Abstract
In this multicenter, single‐arm, phase II study, the efficacy and safety of ibrutinib were examined in Japanese patients with relapsed or refractory mantle cell lymphoma (MCL). Patients (age ≥20 years) with relapsed or refractory MCL who had progressed after receiving at least one prior treatment regimen, were enrolled. Patients were treated with oral ibrutinib (560 mg once daily; 28‐day cycle) until disease progression (or relapse), unacceptable toxicity, or study end. The primary end‐point was overall response rate. Secondary end‐points included duration of response (DOR), time to response, progression‐free survival (PFS), overall survival, and safety. Of the 16 patients who received treatment, 5 patients discontinued the study (progressive disease, 4; sepsis, 1). Median duration of ibrutinib exposure was 6.5 months (range, 2.8–8.3 months). The overall response rate was 87.5% (90% confidence interval, 65.6–97.7; complete response = 2 [12.5%]; partial response = 12 [75.0%]). Median time to response for all responders (n = 14) was 1.8 months (range, 0.7–5.3 months). The median DOR and PFS were not estimable due to censoring (range: DOR, 1.1–6.4+ months; PFS, 2.8–8.0+ months). Overall survival data were immature due to the limited observation period. A total of 8/16 patients (50%) had at least one grade 3 adverse event (AE), and 5 (31.3%) patients reported serious AEs. The most commonly reported AEs were diarrhea and stomatitis (37.5% each), platelet count decrease (31.3%), and anemia (25%). Overall, orally administered single agent ibrutinib was efficacious with an acceptable safety profile in Japanese patients with relapsed or refractory MCL. Clinical trial registration NCT02169180 (ClinicalTrials.gov).
Keywords: Efficacy, ibrutinib, mantle cell lymphoma, overall response rate, safety
Abbreviations
- AE
adverse event
- CI
confidence interval
- CR
complete response
- DOR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- IRC
Independent Review Committee
- MCL
mantle cell lymphoma
- ORR
overall response rate
- OS
overall survival
- PFS
progression‐free survival
- PR
partial response
- PS
performance status
- TTR
time to response
Mantle cell lymphoma is an incurable subtype of B‐cell non‐Hodgkin's lymphoma that often responds poorly to chemotherapy and has a high relapse rate. The hallmark of MCL is the translocation t(11;14) (q13;q32), which causes overexpression of the cell cycle regulator cyclin D1.1 Mantle cell lymphoma is a rare condition and accounts for approximately 2–3% of all malignant lymphoma cases in Japan.2 Intensive chemotherapy followed by autologous stem cell transplant is the treatment of choice for younger and fit patients.3 However, the majority of MCL patients are older (median age of 65 years at diagnosis) and ineligible for intensive treatment approaches.4 Moreover, relapse is common with a high mortality rate among these patients.5 Also, at relapse, the quality and durability of response to salvage treatment is inferior to that achieved with front‐line therapy.6 Several agents like bortezomib, bendamustine, and fludarabine have been approved for the treatment of relapsed or refractory MCL in Japan, which have shown efficacy,7, 8, 9 but the high incidence of toxicities may be a concern.6, 10, 11
Ibrutinib has been approved by the US FDA and European Medical Agency for the treatment of patients with MCL who have received at least one prior therapy.12, 13 Results from pivotal phase II5, 14 and phase III15 studies undertaken in Europe, South and North America, Korea and Taiwan with relapsed MCL, indicated favorable efficacy and safety of ibrutinib.14 A phase I study carried out in Japanese patients with relapsed or refractory B‐cell malignancies, including two MCL patients, showed acceptable efficacy and safety profiles of ibrutinib.16
Therefore, this phase II study was undertaken to evaluate the efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory MCL. We present the data obtained up to the cut‐off date, April 30, 2015.
Materials and Methods
Patients
Japanese patients aged 20 years or older with histologically confirmed, previously treated (1–5 prior lines of therapy) MCL, at least one measurable site of disease (Revised Response Criteria for Malignant Lymphoma),17 and Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1 were enrolled (detailed information on selection criteria is provided in Doc. S1).
The study protocol was approved by the local Independent Ethics Committees of participating institutions, and the study was carried out in accordance with the ethical principles originating in the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the protocol. All participants provided written informed consent to participate in the study.
Study design
This was a multicenter, single‐arm, phase II study carried out at 11 sites in Japan. The study consisted of a screening phase, a treatment phase, and a post‐treatment follow‐up phase (Fig. 1). The 30‐day screening phase was followed by the treatment phase consisting of a 28‐day cycle of self‐administration of the study drug. During this phase, patients received 560 mg oral ibrutinib once daily until disease progression (or relapse if the patient achieved CR), occurrence of unacceptable toxicity, or study end, whichever occurred first. Doses were held or reduced on the basis of the severity and the outcome of toxicity. Dose re‐escalation after reduction for toxicity was not allowed. Patients who discontinued the study treatment entered the post‐treatment follow‐up phase.
Figure 1.
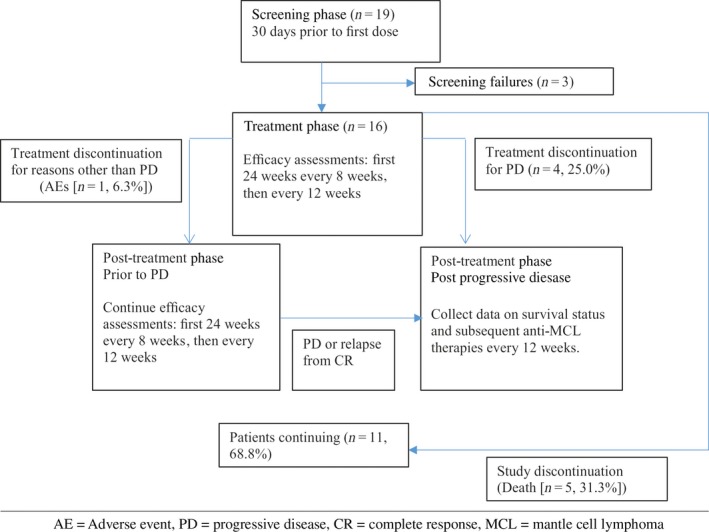
Study design and patient disposition in this phase II study evaluating efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma (MCL).
Mantle cell lymphoma diagnosis of each patient was confirmed by the central pathology review. Disease assessments were scheduled throughout the treatment phase to assess efficacy every 8 weeks up to 24 weeks after start of study treatment, thereafter every 12 weeks. The clinical cut‐off for the primary analysis was defined as the time point at which all patients had completed the efficacy assessment on day 1 of cycle 7 or had been discontinued from treatment prior to completion of the assessment on day 1 of cycle 7. The data collected up to this cut‐off was included in this report. Patients will continue to be followed until study end, defined as 2 years after the last patient was enrolled, the time at which marketing approval is expected to be received from the Ministry of Health, Labor and Welfare in Japan, or the time at which the sponsor terminates the study.
An IRC performing efficacy assessments based on the Revised Response Criteria for Malignant Lymphoma13 and a safety monitoring committee, independent of the sponsor and study sites, were established for the primary end‐point and safety assessments. The primary end‐point of the study was IRC‐assessed ORR. Secondary end‐points were DOR, TTR, PFS, OS, and the safety of ibrutinib.
Concomitant medication
Standard supportive care therapies (e.g., antiemetics and antidiarrheal) needed for the management of symptoms were permitted during the study, as clinically indicated. Systemic chemotherapy, anticancer immunotherapy, systemic use of corticosteroids, experimental therapy, and radiotherapy were prohibited during the study.
Efficacy assessments
The ORR was defined as the proportion of evaluable patients who achieved CR or PR. Other efficacy end‐points included DOR (date of initial documentation of a response [CR or PR] to the date of first documented evidence of progressive disease [or relapse for patients who experienced CR during the study] or death), TTR, PFS (measured from the date of first dose to the date of the first observation of either disease progression, relapse from CR, or death), and OS (time between first dose and death of all cause).
Safety assessments
Safety assessments included physical examinations, vital signs, laboratory safety, ECOG criteria for PS, and monitoring AEs. Adverse events were graded using Common Terminology Criteria for Adverse Events version 4.03.
Statistical analysis
Comparing a clinical threshold value of 20% to the lower bound of the exact 90% CI of the estimate, efficacy was claimed when the lower bound was above the threshold. Assuming a 10% drop‐out rate, 16 patients were to be enrolled to accumulate 14 response‐evaluable patients.5
The efficacy analysis was based on the response‐evaluable population, defined as all enrolled patients who had a confirmed diagnosis of MCL by the central pathology review, received at least one dose of ibrutinib, and had at least one adequate post‐baseline disease assessment. Kaplan–Meier methodology was used to estimate DOR and PFS. Analyses of DOR, TTR, PFS, and OS were carried out at the same cut‐off as the ORR. All treated patients were included in the safety evaluations.
Relative dose intensity (%) was defined as 100 times the total cumulative dose divided by the duration of exposure (days) and 560. The duration of exposure (months) was defined as the last dosing date minus the first dosing date plus 1, divided by 365.25 and multiplied by 12.
Results
Patient group
Of the 19 patients screened, 16 received at least one dose of ibrutinib (Fig. 1). Most of the patients were men (75.0%) with a median age of 72 years (range, 55–83 years). A total of 12 patients (75.0%) had an ECOG PS of 0. The median number of prior treatments was 2.5 (range, 1–4) (Table 1). Four patients (25.0%) had refractory MCL (primary refractory, 1; relapsed, 12; relapsed and refractory, 3). At the time of clinical data cut‐off, 11 patients (68.8%) were still receiving protocol treatment and 5 patients had discontinued the study (progressive disease, 4; sepsis, 1).
Table 1.
Demographics and baseline characteristics of Japanese patients with relapsed or refractory mantle cell lymphoma treated with ibrutinib (all treated population, n = 16)
| Items | Statistics, N = 16 |
|---|---|
| Age, years, n (%) | |
| <65 | 4 (25.0) |
| ≥65 | 12 (75.0) |
| <70 | 7 (43.8) |
| ≥70 | 9 (56.3) |
| Median (range) | 72.0 (55–83) |
| Sex, n (%) | |
| Male | 12 (75.0) |
| Weight, kg | |
| Mean (SD) | 58.34 (11.59) |
| Median (range) | 55.3 (38.4–85.0) |
| Duration of disease, months | |
| Mean (SD) | 59.84 (35.563) |
| Median (range) | 50.64 (9.7–118.0) |
| Number of prior regimens, n (%) | |
| 1 | 6 (37.5) |
| 2 | 2 (12.5) |
| ≥3 | 8 (50.0) |
| Mean (SD) | 2.3 (1.2) |
| Median (range) | 2.5 (1–4) |
| ECOG PS, n (%) | |
| 0 | 12 (75.0) |
| 1 | 4 (25.0) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Extent of exposure
The median duration of ibrutinib exposure until cut‐off was 6.5 months (range, 2.8–8.3 months). The median relative dose intensity was 99.6% (range, 47.9–100.0%).
Efficacy results
The lower limit of exact 90% CI of the observed ORR exceeded the predefined threshold value (20%), indicating the efficacy of ibrutinib. The ORR, as assessed by the IRC, was 87.5% (90% CI, 65.6–97.7; CR = 2 [12.5%]; PR = 12 [75%]). As assessed by the investigator, a total of 15/16 patients achieved objective responses which contributed to 93.8% ORR (90% CI, 73.6–99.7; CR = 1 [6.3%]; PR = 14 [87.5%]) (Table 2).
Table 2.
Efficacy assessment of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma, carried out by the Independent Review Committee (response‐evaluable population, n = 16)
| Parameter | Statistics, n = 16 |
|---|---|
| Primary end‐point | |
| Best overall response, n (%) | |
| CR | 2 (12.5) |
| PR | 12 (75.0) |
| SD | 2 (12.5) |
| PD | 0 (0.0) |
| ORR (CR or PR) | 14 (87.5) |
| Exact 90% CI | (65.6–97.7) |
| Secondary end‐points | |
| Duration of response, months, n (%) | |
| Responder (CR or PR) | 14 |
| Progressed or died, event | 2 (14.3) |
| Censored | 12 (85.7) |
| Median (95% CI) | NE (2.79–NE) |
| Range | (1.1–6.4+) |
| 3‐months duration rate (95% CI) | 0.83 (0.47–0.96) |
| 6‐months duration rate (95% CI) | 0.83 (0.47–0.96) |
| Time to initial response,† months | |
| Responder (CR or PR) | 14 |
| Median | 1.84 |
| Range | 0.7–5.3 |
| Time to CR,‡ months | |
| Responder (CR) | 2 |
| Median | 2.84 |
| Range | 1.8–3.8 |
| Progression‐free survival, months, n (%) | |
| Progressed or died, event | 2 (12.5) |
| Censored | 14 (87.5) |
| Median (95% CI) | NE (NE–NE) |
| Range | (2.8–8.0+) |
| 6‐months PFS rate (95% CI) | 0.88 (0.59–0.97) |
†Derived for subjects who achieved complete response (CR) or partial response (PR). ‡Derived for subjects who achieved CR. +, Censored observation; CI, confidence interval; PD, progressive disease; ORR, overall response rate; NE, not estimable; PFS, progression‐free survival.
The median TTR by IRC assessment for all responders (n = 14) was 1.8 months (range, 0.7–5.3 months). The median DOR and PFS by IRC were not estimable due to censoring (range: DOR, 1.1–6.4+ months; PFS, 2.8–8.0+ months). Similarly, the follow‐up period was not sufficient to estimate the median OS. All patients showed reduction in the sum of the product of the diameters of measurable lesions from baseline (Fig. 2).
Figure 2.

Waterfall chart for maximum reduction in the sum of the products of diameters of measurable lesions (SPD) in Japanese patients with relapsed or refractory mantle cell lymphoma treated with ibrutinib, as determined by the study's Independent Review Committee (response‐evaluable population, n = 16).
Safety results
All patients experienced at least one AE. The most common AEs were diarrhea and stomatitis (n = 6, 37.5% each), decrease in platelet count (n = 5, 31.3%), and anemia (n = 4, 25.0%). Eight patients (50.0%) had at least one AE of toxicity grade 3 or higher. Non‐hematological grade 3 or higher AEs included disease progression (n = 3, 18.8%), sepsis, hypokalemia, hyperuricemia, retinal vascular disorder, arrhythmia, renal impairment, and hypertension (n = 1, 6.3% each). The investigator considered the ongoing hypertension as more likely a causative factor for retinal vascular disorder. Anemia, leukocytosis, neutropenia, and increase in lymphocyte count (n = 1, 6.3% each) were of grade 3 or higher hematological AEs (Table 3). Decrease in lymphocyte count was observed in one patient (6.3%), which was estimated as non‐severe (<grade 3). Hemorrhagic events were reported in 5 (31.3%) patients: contusion (n = 2, 12.5%), anal hemorrhage (n = 1, 6.3%), epistaxis (n = 1, 6.3%), hematuria (n = 1, 6.3%), subcutaneous hemorrhage (n = 1, 6.3%), mouth hemorrhage (n = 1, 6.3%), and petechiae (n = 1, 6.3%). All hemorrhagic events were of grade 1 or 2 and considered non‐serious. Five patients (31.3%) had serious AEs which included disease progression (n = 3, 18.8%), leukocytosis, sepsis, and hyperuricemia (n = 1, 6.3% each). One patient (6.3%) discontinued treatment and two (12.5%) had dose reduction due to at least one AE. No patient discontinued treatment due to hemorrhagic treatment‐emergent AEs. Five patients (31.3%) with AEs died due to disease progression before data cut‐off. There were no notable differences in the incidence of AEs between any of the comparison subgroups (age, sex, the number of prior lines of treatment, and liver function abnormality at baseline).
Table 3.
Treatment‐emergent adverse events (TEAE) occurring in 10% or more Japanese patients with relapsed or refractory mantle cell lymphoma treated with ibrutinib, toxicity grade 3 or higher (all treated population, n = 16)
| All grades, n (%) | Grade 3 or higher, n (%) | |
|---|---|---|
| Total number of subjects with TEAE | 16 (100.0) | 8 (50.0) |
| Gastrointestinal disorders | 10 (62.5) | 1 (6.3) |
| Diarrhea | 6 (37.5) | 0 (0.0) |
| Stomatitis | 6 (37.5) | 1 (6.3) |
| Constipation | 3 (18.8) | 0 (0.0) |
| Dyspepsia | 2 (12.5) | 0 (0.0) |
| Nausea | 2 (12.5) | 0 (0.0) |
| Infections and infestations | 10 (62.5) | 1 (6.3) |
| Upper respiratory tract infection | 3 (18.8) | 0 (0.0) |
| Nasopharyngitis | 2 (12.5) | 0 (0.0) |
| Skin infection | 2 (12.5) | 0 (0.0) |
| Skin and s.c. tissue disorders | 10 (62.5) | 0 (0.0) |
| Dry skin | 3 (18.8) | 0 (0.0) |
| General disorders and administration site conditions | 9 (56.3) | 3 (18.8) |
| Disease progression | 3 (18.8) | 3 (18.8) |
| Fatigue | 3 (18.8) | 0 (0.0) |
| Malaise | 2 (12.5) | 0 (0.0) |
| Investigations | 8 (50.0) | 1 (6.3) |
| Platelet count decreased | 5 (31.3) | 0 (0.0) |
| Alanine aminotransferase increased | 2 (12.5) | 0 (0.0) |
| Aspartate aminotransferase increased | 2 (12.5) | 0 (0.0) |
| Blood bilirubin increased | 2 (12.5) | 0 (0.0) |
| Blood and lymphatic system disorders | 7 (43.8) | 2 (12.5) |
| Anemia | 4 (25.0) | 1 (6.3) |
| Thrombocytopenia | 3 (18.8) | 0 (0.0) |
| Leukocytosis | 2 (12.5) | 1 (6.3) |
| Lymphocytosis | 2 (12.5) | 0 (0.0) |
| Metabolism and nutrition disorders | 5 (31.3) | 2 (12.5) |
| Decreased appetite | 3 (18.8) | 0 (0.0) |
| Hypokalemia | 2 (12.5) | 1 (6.3) |
| Injury, poisoning, and procedural complications | 4 (25.0) | 0 (0.0) |
| Contusion | 2 (12.5) | 0 (0.0) |
| Laceration | 2 (12.5) | 0 (0.0) |
| Nervous system disorders | 4 (25.0) | 0 (0.0) |
| Headache | 2 (12.5) | 0 (0.0) |
| Peripheral neuropathy | 2 (12.5) | 0 (0.0) |
Adverse events coded using MedDRA version 18.0.
Discussion
In this multicenter phase II study, Japanese patients with relapsed or refractory MCL were treated with ibrutinib at 560 mg/day. Prior to study entry, all patients had received rituximab and half of the patients had received bendamustine, including 3 patients with radiotherapy. The dose selected for this study was based on data from a previous pivotal phase II study in Europe, South and North America, Korea and Taiwan and a phase I study in a Japanese patient group.5, 14, 16 Mantle cell lymphoma is predominantly a disease of elderly patients,3, 6 and the age distribution of the study participants (≥65 years, 75%; median, 72 years) represented the real world population. Single agent ibrutinib therapy showed a favorable benefit–risk profile, consistent with other studies reported in non‐Japanese populations.5, 14, 16
The ORR reported in this study (87.5%) was in agreement with the findings of non‐Japanese phase II (n = 111, ORR = 67.0%) and phase III (n = 280, ORR = 71.9%) studies of ibrutinib.14, 15 The response rate achieved was irrespective of baseline characteristics like age, sex, simplified Mantle Cell Lymphoma International Prognostic Index score, number of prior therapies, or baseline refractory disease. These results support the findings of the phase I Japanese study of ibrutinib in patients with B‐cell malignancies, with two MCL patients who responded to ibrutinib treatment (n = 15, combined ORR = 73.3%).16
Bortezomib and lenalidomide, two newer agents approved by the US FDA for the treatment of relapsed or refractory MCL, have reported ORRs of 33.0% (n = 155) (bortezomib) and 28.0% (n = 134) (lenalidomide) in pivotal studies carried out among non‐Japanese patient groups.7, 8 Although not investigated in the same study, the ORR obtained with ibrutinib in this study (i.e., 87.5%) may offer intriguing prospects. However, the shorter follow‐up and the wide range of CI warrant a cautious interpretation of results.
The median time to initial response (1.8 months) reported in this study was consistent with the previous pivotal phase II study (1.9 months).5, 14 The median time to initial response for bortezomib was reported to be 1.3 months and for lenalidomide, 2.2 months.7, 8
The safety profile of ibrutinib was acceptable and consistent with the known safety profile of ibrutinib in B‐cell malignancies with no unexpected AEs being reported in this study. Diarrhea was the most common AE (37.5%), which was consistent with the findings of earlier studies.14, 16 All‐grade bleeding events were reported in 31.3% patients (all ≤ grade 3) compared to an incidence rate of 50.0% reported previously.14 No subdural hematoma events were reported. The most common all‐grade hematological events that occurred in this study were neutropenia (6.3%), anemia (25.0%), and thrombocytopenia (18.8%). The incidence rate of neutropenia was lower than that reported earlier (19.0%) in patients with B‐cell malignancies.16 The incidence rates of anemia (18%) and thrombocytopenia (22%) were comparable with the pivotal phase II study.14 Incidence of any‐grade stomatitis in this study was higher (37.5%) than that reported in the phase I Japanese study of ibrutinib (overall 20.0%),10 however, the majority of stomatitis events were ≤ grade 3. Nevertheless, the smaller sample size is a limitation of the study that may contribute to higher reported rates of AEs.
Bendamustine, a newer cytotoxic i.v. agent, has reported efficacy as a single‐agent therapy for treatment of B‐cell non‐Hodgkin's lymphomas including relapsed or refractory MCL among Japanese patients.10 However, a high number of patients experienced grade 3 or higher hematological AEs compared to the present study (neutropenia [72.0% vs 6.3%] and thrombocytopenia [16.0% vs 0.0%]).10 One patient (6.3%) in this study experienced lymphocytopenia that was not severe.
In this study, orally administered single agent ibrutinib was found to be efficacious with an acceptable safety profile in Japanese patients with relapsed or refractory MCL. The high overall response achieved in this study along with the acceptable toxicity profile is in agreement with phase II and III studies of ibrutinib completed to date. Ibrutinib may provide a less intensive and potentially more effective treatment option for patients who are ineligible for intensive treatment approaches.
Disclosure Statement
T.N. and S.T. are employees of Janssen Pharmaceutical. H.N. received honoraria from Chugai; K.T. from Zenyaku, Eisai, Spectrum, Takeda, and Mundipharma; D.M. from Takeda and Janssen; K.H. from Kyowa Hakko Kirin, ONO, Takeda, and Celgene; and T.U. from Janssen. H.N. received research funding from CMIC, Celgene, Janssen, Mundipharma, Takeda, Servier, and Otsuka; K.T. and D.M. from Chugai, Kyowa Hakko Kirin, ONO, Celgene, Janssen, GlaxoSmithKline, Eisai, Mundipharma, Takeda, Servier, and Abbvie; K.H. from Kyowa Hakko Kirin and Takeda; T. Kinoshita from Chugai, Takeda, ONO, and Gilead Sciences. The study was funded by Janssen Pharmaceutical. The study was designed under the responsibility of Janssen Pharmaceutical, in conjunction with the steering committee. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors met the International Council of Medical Journal Editors’ criteria for authorship, and anyone who met those criteria is listed as an author. All authors have read and approved the final manuscript for submission. The other authors have no conflict of interest.
Supporting information
Doc. S1. Details of patient selection criteria in phase II study of the efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma.
Acknowledgments
The authors thank Norio Komatsu (Juntendo University School of Medicine, Tokyo, Japan) for participating in this study as a member of the Safety Monitoring Committee, Rishabh Pandey (SIRO Clinpharm Pvt. Ltd.) for writing assistance, and Dr. Namit Ghildyal (Janssen Research and Development, LLC) for additional review and editorial assistance. The authors thank all the patients, their families, investigators, review committee members, medical experts, nurses, and clinical research coordinators who participated in this clinical trial.
Cancer Sci 107 (2016) 1785–1790
Funding Information
Janssen Pharmaceutical K.K.
References
- 1. Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood 2009; 114: 1469–76. [DOI] [PubMed] [Google Scholar]
- 2. Lymphoma Study Group of Japanese . The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 2000; 50: 696–702. [DOI] [PubMed] [Google Scholar]
- 3. Stephens DM, Spurgeon SE. Ibrutinib in mantle cell lymphoma patients: glass half full? Evidence and opinion. Ther Adv Hematol 2015; 6: 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dreyling M, Geisler C, Hermine O et al Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol Off J Eur Soc Med Oncol ESMO 2014; 25(Suppl. 3): iii83–92. [DOI] [PubMed] [Google Scholar]
- 5. Wang ML, Rule S, Martin P et al Targeting BTK with ibrutinib in relapsed or refractory mantle‐cell lymphoma. N Engl J Med 2013; 369: 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood 2015; 125(1): 48–55. [DOI] [PubMed] [Google Scholar]
- 7. Fisher RI, Bernstein SH, Kahl BS et al Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 2006; 24: 4867–74. [DOI] [PubMed] [Google Scholar]
- 8. Goy A, Sinha R, Williams ME et al Single‐agent lenalidomide in patients with mantle‐cell lymphoma who relapsed or progressed after or were refractory to bortezomib: Phase II MCL‐001 (EMERGE) Study. J Clin Oncol 2013; 31(29): 3688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FY2006 List of Approved Products: New Drugs [Internet]. [cited 2016 Jul 16]. Available from: https://www.pmda.go.jp/files/000153730.pdf
- 10. Ohmachi K, Ando K, Ogura M et al Multicenter phase II study of bendamustine for relapsed or refractory indolent B‐cell non‐Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci 2010; 101: 2059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dreyling M, Thieblemont C, Gallamini A et al ESMO Consensus conferences: guidelines on malignant lymphoma. Part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T‐cell lymphoma. Ann Oncol Off J Eur Soc Med Oncol ESMO 2013; 24: 857–77. [DOI] [PubMed] [Google Scholar]
- 12. FDA Approval for Ibrutinib [Internet] . National Cancer Institute. [cited 2016 Feb 16]. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-ibrutinib
- 13. European Medicines Agency ‐ Imbruvica [Internet] . [cited 2016 Jul 16]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003791/human_med_001801.jsp&mid=WC0b01ac058001d124
- 14. Wang ML, Blum KA, Martin P et al Long‐term follow‐up of MCL patients treated with single‐agent ibrutinib: updated safety and efficacy results. Blood 2015; 126: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dreyling M, Jurczak W, Jerkeman MS et al Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle‐cell lymphoma: an international, randomised, open‐label, phase 3 study. Lancet 2016; 387: 770–8. [DOI] [PubMed] [Google Scholar]
- 16. Tobinai K, Ogura M, Ishizawa K et al Safety and tolerability of ibrutinib monotherapy in Japanese patients with relapsed/refractory B cell malignancies. Int J Hematol 2016; 103(1): 86–94. [DOI] [PubMed] [Google Scholar]
- 17. Cheson BD, Pfistner B, Juweid ME et al Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc. S1. Details of patient selection criteria in phase II study of the efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma.


