Abstract
The present study determined the prevalence and distribution of gastrointestinal helminths in domestic chickens (Gallus gallus domesticus) between November 2012 and August 2013. One hundred and twenty domestic chickens were purchased from villages in four districts of Phayao province; Mae Chai, Dok Khamtai, Chun and Chiang Kham. Morphological differences were used to identify the helminth species, and HAT-RAPD technique was used to differentiate among closely related species. The results revealed that the total prevalence of infection was 99.2%. Cestode and nematode infections showed the highest prevalence in rainy season, while trematode infections were low and only found in hot season. The species and their prevalence were: Ascaridia galli (50.8%), Heterakis gallinarum (86.7%), Prosthogonimus macrorchis (1.7%), Echinostoma revolutum (0.8%), Raillietina echinobothrida (48.3%), Raillietina tetragona (57.5%), Raillietina cesticillus (12.5%), Raillietina sp. (35.8%), Cotugnia chiangmaii (14.2%) and Cotugnia sp. (32.5%). The prevalence of helminth infections did not differ significantly between male and female chickens. HAT-RAPD analysis, the specific fragment of 400 and 250 bp indicated that Raillietina sp. and Cotugnia sp. found, respectively, differ from other closely related species. This study has confirmed that HAT-RAPD technique can be used to differentiate among related species combined with morphological observations.
Keywords: Gastrointestinal helminth, Domestic chicken, Gallus gallus domesticus, Occurrence, HAT-RAPD, Northern Thailand
1. Introduction
In Thailand, the occurrence of gastrointestinal helminths in domestic chickens has been studied in central, north-eastern and southern areas (Sangvaranond, 1994, Kunchara Na Ayudthaya and Sangvaranond, 1993, Kunchara Na Ayudthaya and Sangvaranond, 1997) but few studies are available in northern area. Phayao is a province in northern Thailand. Most of the people in rural areas of Phayao have animal husbandry. Domestic chickens are a common livestock for agro-farming, and are important for food consumption and commerce in this area. Studies on the occurrence of gastrointestinal helminth parasites in domestic chickens in Phayao province have not been performed.
For species identification, morphological differences are commonly used. However, it is difficult to identify the species level based on the morphology alone. Molecular approach is the most effective and accurate method for genetic characterization of such helminths. High annealing temperature-randomly amplified polymorphic DNA (HAT-RAPD) is a useful procedure to differentiate between closely related and morphologically indistinct species because high annealing temperature gives greater polymorphisms, reproducibility, and resolution (Anuntalabhonchai et al., 2000). This technique has been used successfully for detection and identification of numerous helminths including paramphistome flukes, Haplorchis taichui, Stellantchasmus falcatus (Wongsawad et al., 2009, Wongsawad and Wongsawad, 2010, Puttalakshmamma et al., 2014). The identification of some cestodes in domestic chicken using HAT-RAPD PCR has not been reported from Thailand.
Therefore, the objective of this study was to determine the prevalence and distribution of gastrointestinal helminth infections in domestic chickens from four districts in Phayao province in northern Thailand. Additionally, molecular analysis, HAT-RAPD technique was used to identify morphologically indistinct species among closely related groups combined with morphological characters.
2. Materials and methods
2.1. Study area and parasite collection
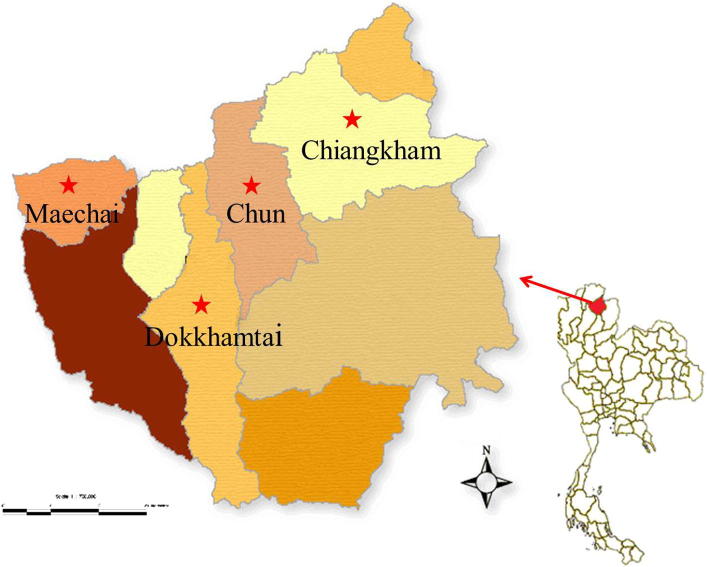
Four districts; Mae Chai, Dok Khamtai, Chun, and Chiang Kham in Phayao province were selected for this research (Fig. 1). These districts are located at an altitude of 300–1550 m above the sea level and mean annual rainfall is 1043.9 mm (high rainfall). The mean minimum and maximum temperatures are 10.8 °C in cool season and 39.5 °C in hot season.
Figure 1.
Four districts which were investigated for helminthic infections in domestic chickens (scale 1:700,000).
Domestic chickens (n = 120, 64 females and 56 males) were purchased from chicken farms in the study areas. For helminth examination, the gastrointestinal tracts were divided into 8 sections: esophagus, crop, proventriculus, duodenum, jejunum, ileum, caeca, and rectum. They were opened by a longitudinal section from the esophagus down to the rectum, rinsed several times with tap water and finally rinsed with 0.85% NaCl. The gastrointestinal helminth recovered was morphologically observed using a light microscope. The species numbers were recorded for calculation of the prevalence and mean intensity of infections. For preparing permanent slides, the specimens were flattened and fixed in 4% formalin. For molecular analysis, the specimens were frozen at −20 °C for later DNA extraction.
2.2. Permanent slide and identification of helminths
The helminth recovered was prepared for morphological investigations. Trematodes and cestodes were stained with acetocarmine or hematoxylin, dehydrated with graded alcohol series, cleared with xylene, and mounted in Permount. Nematodes were dehydrated in a graded alcohol series, cleared with glycerin, and mounted with glycerin-jelly. The species identification was based on Hofstad et al., 1984, Soulsby, 1982, Wongsawad and Jadhav, 1998.
2.3. Statistical analysis
The prevalence and mean intensity of individual helminth species were calculated according to the definitions of Margolis et al. (1982). The chi-square test was used to analyze the association between the prevalence of each helminth species and host sex.
2.4. Molecular analysis
2.4.1. HAT-RAPD PCR
Genomic DNA from all parasites was extracted using 5% Chelex (Fluka) solution as described in Noikong et al. (2014). Extracted DNA was collected and stored at −20 °C until it was used. Six commercially available arbitrary 10-mer primers (Operon Biotechnology, Huntsville, Alabama, USA) were used to perform DNA fingerprint from different species of adult parasites. HAT-RAPD PCR reaction was carried out in a final volume of 20 μl. The reactions were performed in a Thermal Cycler machine (Little Genius, Bioer Technology, Minato-ku, Tokyo, Japan) and PCR protocols were indicated as follows: 1 cycle of 94 °C for 2 min, 40 cycles of 94 °C for 30 s, 48 °C for 30 s, 72 °C for 45 s, and 1 cycle of final extension at 72 °C for 7 min. PCR products were separated on 1.4% TBE agarose gel electrophoresis stained with ethidium bromide and photographed with a Kodak digital camera, Gel Logic 100.
2.4.2. HAT-RAPD data analysis
Data were scored on the basis of the presence or absence of the PCR product. The polymorphism percentage was calculated as per the following formula (Blair et al., 1999):
3. Results
3.1. Parasite species and prevalence
Out of the total 120 domestic chickens examined, 119 (99.2%) were infected with various helminths. Cestode and nematode infections showed the highest prevalence in rainy season follow by hot and cool seasons, respectively, while trematode infections were low and only found in hot season (Fig. 2). There was no statistically significant difference in the prevalence of gastrointestinal helminth infections for one year round. Helminth parasites and their prevalence are summarized in Table 1. Mixed infections were found in 92.4% (110 cases), whereas 7.6% (9 cases) had a single infection. Among the mixed infections, 12.6% (15 cases) had two species, 30.3% (36 cases) had three, 36.1% (43 cases) had four, 10.9% (13 cases) had five and 2.5% (3 cases) had six species. The differences were not significant between the prevalence of helminth infections and the host sex of domestic chickens (P > 0.05).
Figure 2.
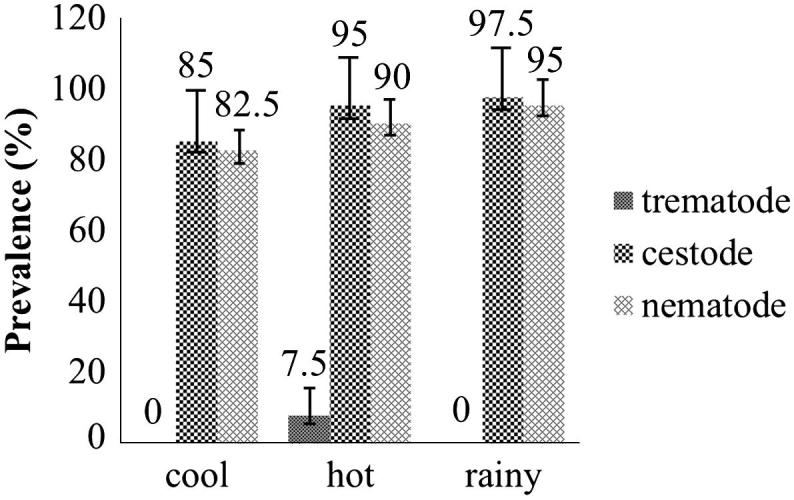
The total prevalence of gastrointestinal helminth in Gallus gallus domesticus from 4 districts of Phayao province during three seasons for one year round.
Table 1.
The prevalence and mean intensity of helminth species in Gallus gallus domesticus from 4 districts.
| Helminth species | District | Host infected |
Total N = 120 |
Prevalence (%) | Mean Intensity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) |
Male N = 56 |
Female N = 64 |
|||||||
| CK N = 30 |
CH N = 30 |
DKT N = 30 |
MC N = 30 |
||||||
| Cestodes | |||||||||
| Raillietina echinobothrida | 53.3 (16) | 13.3 (7) | 46.7 (14) | 70 (21) | 27 | 31 | 58 | 48.3 | 9.5 |
| Raillietina tetragona | 60 (18) | 66.7 (20) | 23.3 (7) | 80 (24) | 38 | 31 | 69 | 57.5 | 9.1 |
| Raillietina cesticillus | 26.7 (8) | 0 | 16.7 (5) | 6.7 (2) | 6 | 9 | 15 | 12.5 | 9.9 |
| Raillietina sp. | 30 (9) | 23.3 (7) | 43.3 (13) | 46.7 (14) | 23 | 20 | 43 | 35.8 | 10.4 |
| Cotugnia chiangmaii | 0 | 16.7 (5) | 36.7 (11) | 3.3 (1) | 7 | 10 | 17 | 14.2 | 23.7 |
| Cotugnia sp. | 10 (3) | 50 (15) | 53.3 (16) | 16.7 (5) | 17 | 22 | 39 | 32.5 | 5.8 |
| Trematodes | |||||||||
| Echinostoma revolutum | 0 | 3.3 (1) | 0 | 0 | 0 | 1 | 1 | 0.8 | 1 |
| Prosthogonimus macrorchis | 0 | 3.3 (1) | 0 | 3.3 (1) | 2 | 0 | 2 | 1.7 | 1 |
| Nematodes | |||||||||
| Ascaridia galli | 40 (12) | 66.7 (20) | 63.3 (19) | 33.3 (10) | 31 | 30 | 61 | 50.8 | 8.7 |
| Heterakis gallinarum | 56.7 (17) | 100 (30) | 100 (30) | 90 (27) | 48 | 56 | 104 | 86.7 | 55.7 |
CK = Chiang Kham, CH = Chun, DKT = Dok Khamtai, MC = Mae Chai, N = number of domestic chicken, () = number of infected domestic chicken.
3.2. Number and distribution of helminth species
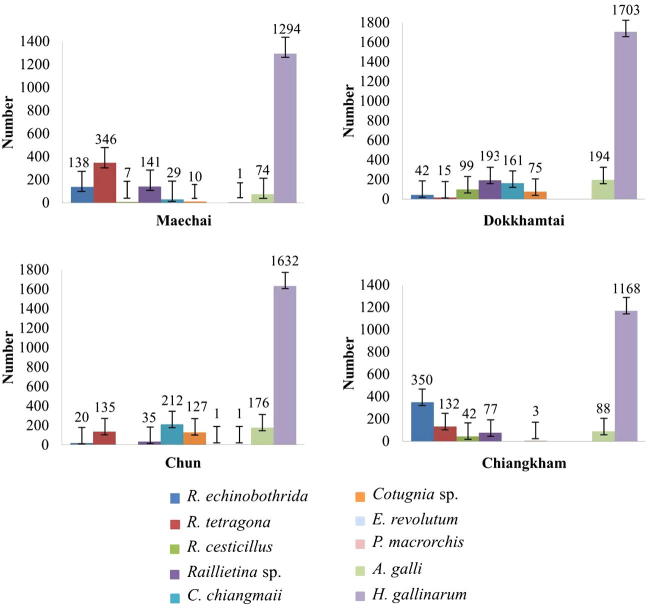
Domestic chickens had various helminth species in their intestinal tract and caeca. Cestodes were isolated mostly from the small intestine (jejunum and ileum) and few in the rectum. Nematodes, A. galli and H. gallinarum were recovered from all intestine parts and caeca, respectively, whereas trematodes were isolated only from the rectum. Two districts, Mae Chai and Chun showed the highest helminth species diversity (9 species), followed by Dok Khamtai (8 species) and Chiang Kham (7 species). Nematode species were distributed in all four districts whereas trematodes were only recovered in Chun and Mae Chai districts. Cestodes, R. cesticillus and C. chiangmaii, were not found in Chun and Chiang Kham district, respectively. A total of 8731 helminths were recovered from 119 of 120 chickens. Total numbers of helminths and mean intensity are summarized in Table 1 and Fig. 3.
Figure 3.
The number and distribution of helminth species in Gallus gallus domesticus from 4 districts.
3.3. Raillietina spp. and Cotugnia spp. with specific fragments
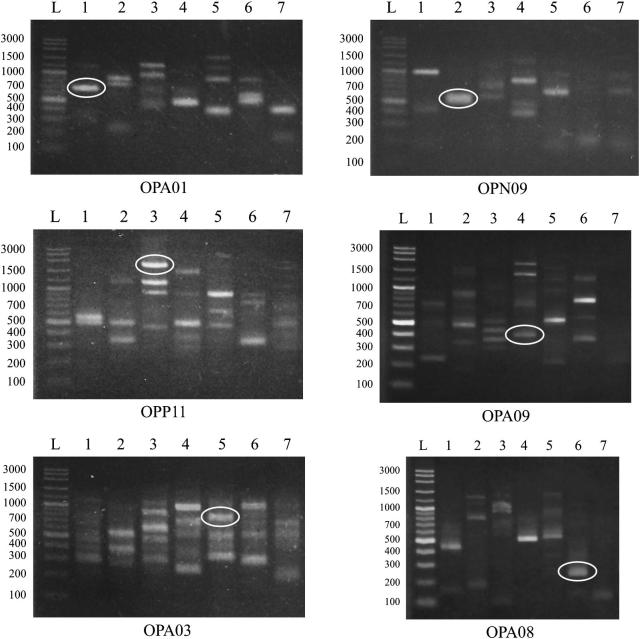
After genomic DNAs were amplified in PCR with 6 arbitrary primers, HAT-RAPD DNA profiles were generated, and 67 and 33 polymorphic markers of Raillietina and Cotugnia, respectively, were also scored. The information regarding monomorphic, polymorphic, and unique band and percentage of polymorphism generated by 6 primers of Raillietina spp. and Cotugnia spp. are shown in Table 2, Table 3, respectively. The highest percentage of polymorphism of Raillietina spp. was 100% from all 6 primers, while that of Cotugnia spp. was from OPA3 and OPN9. Overall, 6 polymorphic markers, 650, 550, 1750, 400, 750, and 250 generated from OPA01, OPN09, OPP11, OPA09, OPA03, and OPA08, respectively, were found to be a R. echinobothrida, R. tetragona, R. cesticillus, Raillietina sp., C. chiangmaii and Cotugnia sp. specific fragment, respectively (Fig. 4). These specific fragments can be selected to design a specific primer for further detection and identification.
Table 2.
Details of monomorphic, polymorphic and unique bands, and percentage of polymorphism generated by 6 primers of Raillietina spp.
| Primer | Sequence of oligo 5′–3′ | Range of fragment size (bp) | Unique bands | Polymorphic bands | Monomorphic bands | Total no. of bands | % of polymorphism |
|---|---|---|---|---|---|---|---|
| OPA1 | TGCCGAGCTG | 210–1200 | 7 | 2 | 0 | 9 | 100 |
| OPA3 | AGTCAGCCAC | 210–1000 | 10 | 3 | 0 | 13 | 100 |
| OPA8 | GTGACGTAGG | 100–1350 | 8 | 2 | 0 | 10 | 100 |
| OPA9 | GGGTAACGCC | 210–1750 | 11 | 3 | 0 | 14 | 100 |
| OPN9 | TGCCGGCTTG | 400–1200 | 10 | 0 | 0 | 10 | 100 |
| OPP11 | AACGCGTCGG | 300–1750 | 9 | 2 | 0 | 11 | 100 |
Table 3.
Details of monomorphic, polymorphic and unique bands, and percentage of polymorphism generated by 6 primers of Cotugnia spp.
| Primer | Sequence of oligo 5′–3′ | Range of fragment size (bp) | Unique bands | Polymorphic bands | Monomorphic bands | Total no. of bands | % of polymorphism |
|---|---|---|---|---|---|---|---|
| OPA1 | TGCCGAGCTG | 350–1500 | 5 | 0 | 1 | 6 | 83.3 |
| OPA3 | AGTCAGCCAC | 290–1000 | 5 | 0 | 0 | 5 | 100 |
| OPA8 | GTGACGTAGG | 150–1400 | 5 | 0 | 1 | 6 | 83.3 |
| OPA9 | GGGTAACGCC | 210–1500 | 7 | 0 | 1 | 8 | 87.5 |
| OPN9 | TGCCGGCTTG | 200–1000 | 3 | 0 | 0 | 3 | 100 |
| OPP11 | AACGCGTCGG | 310–900 | 4 | 0 | 1 | 5 | 80 |
Figure 4.
HAT-RAPD profiles and markers of 650, 550, 1750, 400, 750, and 250 bp fragments generated by OPA01, OPN09, OPP11, OPA09, OPA03, and OPA08, respectively. Lane L, DNA marker (VC ladder plus 100 bp); lane 1, R. echinobothrida; lane 2, R. tetragona; lane 3, R. cesticillus; lane 4, Raillietina sp.; lane 5, C. chiangmaii; lane 6, Cotugnia sp.; lane 7, H. nana.
4. Discussion
There was a high prevalence (99.2%) of gastrointestinal helminths in domestic chickens from all four districts of Phayao province. This study suggests that, domestic chickens managed under free range conditions are heavily infected with helminth parasites. In contrast, previous study revealed that 87.6% of adult chickens in the north-eastern areas had gastrointestinal helminths (Kunchara Na Ayudthaya and Sangvaranond, 1993) and 83.7% in southern areas (Kunchara Na Ayudthaya and Sangvaranond, 1997) of Thailand. Environmental alteration, especially increasing temperature may have affected the occurrence of helminth infections, because the parasites can be transmitted by invertebrate intermediate hosts which are abundant in the tropical region (Fakae and Pual-Abiade, 2003). Additionally, high rainfall in this study area influences the prevalence of these helminths. This finding is similar to previous study that reveals the region with high rainfall has a higher prevalence and diversity than the region with low rainfall (Mukaratirwa and Hove, 2009). The number of helminth species was lower compared to previous reports (26 species (Magwisha et al., 2002), 14 species (Mungube et al., 2008), and 13 species (Hassouni and Belghyti, 2006)). This difference may be due to environmental variation and geographical distribution of helminth parasites and their intermediate hosts.
The results of this study clearly suggest that, domestic chickens (G. gallus domesticus) are susceptible to gastrointestinal helminth in all 3 seasons and especially during the rainy and hot seasons. High availability of intermediate hosts, such as beetles, ants and earthworms, of these parasites occurs during the hot and rainy seasons. Besides, the suitable temperature (range 10–40 °C) and sufficient moisture can affect the parasite survival and egg development to the infective stages (Mungube et al., 2008, Permin and Hansen, 2003). Mixed helminth infections are a common phenomenon of infected chickens. They are often associated with four species followed by three species, scarcely two or five species and rarely six species. This result suggests that parasites and their intermediate hosts, and free-range management system are favorable to their simultaneous development (Magwisha et al., 2002). The current study found no significant association between host sex and the prevalence of helminth infections.
In this study, HAT-RAPD was carried out using 6 primers to differentiate Raillietina and Cotugnia group. High annealing temperature of 48 °C resulted in clearly distinguishable banding patterns and specific fragments to differentiate among Raillietina and Cotugnia groups. The result was similar to that of Wongsawad and Wongsawad, 2010, Puttalakshmamma et al., 2014 performed high annealing temperature of 42–48 °C in HAT-RAPD, which can identify and differentiate some platyhelminths. The HAT-RAPD profiles indicated that Raillietina sp. and Cotugnia sp. were different from other closely related species. The specific fragments derived from HAT-RAPD are expected to be useful for further detection and identification of the larval stages in the intermediate hosts.
In conclusion, gastrointestinal helminths are one of the common parasites causing serious troubles in chicken production and can cause death which affects the economy. Hence, domestic chickens in these areas should be dewormed at regular intervals with an anthelminthic. The management system and hygiene conditions should be improved for better growth. This study has confirmed that HAT-RAPD technique can be used to differentiate among related species combined with morphological observations.
Conflict of interest
We have no conflict of interest related to this study.
Acknowledgements
We would like to thank the Applied Parasitology Research Laboratory, Department of Biology, Faculty of Science and the Applied Technology for Biodiversity Research Unit, Science and Technology Research Institute, Chiang Mai University for their assistance. Special thanks are extended to the Royal Golden Jubilee Ph.D. Program Scholarship and the Graduate School, Chiang Mai University, Thailand for their support. Finally, we would like to thank Mr. Maxwell J.F. for their proof reading of the English language.
Footnotes
Peer review under responsibility of King Saud University.
References
- Anuntalabhonchai, S., Chiangda, J., Chandet, R., Apawat, P., 2000. Genetic diversity within Lychee (Litchi chinensis Soonn.) based on RAPD analysis. In: Int. Symp. Trop. Subtrop. Fruit. 26th Nov–1st Dec. Cairns, Australia, p. 45.
- Blair M.W., Panaud O., McCouch S.R. Inter simple sequence repeat (ISSR) amplification for analysis of microsatellite motif frequency and fingerprinting in rice (Oryza sativa L.) Theor. Appl. Genet. 1999:780–792. [Google Scholar]
- Fakae B.B., Pual-Abiade C.U. Rainy season period prevalence of helminth in the domestic fowl (Gallus gallus) in Nsukka, eastern Nigeria. Niger. Vet. J. 2003;24(1):21–27. [Google Scholar]
- Hassouni T., Belghyti D. Distribution of gastrointestinal helminths in chicken farms in the Gharb region-Morocco. Parasitol. Res. 2006;99:181–183. doi: 10.1007/s00436-006-0145-8. [DOI] [PubMed] [Google Scholar]
- Hofstad M.S., Calnek B.W., Helmboldt C.F., Reid W.M., Yoder H.W. Iowa State University Press; 1984. Diseases of Poultry. [Google Scholar]
- Kunchara Na Ayudthaya C., Sangvaranond A. Internal parasites in the alimentary tracts of adult native chickens in the north-eastern part of Thailand. Kasetsart J. (Nat. Sci.) 1993;27:324–329. [Google Scholar]
- Kunchara Na Ayudthaya C., Sangvaranond A. Internal parasites in the alimentary tracts of adult native chickens in the southern part of Thailand. Kasetsart J. (Nat. Sci.) 1997;31:407–412. [Google Scholar]
- Magwisha H.B., Kassuku A.A., Kyvsgaard N.C., Permin A. Comparison of prevalence and burdens of helminth infection in growers and adult free-range chickens. Trop. Anim. Health Prod. 2002;34:205–214. doi: 10.1023/a:1015278524559. [DOI] [PubMed] [Google Scholar]
- Margolis L., Esch G.W., Holmes J.G., Kuris Schad G.A. The use of ecological terms in parasitology. J. Parasitol. 1982;68:131–133. [Google Scholar]
- Mukaratirwa S., Hove T. A survey of ectoparasites, cestodes and management of free-range indigenous chickens in rural Zimbabwe. Tydskr. S. Afr. Vet. Ver. 2009;80(3):188–191. doi: 10.4102/jsava.v80i3.200. [DOI] [PubMed] [Google Scholar]
- Mungube E.O., Bauni S.M., Tenhagen B.A., Wamae L.M., Nzioka S.M., Muhammed L., Nginyi J.M. Prevalence of parasites of the local scavenging chickens in a selected semi-arid zone of eastern Kenya. Trop. Anim. Health Prod. 2008;40:101–109. doi: 10.1007/s11250-007-9068-3. [DOI] [PubMed] [Google Scholar]
- Noikong W., Wongsawad C., Chai J.Y., Saenphet S., Trudgett A. Molecular analysis of echinostome metacercariae from their second intermediate host found in localized geographic region reveals genetic heterogeneity and possible cryptic speciation. PLOS Negl. Trop. Dis. 2014;8(4):1–7. doi: 10.1371/journal.pntd.0002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permin A., Hansen J.W. Food and Agriculture Organization of the United Nations; Rome, Italy: 2003. The Epidemiology, Diagnosis and Control of Poultry Parasites. [Google Scholar]
- Puttalakshmamma G., Ramani G., Singh K., Patel A., Patel A., Joshi C. Genetic characterization of paramphistomes of buffalo by HAT-RAPD analysis. Turk. J. Vet. Anim. Sci. 2014;38:7–13. [Google Scholar]
- Sangvaranond A. Parasitic helminths of native chickens in the central part of Thailand. Kasetsart J. (Nat. Sci.) 1994;28:402–412. [Google Scholar]
- Soulsby E.J.L. Bailliere Tindal; London: 1982. Helminths, Arthropods and Protoszoa of Demonstrated Animals. [Google Scholar]
- Wongsawad C., Jadhav B.V. A new tapeworm from Gallus gallus domesticus from Thailand. Riv. Parassitol. 1998;15:149–155. [Google Scholar]
- Wongsawad C., Wongsawad P. Molecular markers for identification of Stellantchasmus falcatus and a phylogenic study using the HAT-RAPD method. Korean J. Parasitol. 2010;48(4):303–307. doi: 10.3347/kjp.2010.48.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsawad C., Wongsawad P., Chai J.Y., Anuntalabhonchai S. Haplorchis taichui, Witenberg, 1930: development of a HAT-RAPD marker for the detection of minute intestinal fluke infection. Exp. Parasitol. 2009;123:158–161. doi: 10.1016/j.exppara.2009.06.016. [DOI] [PubMed] [Google Scholar]