Abstract
Potentially life‐threatening, serious hemoptysis is an adverse event associated with bevacizumab in non‐squamous non‐small‐cell lung cancer (NSCLC) trials. Suggested risk factors include central tumor location and cavitation; however, the profile of hemoptysis occurrence in clinical practice is still unclear. A nested case‐control study was conducted to assess the onset profile and risk factors for hemoptysis in bevacizumab‐treated patients in a real‐world setting in Japan. After bevacizumab was approved for NSCLC, physicians registered all NSCLC patients scheduled for bevacizumab therapy, from November 2009 to August 2011. Patients developing grade 2 hemoptysis requiring an injectable hemostatic agent or grade ≥3 hemoptysis were selected as case subjects, matched with four control subjects each. Case report forms were collected after an observation period of 24 weeks. Radiologists assessed blinded thoracic images. Risk factors for hemoptysis were assessed by univariate and stepwise multivariate analysis. Of 6774 patients registered, 23 (0.3%) experienced grade ≥2 drug‐related hemoptysis. A total of 104 patients (21 cases, 83 controls) were analyzed by central reviewers for risk factors of hemoptysis occurrence. In the univariate analysis seven factors were associated with hemoptysis. In the step‐wise multivariate analysis, prior thoracic radiotherapy (P = 0.1844), presence of tumor exposure in the central airway (P = 0.0256) and concomitant radiotherapy (P = 0.1169) were identified as risk factors for hemoptysis. While the incidence of hemoptysis was low in the real‐world setting in Japan, the three risk factors identified, prior thoracic radiotherapy, presence of tumor exposure in the central airway and concomitant radiotherapy, should be considered when selecting patients for bevacizumab treatment. Although technically classed as a clinical trial, a nested case‐control study was a non‐interventional surveillance study analyzing all NSCLC patients receiving bevacizumab in Japan, therefore it was not registered as a phase II/III clinical trial would be.
Keywords: Bevacizumab, hemoptysis, Japanese, non‐small‐cell lung cancer, real‐world
First‐line treatment of non‐small‐cell lung cancer (NSCLC) in patients without driver mutations, is based on the use of platinum‐doublet chemotherapy, which had reached a treatment plateau of approximately 1 year for overall survival (OS).1, 2 Bevacizumab is currently the molecularly targeted therapy being used in the clinical setting in this group of patients. The addition of antiangiogenic agents, such as bevacizumab to existing platinum‐doublet chemotherapy regimens, for those patients has also provided extended survival.3
Tumors depend on angiogenesis to grow; bevacizumab, a monoclonal antibody, acts against vascular endothelial growth factor (VEGF), a key signaling molecule in developmental angiogenesis. Bevacizumab specifically inhibits VEGF ligand–receptor binding and thereby prevents new vessel formation, regresses existing vessels and normalizes tumor vessel permeability. Bevacizumab has proven efficacy in extending OS and progression‐free survival (PFS) when added to platinum‐doublet chemotherapy as first‐line treatment for advanced non‐squamous NSCLC. The first‐line, phase III E4599 trial showed median OS of 12.3 months with bevacizumab plus carboplatin–paclitaxel, compared with 10.3 months with paclitaxel and carboplatin alone (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.67–0.92; P = 0.003).3 In the phase II Japanese JO19907 study of first‐line carboplatin–paclitaxel with or without bevacizumab, median PFS was 6.9 months in the bevacizumab arm and 5.9 months in the control arm (HR 0.61, 95% CI 0.42–0.89).4
Bleeding events such as pulmonary hemorrhage and hemoptysis (the spitting of blood derived from the lungs or bronchial tubes as a result of pulmonary hemorrhage), are among the most common adverse events (AEs) associated with bevacizumab therapy in clinical trials of non‐squamous NSCLC, with some of these events leading to fatal outcomes. In the phase II AVF0757g study, 3.8% of bevacizumab‐treated patients experienced life‐threatening bleeding events.5 Grade ≥3 hemoptysis events were observed in 1.9% of patients in E4599 and in 1.5% and 0.9% of AVAiL patients in the 7.5 and 15 mg/kg bevacizumab populations, respectively; in both of these studies, patients with a history of hemoptysis were excluded from study entry.3, 6 In the SAiL trial grade ≥3 pulmonary hemorrhage/hemoptysis was observed in 0.7% of patients.7 In the phase II Japanese JO19907 trial, 0.8% of bevacizumab‐treated patients experience grade ≥3 hemoptysis.4
A retrospective case control analysis of the E4599 study suggested that tumor cavitation at baseline could be a potential risk factor in bevacizumab‐treated patients who developed hemoptysis, while lesion location, size or vascular involvement did not appear to be significantly associated with severe pulmonary hemorrhage/hemoptysis.8 Other analyses have suggested central tumor location, squamous‐cell histology and involvement with great blood vessels as potential risk factors;9 however, no clinical or radiological features (including cavitation and central tumor location) reliably predict severe pulmonary hemorrhage in bevacizumab‐treated patients.9 In addition, the profile of hemoptysis occurrence with real‐world use of bevacizumab in Japan and risk factors for its occurrence remain unclear. Therefore, a prospective nested case‐control study was conducted to assess the onset profile of hemoptysis (grade 2 cases that used an injectable hemostatic or grade ≥3 cases) and to explore the risk factors for hemoptysis in patients receiving bevacizumab in a real‐world setting in Japan.
Materials and Methods
The objectives of this study were to assess the incidence of hemoptysis and the risk factors for developing hemoptysis during routine clinical use of bevacizumab in Japan. After bevacizumab was approved for NSCLC, all physicians were asked to register all NSCLC patients who were scheduled to receive bevacizumab therapy, from November 2009 to August 2011. Information such as gender and age were collected at registration. Physicians were also asked to report any AEs regardless of grade or severity. Risk factors for hemoptysis were assessed by a case‐control study. The case‐control analysis was undertaken through the selection of control patients to be matched against the case patients, with an observation period of 24 weeks from first bevacizumab dose. Computed tomography (CT) images from within 3 months prior to first bevacizumab dose were collected for analysis. Registered patients who developed grade 2 drug‐related hemoptysis requiring injection of a hemostatic agent or grade ≥3 drug‐related hemoptysis were selected as case subjects. AEs were graded according to the classifications Common Terminology Criteria for Adverse Events version 3.0. Registered patients without hemoptysis or with grade 1 or grade 2 hemoptysis requiring an oral hemostatic agent were matched by gender and age, with priority matching to the same institution, as control subjects. For each case subject, four control subjects were selected.
Central reviewers, including two chest radiologists (experience; 25 years and 20 years) and one medical oncologist, performed blinded assessments of CT images that were taken within 3 months before bevacizumab treatment was started. The following terms were used to assess the CT images: a central lesion was defined as the hilar aspect of the tumor (including the lymph node metastases) being located in the central bronchi rather than the segmental bronchi; a great blood vessel was defined as pulmonary artery (central, not including segmental bronchi), left atrium, pulmonary veins, aorta (including brachiocephalic artery, common carotid artery, subclavian artery) and superior vena cava; peripheral vessels were classed as those other than the great vessels, located ≤2 cm from central bronchi (main and lobar bronchi) or if the primary or metastatic lesion were contiguous with the blood vessel; high degree of macrovascular invasion meant that the angle of tumor contact with a great blood vessel was more than 180° or that deformity of a great blood vessel was caused by the tumor; tumors were classed as having exposure in the central airway down to segmental bronchi if the airway was obstructed by the tumor and exposure could not be ruled out on imaging.
Statistical analysis
The sample size was planned as 105, comprising 21 case patients and 84 control patients. By collecting data from 21 case patients it would be possible to detect risk factors at an odds ratio (OR) of 4 with a two‐sided significance level of 5% and statistical power of 70%.
Risk factors for the development of hemoptysis were analyzed using univariate and multivariate logistic regression, including a step‐wise multivariate model to determine ORs using the Wald χ2 test. In order to develop a comprehensive multivariate analysis model, several steps were taken. First, those variables with ORs derived from univariate analysis that showed convergence were selected. Secondly, representative variables were selected for several sets of variables with high correlation coefficients. Statistical significance was set at P = 0.05 for the univariate analysis and P = 0.20 for the multivariate analysis.
Results
Patients
A total of 6774 patients who were scheduled to receive bevacizumab therapy were registered. Case and control patients were selected from these individuals. The selected set comprised 113 patients (23 cases and 90 controls) from institutions that agreed to complete case report forms. Two case patients were matched with three control patients each, the remaining case patients were each matched with four control patients. A total of 104 patients (21 cases, 83 controls), for whom CT images taken within 3 months of starting bevacizumab treatment were available comprised the analysis set (Fig. 1). Baseline characteristics of patients for whom case report forms were collected are shown in Table 1. Briefly, more males than females were enrolled and the majority of patients had adenocarcinoma histology. Most patients were Eastern Cooperative Oncology Group performance status 0 or 1 and most had stage IIIB/IV disease at baseline. Characteristics for assessing thoracic CT images are shown in Table 2. The characteristics ‘Central lesion’, ‘Tumor involvement of peripheral vessels’ and ‘Tumor exposure in central airway down to segmental bronchi’ were more common in case patients than in control patients.
Figure 1.
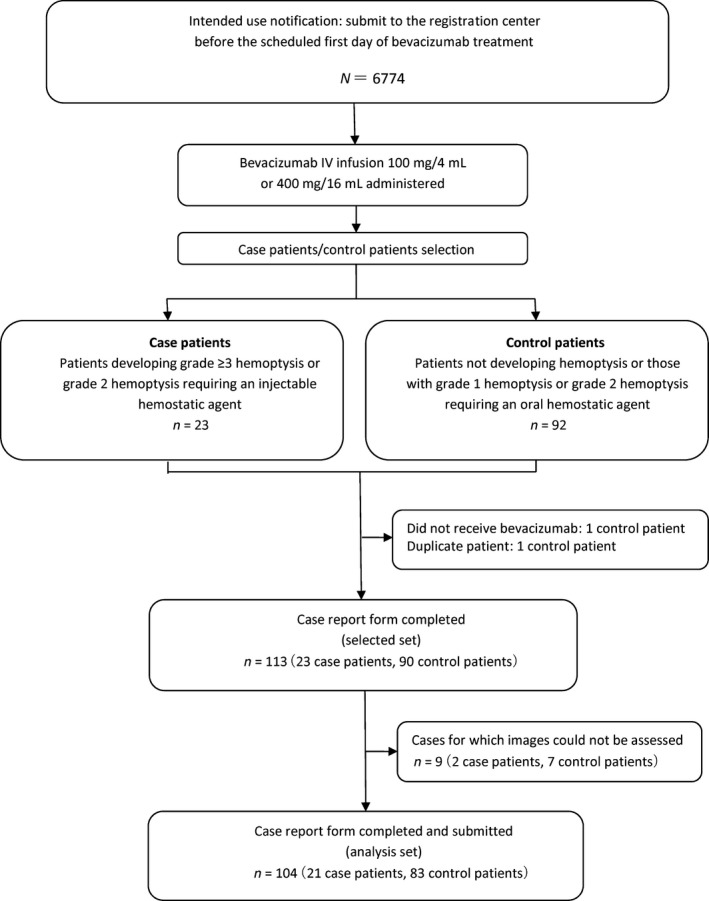
Surveillance study design and patient distribution.
Table 1.
Baseline characteristics
| Characteristic, n (%) | N = 113 (Selected set) | N = 104 (Analysis set) | ||
|---|---|---|---|---|
| Case n = 23 | Control n = 90 | Case n = 21 | Control n = 83 | |
| Gender | ||||
| Male | 18 (78) | 70 (78) | 17 (81) | 66 (80) |
| Female | 5 (22) | 20 (22) | 4 (19) | 17(20) |
| Age | ||||
| <65 years | 13 (57) | 47 (52) | 11(52) | 43 (52) |
| ≥65 years | 10 (43) | 43 (48) | 10 (48) | 40 (48) |
| Histology | ||||
| Adenocarcinoma | 20 (87) | 89 (99) | 18 (86) | 83 (100) |
| Other | 3 (13) | 1 (1) | 3 (14) | 0 (0) |
| ECOG performance status at baseline | ||||
| 0/1 | 22 (96) | 87 (97) | 20 (95) | 80 (96) |
| 2 | 1 (4) | 3 (3) | 1 (5) | 3 (4) |
| Disease stage | ||||
| IIIB/IV | 15(65) | 65 (72) | 15 (71) | 61 (73) |
| Post‐operative recurrence | 4 (17) | 17 (19) | 2 (10) | 14 (17) |
| Other | 4 (17) | 8 (9) | 4 (19) | 8 (10) |
| Previous drug therapy (anticoagulant, aspirin, antiplatelet) | ||||
| No | 9 (39) | 65 (72) | 8 (38) | 60 (72) |
| Yes | 14 (60) | 25 (27) | 13 (62) | 23 (28) |
| Previous thoracic radiotherapy | ||||
| No | 15 (65) | 80 (88) | 14 (67) | 74 (89) |
| Yes | 8 (34) | 10 (11) | 7 (33) | 9 (11) |
| Concomitant thoracic radiotherapy | ||||
| No | 20 (87) | 88 (98) | 18 (86) | 81 (98) |
| Yes | 3 (13) | 2 (2) | 3 (14) | 2 (2) |
| Treatment line | ||||
| First‐line therapy | 13 (57) | 52 (58) | 13 (62) | 50 (60) |
| Second‐line or later line therapy | 10 (43) | 38 (42) | 8 (38) | 33 (40) |
ECOG, Eastern Cooperative Oncology Group.
Table 2.
Characteristics in computed tomography images
| Characteristic, n (%) | N = 104 (Analysis set) | |
|---|---|---|
| Case n = 21 | Control n = 83 | |
| Central lesion | ||
| No | 11 (52) | 71 (86) |
| Yes | 10 (48) | 12 (14) |
| Invasion of great arterial vessels | ||
| No | 18 (86) | 80 (96) |
| Yes | 3 (14) | 3 (4) |
| Invasion of great venous vessels | ||
| No | 19 (90) | 82 (99) |
| Yes | 2 (10) | 1 (1) |
| Tumor involvement of peripheral vessels | ||
| No | 9 (43) | 65 (78) |
| Yes | 12 (57) | 18 (22) |
| Tumor cavitation | ||
| No | 19 (90) | 81 (98) |
| Yes | 2 (10) | 2 (2) |
| Tumor exposure in central airway down to segmental bronchi | ||
| No | 11 (52) | 71 (86) |
| Yes | 10 (48) | 12 (14) |
| Esophageal invasion | ||
| No | 21 (100) | 83 (100) |
| Yes | 0 (0) | 0 (0) |
Incidence and outcomes of hemoptysis
A total of 23/6774 patients registered (0.3%) experienced grade ≥2 hemoptysis events considered to be drug related; a total of 19/6774 patients registered experienced grade ≥3 hemoptysis (grade 2, n = 4; grade 3, n = 4; grade 4, n = 1; grade 5, n = 14). Of the 23 cases, 7 were considered related to bevacizumab treatment, 6 were classed as probably related and 10 cases were considered possibly related to bevacizumab treatment (Table 3). The median time from initial administration of bevacizumab to onset of hemoptysis in the 23 cases was 7.0 weeks. The most common time to onset was 3–6 weeks (30.4%). Of the 23 hemoptysis case patients, 8 (34.8%) recovered, 1 (4.3%) had sequela of impaired consciousness and 14 (60.9%) patients died from hemoptysis. Of the 8 patients who recovered, none continued bevacizumab following the development of hemoptysis, 1 patient received hemostatic injection as treatment and 7 patients received an oral hemostatic agent as treatment. One patient received a blood transfusion and 1 patient had a bronchial artery embolization.
Table 3.
Summary of clinical characteristics of the patients with hemoptysis
| Factor | Events | Case ratio (distribution ratio) |
|---|---|---|
| Total | 23 | 100.0 |
| Volume of bloody sputum | ||
| Large amount | 15 | 65.2 |
| Small amount | 5 | 21.7 |
| Unknown/not recorded | 3 | 13.0 |
| Degree of hemoptysis (grade) | ||
| 2 | 4 | 17.4 |
| 3 | 4 | 17.4 |
| 4 | 1 | 4.3 |
| 5 | 14 | 60.9 |
| Unknown/not recorded | 0 | 0.0 |
| Time from initial administration of bevacizumab to hemoptysis onset (weeks) | ||
| <3 | 3 | 13.0 |
| ≥3 – <24 | 16 | 69.6 |
| ≥24 | 4 | 17.4 |
| Unknown/not recorded | 0 | 0.0 |
| Fundamental statistics | ||
| Mean (standard deviation) | 13.1 (12.33) | |
| Median (range) | 7.0 (1–41) | |
| Hemoptysis outcome | ||
| Recovered/improved | 8 | 34.8 |
| Not recovered | 0 | 0.0 |
| Sequela | 1 | 4.3 |
| Death | 14 | 60.9 |
| Unknown/not recorded | 0 | 0.0 |
| Seriousness | ||
| Non‐serious | 3 | 13.0 |
| Serious | 20 | 87.0 |
| Causal relationship | ||
| Bevacizumab | ||
| Related | 7 | 30.4 |
| Probably related | 6 | 26.1 |
| Possibly related | 10 | 43.5 |
| Factors other than bevacizumab (>1 response possible) | ||
| Primary disease | 15 | |
| Concurrent disease/medical history | 1 | |
| Concomitant drug/concomitant therapy | 5 | |
| Other | 6 | |
Risk factors for occurrence of hemoptysis
In the univariate analysis seven factors remained associated with hemoptysis using the Wald χ2 test. These factors were: prior therapy with anticoagulant, aspirin, non‐steroidal anti‐inflammatory drugs (NSAIDs), or antiplatelet drug; prior thoracic radiotherapy; concomitant therapy with anticoagulant, aspirin, NSAID, or antiplatelet drug; concomitant thoracic radiotherapy; central lesion; tumor involvement of peripheral vessels; and tumor exposure in central airway down to segmental bronchi (Table 4).
Table 4.
Significant risk factors for hemoptysis from the univariate and multivariate analyses (n = 104 assessment)
| Patient baseline factor | Total patients | Case patients | Patient ratio | Conditional logistic regression analysis: (univariate) | Step‐wise logistic regression analysis: (multivariate) | ||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | ||||
| Prior therapy with anticoagulant, aspirin, NSAID, or antiplatelet drug | |||||||
| No | 68 | 8 | 11.8 | ||||
| Yes | 36 | 13 | 36.1 | 6.9541 (1.8493–26.1502) | 0.0041 | – | – |
| Prior thoracic radiotherapy | |||||||
| No | 88 | 14 | 15.9 | ||||
| Yes | 16 | 7 | 43.8 | 4.0201 (1.2285–13.1543) | 0.0214 | 2.7666 (0.6154–12.4366) | 0.1844 |
| Concomitant therapy with anticoagulant, aspirin, NSAID, or antiplatelet drug | |||||||
| No | 70 | 10 | 14.3 | ||||
| Yes | 34 | 11 | 32.4 | 3.0451 (1.0354–8.9557) | 0.0430 | – | – |
| Concomitant thoracic radiotherapy | |||||||
| No | 99 | 18 | 18.2 | ||||
| Yes | 5 | 3 | 60.0 | 5.9999 (1.0025–35.9077) | 0.0496 | 6.1904 (0.6336–60.4794) | 0.1169 |
| Central lesion | |||||||
| No | 82 | 11 | 13.4 | ||||
| Yes | 22 | 10 | 45.5 | 6.2076 (1.8882–20.4077) | 0.0026 | – | – |
| Tumor involvement of peripheral vessels | |||||||
| No | 74 | 9 | 12.2 | ||||
| Yes | 30 | 12 | 40.0 | 6.1545 (1.8720–20.2332) | 0.0027 | – | – |
| Tumor exposure in central airway down to segmental bronchi | |||||||
| No | 82 | 11 | 13.4 | ||||
| Yes | 22 | 10 | 45.5 | 6.2076 (1.8882–20.4077) | 0.0026 | 5.2948 (1.2247–22.8901) | 0.0256 |
NSAID, non‐steroidal anti‐inflammatory drug.
Smoking, performance status, disease stage, metastatic lesions, previous lung disease, concomitant lung disease, prior thoracic radiotherapy, concomitant thoracic radiotherapy, concomitant therapy with anticoagulant, aspirin, NSAID, or antiplatelet drug, treatment line, invasion of great arterial blood vessels, and central tumor exposure were assessed in the multivariate analysis. In the step‐wise model, prior thoracic radiotherapy (OR 2.7666, 95% CI 0.6154–12.4366; P = 0.1844), tumor exposure in the central airway down to segmental bronchi (OR 5.2948, 95% CI 1.2247–22.8901; P = 0.0256) and concomitant thoracic radiotherapy (OR 6.1904, 95% CI 0.6336–60.4794, P = 0.1169) were identified as risk factors for hemoptysis (Table 4).
Discussion
This prospective nested case‐control study investigated the risk factors associated with hemoptysis in bevacizumab‐treated patients in a real‐world setting in Japan. In this study, out of a total of 6774 patients registered, 23 patients (0.3%) experienced grade ≥3 hemoptysis or grade 2 hemoptysis that required an injected hemostatic agent. This was lower than many previously reported rates of hemoptysis. In the Japanese JO19907 trial, 0.8% of bevacizumab‐treated patients experience grade ≥3 hemoptysis.4 Furthermore, incidence of hemoptysis has ranged from 0.9 to 1.9% in non‐Japanese phase III/IV trials.3, 6, 10
Previously reported risk factors for hemoptysis include squamous cell carcinoma, which is an off‐label indication for bevacizumab, and history of hemoptysis, which is a contraindication for bevacizumab, and the fact that bevacizumab use was restricted in patients with these risk factors may account for the low incidence of hemoptysis in this study. Therefore, this study also confirmed that appropriate identification of risk factors is effective at reducing the incidence of hemoptysis.
In an analysis of the phase III E4599 trial, a risk factor related to tumor exposure in the central airway down to the segmental bronchial branch, ‘suspected bronchial tumor invasion’, was also identified as a risk factor for serious hemoptysis in the early stages of the trial.8 Bronchial endoscopy has been suggested as an effective method of diagnosing tumor exposure in the central airway,11 and the authors recommend bronchoscopic evaluation when considering bevacizumab treatment in patients with central lesions. If there is any tumor exposure, physicians should consider avoiding bevacizumab treatment.
While further study is needed to determine the degree to which radiation from concomitant thoracic radiotherapy affects hemoptysis during bevacizumab treatment, thoracic radiotherapy has been reported as a risk factor for hemoptysis.12 Given that serious esophageal fistula has been reported during concomitant bevacizumab and radiotherapy,13 use of bevacizumab in combination with thoracic radiotherapy should be avoided. Further study of prior thoracic radiotherapy is needed because few patients in this study had a history of such therapy and details such as time from radiation exposure to bevacizumab treatment or total radiation dose were not investigated. However, in patients with previous chest radiotherapy, the benefits and risks should be carefully evaluated before proceeding with bevacizumab treatment.
As all patients (both cases and controls) in this study were treated with bevacizumab, and no comparisons with patients not receiving bevacizumab were made, the results are limited to risk factors only associated with bevacizumab administration. Based on the step‐wise multivariate analyses, three important risk factors were identified that were associated with increased risk of developing hemoptysis during bevacizumab treatment: previous thoracic radiotherapy; tumor exposure in the central airway down to the segmental bronchial branch (central lesion); and concomitant thoracic radiotherapy.
Although not risk factors in this surveillance study, the following items have been previously reported as risk factors. A previous retrospective case‐control analysis of the E4599 study suggested that tumor cavitation at baseline could be a potential risk factor for hemoptysis and contrary to our findings, lesion location, size or vascular involvement did not appear to be significantly associated with severe pulmonary hemorrhage/hemoptysis.9 However, because a large‐scale observational study conducted overseas and a pooled analysis of several subsequent clinical studies reported the possibility that tumor cavitation is not a risk factor for hemoptysis, it remains unclear whether cavitation is a risk factor in the Japanese population.( 7, 8, 9, 14 ) Other previously reported risk factors that were not identified in this current analysis include history of hemoptysis and squamous cell carcinoma.11, 14, 15 These were not investigated in this study as a history of hemoptysis was a contraindication and treatment of squamous cell carcinoma is an off‐label indication for bevacizumab. The risk factors of tumors neighboring or being involved with great blood vessels and tumor cavitation, which have also been previously reported,11 were not included in the multivariate analysis model of this study due to a large imbalance of patients in this study.
While the incidence of grade ≥3 hemoptysis was low in the real‐world setting in Japan, it is important to consider previously reported risk factors and the risk factors identified in this study: prior thoracic radiotherapy, presence of tumor exposure in the central airway and concomitant radiotherapy when selecting patients for bevacizumab treatment.
Disclosure Statement
KG, ME and MK have all participated as Computed Tomography Imaging Review Committee members for bevacizumab, reimbursed by Chugai Pharmaceutical Co. Ltd. KG, NY, YO and MF have all participated as Independent Appropriate use Advisory Board members for bevacizumab, reimbursed by Chugai Pharmaceutical Co. Ltd. YO also has a child who is an employee of Chugai Pharmaceutical Co. Ltd. AS is full‐time employee of Chugai Pharmaceutical Co. Ltd.
This trial was designed, funded, and monitored by Chugai Pharmaceutical Co. Ltd. Data were gathered, analyzed, and interpreted by Chugai with input from all authors. The corresponding author had full access to the relevant data and took full responsibility for the final decision to submit the report for publication.
Acknowledgments
Support for third‐party writing assistance for this article was funded by Chugai Pharmaceutical Co. Ltd. We would also like to thank those medical doctors who completed case report forms selected for this study.
Cancer Sci 107 (2016) 1837–1842
Funding Information
This study was funded by Chugai Pharmaceutical Co. Ltd.
Koichi Goto, Masahiro Endo and Masahiko Kusumoto are members in Computed Tomography Imaging Review Committtee for Bevacizumab.
Koichi Goto, Nobuyuki Yamamoto, Yuichiro Ohe and Masahiro Fukuoka are members in Independent Appropriate use Advisory Board for Bevacizumab.
References
- 1. Schiller J, Harrington D, Belani C et al Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002; 346: 92–8. [DOI] [PubMed] [Google Scholar]
- 2. Ohe Y, Ohashi Y, Kubota K et al Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non‐small‐cell lung cancer: four‐Arm Cooperative Study in Japan. Ann Oncol 2007; 18: 317–23. [DOI] [PubMed] [Google Scholar]
- 3. Sandler A, Gray R, Perry M et al Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]
- 4. Niho S, Kunitoh H, Nokihara H et al Randomised phase II study of first‐line carboplatin‐paclitaxel with or without bevacizumab in Japanese patients with advanced non‐squamous non‐small cell lung cancer. Lung Cancer 2012; 76: 362–7. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DH, Fehrenbacher L, Novotny WF et al Randomised phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 2004; 22: 2184–91. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, von Pawel J, Zatloukal P et al Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAIL. J Clin Oncol 2009; 27: 1227–34. [DOI] [PubMed] [Google Scholar]
- 7. Dansin E, Cinieri S, Garrido P et al MO19390 (SAiL): bleeding events in a phase IV study of first‐line bevacizumab with chemotherapy in patients with advanced non‐squamous NSCLC. Lung Cancer 2012; 76: 373–9. [DOI] [PubMed] [Google Scholar]
- 8. Sandler A, Schiller J, Gray R et al Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first‐line advanced, unresectable non‐small‐cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol 2009; 27: 1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reck M, Barlesi F, Crino L et al Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consenus report from a panel of experts. Ann Oncol 2012; 23: 1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crino L, Dansin E, Garrudo P et al Safety and efficacy of first‐line bevacizumab‐based therapy in advanced non‐squamous non‐small‐cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010; 11: 733–40. [DOI] [PubMed] [Google Scholar]
- 11. Okamoto K, Okamoto I, Miyazaki M et al Bronchoscopic findings for bevacizumab‐related pulmonary hemorrhage in advanced non‐small cell lung cancer. Invest New Drugs 2013; 31: 1364–6. [DOI] [PubMed] [Google Scholar]
- 12. Ito M, Niho S, Nihei K et al Risk factors associated with fatal pulmonary hemorrhage in locally advanced non‐small‐cell lung cancer treated with chemoradiotherapy. BMC Cancer 2012; 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spigel D, Hainsworth J, Yardley D et al Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010; 28: 43–8. [DOI] [PubMed] [Google Scholar]
- 14. Sandler A, Johnson D, Brahmer J et al Retrospective study of clinical and radiographic risk factors associated with early‐onset, severe pulmonary hemorrhage in bevacizumab‐treated patients with advanced non‐small cell lung cancer (NSCLC). J Clin Oncol 2008; 26: 15s (Abstr 8074). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hellman M, Chaft J, Rusch V et al Risk of hemoptysis in patients with resected squamous cell and other high‐risk lung cancers treated with adjuvant bevacizumab. Cancer Chemother Pharmacol 2013; 72: 453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


