Abstract
GBS‐01, an extract from the fruit of Arctium lappa L. is an orally administered drug rich in arctigenin, which has been reported to exert antitumor activity by attenuating the tolerance of cancer cells to glucose deprivation. We investigated the maximum tolerated dose of GBS‐01 based on the frequency of the dose‐limiting toxicities (DLTs) and pharmacokinetics in patients with advanced pancreatic cancer refractory to gemcitabine. GBS‐01 was given orally at escalating doses from 3.0 g (containing 1.0 g burdock fruit extract) to 12.0 g q.d. A DLT was defined as a grade 4 hematological toxicity and grade 3 or 4 non‐hematological toxicity appearing during the first 28 days of treatment. Fifteen patients (GBS‐01 dose level 1 [3.0 g], three patients; dose level 2 [7.5 g], three patients; and dose level 3 [12.0 g], nine patients) were enrolled. None of the patients at any of the three dose levels showed any sign of DLTs. The main adverse events were increased serum γ‐glutamyl transpeptidase, hyperglycemia, and increased serum total bilirubin; however, all the toxicities were mild. Of the 15 patients, 1 showed confirmed partial response and 4 patients had stable disease. The median progression‐free and overall survival of the patients were 1.1 and 5.7 months, respectively. The pharmacokinetic study revealed a high bioavailability of arctigenin and rapid conjugation of the drug with glucuronic acid. The recommended dose of GBS‐01 was 12.0 g q.d, and favorable clinical responses were obtained. This trial was registered at UMIN‐CTR (http://www.umin.ac.jp/ctr/index-j.htm), identification number UMIN000005787.
Keywords: Arctigenin, chemotherapy, gemcitabine, natural anticancer compound, pancreatic cancer, phase I trial
The cancer microenvironment is characterized by severe hypoxia and nutrient deprivation, especially of glucose, and this is especially true for hypovascular tumors. Therefore, cancer cells are believed to be highly dependent on glycolysis for energy production, known as the Warburg effect.1 Tumor hypoxia is mainly caused by poor blood supply despite vigorous angiogenesis.2, 3 Severe hypoxia and glucose deprivation have been reported to promote tumor progression and have a big impact on resistance to anticancer drugs.2, 3, 4 Therefore, development of anticancer agents using the novel strategy of targeting the molecular and biochemical processes of adaptation of cancer cells to their very harsh microenvironment of severe hypoxia–hypoglycemia is strongly desired.
We have established a screening method for candidate compounds that can attenuate the tolerance of cancer cells to glucose starvation and hypoxia, and identified many compounds with this effect, including kigamicin D, pyrvinium pamoate, and AG.5 Among these compounds, AG has been shown to exert antitumor activity in many xenograft models of cancer, including PC, and preclinical toxicity evaluation has revealed sufficient acute and subacute safety profiles of AG given at doses up to 100 times the daily dose required for antitumor activity in mice. Therefore, AG is the first of these compounds chosen for clinical trial. Although pure AG is preferable to apply to the clinical trial, we chose GBS‐01, an extract of the fruit of Arctium lappa L., which is registered in the Japanese Pharmacopoeia as a traditional herbal medicine. Repositioning of GBS‐01 to an anticancer drug seems feasible and swift for clinical application.
Pancreatic cancer is reported to be characterized by hypovascularity and severe hypoxia of the cancer tissue,2 with only limited benefit of chemotherapy achieved so far, especially in patients with advanced PC who are refractory to GEM therapy, regarded as one of the standard treatments for advanced PC.6, 7 Thus, a phase I trial of GBS‐01 as monotherapy was carried out in advanced PC patients who were refractory to treatment with GEM.
Patients and Methods
Patients
Patients eligible for study entry had unresectable advanced PC refractory to GEM. The eligibility criteria were as follows: (i) cytologically or histologically proven invasive ductal adenocarcinoma or adenosquamous carcinoma; (ii) refractory to GEM‐based chemotherapy as determined by radiologically or clinically confirmed PD, recurrence during or within 12 weeks of completion of adjuvant chemotherapy, or appearance of unacceptable toxicities; (iii) age ≥20 years; (iv) Eastern Cooperative Oncology Group performance status 0–2; (v) at least one measurable lesion according to RECIST version 1.1;8 (vi) adequate oral intake; (vii) >14 days’ interval from prior chemotherapy; (viii) satisfactory hematological functions (hemoglobin ≥8.5 g/dL, leukocytes ≥3000/mm3, neutrophils ≥1500/mm3, platelets ≥75 000/mm3); (ix) adequate hepatic function (serum total bilirubin ≤2.0 or ≤3.0 mg/dL with biliary drainage, serum AST and ALT ≤100 or ≤150 U/L with biliary drainage); and (x) adequate renal function (serum creatinine ≤1.5 mg/dL). Written informed consent was obtained from all patients.
The exclusion criteria were: (i) moderate or massive pleural effusion or ascites; (ii) symptomatic brain metastasis or a history of brain metastasis; (iii) synchronous or asynchronous other cancer within 3 years except carcinoma in situ or intramucosal carcinoma; (iv) active infection; (v) psychiatric disorder; (vi) serious medical condition such as intestinal paralysis; (vii) intestinal obstruction; (viii) poorly controlled diabetes; (ix) heart failure, renal failure, hepatic failure, or active gastrointestinal ulcer; and (x) pregnant or breast‐feeding.
The pretreatment evaluation consisted of a complete history and physical examination and baseline assessments of organ functions. In addition, contrast‐medium enhanced computed tomography or MRI of the abdomen and computed tomography of the chest were carried out for pretreatment staging to assess the local extent of the tumor and to exclude the presence of distant metastases. All patients with obstructive jaundice underwent percutaneous transhepatic or endoscopic retrograde biliary drainage before the treatment.
Study design
This was an open‐label, single institutional, single‐arm phase I study to investigate the maximum tolerated dose of GBS‐01 based on the frequency of DLTs in patients with advanced PC refractory to GEM therapy. GBS‐01 is an extract of the fruit of Arctium lappa (Japanese name, Goboushi). The extraction method to obtain abundant quantities of AG was developed by Kracie Pharma, Ltd., which manufactures GBS‐01 as an oral drug. The content of AG and arctiin, a glucoside of AG, was measured by HPLC–mass spectrometry as described previously,9 to maintain the quality and consistency of product. The content of AG and arctiin in GBS‐01 was 59.4 mg and 68.5 mg/g extract, respectively. GBS‐01 was given orally once daily after breakfast on consecutive days. Treatment was repeated until the appearance of disease progression, unacceptable toxicities, or the patient's refusal to continue treatment. Three dose levels of GBS‐01 were set: dose level 1, 3.0 g q.d.; dose level 2, 7.5 g q.d.; and dose level 3, 12.0 g q.d. GBS‐01 at dose level 1 (3.0 g/day) contains 1.0 g burdock fruit extract, which in turn contains 68.5 mg arctiin and 59.4 mg AG. The starting dose was dose level 1. In the practice of Chinese herbal medicine, GBS‐01 is usually prescribed as burdock fruit at the maximum dose of 12.0 g/day (level 3). Patients were instructed to maintain daily records of their intake of GBS‐01 and of any signs or symptoms that they experienced.
There were initially three patients at each dose level. If no DLT was observed in the initial three patients, the dosage was escalated in successive cohorts. If DLT was observed in one or two of the initial three patients, three additional patients were evaluated at the same dose level. If only one or two of six patients experienced DLT, the dose escalation protocol was continued. However, if three or more patients experienced DLT at a given dose level, then the previous dose level was considered as the maximum tolerated dose. At the final dose level, a total of six patients were scheduled, for accurate assessment of the DLTs. A DLT was defined as any of the adverse events listed below occurring within 28 days of initiation of GBS‐01 treatment, for which a causal relationship to the drug could not be denied: grade 3 febrile neutropenia, grade 4 neutropenia, platelets <25 000/mm3, thrombocytopenia requiring transfusion by investigator's judgment, serum AST/ALT/γ‐GTP/alkaline phosphatase ≥10 times the upper limit of normal, serum creatinine ≥2.0 mg/dL, grade 3 or 4 non‐hematological toxicities, excluding hyperglycemia and electrolyte abnormalities, or need for treatment interruption for longer than 15 consecutive days.
When grade 3 febrile neutropenia, grade 4 hematological toxicity, serum AST/ALT/γ‐GTP/alkaline phosphatase ≥10 times the upper limit of normal, and/or grade 3 or higher non‐hematological toxicities were observed, treatment with GBS‐01 was suspended. Treatment was resumed at a dose one level lower when the toxicities improved by one grade or over. In patients treated with dose level 1 of GBS‐01, the treatment was discontinued. After this treatment, the patients were allowed to receive other anticancer treatments at their physician's discretion.
Study assessment
The primary end‐point of this trial was the frequency of DLTs, and the secondary end‐points were the frequency of adverse events, PKs, and antitumor activity. Treatment‐related toxicities were assessed using the Common Terminology Criteria for Adverse Events, version 4.0. During the period of assessment of the DLTs, physical examination, complete blood count with differential leukocyte counts, and serum chemistry were carried out at least once a week; thereafter, these assessments were carried out every 2 weeks at least. Tumor response was evaluated every 4 weeks until tumor progression, according to RECIST version 1.1. Progression‐free survival was defined as the time from the date of enrolment in the study to the first documentation of disease progression or death. Overall survival was measured from the date of enrolment in the study to the date of death or date of the last follow‐up. All time‐to‐event distributions were estimated by the Kaplan–Meier method. Serum levels of carbohydrate antigen 19‐9 were measured at the baseline and every 4 weeks.
This phase I study was carried out with the approval of the institutional review board of the National Cancer Center (Kashiwa, Japan) and in accordance with the Declaration of Helsinki and Ethical Guidelines for Clinical Research (Ministry of Health, Labor and Welfare, Japan). The trial was registered at UMIN‐CTR (http://www.umin.ac.jp/ctr/index.htm), identification number (UMIN000005787). Patient registrations and data collection were managed by the Clinical Trial Section at the National Cancer Center Hospital East (Kashiwa, Japan). The quality of the data was ensured by a careful review undertaken by staff of the data center (K.T. and H.H.) and the coordinating investigator of this study (M.I.). All data were fixed on April 15, 2014, and all the analyses in this study were carried out by a statistician (S.N.) using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Pharmacokinetic analysis
Arctiin is metabolized to AG by β‐glucosidase, and AG is conjugated with glucuronide by UDP‐glucuronosyltransferase in the liver and intestine, to form AGG. Plasma concentrations and urinary excretion of AG and AGG were measured for assessment of the PK. Blood samples for the plasma AG and AGG measurements were drawn predose, and at 0.5, 1, 1.5, 2, 3, 4, 6, and 12 h after treatment with GBS‐01 on days 1 and 8. In addition, the trough concentrations of plasma AG and AGG were measured before treatment with GBS‐01 on days 2, 5, and 9. Urinary excretion of AG and AGG was assessed in 24‐h urine sample collections on days 1 and 8. The AG and AGG concentrations were determined by the HPLC–mass spectrometry method, as described previously.9 Arctiin can be measured by the present HPLC–mass spectrometry method and the detection limit was 10 ng/mL. The PK parameters were calculated using the non‐linear least‐squares computer program Phoenix WinNonlin (Pharsight Corporation, Certara, St. Louis, MO, USA).
Results
Patient characteristics
Fifteen patients were enrolled in this trial between June 2011 and May 2012 at the National Cancer Center Hospital East. Table 1 shows the characteristics of the 15 patients (level 1, three patients; level 2, three patients; and level 3, nine patients). At dose level 3, three patients could not be given the drug for the entire 28‐day period, the period of DLT assessment, because of early disease progression. Therefore, these three patients were replaced at level 3, increasing the number of patients enrolled at dose level 3 to a total of nine. All patients were refractory to S‐1 as well as GEM therapy. As subsequent treatments, five patients were enrolled into clinical trials of new agents and one patient underwent intra‐arterial chemotherapy, while the remaining received supportive care only.
Table 1.
Characteristics of patients with advanced pancreatic cancer refractory to gemcitabine treated with GBS‐01 (n = 15)
| Characteristic | No. of patients | % |
|---|---|---|
| Age, years | ||
| Median | 65 | |
| Range | 44–77 | |
| Gender | ||
| Male | 10 | 67 |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 9 | 60 |
| 1 | 6 | 40 |
| Biliary drainage | ||
| Present | 6 | 40 |
| Pathology | ||
| Adenocarcinoma | 15 | 100 |
| Tumor location | ||
| Head | 10 | 67 |
| Body or tail | 5 | 33 |
| History of resection | ||
| Present | 9 | 60 |
| History of chemotherapy | ||
| Gemcitabine‐based regimen | 15 | 100 |
| S‐1 based regimen | 15 | 100 |
| Number of prior chemotherapy | ||
| 1 | 3 | 20 |
| 2 | 9 | 60 |
| 3 | 3 | 20 |
| Clinical stage | ||
| Local relapse | 1 | 7 |
| Distant metastases | 14 | 93 |
| Metastatic site | ||
| Liver | 12 | 80 |
| Lymph node | 5 | 33 |
| Peritoneal | 3 | 20 |
| Lung | 3 | 20 |
| Subcutaneous | 1 | 7 |
| Serum CA19‐9, U/mL | ||
| Median | 10 230 | |
| Range | 48.1–45 450 | |
CA19‐9, carbohydrate antigen 19‐9.
Toxicity
The toxicities observed in the 15 enrolled patients are listed in Table 2. The main adverse events associated with this agent were increased γ‐GTP, hyperglycemia, and increased total bilirubin. Although some grade 3 adverse events were observed in this patient series, all of these were considered to be due to tumor progression or concomitant disease. Therefore, no DLT was observed at any of the dose levels, and all the adverse events associated with this agent were extremely mild. The recommended dose of GBS‐01 was therefore determined to be 12.0 g q.d. (appropriately equivalent to 4.0 g burdock fruit extract).
Table 2.
Adverse events during phase I trial of GBS‐01 in patients with advanced pancreatic cancer refractory to gemcitabine
| CTC‐AE4.0† | Level 1 (n = 3) | Level 2 (n = 3) | Level 3 (n = 9) | All levels (n = 15) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | G1–2 (%) | G3–4 (%) | |
| Leukopenia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 27 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 13 | 0 |
| Anemia | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 53 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 7 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 27 | 0 |
| Nausea | 0 | 0 | 0 | – | 0 | 1 | 0 | – | 2 | 3 | 0 | – | 40 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 27 | 0 |
| Anorexia | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 4 | 1 | 0 | 53 | 7 |
| Fatigue | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 27 | 13 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 20 | 0 |
| T‐Bil increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 13 | 7 |
| AST increased | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 6 | 1 | 1 | 0 | 67 | 7 |
| ALT increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 33 | 7 |
| ALP increased | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 6 | 1 | 1 | 0 | 73 | 7 |
| Γ‐GTP increased | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 5 | 0 | 33 | 40 |
| Cr increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 7 | 0 |
| Hyperglycemia | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 3 | 4 | 2 | 0 | 67 | 33 |
†Grading according to the Common Terminology Criteria for Adverse Events, version 4.0. GBS‐01 dose level 1, 3.0 g q.d. (n = 3); dose level 2, 7.5 g q.d. (n = 3); dose level 3, 12.0 g q.d. (n = 9). –, . γ‐GTP, serum γ‐glutamyl transpeptidase; ALP, serum alkaline phosphatase; ALT, serum alanine aminotransferase; AST, serum aspartate aminotransferase; Cr, serum creatinine; T‐Bil, serum total bilirubin.
Efficacy
All the patients were included in the response evaluation. Of the 15 enrolled patients, one showed PR and four had SD during the course of observation. The response rate was 6.7% (95% CI, 0.2–32%) and the disease control rate was 33.3% (95% CI, 12–60%). The median progression‐free and overall survival times of the patients were 1.1 months (95% CI, 0.9–1.9 months) and 5.7 months (95% CI, 1.9–8.4 months), respectively (Fig. 1).
Figure 1.
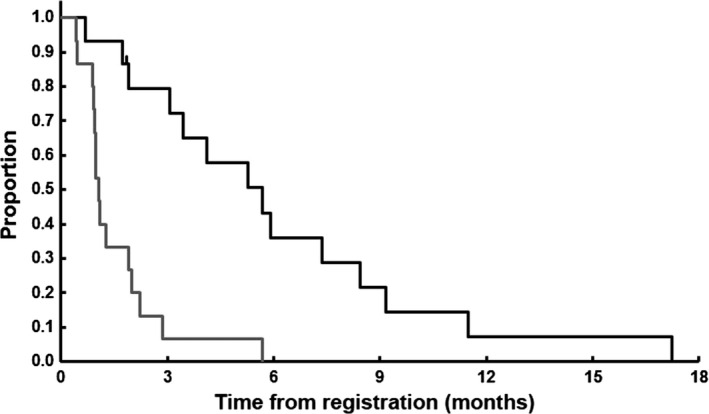
Overall survival (black line) and progression‐free survival curves (gray line) of the 15 patients with gemcitabine‐refractory pancreatic cancer who were treated with GBS‐01. Censored cases are shown by tick marks.
Case showing PR
In this trial, PR was obtained in one patient (Fig. 2). The patient was a 54‐year‐old man who had been diagnosed as having advanced PC with liver, abdominal lymph node, and pulmonary metastases, and had received first‐line treatment with the drug combination of GEM plus S‐1. Fourteen months after the commencement of this treatment, evidence of tumor progression was detected. The patient was enrolled at dose level 2 in this study, and GBS‐01 was given at 7.5 g/day. Three months after the start of GBS‐01 treatment, his liver metastases had clearly reduced in size, and PR was confirmed at 4 months after the start of GBS‐01 treatment. The serum carbohydrate antigen 19‐9 level also gradually decreased. The PR was sustained for 3 months; however, the disease began to progress again, and at 6 months after GBS‐01 treatment was commenced, the patient abandoned the treatment. The PK profiles of AG and AGG are shown in Figure S1.
Figure 2.
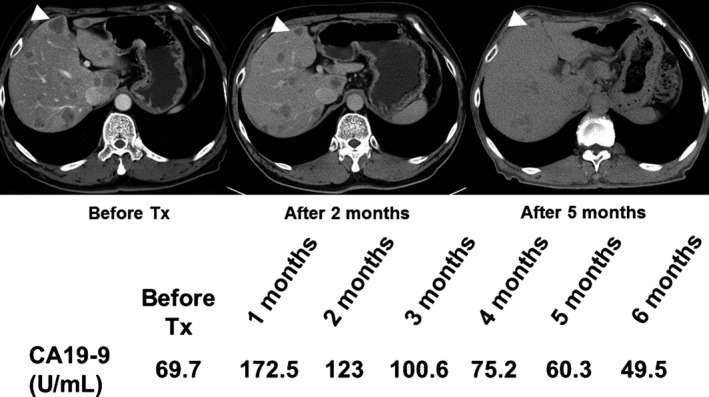
Serial computed tomographic images of a patient with advanced pancreatic cancer refractory to gemcitabine who showed partial response to treatment (Tx) with GBS‐01. CA19‐9, carbohydrate antigen 19‐9; mo, months.
Pharmacokinetics
A representative PK profile of AG and AGG from three patients given 12.0 g GBS‐01 who showed SD (no. 9) and PD (nos. 12 and 15) are shown in Figure 3(a). The Cpmax of AG was observed at 1 h after administration of GBS‐01, and that of AGG was observed between 1.5 and 3 h after administration. In most cases, second peaks of AG and AGG were observed, indicating enterohepatic circulation. The actual concentration of AGG was more than 100 times higher than that of AG, even in the early phase, indicating that AG is rapidly conjugated with glucuronic acid, probably at the first pass. Actually, the AUC of AGG was almost 1000 times higher than that of AG, as shown in Table 3. From these results, it was clarified that GBS‐01 mostly exists as AGG in the plasma. In the dose‐escalation phase of the study, following single‐ and multiple‐dose administration of GBS‐01, the mean AUC0–t and peak (Cpmax) exposures of AGG increased, in general, with increasing dose. Judging from the urinary excretion and plasma levels of AG and AGG, it was inferred that orally administered arctigenin has high bioavailability. Arctiin was not detected at any time point in any patient, indicating that arctiin might be converted to arctigenin during absorption. This hypothesis was also supported by high recovery of AGG and AG in the urine.
Figure 3.
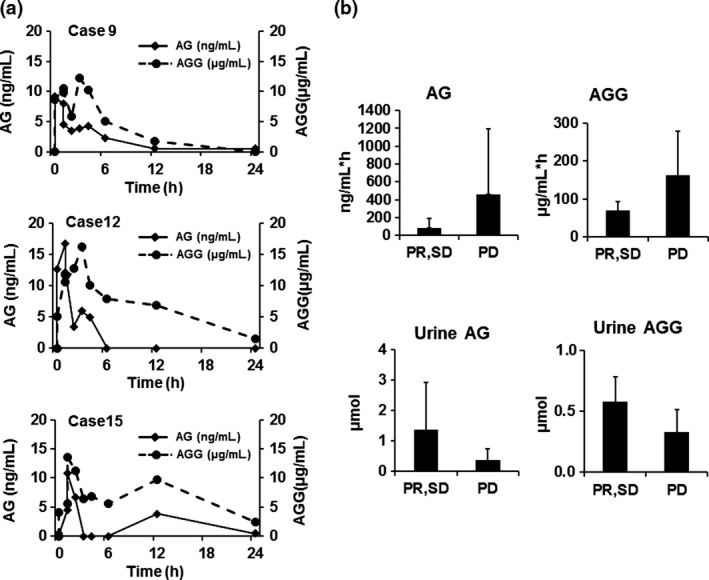
Pharmacokinetic profiles of arctigenin (AG) and arctigenin glucuronide (AGG) and their relation to clinical outcome. (a) Representative pharmacokinetic profile on day 1 of three patients treated with 12.0 g GBS‐01 (level 3) who showed stable disease (case 9) and progressive disease (cases 12 and 15). Solid line, plasma concentration of AG; dotted line, plasma concentration of AGG. (b) Correlation analyses of plasma AG area under the curve, AGG area under the curve, urinary output of AG in 24 h, and urinary output of AGG in 24 h. Patients were stratified into two groups, partial response/stable disease (PR/SD) and progressive disease (PD), according to their clinical responses. Bars show mean and error bars show standard error.
Table 3.
Pharmacokinetics of arctigenin (AG) and arctigenin glucuronide (AGG) in patients with advanced pancreatic cancer receiving GBS‐01. (A) Single dose pharmacokinetics parameter of AG; (B) Single dose pharmacokinetics parameter of AGG; (C) Multiple dose pharmacokinetics parameter of AG at level 3; (D) Multiple dose pharmacokinetics parameter of AGG at level 3; (E) Contents of AG and AGG in urine on day 1 of treatment
| Cpmax, ng/mL | T max, h | AUC0–t, ng·h/mL | t 1/2, h | |
|---|---|---|---|---|
| (A) | ||||
| Level 1 (n = 3) | 16.97 ± 3.44 | 1.00 ± 0.50 | 248.58 ± 206.30 | 7.18 |
| Level 2 (n = 3) | 24.83 ± 8.51 | 0.50 ± 0.00 | 142.36 ± 57.23 | 3.06 ± 2.94 |
| Level 3 (n = 9) | 66.56 ± 26.81 | 0.87 ± 0.62 | 487.97 ± 368.86 | 5.68 ± 6.34 |
| (B) | ||||
| Level 1 (n = 3) | 4.40 ± 0.23 (×103) | 1.04 ± 0.41 | 44.10 ± 4.79 (×103) | 5.13 ± 0.72 |
| Level 2 (n = 3) | 9.05 ± 2.06 (×103) | 0.67 ± 0.24 | 62.71 ± 21.24 (×103) | 15.93 ± 9.37 |
| Level 3 (n = 9) | 15.91 ± 6.94 (×103) | 1.60 ± 0.61 | 157.07 ± 74.44 (×103) | 6.98 ± 5.66 |
| (C) | ||||
| Day 1 (n = 9) | 66.56 ± 26.81 | 0.87 ± 0.62 | 487.97 ± 368.86 | 5.68 ± 6.34 |
| Day 8 (n = 9) | 82.92 ± 47.58 | 1.06 ± 0.88 | 694.92 ± 260.70 | 4.42 ± 3.91 |
| (D) | ||||
| Day 1 (n = 9) | 15.91 ± 6.94 (×103) | 1.60 ± 0.61 | 157.07 ± 74.44 (×103) | 6.98 ± 5.66 |
| Day 8 (n = 9) | 15.30 ± 7.20 (×103) | 2.08 ± 1.54 | 176.67 ± 100.09 (×103) | 7.29 ± 4.57 |
| AG, μmol† | AGG, μmol† | Total dose of AG + AGG of orally administered GBS‐01, μmol† | |
|---|---|---|---|
| (E) | |||
| Level 1 | 0.12 ± 0.05 | 148.8 ± 16.51 | 287 |
| Level 2 | 1.26 ± 1.05 | 444.1 ± 121.64 | 718 |
| Level 3 | 1.58 ± 2.47 | 428.59 ± 265.29 | 1148 |
†Expressed as a molar concentration for comparison. Molecular weight: AG, 372.41; AGG, 548. GBS‐01 dose level 1, 3.0 g q.d. (n = 3); dose level 2, 7.5 g q.d. (n = 3); dose level 3, 12.0 g q.d. (n = 9). All values shown as mean ± SD. AUC0 –t, area under the plasma concentration–time curve from 0 h to time; Cpmax, peak exposure; T max, time to maximum concentration.
Regarding the relation between the PK and clinical efficacy, we compared the mean AUCs of AG and AGG between patients showing PR and SD and those showing PD, but observed no statistically significant difference. The Cpmax as well as the AUC values of AG and AGG in a patient who showed PR were lower than those in any other patient groups at the same level of dosing. Interestingly, the urinary excretion of AG was higher in patients with a favorable clinical outcome than in the remaining patients (Fig. 3b).
Discussion
GBS‐01 is an orally available drug rich in AG that is extracted from the fruit of Arctium lappa, a traditional herbal medicine. It exerts antitumor activities through the novel mechanism of attenuating the tolerance of cancer cells to glucose deprivation (anti‐austerity).5, 10, 11 It has also been shown to have the ability to eliminate the tolerance of cancer cells to nutrient starvation in PC cell lines such as PANC‐1, CAPAN1, and MiaPaCa2. Furthermore, AG has been shown to strongly suppress tumor growth in mouse xenograft models. Pancreatic cancer is known to be a hypovascular tumor with extremely hypoxic conditions due to low blood perfusion. Pancreatic cancer cells have been reported to be relatively tolerant to nutrient starvation and to have lower oxygen saturation and nutrient levels than normal pancreatic cells.1, 2, 3, 4 Thus, GBS‐01 has potent antitumor activity even in patients with advanced PC that might show resistance to ordinary chemotherapy because of tissue hypoxia and low perfusion. Therefore, a phase I trial of GBS‐01 was undertaken in advanced PC patients who are refractory to GEM therapy.
In this study, the recommended dose of GBS‐01 was determined to be 12.0 g q.d. (approximate equivalent to 4.0 g burdock fruit extract), because no DLT was seen at any of the three dose levels. There were very few adverse events during treatment, therefore, GBS‐01 was found to have an extremely mild toxicity profile. Considering the mild toxicity profile, further dose escalation was discussed, however, we decided against it for the following reasons. First, in the practice of Chinese herbal medicine, the 12.0 g/day dose (dose level 3) is the maximum prescribed dose. Second, it is not easy to take this orally administered drug, since the formulation is very voluminous and has a bitter taste. Finally, a PR was already seen at dose level 2 in one patient.
GBS‐01 contains arctiin and AG. Arctiin is metabolized into AG by β‐glucosidase, and AG is glucuronidated into AGG in the liver and intestine by UDP‐glucuronosyltransferases such as 1A1, 1A9, and 2B7 (our unpublished data). The PK study revealed that AG is efficiently absorbed and rapidly conjugated with glucuronic acid to form AGG. The plasma concentration of AGG was approximately 100‐ to 1000‐fold higher than that of AG, even in the early phase, indicating glucuronidation in the first pass. The urinary excretion of AG plus AGG was more than half of the given dose in more than half of patients. Furthermore, the effective trough plasma concentration of AGG (≥0.1 μg/mL), which has been clarified in a preclinical study,2 was maintained for a prolonged period in all patients at dose level 3. Based on the PK parameter analysis, GBS‐01 at the dose of 12.0 g q.d., which was determined as the recommended dose in this study, was set as the dose anticipated to exert optimal tumor efficacy. In the present study, neither the plasma AG nor the plasma AGG level was correlated with the clinical outcome. Although further analysis is warranted in a larger number of patients, we propose the following mechanism to explain this finding. Arctigenin is rapidly conjugated to form AGG, and AGG is not active per se. Arctigenin glucuronide can be reactivated by β‐glucuronidase, which is often highly expressed in regions of inflammation. Rapid conjugation of AG may also be related to the safely profile of the drug. The antitumor effect is not a systemic, but local reaction, therefore, the critical factor for the antitumor effect of the drug might be local reactivation, which is not necessarily related to the plasma concentration. In addition, in the present study, the urinary excretion of AG was higher in patients with a favorable outcome, although the plasma concentrations of AG and AGG were lower (not statistically significantly) than those in the other patient groups. This result also suggests that the reactivation of AGG to AG may play a role in the clinical effectiveness of GBS‐01.
In the present trial, all patients were refractory to S‐1 as well as GEM. S‐1 is an orally administered fluoropyrimidine agent, and its beneficial effect on survival has been established in the first‐line treatment setting for advanced PC12 and in the adjuvant setting after resection of PC.13 In addition, recent phase III trials have shown significant beneficial effects of the fluoropyrimidine‐based regimen of 5‐fluorouracil/leucovorin, irinotecan, and oxaliplatin over GEM alone.14 In general, advanced PC patients who are refractory to treatment with GEM‐ and fluoropyrimidine‐based regimens have far‐advanced tumors, and tumor shrinkage can hardly be expected. However, a PR was confirmed in an advanced GEM plus S‐1‐ refractory PC patient following treatment with GBS‐01. In addition, the disease control rate (33.3%) and overall survival (median, 5.68 months) obtained following treatment with this drug were also promising. Therefore, GBS‐01 might have favorable efficacy, even in advanced PC patients who are refractory to GEM‐ and fluoropyrimidine‐based regimens.
The mechanism of action of GBS‐01 remains to be clearly determined. In our previous work, AG was found to inhibit the phosphatidylinositol 3‐kinase–protein kinase B pathway activation induced by glucose starvation and this inhibition was suspected to be the cause of glucose deprivation‐specific cancer cell death (anti‐austerity). Recent work has also revealed that AG inhibits mitochondrial complex I activity, and we have found that reactive oxygen species is generated through inhibition of mitochondrial complex I by AG, suggesting the anti‐austerity activity of the drug (our unpublished data). With the inhibition of mitochondrial complex I, it was assumed that the tumor cells shifted from aerobic respiration to anaerobic respiration and that this shift induced an increase in the serum lactate level.15 To elucidate the pharmacodynamic effects of AG on the metabolism in patients treated with GBS‐01, we examined serum lactate levels in patients enrolled in this phase I study.16 We found elevated serum lactate levels in seven patients (47%) at 2 h after treatment with GBS‐01, although the elevation was transient and continued administration of GBS‐01 did not have a cumulative effect on lactate accumulation. None of the patients showed any signs or symptoms of lactic acidosis. Thus, GBS‐01 may have a rather similar mode of action to metformin, which is an oral antidiabetic drug in the biguanide class that inhibits mitochondrial complex I, rarely induces lactic acidosis, and seems to exert preferential antitumor efficacy against cancer stem cells. Actually, we recently found that AG has an activity to PC stem cell fraction (Chiba et al. to be published). In the present study, we focused on the safety, bioavailability, and PK profile, but getting proof of concept is also needed in future studies. Monitoring change in metabolism in cancer tissue is not easy at present in clinical examinations, but metabolome analyses and surrogate markers are being developed and their incorporation into clinical study is planned in the future.
In conclusion, the recommended dose of GBS‐01 was 12.0 g q.d. (containing approximately 4.0 g burdock fruit extract), and the clinical safety and possible benefit of GBS‐01 monotherapy were clarified in patients with advanced PC refractory to GEM therapy. A multicenter phase II trial (UMIN000010111) is, therefore, underway to evaluate the efficacy and safety of this agent in PC patients who are refractory to GEM and fluoropyrimidine therapy. Gemcitabine exerts antitumor effects against cancer cells under oxygen‐ and glucose‐rich conditions, whereas GBS‐01 has the ability to eliminate the tolerance of cancer cells to nutrient starvation. Therefore, a combination of GEM with GBS‐01 may also be a promising regimen for patients with PC. Further clinical trials are needed to elucidate the usefulness of this agent used alone or in combination with other agents.
Disclosure Statement
Ryuji Takahashi and Satoshi Yomoda are employees of Kracie Pharmaceutical company; Nobuo Mochizuki and Satoshi Kishino received a grant from Kracie Pharmaceutical company. The other authors have no conflict of interest. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations
- AG
arctigenin
- AGG
arctigenin glucuronide
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the plasma concentration–time curve
- CI
confidence interval
- Cpmax
peak exposure
- DLT
dose‐limiting toxicity
- GEM
gemcitabine
- PC
pancreatic cancer
- PD
progressive disease
- PK
pharmacokinetics
- PR
partial response
- RECIST
Response Evaluation Criteria In Solid Tumors
- SD
stable disease
- γ‐GTP
γ‐glutamyl transpeptidase
Supporting information
Fig. S1. Pharmacokinetic profiles of a patient showing partial response (Case. 5). Solid line, plasma concentration of arctigenin (AG); dotted line, plasma concentration of arctigenin glucuronide (AGG).
Acknowledgments
This research was presented as an abstract at the 49th Annual Meeting of the American Society of Clinical Oncology, 31 May–4 June 2013, Chicago, IL, USA. This study was supported by grants from the Ministry of Health, Labor and Welfare, Japan, and the Project for Development of Innovative Research on Cancer Therapeutics from the Ministry of Education, Culture, Sports, Science and Technology, Japan. GBS‐01 was supplied gratis by Kracie Pharma, Ltd.
Cancer Sci 107 (2016) 1818–1824
Funding Information
Ministry of Health, Labor and Welfare, Japan; Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Warburg O. On respiratory impairment in cancer cells. Science 1956; 124: 269–70. [PubMed] [Google Scholar]
- 2. Vaupel P, Müller‐Klieser W, Otte J, Manz R, Kallinowski F. Blood flow, tissue oxygenation, and pH‐distribution in malignant tumors upon localized hyperthermia. Basic pathophysiological aspects and the role of various thermal doses. Strahlentherapie 1983; 159: 73–81. [PubMed] [Google Scholar]
- 3. Semenza GL. HIF‐1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr 2007; 39: 231–4. [DOI] [PubMed] [Google Scholar]
- 4. Hirayama A, Kami K, Sugimoto M et al Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time‐of‐flight mass spectrometry. Cancer Res 2009; 69: 4918–25. [DOI] [PubMed] [Google Scholar]
- 5. Awale S, Lu J, Kalauni SK et al Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 2006; 66: 1751–7. [DOI] [PubMed] [Google Scholar]
- 6. Burris HA III, Moore MJ, Andersen J et al Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 7. Saif MW, Chabot J. Chemotherapy: metastatic pancreatic cancer–is FOLFIRINOX the new standard? Nat Rev Clin Oncol 2011; 8: 452–3. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 9. Liu S, Chen K, Schliemann W, Strack D. Isolation and identification of arctiin and arctigenin in leaves of burdock (Arctium lappa L.) by polyamide column chromatography in combination with HPLC‐ESI/MS. Phytochem Anal 2005; 16: 86–9. [DOI] [PubMed] [Google Scholar]
- 10. Esumi H, Izuishi K, Kato K et al Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′‐AMP‐activated protein kinase‐dependent manner. J Biol Chem 2002; 277: 32791–8. [DOI] [PubMed] [Google Scholar]
- 11. Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res 2000; 60: 6201–7. [PubMed] [Google Scholar]
- 12. Ueno H, Ioka T, Ikeda M et al Randomized phase III study of gemcitabine plus S‐1, S‐1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31: 1640–8. [DOI] [PubMed] [Google Scholar]
- 13. Uesaka K, Boku N, Fukutomi A, et al Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet 2016; 388: 248‐57. [DOI] [PubMed] [Google Scholar]
- 14. Conroy T, Desseigne F, Ychou M et al FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 15. Owada S, Fujioka R, Kawashima T et al The 36th annual meeting of the molecular biology society of Japan 2013. (Abstract)
- 16. Fujioka R, Owada S, Kawashima T et al Mechanisms of Action of an Antiausterity Agent Arctigenin and Clinical Pharmacodymanic Analysis. The 36th annual meeting of the molecular biology society of Japan 2013. (Abstract)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Pharmacokinetic profiles of a patient showing partial response (Case. 5). Solid line, plasma concentration of arctigenin (AG); dotted line, plasma concentration of arctigenin glucuronide (AGG).


