Abstract
Interstitial lung disease is a serious drug‐related condition that can cause life threatening organ failure. The incidence and risk factors of drug‐induced interstitial lung disease (DILD) are unknown in oncology phase I trials. This study analyzed clinical information from 8906 patients with malignancies who were enrolled in 470 phase I trials sponsored by the Cancer Therapy Evaluation Program, National Cancer Institute, from 1988 to 2014. Logistic and Cox statistical analyses were utilized to determine clinical differences between patients who developed DILD and patients who did not. In this study, the overall incidence rate of patients with pulmonary toxicity was 2.7%. The overall incidence rate for DILD was 0.77%, whereas for grade 3 or 4 DILD it was 0.31%. Median time to occurrence of DILD was 1.4 months. The Cox hazard analysis indicated smaller body surface area and a combination of thoracic radiation with investigational drug regimens were significant risk factors for time to occurrence of interstitial lung disease. Investigators should carefully monitor for DILD in oncology patients enrolled in phase I trials with identified risk factors. A 6‐month observation period would be sufficient to detect the onset of most DILD in such patients.
Keywords: Drug induced interstitial lung disease, investigational new drug, oncology, phase I trial pulmonary toxicity
Drug‐induced lung injury involves single or multiple structures of the respiratory system, including airways, lung parenchyma, mediastinum, pleura, pulmonary vasculature and the neuromuscular system. The most common form of drug‐induced lung injury is drug‐induced interstitial lung disease (DILD), which often manifests as a dry cough, fever and dyspnea. DILD is caused by various types of drugs, particularly antineoplastic agents, antimicrobial agents and antirheumatic agents. The pathogenesis of DILD is still unknown; however, it is thought to be a drug‐induced direct lung injury or an immune‐mediated reaction. DILD is a serious adverse drug reaction that is life threatening and can lead to permanent respiratory failure requiring chronic oxygen therapy or even death.
In regards to antineoplastic agents for solid or hematologic malignancies, bleomycin is a well‐known causative agent for DILD, with a reported incidence rate of 10%. Other cytotoxic agents (CA) and molecular targeted agents (MTA) have been generally reported as having an associated incidence of DILD from approximately 0.5–1%.1 Many other studies for specific anticancer agents, particularly those associated with epidermal growth factor receptor tyrosine kinase inhibitors, including gefitinib and erlotinib, evaluate DILD incidence rate and identification of risk factors based on data from post‐marketing reports.2, 3, 4, 5, 6 Documented cases of DILD in the scientific literature have focused on rapid onset of disease developing within 3 months of treatment initiation.2, 3, 4, 5 However, DILD associated with other MTA or CA have not been investigated in detail. In addition, there are patients who develop DILD after long‐term treatment (e.g. 3 or 4 months after starting treatment) in clinical practice.
In early phase drug development, animal toxicology preclinical studies may provide some information regarding potential risk of DILD for each new investigational drug. However, the information is limited in its usefulness because DILD is not a frequent adverse event and there is a difference between human and animal dose exposure levels in preclinical studies. Consequently, the importance of understanding DILD occurrences in phase I trials is attributable to the associated risks to patient accrual and determination of maximum tolerated dose level.
Identification of risk factors associated with the occurrence of DILD is potentially very useful and can alert investigators involved in phase I trials to closely monitor specific enrolled patients, even in the absence of information regarding DILD occurring in preclinical studies. Determining the time to occurrence of DILD using a large database of phase I trials, including various agents and treatment combinations, is also potentially valuable to outline sufficient observation periods in phase I trials. In addition, determining the time to occurrence of DILD may enable investigators to understand the potential risk of DILD for each investigational drug during further phases of clinical development.
To explore this issue, this study investigated incidence, grade of DILD at time of detection, and time to occurrence of DILD along with associated risk factors related to its occurrence in phase I trials for malignancies. Case reports from the database of phase I trials sponsored by the Cancer Therapy Evaluation Program (CTEP), National Cancer Institute (NCI), National Institutes of Health were used in the analyses.
Materials and Methods
Data source
In this study, we obtained a dataset of 28 771 patients enrolled in 470 protocols of phase I trials sponsored by CTEP. These trials were conducted between November 1982 and September 2014. Due to incomplete demographic data for patients (e.g. age, sex, race, body surface area [BSA], treatment in each protocol, performance status [PS], cancer type and LDH levels before treatment), we excluded 19 865 patients and included the remaining 8906 patients as study subjects. From these 8906 patients, 69 patients had developed DILD (termed Group A). Patients with pulmonary toxicities (termed Group B) included a total of 171 patients with 172 adverse events: 149 who developed pneumonia, 19 who developed pneumothorax and four patients who developed pulmonary infiltration other than DILD. The remaining 8666 patients did not develop any pulmonary‐related toxicities (termed Group C) (Fig. 1).
Figure 1.
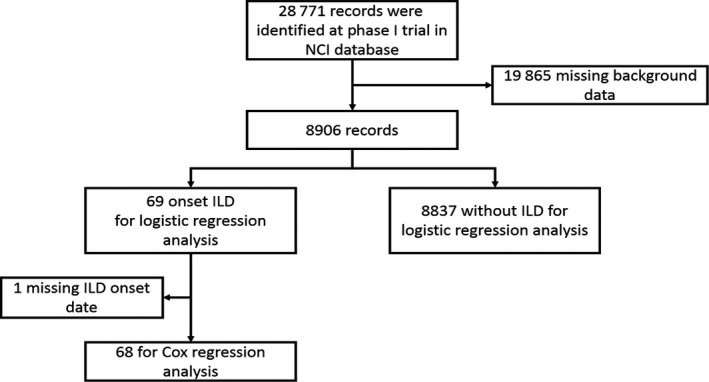
CONSORT flow diagram of the study population.
Statistical analyses
Continuous and discrete variables for the patients' characteristics were summarized using descriptive statistics. The distribution of continuous and discrete variables among Group A (DILD group), Group B (pulmonary toxicity group) and Group C (control group) were compared using the Wilcoxon and Fisher's exact tests, respectively. For Group A (DILD group, 69 patients), the number of patients by each grade and development time is visually displayed using the interpolation method. The survival curve for the time to DILD onset was estimated using the Kaplan–Meier method. Variables affecting the time to DILD development were identified using the multivariate Cox regression analysis that estimates the hazard ratio (HR) with a 95% confidence interval (CI). Moreover, the impact of variables on the onset of DILD was evaluated using multivariate logistic regression analysis that estimates odds ratios (OR) with a 95% CI. For each variable, the OR of Group A (DILD group) relative to the remaining two groups was estimated. P‐values <0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Patients' characteristics
Patients' characteristics are shown in Table 1. Patients received MTA only, CA only, or a combination of the two agents (i.e. combination treatments including MTA followed by CA or CA followed by MTA). In addition, combination or sequential therapies that included MTA + MTA or CA + CA were also used. The proportion of patients with PS ≥1 was higher in Group A (DILD group) than in the other two groups by 10%. In Group A (DILD group) and Group B (pulmonary toxicity group), many more patients were treated with MTA + CA or MTA + MTA. The proportion of patients receiving concurrent radiation therapy in Group A (DILD group) was higher than those observed in either Group B (pulmonary toxicity group) or Group C (control group).
Table 1.
Patient characteristics
| Group A (N = 69) | Group B (N = 171) | Group C (N = 8666) | P‐value | All (N = 8906) | |
|---|---|---|---|---|---|
| Age | |||||
| <65 | 48 (69.6%) | 101 (59.1%) | 6150 (71.0%) | 0.0032 | 6299 (70.7%) |
| ≥65 | 21 (30.4%) | 70 (40.9%) | 2516 (29.0%) | 2607 (29.3%) | |
| Sex | |||||
| Male | 43 (62.3%) | 98 (57.3%) | 4849 (56.0%) | 0.5375 | 4990 (56.0%) |
| Female | 26 (37.7%) | 73 (42.7%) | 3817 (44.0%) | 3916 (44.0%) | |
| Race | |||||
| White | 60 (87.0%) | 151 (88.3%) | 7481 (86.3%) | 0.4833 | 7692 (86.4%) |
| Black | 6 (8.7%) | 12 (7.0%) | 725 (8.4%) | 743 (8.3%) | |
| Native Hawaiian or other Pacific Islander | 0 (0.0%) | 1 (0.6%) | 37 (0.4%) | 38 (0.4%) | |
| Asian | 2 (2.9%) | 5 (2.9%) | 211 (2.4%) | 218 (2.4%) | |
| American Indian or Alaskan Native | 1 (1.4%) | 0 (0.0%) | 18 (0.2%) | 19 (0.2%) | |
| Mixed | 0 (0.0%) | 1 (0.6%) | 14 (0.2%) | 15 (0.2%) | |
| Unknown | 0 (0.0%) | 1 (0.6%) | 180 (2.1%) | 181 (2.0%) | |
| Race | |||||
| White | 60 (87.0%) | 151 (88.3%) | 7481 (86.3%) | 0.9517 | 7692 (86.4%) |
| Black | 6 (8.7%) | 12 (7.0%) | 725 (8.4%) | 743 (8.3%) | |
| Others | 3 (4.3%) | 8 (4.7%) | 460 (5.3%) | 471 (5.3%) | |
| Body surface area | 0.3414 | ||||
| Median | 1.9 | 1.8 | 1.9 | 1.9 | |
| Range | 0.6–2.4 | 1.3–2.6 | 0.1–3.1 | 0.1–3.1 | |
| Treatment | |||||
| M | 10 (14.5%) | 23 (13.5%) | 1576 (18.2%) | <0.0001 | 1609 (18.1%) |
| C | 13 (18.8%) | 37 (21.6%) | 2314 (26.7%) | 2364 (26.5%) | |
| M + C | 21 (30.4%) | 63 (36.8%) | 1301 (15.0%) | 1385 (15.6%) | |
| M + M | 9 (13.0%) | 18 (10.5%) | 739 (8.5%) | 766 (8.6%) | |
| C + C | 5 (7.2%) | 11 (6.4%) | 767 (8.9%) | 783 (8.8%) | |
| Others | 11 (15.9%) | 19 (11.1%) | 1969 (22.7%) | 1999 (22.4%) | |
| Concurrence of radiation therapy | |||||
| No | 63 (91.3%) | 170 (99.4%) | 8472 (97.8%) | 0.0005 | 8705 (97.7%) |
| Yes | 6 (8.7%) | 1 (0.6%) | 194 (2.2%) | 201 (2.3%) | |
| Previous radiation therapy | |||||
| No | 45 (65.2%) | 114 (66.7%) | 5451 (62.9%) | 0.1394 | 5610 (63.0%) |
| Lung | 2 (2.9%) | 3 (1.8%) | 71 (0.8%) | 76 (0.9%) | |
| Any site in the body except lung | 22 (31.9%) | 54 (31.6%) | 3144 (36.3%) | 3220 (36.2%) | |
| Coexistence of lung disease† | |||||
| No | 65 (94.2%) | 159 (93.0%) | 8427 (97.2%) | 0.0014 | 8651 (97.1%) |
| Yes | 4 (5.8%) | 12 (7.0%) | 239 (2.8%) | 255 (2.9%) | |
| Smoking history | |||||
| No | 67 (97.1%) | 169 (98.8%) | 8558 (98.8%) | 0.4686 | 8794 (98.7%) |
| Ex‐smoker or current smoker | 2 (2.9%) | 2 (1.2%) | 108 (1.2%) | 112 (1.3%) | |
| Coexistence of lung lesion‡ | |||||
| No | 48 (69.6%) | 122 (71.3%) | 5993 (69.2%) | 0.8263 | 6163 (69.2%) |
| Yes | 21 (30.4%) | 49 (28.7%) | 2673 (30.8%) | 2743 (30.8%) | |
| ECOG‐PS | |||||
| 0 | 15 (21.7%) | 63 (36.8%) | 2895 (33.4%) | 0.2073 | 2973 (33.4%) |
| 1 | 48 (69.6%) | 93 (54.4%) | 5128 (59.2%) | 5269 (59.2%) | |
| ≥2 | 6 (8.7%) | 15 (8.8%) | 643 (7.4%) | 664 (7.5%) | |
| ECOG‐PS | |||||
| 0 | 15 (21.7%) | 63 (36.8%) | 2895 (33.4%) | 0.077 | 2973 (33.4%) |
| ≥1 | 54 (78.3%) | 108 (63.2%) | 5771 (66.6%) | 5933 (66.6%) | |
| Solid or hematological malignancy | |||||
| Solid tumor | 46 (66.7%) | 95 (55.6%) | 7399 (85.4%) | <0.0001 | 7540 (84.7%) |
| Hematology | 23 (33.3%) | 76 (44.4%) | 1267 (14.6%) | 1366 (15.3%) | |
| LDH | 0.0604 | ||||
| Median | 284 | 305 | 248 | 249 | |
| Range | 95.0–1611.0 | 80.0–3993.0 | 0.0–17 945.0 | 0.0–17 945.0 | |
| LDH | |||||
| Normal | 32 (46.4%) | 74 (43.3%) | 4297 (49.6%) | 0.2309 | 4403 (49.4%) |
| Elevated | 37 (53.6%) | 97 (56.7%) | 4369 (50.4%) | 4503 (50.6%) | |
†Coexistence of pulmonary disease was defined as patient with lung complication or past‐history such as chronic pulmonary lung disease, bronchial asthma, pulmonary embolism, sarcoidosis, pneumothorax, asbestosis and infection. ‡Coexistence of lung lesion was defined as patient with primary lung tumor and/or lung metastases. C, Cytotoxic agent; ECOG; Eastern Cooperation Oncology Group; LDH, lactate dehydrogenase; M, molecularly targeted agent; PS, performance status.
Analysis of drug‐induced interstitial lung disease
The median of time to DILD onset was 1.4 months (95% CI, 0.9–1.7 months). The number of patients with grade 1, 2, 3, 4 or 5 DILD was 7 (10.3%), 26 (38.2%), 24 (35.3%), 4 (5.9%) and 7 (10.3%), respectively. Figure 2 shows the number of DILD patients by each grade and month at onset of DILD. We found that onset of disease in the majority of DILD patients with grade 1 or 2 occurred within 2 months of initiating treatment, while those with grades >3 frequently occurred after 3 months. Table 2 shows the results of multivariate Cox regression analysis for 68 patients. A decrease in BSA of 0.1 m2 significantly affected DILD development (P = 0.0249).
Figure 2.
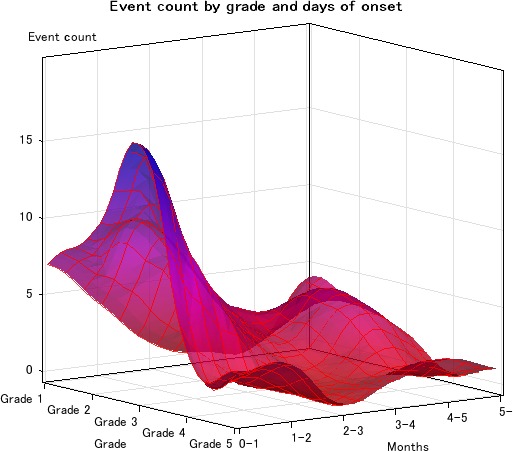
Distribution of drug‐induced interstitial lung disease (DILD) toxicity grade and onset from first day of treatment.
Table 2.
Multivariate Cox regression analysis for 68 patients who developed DILD
| Characteristics | Category | N | Multivariate | |||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% lower CI for hazard ratio | 95% upper CI for hazard ratio | P‐value | |||
| Age | <65 | 47 | 1 | NA | NA | NA |
| ≥65 | 21 | 1.576 | 0.763 | 3.252 | 0.2187 | |
| Sex | Male | 42 | 1 | NA | NA | NA |
| Female | 26 | 0.771 | 0.371 | 1.598 | 0.4838 | |
| Race | White | 59 | 1 | NA | NA | NA |
| Black | 6 | 2.407 | 0.827 | 7.003 | 0.1069 | |
| Others | 3 | 0.521 | 0.104 | 2.600 | 0.4264 | |
| Body surface area | 0.1 m2 decrease | 68 | 1.157 | 1.019 | 1.313 | 0.0249 |
| Treatment | M | 9 | 1 | NA | NA | NA |
| C | 13 | 0.621 | 0.159 | 2.421 | 0.4926 | |
| M + C | 21 | 0.450 | 0.130 | 1.554 | 0.2066 | |
| M + M | 9 | 0.473 | 0.095 | 2.348 | 0.36 | |
| C + C | 5 | 1.166 | 0.276 | 4.931 | 0.8345 | |
| Others | 11 | 0.847 | 0.191 | 3.759 | 0.8269 | |
| Concurrence of radiation therapy | No | 62 | 1 | NA | NA | NA |
| Yes | 6 | 0.311 | 0.085 | 1.137 | 0.0775 | |
| Previous history of thoracic radiation therapy | No | 66 | 1 | NA | NA | NA |
| Yes | 2 | 7.279 | 0.957 | 55.373 | 0.0552 | |
| Coexistence of pulmonary disease† | No | 64 | 1 | NA | NA | NA |
| Yes | 4 | 0.921 | 0.176 | 4.807 | 0.922 | |
| Smoking history | No | 66 | 1 | NA | NA | NA |
| Ex‐smoker or current smoker | 2 | 0.779 | 0.074 | 8.239 | 0.8358 | |
| Coexistence of lung lesion‡ | No | 47 | 1 | NA | NA | NA |
| Yes | 21 | 1.477 | 0.694 | 3.140 | 0.3113 | |
| PS | 0 | 14 | 1 | NA | NA | NA |
| ≥1 | 54 | 1.440 | 0.653 | 3.178 | 0.3661 | |
| Solid or hematological malignancy | Solid tumor | 46 | 1 | NA | NA | NA |
| Hematology | 22 | 1.849 | 0.827 | 4.138 | 0.1345 | |
| LDH | Normal | 32 | 1 | NA | NA | NA |
| Elevated | 36 | 1.693 | 0.905 | 3.168 | 0.0995 | |
†Coexistence of pulmonary disease was defined as patient with lung complication or past‐history such as chronic pulmonary lung disease, bronchial asthma, pulmonary embolism, sarcoidosis, pneumothorax, asbestosis and infection. ‡Coexistence of lung lesion was defined as patient with primary lung tumor and/or lung metastases. C, Cytotoxic agent; DILS, drug‐induced interstitial lung disease; LDH, lactate dehydrogenase; M, molecular targeted drug; PS, performance status; NA, not applicable.
Risk factors affecting the development of DILD
Table 3 shows the results of multivariate logistic regression analysis. The proportion of DILD patients receiving MTA + CA therapy was significantly higher than those receiving MTA therapy alone (i.e. the single use of an MTA) (P = 0.0164). A similar result was observed in patients receiving MTA + MTA therapy, but it did not reach statistical significance (P = 0.0952). The risk of DILD development was similar between patients receiving MTA therapy and CA therapy because the HR for CA relative to MTA was 1.035 (95% CI, 0.449–2.384, P = 0.9359). In addition, the proportion of patients with DILD and other risk factors such as concurrent radiation therapy, PS >1, or hematologic cancer was also significantly higher in Group A (DILD group) than in Group B (pulmonary toxicity group) and Group C (control group). Details of drugs that induced DILD are shown in Table 4. There were 110 identified cases of DILD that were linked to 24 different drugs. Among the categories of drugs involved, mTOR inhibitors, HDAC inhibitors and cytidine antimetabolite agents were the most frequently cited.
Table 3.
Multivariate logistic regression analysis for prevalence of DILD
| Characteristics | Category | N | Multivariate | |||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI lower | 95% CI upper | P‐value | |||
| Age | <65 | 6299 | 1 | NA | NA | NA |
| ≥65 | 2607 | 0.822 | 0.483 | 1.399 | 0.4709 | |
| Sex | Male | 4990 | 1 | NA | NA | NA |
| Female | 3916 | 0.608 | 0.347 | 1.064 | 0.0813 | |
| Race | White | 7692 | 1 | NA | NA | NA |
| Black | 743 | 0.897 | 0.382 | 2.103 | 0.8017 | |
| Others | 471 | 0.706 | 0.217 | 2.293 | 0.5619 | |
| Body surface area | 0.1 m2 decrease | 8906 | 1.096 | 0.990 | 1.213 | 0.0784 |
| Treatment | M | 1609 | 1 | NA | NA | NA |
| C | 2364 | 1.035 | 0.449 | 2.384 | 0.9359 | |
| M + C | 1385 | 2.553 | 1.188 | 5.486 | 0.0164 | |
| M + M | 766 | 2.175 | 0.873 | 5.420 | 0.0952 | |
| C + C | 783 | 1.173 | 0.397 | 3.466 | 0.7728 | |
| Others | 1999 | 0.646 | 0.217 | 1.929 | 0.4342 | |
| Concurrence of radiation therapy | No | 8705 | 1 | NA | NA | NA |
| Yes | 201 | 11.392 | 3.408 | 38.076 | <0.0001 | |
| Previous history of thoracic radiation therapy | No | 8830 | 1 | NA | NA | NA |
| Yes | 76 | 3.166 | 0.734 | 13.662 | 0.1223 | |
| Coexistence of pulmonary disease† | No | 8651 | 1 | NA | NA | NA |
| Yes | 255 | 1.527 | 0.513 | 4.544 | 0.4468 | |
| Smoking history | No | 8794 | 1 | NA | NA | NA |
| Ex‐smoker or current smoker | 112 | 1.605 | 0.349 | 7.393 | 0.5434 | |
| Coexistence of lung lesion‡ | No | 6163 | 1 | NA | NA | NA |
| Yes | 2743 | 1.342 | 0.749 | 2.406 | 0.3233 | |
| PS | 0 | 2973 | 1 | NA | NA | NA |
| ≥2 | 5933 | 1.872 | 1.044 | 3.356 | 0.0353 | |
| Solid or hematological malignancy | Solid tumor | 7540 | 1 | NA | NA | NA |
| Hematology | 1366 | 2.894 | 1.593 | 5.258 | 0.0005 | |
| LDH | Normal | 4403 | 1 | NA | NA | NA |
| Elevated | 4503 | 1.146 | 0.704 | 1.864 | 0.5837 | |
†Coexistence of pulmonary disease was defined as patient with lung complication or past‐history such as chronic pulmonary lung disease, bronchial asthma, pulmonary embolism, sarcoidosis, pneumothorax, asbestosis and infection. ‡Coexistence of lung lesion was defined as patient with primary lung tumor and/or lung metastases. C, cytotoxic agent; CI, confidence interval; DILS, drug‐induced interstitial lung disease; LDH, lactate dehydrogenase; M, molecular targeted drug; PS, performance status; NA, not applicable.
Table 4.
Details for drugs that induced DILD
| Category | Target | n | Category | Target | n | ||
|---|---|---|---|---|---|---|---|
| M (n = 52) | TKI | AKT | 1 | C (n = 58) | Tubulin inhibitor | Taxane | 6 |
| mTOR | 8 | Kinase | 1 | ||||
| Her1 (EGFR) | 2 | Others | 2 | ||||
| VEGF | 4 | Topoisomerase inhibitor | I | 9 | |||
| mAb | CTLA4 | 2 | II | 5 | |||
| IGF‐1R | 4 | Antimetabolite agents | Cytidine | 13 | |||
| Immunotoxin | 2 | Pyrimidine fluoride | 5 | ||||
| CDK inhibitor | 6 | Platinum | 8 | ||||
| Angiopoietin inhibitor | 2 | Antitumor antibiotics | 5 | ||||
| Proteasome inhibitor | 1 | Nitrosourea | 1 | ||||
| Farnesyltransferase inhibitor | 7 | PKC inhibitor | 2 | ||||
| PARP inhibitor | 4 | DNA minor groove binding agent | 1 | ||||
| HDAC inhibitor | 9 | Radiation therapy | 6 | ||||
AKT, serine/threonine‐specific protein kinase; C, cytotoxic agent; CDK, cyclin dependent kinase; CTLA, cytotoxic T‐lymphocyte‐associated protein; EGFR, epidermal growth factor receptor; HDAC, histone deacetylase; IGF‐1R, insulin like growth factor‐1 receptor; M, molecular targeted agent; mAb, monoclonal antibody; mTOR, mammalian target of rapamycin; PARP, poly ADP ribose polymerase; PKC, phosphokinase C; TKI, tyrosine kinase inhibitoror; VEGF, vascular endothelial growth fact.
Discussion
To our knowledge, this analysis summarizes the largest series of adverse events associated with DILD in phase 1 clinical trials to date and facilitates a detailed analysis of DILD that occurred with different types of treatment. Prevalence of DILD in our study was 0.77% for all grades, which is lower than that reported in the general population (2.5–3%).7 Interestingly, the analysis showed no difference in the incidence of DILD between CA and MTA. As for combination drug therapy, MTA + MTA and MTA + CA trended toward a greater risk than the respective monotherapies. Patients with an unfavorable PS (>2) or a hematological malignancy were also shown to be at a higher risk of developing DILD. No increased risk was observed in Group B (respiratory toxicity group) because patients with known lung fibrosis are excluded from entering phase 1 clinical trials. In addition, our results indicate that a decrease in BSA is closely associated with the onset of DILD.
Our study demonstrates that combination therapies, as a cytotoxic agent plus a molecular targeted agent or combination with radiation, are associated with a greater risk for inducing DILD than monotherapies. In particular, the odds ratio for patients receiving radiation therapy in combination with MTA or CA was 11.39 (95% CI; 3.408–38.076). A previous study of advanced lung cancer showed that 87% of participants treated with conventional chemoradiation therapy experienced complicated pneumonitis at any grade.8 This phenomenon is attributable to radiotherapy being directly toxic to parenchymal lung cells and inducing the inflammatory process, which was exacerbated by drug therapy.7 In addition, it is well known that increases in drug dose and combinations of different drugs increase the incidence of DILD.8 Thus, special caution is needed for patients with low BSA in addition to the abovementioned risk factors who are receiving combination therapy.
Here, we describe the different types of drugs that might have resulted in DILD. However, the data shown are limited and the study did not cover all the drugs available clinically. Nonetheless, caution may be required for the continued development of agents identified in this report to have a causal relationship with DILD, or any anti‐cancer medication within the same class as these agents. Moreover, the data included several agents that are currently well recognized as higher risk agents for inducing DILD, such as mTOR inhibitors, EGFR tyrosine kinase inhibitors, and a PARP inhibitor that has been recently suggested as an agent with potential risks.9
A biphasic peak in incidence of DILD was observed in our study. In general, a lower grade of DILD occurred within 1 or 2 months of receiving the drug treatment, whereas a higher grade DILD occurred later than 3 months after receiving treatment. The incidence of DILD was <0.5% after 3 months. Furthermore, the incidence, prognosis and peak time of onset varied by drug. For instance, gefitinib induced an acute and highly lethal form of DILD; most cases of DILD occurred within 2 months of receiving treatment.10 In contrast, onset of DILD after treatment with everolimus was gradual and most cases were lower than grade 2; median time to occurrence was 108 days.11 Phase 1 investigators should monitor patients for signs of DILD throughout their treatment course as extremely rare cases of DILD may occur later than 6 months after initiating treatment.
There are several limitations to our study. First, diagnosing asymptomatic DILD is challenging; therefore, an underestimation of DILD prevalence in this study is possible. Second, we excluded studies in the database that lacked information related to DILD, meaning we potentially missed studies with a lower incidence.
A strength of our study is the uniqueness of our database. Individual patient information was collected prospectively and the study included drugs that were not further developed. Furthermore, the data were analyzed retrospectively. Previously, the majority of reports were from post‐marketing or larger clinical trials. To the best of our knowledge, this is the first study to evaluate the incidence and risk factors for DILD in the earliest phase of drug development. Moreover, our study had a large sample size that provides statistical power with regard to examining effects related to rare adverse events.
In summary, this large analysis of phase I clinical trials provides epidemiologic evidence that risk factor profiles for DILD include concurrent therapy (including radiation), poor PS and hematologic malignancy. In addition, a decrease in BSA is associated with time to development of DILD. Most severe cases occurred 3 months after initiation of therapy. We suggest that a 6‐month observation period is sufficient for detecting the onset of most DILD cases.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
We would like to thank Mr Gary Smith at CTEP National Cancer Institute at National Institutes of Health and Indrani Chatterjee at Theradex for their generous provision of database support.
Cancer Sci 107 (2016) 1830–1836
Funding Information
No sources of funding were declared for this study.
Contributor Information
Kan Yonemori, Email: kyonemor@ncc.go.jp.
Naoko Takebe, Email: takeben@mail.nih.gov.
References
- 1. Hirakawa A, Yonemori K, Kuwatsuka Y et al A descriptive analysis of post‐chemotherapy development of interstitial lung disease using spontaneous reporting data in Japan. Curr Drug Saf 2014; 9: 220–6. [DOI] [PubMed] [Google Scholar]
- 2. Ando M, Okamoto I, Yamamoto N et al Predictive factors for interstitial lung disease, antitumor response, and survival in nonsmall‐cell lung cancer patients treated with gefitinib. J Clin Oncol 2006; 24: 2549–56. [DOI] [PubMed] [Google Scholar]
- 3. Hotta K, Kiura K, Tabata M et al Interstitial lung disease in Japanese patients with non‐small cell lung cancer receiving gefitinib: an analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J 2005; 11: 417–24. [DOI] [PubMed] [Google Scholar]
- 4. Hotta K, Kiura K, Takigawa N et al Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non‐small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol 2010; 5: 179–84. [DOI] [PubMed] [Google Scholar]
- 5. Kudoh S, Kato H, Nishiwaki Y et al Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case control study. Am J Respir Crit Care Med 2008; 177: 1348–57. [DOI] [PubMed] [Google Scholar]
- 6. Nakagawa M, Nishimura T, Teramukai S et al Interstitial lung disease in gefitinib‐treated Japanese patients with non‐small cell lung cancer – a retrospective analysis: JMTO LC03‐02. BMC Res Notes 2009; 2: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, Berghaus T. Drug induced interstitial lung disease. Open Respir Med J 2012; 6: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez EG, Peloche MGB, Quiroz CFA et al Prediction parameters for radiation pneumonitis in patients with stage III NSCLC. J Clin Oncol 2014; 32: Abstract e18538. [Google Scholar]
- 9. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer Aug 2004; 91(Suppl 2): S18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kapoor K, Singla E, Sahu B, Naura AS. PARP inhibitor, olaparib ameliorates acute lung and kidney injury upon intratracheal administration of LPS in mice. Mol Cell Biochem Feb 2015; 400: 153–62. [DOI] [PubMed] [Google Scholar]
- 11. Cohen MH, Williams GA, Sridhara R et al United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res 2004; 10: 1212–8. [DOI] [PubMed] [Google Scholar]


