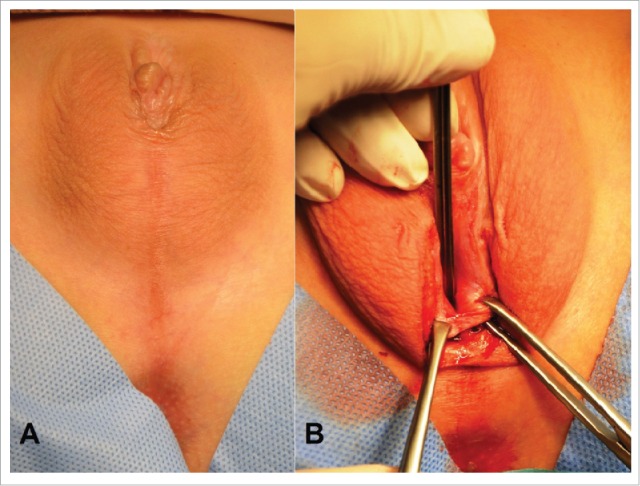
Androgen insensitivity syndrome, incomplete form. (A) External genital view after clitororeduction. The urogenital sinus opens near of the base clitoris. (B) The Y-shaped introitoplasty. The vertical cutting of the urogenital sinus opens the orificium. The internal mucosa was turned outwards, and located edge-to-edge with the skin incision before suturing. The widely shaped vaginal introitus was formed.
Postoperative examination showed positive cosmetic results, feminine-appearing genitalia and preservation of the erogenous sensibility of the clitoris, vestibulum vaginae and labia minora.
The second-line surgery consists of the creation of a neovagina, and this is performed for patients with Turner syndrome, AIS, incomplete 46,XY gonadal dysgenesis, and ovotesticular DSD with uterovaginal aplasia.
Systematization of the clinical features and anatomy of the external genitalia of female patients with DSD is presented in Table 1, including some clinico-anatomical differences in DSD variants.
The complete diagnosis of patterns of intersex disorders is provided by investigation of the karyotype, gonadal morphology, internal genital anatomy (Table 1), with indication of external genital virilization stages by Prader (Table 2).
The major groups of DSD females described below in the sequence, as they specified in the Table 1:
-
✓
46,XX karyotype – congenital adrenal hyperplasia;
-
✓
X chromosome monosomy in the 3 variants of Turner syndrome;
-
✓
46,XY karyotype - androgen insensitivity syndrome and gonadal dysgenesis;
-
✓
Ovotesticular DSD.
Some clinical cases of ambiguity have presented as most demonstrative.
Patients with congenital adrenal hyperplasia (CAH) have normal ovaries, uterus and vagina.
The 6 patients of them had uterovaginal malformations; 4 had septate uterus and underwent hysteroresection, and 2 had duplicated uterus and vagina.
The 9 patients with ambiguous external genitalia had a clitoris larger than 5-6 cm, and 2 patients had Prader degree V masculinization.
Surgical treatment includes clitororeduction and introitoplasty.
Clinical case. Patient 1, 18 y aged (Fig. 1A). External genitalia was ambiguous, corresponds to Prader stage III. She had congenital uterine anomaly: subseptate uterus with normal vagina.
Hormonal measurement before gonadectomy revealed severe hyperandrogenia: LG – 31.7↑ (3.0-10.0) Eq/l, FSH - 29↑ (3.0-8.0) Eq/l, Prl – 297 (120-500) mEq/l, Cortisol - 625 (200-500) nml/l, DHEAS – 22.8 (0.9-11.7) mcml/l, E2 – 62 (150-480) nml/l, 17-OHP – 143 (6-16) nml/l, T – 25,4↑ (1.0-2.5) nml/l.
Surgical treatment includes clitororeduction and introitoplasty (Fig. 1B). Hysteroresectoscopic metroplasy also performed.
Turner syndrome is characterized by X chromosome monosomy and has 3 distinct forms, for which surgical treatment differs.
-
Ø
The classical form (45,X) is characterized by fibrous streak in the gonads and persistence of Mullerian ducts derivates (PMDS). The external genitalia appear female (Prader stage 0).
The indicated surgical treatment is creation of a neovagina (colpopoesis) only.
-
Ø
Cases of 45,X/46,XX mosaicism are characterized by ovarian dysgenesis and a normal uterus and vagina. The external genitalia appear female (Prader stage 0). Most of these patients ovulate and may be fertile. Surgery is not required.
-
Ø
Cases of 45,X/46,XY mosaicism are characterized by a streak gonad or streak testes (or ovotestis). These cases are considered PMDS. The external genitalia are ambiguous.
Surgical treatment consists of laparoscopy and gonadectomy, feminizing genitoplasty (clitororeduction and introitoplasty), and colpopoesis.
The 46,XY gonadal (testicle) dysgenesis may present in one of 2 forms.
-
Ø
In the complete form, the uterus and vagina are present, and the external genitalia appear female (Prader stage 0).
Surgical treatment consists of laparoscopy and gonadectomy. These patients may become pregnant by in vitro fertilization (IVF) using donor oocytes.
-
Ø
The incomplete form presented of PMDS (or uterus and vagina). The external genitalia are ambiguous.
Surgical treatment consists of laparoscopy and gonadectomy, feminizing genitoplasty (clitororeduction and introitoplasty), and colpopoesis.
Clinical case. Patient 2, 14 y aged (Fig. 2A). She has normal shaped uterus and vagina. Severe clitoromegaly (approximately 5-6 sm), that corresponds to Prader III–IV stages. Vaginal vestibulum had normal orificium (opening).
Hormonal measurement before gonadectomy: LG – 40.7↑ (3.0-10.0) Eq/l, FSH - 109↑ (3.0-8.0) Eq/l, Prl – 297 (120-500) mEq/l, Cortisol - 425 (200-500) nmi/l, DHEAS – 6.3 (0.9-11.7) mcml/l, E2 – 273 (150-480) nml/l, 17-OHP – 4.3 (6-16) nml/l, T – 6.5↑ (1.0-2.5) nml/l.
Surgical treatment: gonadectomy and clitoreduction was performed.
On the Fig. 2B presented the operation stage of resection of the corpora cavernosa with preserving the glans clitoridis connected with dorsal and ventral neurovascular bundles.
The external genitalia in 3 week after operation (Fig. 2C)
Clinical case. Patient 3, 19 y aged. She was assigned as a male newborn, but in 17 y reassigned to female gender. Gonads appear as streak-testes, located in the abdomen and removed laparoscopically. Uterus and vagina are absent.
Hormonal measurement before gonadectomy revealed hypergonadotropic hypogonadism, low level of androgens: LG – 13.8↑ (3.0-10.0) Eq/l, FSH – 29.2↑ (3.0-8.0) Eq/l, Prl – 140 (120-500) mEq/l, Cortisol - 245 (200-500) nmi/l, DHEAS – 1.6 (0.9-11.7) mcml/l, E2 – 65.6↓ (150-480) nml/l, 17-OHP – 5.0↓ (6-16) nml/l, T – 0.37↓ (1.0-2.5) nml/l.
External genitalia have male phenotype with micropenis. Complete fusion of the labial folds persisted, the urogenital sinus transforming to the penile urethra, with single orifice at the top of glans penis. This stage corresponds to Prader V degree (Fig. 3).
FIGURE 3.
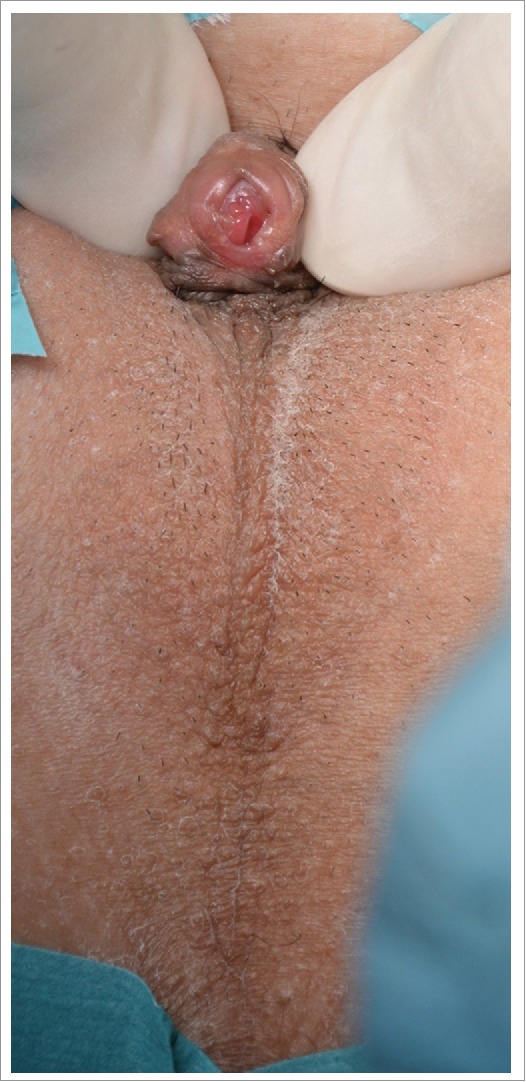
46,XY gonadal (testicle) dysgenesis. External genitalia corresponds to Prader stage V. Micropenis and complete labial fusion to penile (masculine) urethra.
First line surgery performed gonadectomy and introitoplasty; second line – creation of neovagina.
Androgen insensitivity syndrome may present in one of 2 forms.
-
Ø
The complete form presented of rudimental structures of PMDS (Fallopian tubes and uterine rudiments), and the external genitalia appear female (Prader stage 0).
Surgical treatment consists of laparoscopy, gonadectomy, and colpopoesis.
-
Ø
The incomplete form presented of rudimental structures of PMDS, and the external genitalia appear ambiguous.
Surgical treatment consists of laparoscopy, gonadectomy, feminizing genitoplasty, and colpopoesis.
Clinical case. Patient 4, 14 y aged. Androgen insensitivity syndrome, incomplete form. Corresponds to Prader stage IV. The clitororeduction was performed before (Fig. 4A). The urogenital sinus opens near of the base clitoris. The Y-shaped introitoplasty realized on the second line (Fig. 4B).
The key differences between AIS and 46,XY gonadal dysgenesis are that AIS has only rudimental PMDS, while some patients with testicle dysgenesis have a uterus and vagina and thus may become pregnant (by IVF with donor oocytes). The external genitalia in both cases may be female or ambiguous.
Ovotesticular DSD is diagnosed by various karyotype and has PMDS with ambiguous external genitalia. Surgical treatment consists of laparoscopy, gonadectomy, feminizing genitoplasty, and creation of a neovagina (colpopoesis).
Ultrasound and MRI investigations revealed the 10.3% of intersexual patients (especially those with CAH) had uterovaginal anomalies that required metroplasty.
Uterovaginal anomalies comparatively with female genital ducts origination are discussed in “New theory of uterovaginal embryogenesis.”20
Systematization of DSD by distinguishing gonadal, internal and external genital morphology should facilitate differential diagnosis and the choice of an appropriate surgical correction.
The validity of the current classification systems of female genital malformations has been challenged. The proposed VCUAM (vagina cervix uterus adnex - associated malformations) classification makes it possible to estimate the anatomy of pathological uterovaginal malformations.21,22 The structure of the system used to reflect oncologic tumors in the TNM classification served as the basis for establishing a new classification of genital malformations.23
We encourage the use of the DSD systematization to guide diagnosis and management in gynecological practice. Applying the “karyotype-gonadal morphology-internal genital anatomy-external genital virilization stages” facilitates diagnosis and appropriate surgical management. This systematization may be extended horizontally to include “reproduction” and “hormonal treatment,” and it may be continued vertically to describe new atypical anomalies.
DISCUSSION
Sex assignment in newborns depends on the anatomy of the external genitalia, despite this stage being the final stage in embryogenesis.
Systematization of intersex disorders distinguishes the karyotype, gonadal morphology, and genital anatomy to provide a differential diagnosis and guide appropriate surgical management (Table 1).
Patients with CAH, Turner syndrome (45,X/46,XX) and some of 46,XY gonadal (testicle) dysgenesis have uterus and vagina (Table 1).
The precise ultrasound (or MRI) investigations of internal organ's anatomy important for intersexual females, because 10.3% of them had uterovaginal anomalies, required additional surgical correction.
The estimation of external genital virilization degree carried out by Prader stages in all patients (Table 2). The modified feminizing clitoroplasty with preservation of the dorsal and ventral neurovascular bundles to retain erogenous sensitivity was performed only in females with severe virilization degree, according to Prader stages III-V.
The outgrowth of the genital tubercle and the fusion of the urethral fold proceed in an ordered fashion.
In presented cases of ambiguity in 46,XY gonadal (testicle) dysgenesis revealed discordance between virilization degree between clitoris size and urogenital fusion of labial fold. For example, Patient 2 (Fig. 2A) has severe clitoromegaly and normal vaginal opening; but Patient 3 (Fig. 3) has micropenis (looks like normal clitoris) and complete labial fusion to penile (masculine) urethra. These cases demonstrate the deviations of external organs development pathways, due to different derivations, influences of growth factors and receptors.
During feminizing surgery detected some nuances: the branches of clitoris located deep inside in the labial folds and surround of vestibulum vaginae.
Androgens and estrogens are typically associated with sexual differentiation of the genitalia and secondary sex characters.
Alfred Jost's model of sexual differentiation considers that in the absence or inactivity of androgen, the fetus remains in the indifferent stages and becomes phenotypically female (independently of karyotype or gonadal morphology).3
Androgens (testosterone and dihydrotestosterone) and estradiol signal by means of androgen receptors (AR) and estrogen receptors (ERa and ERb), respectively. While good progress has been made in identifying the molecules involved in the initiation of limb budding, the initiation of genital outgrowth is not well understood. An interaction between the endoderm and the ectoderm at the cloacal membrane may be an important step in induction of budding.5,6,24,25,26
Longstanding dogma is that the sexually indifferent genital tubercle is masculinized by androgens and that feminization is a default state that occurs in the absence of androgen activity, though recent studies of ER mutants have falsified this hypothesis.5
Thus, in the absence of ERa activity, female external genitalia are partially masculinized, suggesting that estrogen is required for inhibition of clitoral growth in females. This raises the intriguing possibility that basal levels of androgen in females can lead to masculinization of the genital tubercle, and estrogen is required to counter the influence of androgen.5,6,26,27
Mutations in the androgen receptor (as in the testicular feminization or Tfm mutation), by contrast, cause feminization of the external genitalia, such that Tfm mutant male genitalia are indistinguishable from female genitalia.26
Mutations in the gene that encodes 5a-reductase 2, which converts testosterone to dihydrotestosterone, also disrupt masculinization of the genital tubercle and cause defects ranging from hypospadias and micropenis to complete feminization of the external genitalia.8,27
Together, these results suggest that the balance of androgens to estrogen is a critical factor in determining sexual differentiation of the genitalia.
Martin Cohn reported, that surgical manipulations of the genital tubercle identified cell populations with functions that appear to be analogous to the apical ectodermal ridge (AER).5
Petiot et al. showed that FgfR2, which is essential for urethral tube closure, contains an androgen response element in its promoter, and antagonism of AR results in down-regulation of FgfR2 in the urethral plate and prepuce.25
Intersexuality (ambiguity) in 46,XY patients results from disruptors in the pathways of sex steroid hormones and receptors; in 46,XX females this arises from the effects of excess androgen. These processes develop differently but result in the same ambiguity.
Systematic clinical analysis and a literature review revealed the following questions:
-
○
From where does the genital tubercle originate?
-
○
According to the current view, the indifferent genital tubercle originates from mesenchymal tissue, but mesenchymal cells are arranged across the embryonal body and do not have specific androgen receptors.
-
○
Why do androgens cause virilization, and particularly, why does this hormonal influence promote the outgrowth and elongation of the genital tubercle?
SPECULATION ABOUT THE DERIVATION OF THE GENITAL TUBERCLE
According to the new hypothesis (Fig. 5), during the indifferent stages, the 5 sacral somites have to recede from their segmentation and disintegrate. The sclerotomes fuse to pelvic bones, which form the arcus, and they join together end-to-end in the midline of the ventral body with the pubic symphysis.
FIGURE 5.
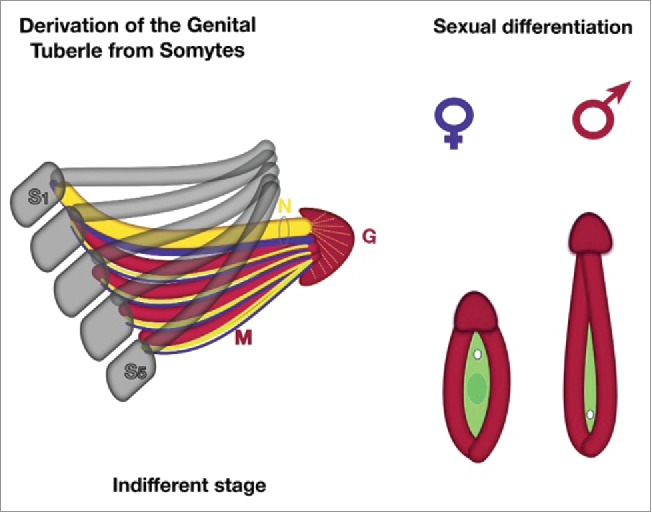
New theory about derivation of external genitalia (schematic). On the indifferent stages the 5 sacral somites (S1-S5) have to recede of their segmentation and desintegrate. The sclerotomes (gray color) fuse to pelvic bones, which form the arcus, they conjoin together end-to-end in the midline of ventral body with pubic symphysis. The fused 5 sacral myotomes (M, red color) with its genuine neurotomes (blue) and angiotomes (yellow), covered by dermatome growing below along of pubic bones and fuse together endways on pubic area. The endwise conjoined myotomes form the corpora cavernosa of genital tubercle, following with dorsal neuro-vascular bundles (bold yellow and blue in the ring, labeled N). The top of myotomes ends become glans tubercle (G) covered of ectodermal layer. During sexual differentiation: myotomes are forming the genital swelling (red), genital tubercle become glans penis or clitoridis, urogenital fold (green) originating from cloaca (hindgut) of embryo. Female pattern or indifferent stage – small tubercle with opened vestibulum vaginae. The genital swellings form the labial folds; the branches of the genital tubercle (corpora cavernosa) surround the urogenital groove and located deep inside in the genital swellings. Male pattern – fusion of labial folds into the urogenital sinus (or penile urethra) extends along the elongated phallus. The branches of the genital tubercle (corpora cavernosa) conjoin together.
The 5 fused sacral myotomes with their genuine neurotomes (innervation) and angiotomes (vascularization), which are covered by dermatome growing below along the pubic bones, fuse together endways on the pubic area. These conjoined myotomes form the corpora cavernosa of the genital tubercle and follow the autonomic neuro-vascular bundles (Fig. 5).
The tops of the myotomes' ends become the glans tubercle, which is covered by an ectodermal layer. The glans penis (clitoris) is innervated by the somatic pudendal nerve (S1-S5). This nerve passes under the pubis symphysis to travel just below the Buck fascia to supply sensory innervation through dorsal neurovascular bundle. The neuronal axons terminate superficially on the glans of the genital tubercle's surface.
The sensory innervation maintains a segmental pattern that reflects the embryological origin of each dermatome's innervation. The genital neural tract originates from the sacral vertebral segments.
In the female embryo, the fused myotomes (analogs of the male corpora cavernosa) surround the vestibulum vaginae, forming the musculus bulbocavernosus and the ischiocavernosus deep in the genital swellings. Medially, they join into the glans clitoridis (same as the glans penis). The glans clitoridis has less surface area (compared to the glans penis), and thus the neurons are much tougher.
In the male embryo, the genital tubercle elongates and fuses to form the penis (phallus), and the genital swellings fuse to become the scrotum. The urogenital groove is transformed into the penile urethra, which terminates with an orificium on the glans penis.
The sclerotomes of 8-10 coccygeal pairs are reduced, and the myotomes form the pelvic diaphragm and striated muscles of the anus.
The genital tubercle derives from all of the following 3 germ layers:
The glans of the tubercle (glans penis, clitoridis) is covered by ectoderm, which is derive from the apical ectodermal ridge.
The corpora cavernosa derives from mesodermal somites.
The urogenital groove originates from the cloacal endoderm (part of the hindgut), which forms the urethral plate epithelium.
During the undifferentiated stages, the early genital tubercle has a superficial affinity to the limb bud, but there is an important distinction between the two.
The upper limb buds derive from C3-Th1, while the lower limb buds derive from L2-S1 somitomeres. Whereas the limb bud is composed of mesoderm covered by surface ectoderm, the genital tubercle is derived from all 3 germ layers.
The genital tubercle derives from S1-S5 somitomeres and receives sensory innervation from the pudendal nerve, which provides the nervus dorsalis of the tubercle (penis and clitoridis).
The new theory is that the genital tubercle originates from conjoined genitogenous ribs, and this is the key difference between this process and limb budding.
It is likely that the initiation of derivation of the genital tubercle during the indifferent stages depends on growth factors that play a role in limb development from (5) sacral somitomeres. Signals for the development of genital somites derive from the notochord and neuromeres, which are stimulated by nerve growth factor. The apical ectodermal ridge (like in limb growth) exerts an inductive proliferation of the adjacent tubercle's mesoderm (corpora cavernosa). Androgens stimulate the masculinization of the indifferent genital tubercle by hypertrophy of the corpora cavernosa and through an inductive influence on the neurotomes with activation of nerve growth factor.
Patrick Tschopp et al. (2014),29 Anna Herrera and Martin Cohn (2014)30 considers, the paired genital swellings on either side of the cloacal membrane merge beneath the surface of ectoderm to form a single genital tubercle anterior to the cloaca.
I suppose, the genital swellings form the labial folds on females; they brought at the ventral midline and fuse together into the penile urethra on male embryo. The branches of the genital tubercle that derived from 5 fused myotomes (corpora cavernosa) surround the urogenital groove and located deep inside in the genital swellings.
The fusion of the labial folds into the urogenital sinus (or the penile urethra in the male embryo) extends along the elongated phallus. Typically, the process of elongation (outgrowth) of the genital tubercle and fusion of the urethral fold are proceeding together (like Prader stages). These processes may be discordant, as the genital tubercle (ectodermal and mesodermal) and the urogenital groove (endodermal) originate from different germ layers, and thus they are stimulated by different pathways of growth factors and receptors.
Erogenous sensitivity is perceived through numerous sensor neurons within the 5 sacral somitomeres. The embryonal precursors of genital erogenous sensation have principal differences: the glans clitoridis (penis) has somatic sensitivity through the nervus dorsalis clitoridis (penis) from 5 sacral S1-S5 neurotomes; while the corpora cavernosa has autonomic innervation from pudendal nerves, that provide the erectile function; and the vestibulum (female) vaginae and (male) penile urethra have visceral innervation originating from the hindgut. The hymen is a mucous membrane located between the fused mesonephric ducts and the urogenital sinus (part of the cloaca), and it has high sensitivity originating from the autonomic and visceral nervous systems. The clitoris, vestibulum vaginae, and hymenal fold are erotically important organs that contribute to female erogenous sensation, arousal and orgasm.28
Presumably, sexual differentiation of external genitalia is final in gender embryogenesis, but surprisingly derivation of the indifferent genital tubercle from 5 sacral somites occurs before gonadal and internal organs development.
Speculation about genital organs origin may be useful for future embryological investigations.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- [1].Sadler TW. “Langman's Medical Embryology.” XII-th edn, Baltimore: Lippincott Williams&Wilkins, 2012; 232-259. [Google Scholar]
- [2].Hill MA. Embryology BGD Lecture - Sexual Differentiation. Retrieved October16, 2014 URL: http://php.med.unsw.edu.au/embryology/index.php?title=BGD_Lecture_-_Sexual_Differentiation [Google Scholar]
- [3].Jost AA. The new look at the mechanism controlling sex differentiation in mammals. John Hopkins Med J 1972; 130:28-36; PMID:4481103 [PubMed] [Google Scholar]
- [4].Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev 2007; 87(1):1-28; PMID:17237341; http://dx.doi.org/ 10.1152/physrev.00009.2006 [DOI] [PubMed] [Google Scholar]
- [5].Cohn MJ. Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Dev Dyn 2011; 240(5):1108-15; PMID:21465625; http://dx.doi.org/ 10.1002/dvdy.22631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Munger SC, Natarajan A, Looger LL, Ohler U, Capel B. Fine time course expression analysis identifies cascades of activation and repression and maps a putative regulator of mammalian sex determination. PLoS Genet 2013; 9(7):e1003630; PMID:23874228; http://dx.doi.org/ 10.1371/journal.pgen.1003630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mieszczak J, Houk CP, Lee PA. Assignment of the sex of rearing in the neonate with a disorder of sex development. Curr Opin Pediatr 2009; 21:541-7; PMID:19444113; http://dx.doi.org/ 10.1097/MOP.0b013e32832c6d2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sax L. How common is intersex?: a response to Anne Fausto-Sterling. J Sex Res 2002; 39:174-8; PMID:12476264; http://dx.doi.org/ 10.1080/0022-4490209552139 [DOI] [PubMed] [Google Scholar]
- [9].Hughes IA, Houk C, Ahmed SF, Lee PA, LWPES Consensus Group, ESPE Consensus Group . Consensus statement on management of intersex disorders. Arch Dis Child 2006; 91:554-63; PMID:16624884; http://dx.doi.org/ 10.1136/adc.2006.098319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brain CE, Creighton SM, Mushtaq I, Carmichael PA, Barnicoat A, Honour JW, Larcher V, Achermann JC. Holistic management of DSD. Best Pract Res Clin Endocrinol Metab 2010; 24:335-54; PMID:20541156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pasterski V, Prentice P, Hughes IA. Consequences of the Chicago consensus on disorders of sex development (DSD): current practices in Europe. Arch Dis Child 2010; 95:618-23; PMID:19773218; http://dx.doi.org/ 10.1136/adc.2009.163840 [DOI] [PubMed] [Google Scholar]
- [12].Houk CP, Lee PA. Update on disorders of sex development. Curr Opin Endocrinol Diabetes Obes 2012; 19:28-32; http://dx.doi.org/ 10.1097/MED.0b013e328-34edacb [DOI] [PubMed] [Google Scholar]
- [13].Ahmed F, Achermann J, Arlt W, Balen AH, Conway G, Edwards ZL, Elford S, Hughes IA, Izatt L, Krone N, et al.. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin Endocrinol (Oxf) 2011; 75(1):12-26; PMID:21521344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahmed SF, Gardner M, Sandberg DE. Management of children with disorders of sex development: new care standards explained. Psychol Sex 2014; 5(1):5-14; http://dx.doi.org/ 10.1080/19419899.2013.83-1211 [DOI] [Google Scholar]
- [15].Prader A. Der genitalbefund beim pseudohermaproditus feminus des kongenitalen adrenogenitalen syndromes. Helv Pediatr Acta 1954; 9; 231-48. [PubMed] [Google Scholar]
- [16].Prader A, Gurtner HP. The syndrome of male pseudohermaphrodism in congenital adrenocortical hyperplasia without overproduction of androgens (adrenal male pseudohermaphrodism). Helv Paediatr Acta 1955; 10:397-412; PMID:13285832 [PubMed] [Google Scholar]
- [17].Creighton SM. Long-term outcome of feminization surgery: the London experience. BJU Int 2004; 93(suppl. 3)44-6; PMID:15086441; http://dx.doi.org/ 10.1111/j.1464-410X.2004.04708.x [DOI] [PubMed] [Google Scholar]
- [18].Sugiyama Y, Mizuno H, Hayashi Y, Imamine H, Ito T, Kato I, Yamamoto-Tomita M, Aoyama M, Asai K, Togari H. Severity of virilization of external genitalia in Japanese patients with salt-wasting 21-hydroxylase deficiency. Tohoku J Exp Med 2008; 215(4):341-8; PMID:18679008; http://dx.doi.org/ 10.1620/tjem.215.341 [DOI] [PubMed] [Google Scholar]
- [19].Makiyan Z. Studies of gonadal sex differentiation. Organogenesis 2016; 12(1):42-51; PMID:26950283; http://dx.doi.org/ 10.1080/15476278.2016.1145318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Makiyan Z. New theory of uterovaginal embryogenesis. Organogenesis 2016; 12(1):33-41; PMID:269-00909; http://dx.doi.org/ 10.1080/15476278.2016.1145317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oppelt P, Renner SP, Brucker S, Strissel PL, Strick R, Oppelt PG, Doerr HG, Schott GE, Hucke J, Wallwiener D, et al.. The VCUAM (Vagina Cervix Uterus Adnex-associated Malformation) classification: a new classification for genital malformations. Fertil Steril 2005; 84(5):1493-7; PMID:16275249; http://dx.doi.org/ 10.1016/j.fertnstert.2005.05.036 [DOI] [PubMed] [Google Scholar]
- [22].Grimbizis GF1, Gordts S, Di Spiezio Sardo A, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Brölmann H, Gianaroli L, et al.. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod 2013; 28(8):2032-44; PMID:23771171; http://dx.doi.org/ 10.1093/humrep/det098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wittekind C, Greene FL, Hutter RVP, Klimpfinger M, Sobin LH (eds). TNM atlas: illustrated guide to the TNM/pTNM classification of malignant tumours (5th ed.), Springer, Heidelberg 2004. [Google Scholar]
- [24].Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L.. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 2009; 136(23):3959-67; PMID:19906863; http://dx.doi.org/ 10.1242/dev.039768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 2005; 132:2441-50; PMID:15843416; http://dx.doi.org/ 10.1242/dev.01778 [DOI] [PubMed] [Google Scholar]
- [26].Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol 2010; 184:1604-9; PMID:20728117; http://dx.doi.org/ 10.1016/j.juro.2010.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sinnecker GH, Hiort O, Dibbelt L, et al.. Phenotypic classification of male pseudohermaphroditism due to steroid 5 alphareductase 2 deficiency. Am J Med Genet 1996; 63:223-30; PMID:87-23114; http://dx.doi.org/ 10.1002/(SICI)1096-8628(19960503)63:1%3c223::AIDAJMG39%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- [28].Makiyan Z. Female sexuality. Lulu Publisning, USA: 2013. ISBN-9781304000125 http://www.lulu.com/shop/zohrab-makiyan/female-sexuality/ebook/product-21025371.html [Google Scholar]
- [29].Tschopp P, Sherratt E, Sanger T, Groner A, Aspiras A, Hu J, Pourquié O, Gros J, Tabin C.. A relative shift in cloacal location repositions external genitalia in amniote evolution. Nature 2014; 516(7531):391-4; PMID:8723114; http://dx.doi.org/ 10.1038/nature-13819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Herrera AM, Cohn MJ. Embryonic origin and compartmental organization of the external genitalia. Scientific Reports 2014; 4:6896; PMID:25372631; http://dx.doi.org/ 10.1038/srep06896 [DOI] [PMC free article] [PubMed] [Google Scholar]


