Abstract
We proposed to compare the outcomes of first‐line epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR‐TKI) alone with EGFR‐TKI plus whole‐brain radiotherapy (WBRT) for the treatment of brain metastases (BM) in patients with EGFR‐mutated lung adenocarcinoma. A total of 1665 patients were screened from 2008 to 2014, and 132 were enrolled in our study. Among the 132 patients, 72 (54.5%) harbored a deletion in exon 19, 97 (73.5%) showed multiple intracranial lesions, and 67 (50.8%) had asymptomatic BM. Seventy‐nine patients (59.8%) were treated with EGFR‐TKI alone, 53 with concomitant WBRT. The intracranial objective response rate was significantly higher in the EGFR‐TKI plus WBRT treatment group (67.9%) compared with the EGFR‐TKI alone group (39.2%) (P = 0.001). After a median follow‐up of 36.2 months, 62.1% of patients were still alive. The median intracranial TTP was 24.7 months (95% CI, 19.5–29.9) in patients who received WBRT, which was significantly longer than in those who received EGFR‐TKI alone, with the median intracranial TTP of 18.2 months (95% CI, 12.5–23.9) (P = 0.004). There was no significant difference in overall survival between WBRT and EGFR‐TKI alone groups, (median, 48.0 vs 41.1 months; P = 0.740). The overall survival is significantly prolonged in patients who had an intracranial TTP exceeding 22 months compared to those who developed intracranial progression <22 months after treatment, (median, 58.0 vs 28.0 months; P = 0.001). For EGFR‐mutated lung adenocarcinoma patients with BM, treatment with concomitant WBRT achieved a higher response rate of BM and significant improvement in intracranial progression‐free survival compared with EGFR‐TKI alone.
Keywords: Brain metastasis, EGFR mutation, lung adenocarcinoma, prognosis, radiotherapy
Abbreviations
- BM
brain metastases
- CI
confidence interval
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- NSCLC
non‐small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression‐free survival
- SRS
stereotactic radiosurgery
- TKI
tyrosine kinase inhibitor
- TTP
time to progression
- WBRT
whole brain radiotherapy
Lung cancer is—and has been for the last several decades—the leading cause of cancer‐related mortality worldwide.1 The last decade has revealed EGFR abnormalities present within NSCLC and led to the development of EGFR‐TKI, in what is now commonly referred to as precision oncology.2
Epidermal growth factor receptor mutation positivity is a good prognostic marker and patients with EGFR mutant lung cancer tend to have a longer survival. However, patients with EGFR mutated NSCLC have a predilection to develop BM; the incidence of EGFR mutation positivity among patients with BM is high, with a range of from 44 to 63%.3
Given the activity of EGFR‐TKIs in the central nervous system,4 it is theoretically reasonable to treat NSCLC patients with BM with EGFR‐TKIs, and studies have reported the efficacy of EGFR‐TKIs in NSCLC patients with BM. In a phase II trial, NSCLC patients with BM who have activating mutations of EGFR treated with EGFR‐TKI had a longer intracranial median PFS of 15.2 months than did those with wild‐type EGFR (median PFS, 4.4 months) (P < 0.02).5 In a prospective study in NSCLC patients harboring either exon 19 or 21 mutation, oral EGFR‐TKIs resulted in an intracranial disease control rate of 93%, with 83% of patients reaching a partial response and 11% showing stable disease.6
Epidermal growth factor receptor–TKIs can significantly improve the duration of disease control for patients with oncogene‐driven NSCLC,7, 8 and the control of BM has emerged as an important therapeutic issue. Radiotherapy is the principal treatment method for patients with BM. However, whether additional brain‐directed therapy can improve the control of BM from EGFR‐mutated NSCLC patients has not been determined prospectively. We therefore carried out a retrospective study to compare the efficacy of first‐line EGFR‐TKI in combination with radiotherapy versus EGFR‐TKI alone in patients with EGFR‐mutant lung adenocarcinoma with BM.
Patients and Methods
Patients
The study group consisted of patients with stage IV lung adenocarcinoma, as confirmed by pathological analysis. None of the patients had received previous systemic therapy. Eligible patients also were required to be 18–75 years of age, with an Eastern Cooperative Oncology Group performance status of 0–2, harboring EGFR mutation, initially presenting with brain metastasis, adequate hematological and biochemical values, and first‐generation EGFR‐TKI as first‐line therapy. Patients were required to have extracranial and intracranial lesions that could be measured according to the Response Evaluation Criteria in Solid Tumors (version 1.1).9 The study was approved by the institutional review board of Zhengzhou University (Zhengzhou, China) and complied with the Declaration of Helsinki. Informed consent was exempted by the board due to the retrospective nature of this research.
Treatment and assessment
All patients included in this analysis received 250 mg gefitinib or 150 mg erlotinib orally once daily. Baseline brain imaging was detected using either CT and/or MRI. Whole brain radiotherapy was delivered at a dose of 30 Gy/10f for 5 days per week, up to 2 weeks, and concomitant WBRT was given in patients with brain metastatic lesion >3 cm in diameter or those who had symptoms like dizziness, headache, nausea, and vomiting. Systemic lesions and BM were monitored as target lesions, and tumor assessments were carried out every 6 weeks from the date of first dose.
Herman et al.10 established a battery of validated, language‐specific, and population‐normalized neurocognitive function tests evaluating memory, fine motor coordination, and executive functions in BM patients. Memory impairment was assessed using this instrument among the patients in our study.
Physical examination, thoracic/abdominal CT and contrast‐enhanced MRI, or CT of the brain were carried out 4 weeks after initial treatment. These tests were subsequently performed every 2 months for the first year, and 3 months thereafter.
Statistical analysis
Intracranial and systemic ORRs were evaluated at 12 weeks after the start of treatment. Intracranial TTP was defined as the time from the first dose to the first documentation of intracranial tumor progression. Overall survival was calculated from the date of treatment until death from any cause or last follow‐up. Time to progression and OS were analyzed using the Kaplan–Meier method and differences between the curves were analyzed using the log–rank test. Analysis of variance or the χ2‐test was used to compare clinicopathologic characteristics. Multivariate analysis (Cox regression model) was used to determine the independent prognostic factors. All statistical analyses were carried out using spss software (version 16.0.1; SPSS, Chicago, IL, USA) and the correlation was significant at the 0.05 level (two‐tailed).
Results
Baseline characteristics of patients
From September 1, 2008, to September 1, 2014, we screened 1665 patients with stage IV lung adenocarcinoma and 132 met the eligibility criteria (Fig. 1). Baseline clinicopathologic characteristics of the 132 patients are listed in Table 1. Among the 132 patients, 72 (54.5%) harbored a deletion in exon 19, 97 (73.5%) showed multiple intracranial lesions, and 67 (50.8%) had asymptomatic BM. Seventy‐nine patients (59.8%) were treated with EGFR‐TKI alone, 53 with concomitant WBRT.
Figure 1.
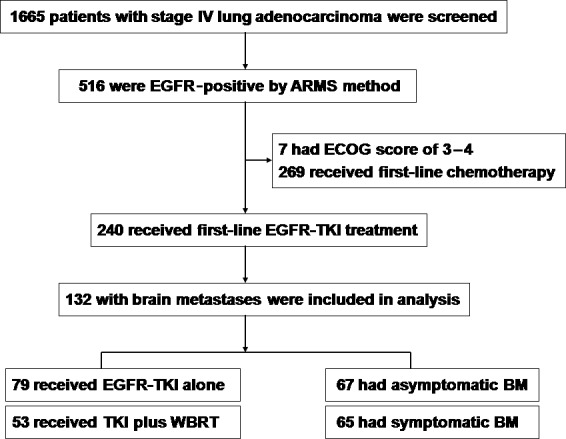
Patient disposition in a group with epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma and brain metastases (BM) treated with EGFR–tyrosine kinase inhibitor (TKI) alone or with whole‐brain radiotherapy (WBRT). ARMS, amplification mutation refractory system; ECOG, Eastern Cooperative Oncology Group.
Table 1.
Baseline demographic data of patients with epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma and brain metastases (BM) treated with EGFR–tyrosine kinase inhibitor (TKI) alone or with whole‐brain radiotherapy (WBRT)
| Characteristic | EGFR‐TKI alone (n = 79) | EGFR‐TKI plus WBRT (n = 53) | P‐value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 52 | 52 | |||
| Range | 29–75 | 31–74 | |||
| Age distribution, years | |||||
| <65 | 63 | 79.7 | 44 | 83.0 | 0.585 |
| ≥65 | 16 | 20.3 | 9 | 17.0 | |
| Gender | |||||
| Male | 27 | 34.2 | 24 | 45.3 | 0.112 |
| Female | 52 | 65.8 | 29 | 54.7 | |
| Smoking status | |||||
| Never | 61 | 77.2 | 36 | 67.9 | 0.154 |
| Former | 18 | 22.8 | 17 | 32.1 | |
| Primary tumor location | |||||
| Left lung | 30 | 38.0 | 20 | 37.7 | 1.000 |
| Right lung | 49 | 62.0 | 33 | 62.3 | |
| EGFR mutation status | |||||
| Del‐19 | 44 | 55.7 | 28 | 52.8 | 0.670 |
| L858R | 35 | 44.3 | 25 | 47.2 | |
| BM number | |||||
| ≤3 | 24 | 30.4 | 11 | 20.8 | 0.144 |
| >3 | 55 | 69.6 | 42 | 79.2 | |
| Intracranial symptoms | |||||
| Without | 51 | 65.6 | 16 | 30.2 | 0.001 |
| With | 28 | 34.4 | 37 | 69.8 | |
Disease control
Intracranial and systemic disease responses to therapy were evaluated for all 132 patients. The systemic ORR at 12 weeks was 62.9% (83 of 132); and 7 patients achieved complete response and 76 achieved partial response. In the patients treated with EGFR‐TKI alone, the intracranial ORR at 12 weeks was 39.2% (31 of 79), and those treated with concomitant WBRT had an intracranial ORR of 67.9% (36 of 53) (P = 0.001).
Intracranial progression
Data cut‐off for this retrospective analysis was April 1, 2015, and the median follow‐up was 36.2 months. Thirty‐three patients (25.0%) were alive without evidence of disease progression, 49 (37.1%) were alive with disease, and 50 (37.9%) were dead due to disease progression.
Of the 132 patients, intracranial progression was detected in 74 patients (56.1%). The median intracranial TTP was 22.3 months (95% CI, 19.1–25.5). For patients treated with EGFR‐TKI alone, intracranial progression developed in 64.6% of them (51 of 79), while intracranial progression for patients treated with WBRT occurred in 43.4% of patients (23 of 53).
The median intracranial TTP was 24.7 months (95% CI, 19.5–29.9) in patients who received WBRT, which was significantly longer than in those who received EGFR‐TKI alone with the median intracranial TTP of 18.2 months (95% CI, 12.5–23.9; P = 0.004), as shown in Figure 2(a).
Figure 2.
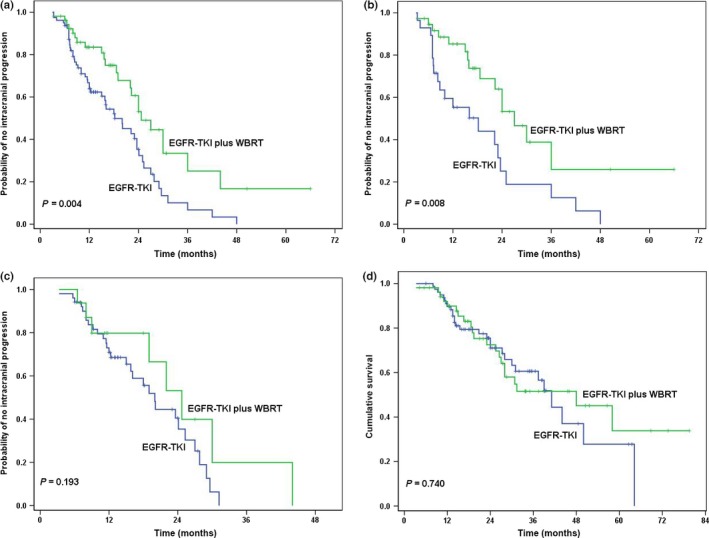
Survival curves in patients with epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma and brain metastases (BM), according to treatment with EGFR–tyrosine kinase inhibitor (TKI) alone or with whole‐brain radiotherapy (WBRT). (a) Intracranial time to progression (TTP). (b) Intracranial TTP in patients with symptomatic brain metastases. (c) Intracranial TTP in patients with asymptomatic brain metastases. (d) Overall survival.
For patients presented with symptomatic BM, the median intracranial TTP was 27.0 months (95% CI, 20.3–33.7) in 37 patients who received WBRT, much longer than in 28 patients who received EGFR‐TKI alone, who had a median intracranial TTP of 18.2 months (95% CI, 6.8–29.6) (P = 0.008), as shown in Figure 2(b).
For patients who had asymptomatic BM, no statistical difference of intracranial TTP appeared between 16 patients who received WBRT and 51 patients who received EGFR‐TKI alone, the median intracranial TTP was 24.7 months (95% CI, 17.5–31.9) and 20.0 months (95% CI, 16.9–23.1), respectively (P = 0.193) (Fig. 2c).
When the treatment strategy in combination with patient and tumor characteristics were subjected to Cox multivariate regression analysis, WBRT (P = 0.004) was an independent predictor of intracranial TTP, and multiple BM was a potential independent predictor unfavorably influencing intracranial TTP (Table 2).
Table 2.
Multivariate analysis of intracranial progression‐free survival in patients with epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma and brain metastases (BM) treated with EGFR–tyrosine kinase inhibitor (TKI) alone or with whole‐brain radiotherapy (WBRT), according to the Cox regression model
| B | SE | P‐value | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Sex, male/female | −0.229 | 0.438 | 0.600 | 0.795 | 0.337 | 1.874 |
| Age, <65/≥65 years old | 0.187 | 0.368 | 0.611 | 1.206 | 0.587 | 2.478 |
| Tumor location, left/right lung | −0.050 | 0.268 | 0.853 | 0.952 | 0.563 | 1.609 |
| EGFR mutation status, Del‐19/L858R | −0.324 | 0.243 | 0.182 | 0.723 | 0.450 | 1.164 |
| Smoking status, never/former | −0.435 | 0.484 | 0.369 | 0.647 | 0.251 | 1.672 |
| BM number, ≤3/>3 | 0.538 | 0.291 | 0.064 | 1.713 | 0.968 | 3.030 |
| Intracranial symptoms, without/with | 0.013 | 0.263 | 0.959 | 1.014 | 0.605 | 1.698 |
| Treatment strategy, TKI alone/WBRT | −0.812 | 0.282 | 0.004 | 0.444 | 0.255 | 0.772 |
B, regression coefficient; CI, confidence interval; OR, odds ratio; SE, standard error.
Overall survival
By the data cut‐off date for this analysis, the median OS for the 132 patients was 41.1 months (95% CI, 26.7–55.5).
For patients treated with EGFR‐TKI alone, 29 (36.7%) died, and the median OS was 41.1 months (95% CI, 35.2–47.0) in this group; 21 patients (39.6%) died in the group treated with WBRT, and the median OS was 48.0 months (95% CI, 25.0–71.0) in this group. There was no significant difference in OS between WBRT and EGFR‐TKI alone groups (P = 0.740) (Fig. 2d).
To assess the correlation between intracranial progression and OS, the median intracranial TTP of 22.3 months was selected as the cut‐off point. The median OS was 58.0 months (95% CI, 36.2–79.8) in patients who had an intracranial TTP exceeding 22 months, significantly longer than in those who developed intracranial progression <22 months after treatment, who had a median OS of 28.0 months (95% CI, 15.8–40.2; P = 0.001).
Using Cox multivariate regression analysis, intracranial TTP >22 months was a statistically significant factor favorably influencing OS (95% CI, 0.111–0.552; P = 0.001). Intracranial symptoms was also an independent unfavorable factor for OS (95% CI, 1.136–4.174; P = 0.019).
Neurocognitive toxicity
We compared memory functions in the two groups of surviving patients at early (6 months), medium (1 year), and late (>2 years) time points. As shown in Table 3, at early follow‐up, significantly more patients in the EGFR‐TKI plus WBRT group had impairments in memory (delayed recall and recall) and learning (recognition) than in the EGFR‐TKI alone group. On the contrary, in the >2 years survivors, the percentage of those suffering from impaired memory function had declined by more than half.
Table 3.
Comparison of memory impairment in patients with epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma and brain metastases treated with EGFR–tyrosine kinase inhibitor (TKI) alone or with whole‐brain radiotherapy (WBRT)
| 6 months | 1 year | >2 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recall | Delayed recall | Recognition | Recall | Delayed recall | Recognition | Recall | Delayed recall | Recognition | |
| EGFR‐TKI alone | 4% (3/78) | 5% (4/78) | 1% (1/78) | 1% (1/67) | 4% (3/67) | 1% (1/67) | 0 (0/30) | 3% (1/30) | 0 (0/30) |
| TKI plus WBRT | 12% (6/50) | 18% (9/50) | 8% (4/50) | 9% (4/43) | 14% (6/43) | 7% (3/43) | 4% (1/25) | 8% (2/25) | 4% (1/25) |
Discussion
We first reported the direct comparison of therapeutic effects between first‐line EGFR‐TKI and WBRT in EGFR‐mutated lung adenocarcinoma patients with BM. The survival is notably long in such molecularly selected populations, with a median survival time of nearly 3.5 years. The survival rates were similar among patients treated with WBRT versus EGFR‐TKI alone, but concomitant WBRT achieved a significantly increased intracranial TTP, with 1‐year and 3‐year intracranial control rates of 83.5% and 33.4%.
Evidence showed that patients with EGFR‐mutant lung cancers are more likely to develop BM than those with wild‐type tumors,11, 12 but EGFR mutations are associated with increased incidence rates of response both in primary tumors and BM; good long‐term survival outcomes were often observed in NSCLC patients with BM who have activating mutations of EGFR. The median OS for the molecularly selected patients with NSCLC and BM was 41.1 months, which is comparable with other trials that segregated patients by EGFR mutation.13, 14
In a recent study by Gerber et al., better outcomes were noted in mutant lung adenocarcinoma patients harboring EGFR mutations with BM who were treated with SRS. That group had significantly longer OS than the patient group treated with erlotinib, with a median of 64 months (P = 0.006). In addition, patients treated with SRS had better performance status and fewer BM compared with those receiving erlotinib.15 Our multivariate analysis also found that brain oligometastases (≤3 lesions) was a potential independent predictor favorably influencing intracranial TTP. Of note, our results showed that patients who had an intracranial TTP exceeding 22 months achieved a significantly increased median survival of 58 months, from which we could deduce that longer intracranial control would translate into improved OS. As WBRT can be associated with neurocognitive toxicity, newer generation TKIs with improved central nervous system activity, including in leptomeningeal diseases, might bring promising results in the reduction of side‐effects and improvement of long‐term outcomes.
Studies showed that EGFR‐TKIs are valid options among patients with asymptomatic BM that have arisen from NSCLC harboring sensitizing EGFR mutations. The response rate is approximately 70%, median PFS ranges from 6.6 to 23.2 months, and OS varies from 12.9 to 19.8 months.6, 12 However, WBRT alone or in combination with surgery and SRS, has been the standard of care for BM. Studies found that EGFR‐TKI radiosensitizes NSCLC cells by reducing proliferation, inhibiting anti‐apoptotic pathways, and suppressing cellular DNA repair capacity.16, 17 Doherty et al.18 evaluated the impact of EGFR‐TKIs and radiotherapy for 139 NSCLC patients harboring EGFR mutations with BM. Initial BM treatment consisted of systemic therapy alone in 19 patients (17 receiving TKI, 2 receiving chemotherapy), SRS +/− surgery in 27 patients, and WBRT +/− SRS/surgery in 88 patients. The median intracranial TTP was 18, 16, and 40 months, respectively (P = 0.12). Median OS was 26, 27, and 34 months, respectively (P = 0.49). The results indicate a trend towards longer survival in patients with BM from EGFR‐driven NSCLC who received WBRT. Zeng et al.19 compared the efficacy of gefitinib alone with gefitinib plus WBRT in patients with BM from NSCLC. They found that the median TTP of BM and median OS was 10.6 months and 23.4 months, respectively, in the gefitinib–WBRT group, and 6.6 months and 14.8 months, respectively, in the gefitinib alone group (P < 0.01). A phase II trial carried out in a molecularly unselected population also showed that the combination of erlotinib and WBRT was both safe and efficacious, with an ORR of 86% and no increase in neurotoxicity.20
Li et al.21 suggested that good control of intracranial disease by radiotherapy is associated with stabilization and improvement of neurocognitive function. However, WBRT might specifically impair hippocampus‐related functions such as memory and learning, and we found that a proportion of patients who received EGFR‐TKI plus WBRT showed impaired memory. The optimal time to add WBRT to EGFR‐TKI treatment remains unknown. A recent study found that continuous EGFR‐TKI treatment following radiotherapy for NSCLC patients with isolated central nervous system failure remained effective, with a response rate and disease control rate of central nervous system lesions at 41% and 76%, respectively.22 In EGFR‐mutant lung adenocarcinoma patients who had asymptomatic BM, our findings illustrated that the median intracranial TTP was comparable between patients who received WBRT and those who received EGFR‐TKI alone. Patients with sensitizing EGFR mutations can thus be primarily treated with targeted therapy, and radiotherapy could be considered when intracranial progression is shown. The ongoing prospective trial called TRACTS (ClinicalTrials.gov registration NCT01763385), comparing concurrent WBRT and erlotinib to erlotinib alone with WBRT at time of progression, will provide further insight into the optimal time to add brain radiotherapy.
To our knowledge, there are few published reports describing the management of EGFR‐mutant NSCLC patients with symptomatic central nervous system metastases. In the present study, more patients presented with symptomatic BM in the brain radiotherapy group compared to those who received EGFR‐TKI alone, and they had a greater burden of intracranial disease. For patients with symptomatic BM, results showed that the median intracranial TTP was 8.8 months longer in patients who received WBRT than in those who received EGFR‐TKI alone (27.0 months vs 18.2 months; P = 0.008). In addition, multivariate survival analysis indicated that symptomatic BM was an independent unfavorable prognostic factor for OS (P = 0.019). Our findings are limited by the retrospective nature of the analysis, however, WBRT might be administered at the beginning of EGFR‐TKI therapy to prolong duration of intracranial control and to improve the survival of EGFR‐mutant lung adenocarcinoma patients with symptomatic BM.
Our study has the following limitations: (i) the data represent patients from a single institution; (ii) the patient group is relatively small; and (iii) owing to its retrospective nature, some data are not available, such as baseline albumin, weight change during treatment, and treatment‐related rash. The missing information might hamper the prognostic evaluation. Finally, baseline brain imaging and intracranial progression were detected by CT in some patients, which can affect the accuracy of evaluation for intracranial lesions.
In summary, concomitant WBRT significantly improved intracranial lesion control and prolonged intracranial TTP compared with EGFR‐TKI treatment alone. A long OS for patients with EGFR‐mutant lung adenocarcinoma and BM was observed in our study, and the OS was equivalent between the WBRT and EGFR‐TKI alone treatment groups. To protect against radiation‐induced neurotoxicity, prospective studies are needed to identify the subsets of patients treated with EGFR‐TKI for whom radiotherapy can be omitted.
Disclosure Statement
The authors have no conflict of interest.
Cancer Sci 107 (2016) 1800–1805
Funding Information
Supported by National Natural Science Foundation of China.
This research was presented at the 16th World Conference on Lung Cancer (Abstract ID: 1566), Denver, CO, USA, September 6–9, 2015.
References
- 1. Govindan R. Overcoming resistance to targeted therapy for lung cancer. N Engl J Med 2015; 372: 1760–1. [DOI] [PubMed] [Google Scholar]
- 2. Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol 2013; 31: 1803–5. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt VR, Kedia S, Kessinger A, Ganti AK. Brain metastasis in patients with non‐small‐cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol 2013; 31: 3162–4. [DOI] [PubMed] [Google Scholar]
- 4. Yi HG, Kim HJ, Kim YJ et al Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non‐small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer 2009; 65: 80–4. [DOI] [PubMed] [Google Scholar]
- 5. Wu YL, Zhou C, Cheng Y et al Erlotinib as second‐line treatment inpatients with advanced non‐small‐cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG‐0803). Ann Oncol 2013; 24: 993–9. [DOI] [PubMed] [Google Scholar]
- 6. Park SJ, Kim HT, Lee DH et al Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non‐small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012; 77: 556–60. [DOI] [PubMed] [Google Scholar]
- 7. Yusuf SW, Kim P, Durand JB. Erlotinib or gefitinib for non‐small‐cell lung cancer. N Engl J Med 2011; 364: 947–55. [DOI] [PubMed] [Google Scholar]
- 8. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 10. Herman MA, Tremont‐Lukats I, Meyers CA. Neurocognitive and functional assessment of patients with brain metastases: a pilot study. Am J Clin Oncol 2003; 26: 273–9. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto S, Takahashi K, Iwakawa R et al Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 2006; 119: 1491–4. [DOI] [PubMed] [Google Scholar]
- 12. Jamal‐Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptormutant non‐small cell lung cancer metastatic to the brain. Clin Cancer Res 2012; 18: 938–44. [DOI] [PubMed] [Google Scholar]
- 13. Mitsudomi T, Morita S, Yatabe Y et al Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first‐line treatment for patients with non‐small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2012; 30: (suppl; abstr 7521). [Google Scholar]
- 14. Heon S, Yeap BY, Britt GJ et al Development of central nervous system metastases in patients with advanced non‐small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010; 16: 5873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerber NK, Yamada Y, Rimner A et al Erlotinib versus radiation therapy for brain metastases in patients with EGFR‐mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys 2014; 89: 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka T, Munshi A, Brooks C et al Gefitinib radiosensitizes nonsmall cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res 2008; 14: 1266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chinnaiyan P, Huang S, Vallabhaneni G et al Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005; 65: 3328–35. [DOI] [PubMed] [Google Scholar]
- 18. Doherty MK, Korpanty G, Tomasini P. Treatment of EGFR/ALK‐Driven non‐Small cell lung cancer (NSCLC) brain metastases: impact of first‐line whole brain radiotherapy on outcome. J Thorac Oncol 2015; 10(Suppl. 2): S279. [Google Scholar]
- 19. Zeng YD, Zhang L, Liao H et al Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non‐small‐cell lung cancer: a Retrospective Study. Asian Pac J Cancer Prev 2012; 13: 909–14. [DOI] [PubMed] [Google Scholar]
- 20. Welsh JW, Komaki R, Amini A et al Phase II trial of erlotinib plus concurrent whole‐brain radiation therapy for patients with brain metastases from non‐small‐cell lung cancer. J Clin Oncol 2013; 31: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole‐brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol 2007; 25: 1260–6. [DOI] [PubMed] [Google Scholar]
- 22. Shukuya T, Takahashi T, Naito T et al Continuous EGFR‐TKI administration following radiotherapy for non‐small cell lung cancer patients with isolated CNS failure. Lung Cancer 2011; 74: 457–61. [DOI] [PubMed] [Google Scholar]


