Abstract
High mobility group box 1 (HMGB1) is a member of the family of damage‐associated molecular patterns, which cause inflammation and trigger innate immunity through Toll‐like receptors 2/4 and the receptor for advanced glycation end products. We examined the effect of glycyrrhizin, a selective inhibitor of HMGB1, on the induction of CTLs in mice. B6 mice, either OT‐1 spleen cell‐transferred or untransferred, were immunized with an s.c. injection of OVA 257–264 peptide with topical imiquimod, and glycyrrhizin was mixed with the antigen peptide. Proliferation of OT‐1 cells after immunization was enhanced by glycyrrhizin. The effect of glycyrrhizin was confirmed in other adjuvant systems, such as CpG oligonucleotide and monophosphoryl lipid A, but glycyrrhizin was not effective in Freund's incomplete adjuvant system. The augmenting effects of glycyrrhizin were also observed in other synthetic HMGB1 inhibitors, gabexate mesilate, nafamostat, and sivelestat. Thus, the effects are common to the HMGB1 inhibitors. Induction of CTLs detected by γ‐interferon enzyme‐linked immunospot assay was similarly augmented by glycyrrhizin. In a therapeutic vaccine model, glycyrrhizin inhibited the growth of s.c. transplanted EG.7 tumors. Expression of inflammatory cytokines in the skin inoculation site was downregulated by glycyrrhizin. These results suggest that HMGB1 inhibitors might be useful as a co‐adjuvant for peptide vaccination with an innate immunity receptor‐related adjuvant.
Keywords: Antitumor, cytotoxic T‐lymphocytes, glycyrrhizin, high mobility group box 1, peptide vaccine
Immunotherapy is one of the most promising methods for cancer treatment. Many clinical trials of cancer vaccines are currently ongoing, with the majority using Montanide ISA51VG, a clinical grade of Freund's incomplete adjuvant, for their vaccine formulations.1 To date, however, the clinical impact of most cancer vaccines has not been sufficient due to the immune suppressive status in most patients with cancer. Therefore, the development of new vaccination systems, such as new adjuvants, co‐adjuvants or immunomodulators, and delivery systems to break the immune suppression and induce a strong immune response is needed. Toll‐like receptors (TLRs) are potent targets of the new adjuvants and several TLR‐directed adjuvants have been developed.2, 3 The induction of type‐1 helper T cell (Th1)‐mediated CTLs is important for cancer treatment, although most of the TLR‐directed adjuvants induce activation of both Th1 and Th2 through MyD88 and its downstream nuclear factor‐κB activation.4, 5 The subsequently produced inflammatory cytokine, such as tumor necrosis factor‐α (TNF‐α), often play a role in tumor progression in the tumor microenvironment.6, 7 Therefore, the control of inflammatory cytokines is also important for the induction of antitumor immunity. Cyclooxygenase inhibitors, a type of conventional non‐steroidal anti‐inflammatory drugs, have been reported to have a co‐adjuvant effect.8, 9 Glycyrrhizin is a plant‐derived anti‐inflammatory drug approved as a pharmaceutical in several Asian countries, including Japan, and its mode of action has been identified as selective inhibition of high mobility group box 1 (HMGB1).10, 11 High mobility group box 1 is a member of the family of damage‐associated molecular patterns (DAMPs), a type of intrinsic danger signal that causes inflammation and triggers innate immunity through TLR2/4 and the receptor for advanced glycation end products (RAGE).12, 13, 14, 15 High mobility group box 1 has also been reported to exert an immunosuppressive effect through binding to the T cell immunoglobulin domain and mucin domain 3 (TIM‐3), an immune checkpoint molecule on activated T cells.12, 16, 17 Glycyrrhizin directly binds to HMG boxes of HMGB1 and inhibits binding of HMGB1 to the cellular receptors expressed on various types of cells, including dendritic cells (DCs) and T cells.12 In the present study, we examined the co‐adjuvant effect of glycyrrhizin in various adjuvant systems for the induction of CTLs by peptide vaccination.
Materials and Methods
Mice and tumor cells
Female C57BL/6J (B6) mice obtained from CLEA Japan (Tokyo, Japan) were used for all experiments at 8–12 weeks old. OT‐1 T‐cell receptor transgenic mice were obtained from Taconic (Hudson, NY, USA) and bred in our animal facility. All mice were maintained under specific pathogen‐free conditions according to the guidelines for animal care at Kurume University (Kurume, Japan). Experimental protocols for the in vivo animal studies were approved by the Ethics Committee for Animal Experiments of Kurume University.
E.G7‐OVA (E.G7) cells were obtained in September, 2014, from ATCC (Manassas, VA, USA) and maintained in RPMI‐1640 supplemented with 50 μM 2‐mercaptoethanol, 0.4 mg/mL G418, and 10% FBS (TRACE Scientific, Melbourne, Australia). EL‐4 was obtained in September, 2015, from JCRB Cell Bank (Osaka, Japan) and maintained in 10% FBS RPMI‐1640. Both the cell lines were grown, batch‐frozen, and used in the experiment.
Reagents
SIINFEKL, an H‐2Kb‐restricted CTL epitope peptide derived from ovalbumin 257–264 (OVA257–264), was purchased from American Peptide (Sunnyvale, CA, USA). Glycyrrhizin (Eisai, Tokyo, Japan), gabexate mesilate (Alfresa, Osaka, Japan), nafamostat mesilate (Shionogi, Osaka, Japan), and sivelestat sodium hydrate (Ono Pharmaceutical, Osaka, Japan) were used as HMGB1 inhibitors. Beselna cream containing 5% imiquimod (Mochida Pharmaceuticals, Tokyo, Japan), CpG oligodeoxynucleotide (CpG‐ODN) (ODN2395; InvivoGen, San Diego, CA, USA), monophosphoryl lipid A (MPL) (InvivoGen), and Montanide ISA51VG (Seppic, Paris, France) were used as adjuvants.
Immunization protocol
The back skin of B6 mice was shaved 1–3 days before the immunization. Beselna cream containing 5% imiquimod (approximately 25mg cream/head) was topically applied on the back skin of mice under anesthesia. After 30 min, an antigen solution consisting of OVA257–264 (0.3 μg for the carboxyfluorescein succinimidyl ester (CFSE) proliferation assay and 5 μg for the other assays) with glycyrrhizin or other HMGB1 inhibitors in a total volume of 0.1 mL was s.c. injected to the same region.
Proliferation assay
Antigen‐induced proliferation of T cells was evaluated by transfusion of OT‐1 cells. Spleen cells from OT‐1 mice were labeled with 5 μM CSFE (Molecular Probes, Eugene, OR, USA) at 37°C for 15 min. After washing the cells twice, 3 × 106 cells (0.2 mL) were i.v. transfused to each B6 mouse (three to four mice/group). One day after the transfusion, the OT‐1 cell‐transfused B6 mice were immunized with OVA257–264 plus various adjuvants and HMGB1 inhibitors. Three days after the vaccination, the spleen cells from the vaccinated B6 mice were stained with anti‐mouse CD8a‐PerCP/Cy5.5 (BioLegend, San Diego, CA, USA) and analyzed for the proliferation of the OT‐1 CD8 T cells by using a FACS CantoII system (BD Biosciences, Franklin Lakes, NJ, USA).
Enzyme‐linked immunospot assay
The CTL responses were evaluated by γ‐interferon (IFN‐γ) enzyme‐linked immunospot (ELISPOT) assay. Five mice in each treatment group were immunized once per week for 2 weeks. Spleen cells were collected from the vaccinated B6 mice 1 week after the last vaccination and resuspended in red blood cell lysis buffer, then resuspended at 107 cells/mL in X‐VIVO15 (Lonza, Walkersville, MD, USA) supplemented with 10 mM HEPES, 2 mM L‐alanyl‐L‐glutamine (nacalai tesque, Kyoto, Japan), and 0.05 mM 2‐mercaptoethanol. Spleen cells (106 in 0.1 mL) were seeded in each well of a 96‐well Multi‐Screen Filter Plate (MSHAS4510; Merck Millipore, Darmstadt, Germany) coated by anti‐mouse IFN‐γ antibody (AN18; Mabtech, Nacka Strand, Sweden). An equal volume of X‐VIVO 15 with or without 20 μg/mL OVA257–264 was added to each well. After 18 h of incubation at 37°C, the plate was incubated with biotin‐conjugated anti‐mouse IFN‐γ (R4‐6A2; Mabtech) at room temperature for 2 h, followed by 1 h of incubation with ExtrAvidin alkaline phosphatase (Sigma‐Aldrich, St. Louis, MO, USA) at room temperature. γ‐Interferon spots were visualized by SIGMAFAST BCIP/NBT (Sigma‐Aldrich). The ELISPOT plates were analyzed by ImmunoCapture version 6 (Cellular Technology, Shaker Heights, OH, USA).
Therapeutic vaccine model against transplanted tumor
EG.7 cells (1 × 106 cells in 0.1 mL) were s.c. injected to the left back skin of shaved B6 mice. When the tumor mass grew to 7–8 mm in diameter, vaccination was started. OVA257–264 (5 μg) with glycyrrhizin in a total volume of 0.1 mL was s.c. injected to the underside of the back skin of the mice, following the topical application of imiquimod. The vaccination was carried out once a week for 3 weeks. Tumor growth was measured every 2 or 3 days. Tumor size was expressed as follows: tumor size (mm2) = length (mm) × width (mm).
Real‐time PCR
The levels of various cytokines and inflammation‐related genes were measured by real‐time PCR. The skin inoculation site (10 mm × 15 mm) and the spleen specimens were obtained from mice at 6, 12, and 24 h after the inoculation. The specimens were soaked in RNAlater (Ambion, Austin, TX, USA) and stored at 4°C. Extraction and purification of RNA was undertaken using an RNeasy Plus Universal Mini Kit with the TissueRuptor system (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. cDNAs were synthesized from 500 ng total RNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Real‐time PCR was carried out using Thunderbird SYBR qPCR Mix (Toyobo) on a StepOnePlus (Applied Biosciences, Foster City, CA, USA).
Primer sequences used for the PCR were as follows: interleukin (IL)‐1β, forward 5′‐CTTCAGGCAGGCAGTATCACTCAT‐3′ and reverse 5′‐TCTAATGGGAACGTCACACACCAG‐3′; IL‐6, forward 5′‐CCTCTCTGCAAGAGACTTCC‐3′ and reverse 5′‐CTCCGGACTTGTGAAGTAGG‐3′; IL‐10, forward 5′‐TACAGCCGGGAAGACAATAA‐3′ and reverse 5′‐AAGGAGTCGGTTAGCAGTAT‐3′; TNF‐α, forward 5′‐CCCTCACACTCAGATCATCTTCT‐3′ and reverse 5′‐GCTACGACGTGGGCTACAG; COX‐2, forward 5′‐CCTGCTGCCCGACACCTTCA‐3′ and reverse 5′‐AGCAACCCGGCCAGCAATCT‐3′; arginase 1 (Arg1), forward 5′‐CTCCAAGCCAAAGTCCTTAGAG‐3′ and reverse 5′‐AGGAGCTGTCATTAGGGACATC; IFN‐γ, forward 5′‐GTGAGGAAATACTTCCACAGGATCAC‐3′ and reverse 5′‐GCTCTCCAGACTTCTGCTCTG‐3′; and GAPDH, forward 5′‐AGGTCGGTGTGAACGGATTTG‐3′ and reverse 5′‐TGTAGACCATGTAGTTGAGGTCA‐3′.
Statistical analysis
The statistical analyses were carried out using JMP version 11 software (SAS Institute, Cary, NC, USA).
Results
Effect of glycyrrhizin on antigen‐induced proliferation of OT‐1 T cells
Spleen cells of OT‐1 mice, labeled with CFSE, were transfused to B6 mice. One day later the mice were immunized by s.c. injection of OVA257–264 peptide with topical imiquimod, a TLR7 agonist, as an adjuvant. Glycyrrhizin was mixed with the antigen peptide. Proliferation of OT‐1 CD8 T cells was examined 2 or 3 days after the immunization by FACS analyses (Fig. 1). In this experiment, OVA plus imiquimod induced proliferation of most OT‐1 CD8 T cells, therefore, no difference was observed in total proliferated cell contents. However, if compared in terms of ≥3‐times divided cell contents, glycyrrhizin significantly enhanced the immunization with OVA plus imiquimod. The enhancing effect of glycyrrhizin was not observed in the OVA257–264 immunized group without imiquimod. These results suggest that glycyrrhizin has no adjuvant effect when given alone, but does have an effect as a co‐adjuvant.
Figure 1.
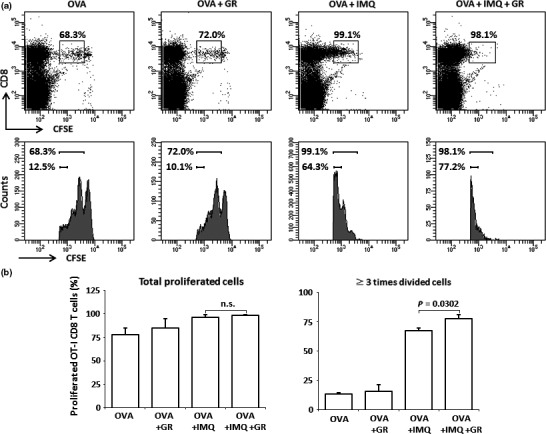
Carboxyfluorescein succinimidyl ester (CFSE)‐labeled spleen cells of OT‐1 mice were transfused to B6 mice. One day later the mice were immunized by s.c. injection of OVA 257–264 peptide (OVA) with topical imiquimod (IMQ) (four mice/group). Three days after the immunization, the proliferation of OT‐1 CD8 T cells was examined by FACS analyses. (a) Representative staining patterns and histograms of each treatment group are shown. Boxes and the upper bars (histograms) indicate the proliferated OT‐1 CD8 T cells. Lower bars in the histograms indicate the OT‐1 CD8 T cells that were divided at least three times. (b) Contents of total OT‐1 CD8 T cells (left) and OT‐1 CD8 T cells divided at least three times (right) are shown. Data are representative of four experiments. GR, glycyrrhizin; n.s., not significant.
To examine whether glycyrrhizin would enhance antigen‐induced T‐cell proliferation in other adjuvant systems, MPL, CpG‐ODN (ODN2395), and Freund's incomplete adjuvant (Montanide ISA51VG) (Fig. 2). Monophosphoryl lipid A and CpG‐ODN are TLR4 and TLR9 agonists, respectively, whereas Freund's incomplete adjuvant is a kind of antigen deposit system in which antigen peptides are slowly released from water in oil emulsion of a peptide/adjuvant mixture. The effect of glycyrrhizin was confirmed in both CpG‐ODN and MPL, but was not observed in the Freund's incomplete adjuvant system.
Figure 2.
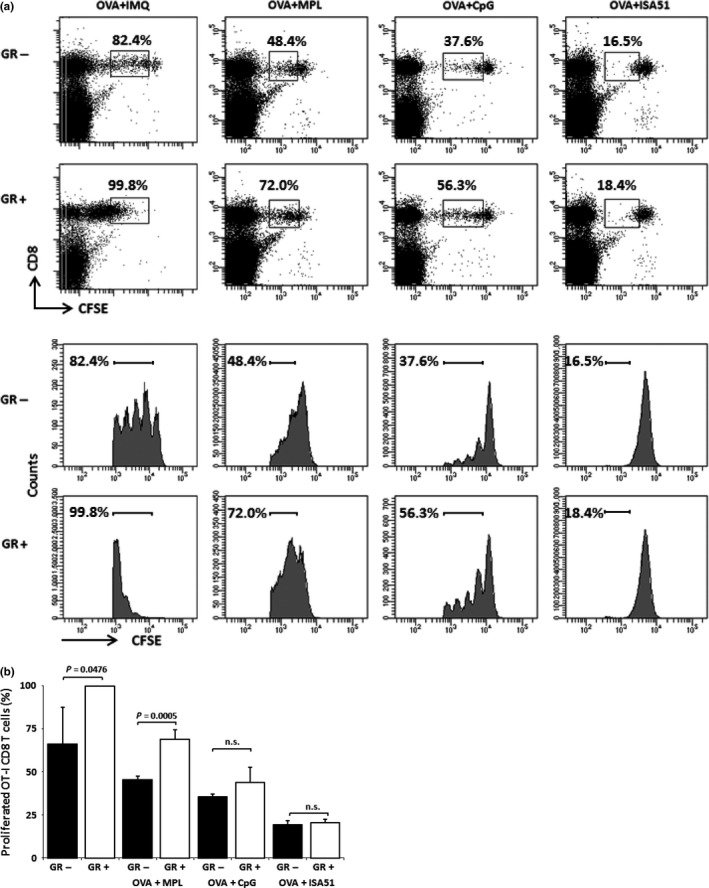
Carboxyfluorescein succinimidyl ester (CFSE)‐labeled OT‐1 cell‐transfused B6 mice were immunized by s.c. injection of OVA 257–264 peptide (OVA) with various adjuvants (four mice/group), and proliferation of OT‐1 CD8 T cells was examined 3 days after the immunization by FACS analyses. The descriptions of (a) Representative staining patterns of each treatment group are shown. (b) Contents of total proliferated OT‐I CD8 T cells are shown. Experiments were carried out twice for CpG oligodeoxynucleotide (CpG) and once for the other adjuvants. Combined data are shown. GR, glycyrrhizin; IMQ, imiquimod; ISA51, Montanide ISA51VG; MPL, monophosphoryl lipid A; n.s., not significant.
Co‐adjuvant effect found in glycyrrhizin also observed in other HMGB1 inhibitors
Glycyrrhizin is a selective inhibitor of HMGB1. Therefore, we next investigated whether the effect of glycyrrhizin on OVA257–264‐induced T‐cell proliferation would also be observed in other synthetic HMGB1 inhibitors, that is, gabexate mesilate, nafamostat, and sivelestat. As shown in Figure 3, gabexate mesilate and nafamostat significantly enhanced OVA257–264‐induced OT‐1 CD8 T‐cell proliferation, and sivelestat also showed a trend toward enhancement, although this effect was not significant. Gabexate mesilate and nafamostat possess a serine protease inhibitor and sivelestat has neutrophil elastase inhibitor activities, whereas glycyrrhizin has no such activities. These facts suggest that the enhancing effect found in glycyrrhizin is also observed in other HMGB1 inhibitors.
Figure 3.
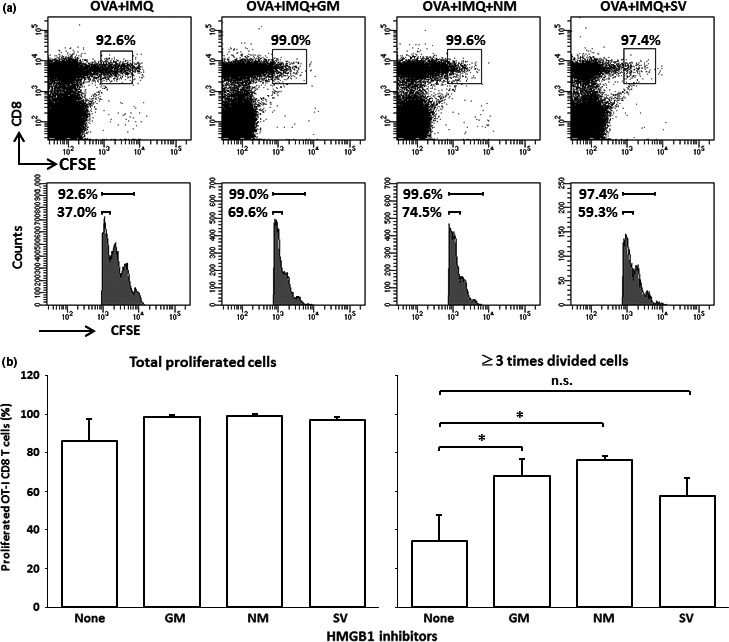
Carboxyfluorescein succinimidyl ester (CFSE)‐labeled OT‐1 cell‐transfused B6 mice were immunized by s.c. injection of OVA 257–264 peptide (OVA) with topical imiquimod (IMQ) and high mobility group box 1 (HMGB1) inhibitors (200 μg/mouse) (four mice/group), and the proliferation of OT‐1 CD8 T cells was examined 3 days after the immunization by FACS analyses. (a) Representative staining patterns of each treatment group are shown. The boxes and the upper bar (histograms) indicate the proliferated OT‐I CD8 T cells. Lower bars in the histograms indicate the OT‐I CD8 T cells that were divided at least three times. (b) Contents of total OT‐I CD8 T cells (left) and OT‐I CD8 T cells divided at least three times (were) are shown. Data are representative of two experiments. *P < 0.05 by anova. GM, gabexate mesilate; IMQ, imiquimod; NM, nafamostat mesilate; SV, sivelestat.
Effect of glycyrrhizin on in vivo CTL induction
The OVA257–264‐induced CTL induction observed in B6 mice was analyzed by ELISPOT assay. B6 mice were immunized with s.c. injection of OVA257–264 mixed with or without glycyrrhizin after imiquimod was topically applied. Spleen cells were obtained 1 week after the last immunization and subjected to IFN‐γ ELISPOT assay without in vitro expansion of antigen‐specific T cells. The numbers of IFN‐γ‐producing cells in response to the OVA257–264 in each mouse are shown in Figure 4. If the values were higher than the mean + 2SD of the IFN‐γ‐producing cell number of mice immunized with OVA257–264 alone, the response was considered to be augmented; the rates of augmented versus total mice are also shown. The ratio of augmented to total mice in the OVA257–264 plus imiquimod plus glycyrrhizin group was significantly higher than that in the OVA257–264 plus imiquimod group (4/5 vs 2/5; P < 0.05). Such an enhancing effect of glycyrrhizin was not observed in the OVA257–264 immunized group without imiquimod.
Figure 4.
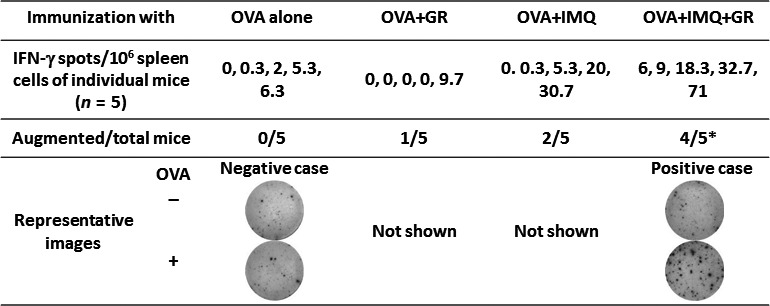
B6 mice were immunized once a week for 2 weeks with OVA 257–264 peptide (OVA) (five mice/group). One week after the last immunization, spleen cells were subjected to γ‐interferon (IFN‐γ) enzyme‐linked immunospot assay (ELISPOT). If the values were higher than the mean + 2SD of the number of IFN‐γ‐producing cells in mice immunized with OVA 257–264 alone (8.58/106 spleen cells), the response was considered to be augmented. Representative ELISPOT images of negative and positive cases are shown. Data are representative of two experiments. *P < 0.05 by Fisher's exact test. GR, glycyrrhizin; IMQ, imiquimod.
Glycyrrhizin augments in vivo antitumor effects induced by peptide vaccination
The co‐adjuvant effect of glycyrrhizin was further examined by means of an in vivo therapeutic model. EG.7 tumor cells were s.c. transplanted in B6 mice. After it was confirmed that the transplanted EG.7 cells had established a solid tumor mass, the mice were immunized with OVA257–264 with/without imiquimod and glycyrrhizin. The tumor growth and overall survival of each group of mice are shown in Figure 5(a). Tumor growth appeared to be inhibited in the group receiving OVA257–264/imiquimod plus glycyrrhizin compared to the growth in the groups receiving OVA257–264/imiquimod or OVA257–264 alone. The plot of the tumor growth of individual mice between days 14 and 26 in the OVA plus imiquimod group and the OVA plus imiquimod with glycyrrhizin group shows that the growth was significantly different between the two groups. Tumor specificity was subsequently examined. EG.7 or parental EL‐4 (OVA‐negative) cells were transplanted to B6 mice and imiquimod plus glycyrrhizin treatment with or without OVA257–264 vaccination was started after establishment of the tumor mass. As shown in Figure 5(b), growth inhibition of the tumor by OVA vaccination was only observed in EG.7‐transplanted mice, and was not observed in EL‐4‐transplanted mice. Therefore, the tumor‐growth inhibition observed in this study was antigen‐specific.
Figure 5.
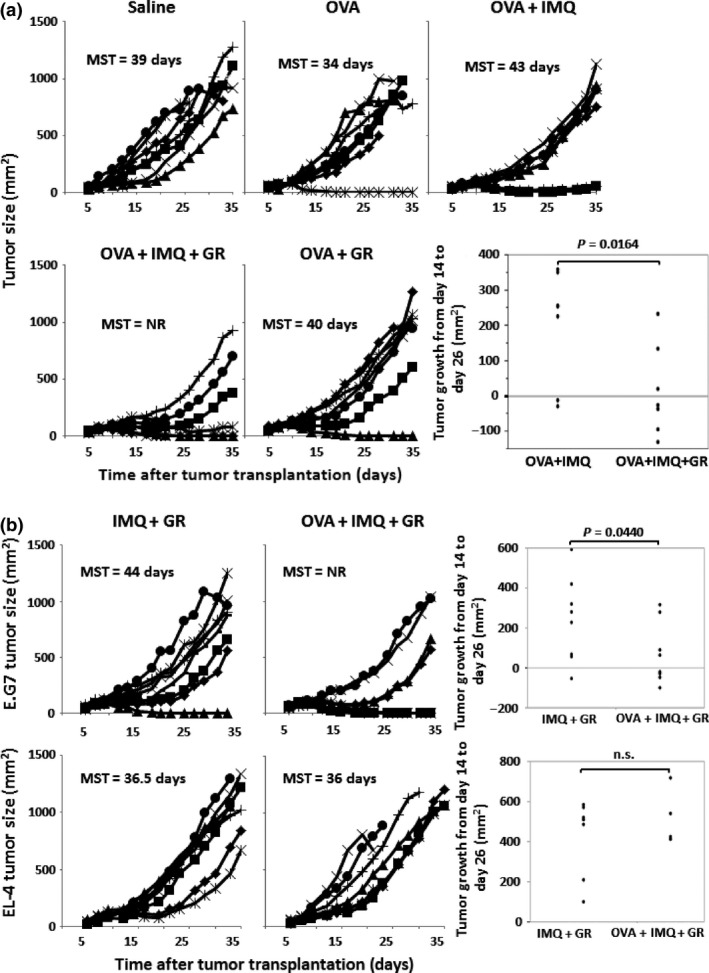
(a) EG.7 tumor cells were s.c. transplanted in B6 mice (day 0). After it was confirmed that the transplanted EG.7 cells had established a solid tumor mass, the mice were weekly immunized with OVA 257–264 (OVA) with/without imiquimod (IMQ) and glycyrrhizin (GR) (days 7, 14, and 21) (seven mice/group). The tumor growth and median overall survival (MST) of each group of mice are shown. The tumor growths from day 14 to day 26 in the group given OVA plus imiquimod with or without glycyrrhizin are also shown. Statistical significance was analyzed by anova (one‐sided test). (b) EG.7 or parental EL‐4 tumor cells were s.c. transplanted in B6 mice (day 0) and the mice were weekly treated with imiquimod and glycyrrhizin with/without OVA 257–264 (days 7, 14, and 21) (seven or eight mice/group). The tumor growth of each group of mice is shown. Data are representative of two experiments. NR, not reached.
Effect of glycyrrhizin on the expression of immune‐related genes
The message levels of various immune‐related genes at the inoculation skin site and the spleen after 6–24 h of immunization were further analyzed by real‐time PCR (Fig. 6). The following cytokines were downregulated at the skin immunization sites of the glycyrrhizin‐treated group compared with those of the glycyrrhizin‐untreated OVA plus imiquimod group: IL‐1β, IL‐6, IL‐10, COX‐2, IFN‐α, and TNF‐α. The Arg1 levels were suppressed by imiquimod and the levels of Arg1 in the OVA plus imiquimod plus glycyrrhizin‐treated group were still lower than those in the group treated with OVA alone (Fig. 6a). Injection of glycyrrhizin alone did not alter the immune‐related cytokine levels (data not shown). The expression profiles of the genes in the spleen or skin were similar among the different treatment groups (data not shown), with the exception that the IL1B, IFNA, and IL6 genes were transiently upregulated in the spleens of the glycyrrhizin‐treated group compared with those of the glycyrrhizin‐untreated OVA plus imiquimod group (Fig. 6b).
Figure 6.
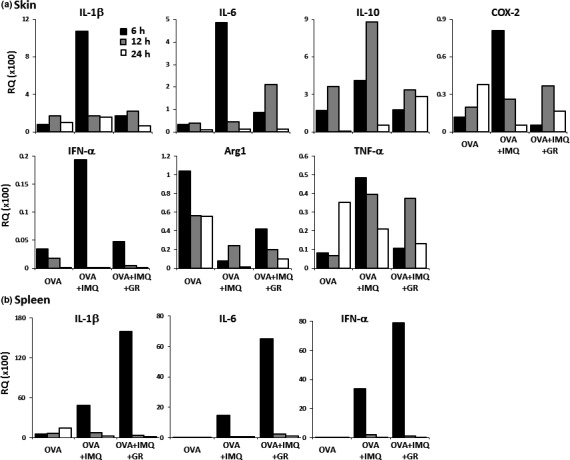
Message levels of various immune‐related genes in mice immunized with OVA 257–264 peptide (OVA), measured at the inoculation skin site (a) and the spleen (b) 6 h (black column), 12 h (gray), and 24 h (white) after immunization, were analyzed by real‐time PCR. Samples of mRNA were extracted from the pooled tissues of three mice from each treatment group. The expression levels were calculated relative to GAPDH expression in untreated mouse skin or spleen. (b) Message levels of interleukin‐1β (IL‐1β; pro‐inflammatory cytokine), interferon‐α (IFN‐α; upregulator of CTL induction), and IL‐6 (pro‐inflammatory cytokine) in the spleen are shown. The expression profile of the other genes among the treatment groups in the spleen was similar to that of the skin (data not shown). Data are representative of two experiments. Arg1, arginase 1, mediator of immunosuppressive M2 macrophages and myeloid‐derived suppressor cells; COX‐2, responsible for inflammation mediators such as prostaglandin E2; GR, glycyrrhizin; IL‐10, immunosuppressive cytokine; IMQ, imiquimod; TNF‐α, tumor necrosis factor‐α, pro‐inflammatory cytokine.
Discussion
We found that glycyrrhizin augmented the induction of CTLs by peptide vaccination with imiquimod. The effect of glycyrrhizin was confirmed in other adjuvant systems, such as CpG‐ODN and MPL, and the effect was common to a group of HMGB1 inhibitors, namely, gabexate mesilate, nafamostat, and sivelestat.10 However, glycyrrhizin, as well as two of the HMGB1 inhibitors, gabexate mesilate and sivelestat, did not augment the T‐cell response induced by peptide vaccination when given without adjuvant. This indicates that glycyrrhizin is a co‐adjuvant and has no adjuvant activity when given alone.
One of the possible mechanisms of the co‐adjuvant activity of glycyrrhizin is the alteration of immune‐related cytokine profiles. Cytokine analysis indicated that the injection of glycyrrhizin alone did not alter the immune‐related cytokine levels, but glycyrrhizin downregulated the induction of inflammatory cytokines by imiquimod. Immunosuppressive effects of inflammatory cytokines, have been reported, for example, suppression of the Th1 response by IL‐10 and CTL by COX‐2. Therefore, inhibition of these inflammatory cytokines by glycyrrhizin may result in the augmentation of CTL induction. Indeed, the augmenting effect of glycyrrhizin was not observed in Freund's incomplete adjuvant system, which is an antigen deposit system that does not contain cytokine inducers such as innate immunity receptor agonists. Although most of the inflammatory cytokines negatively affect antitumor immunity, IL‐1β and IFN‐α are known to be useful for the induction of antitumor immunity. Glycyrrhizin had opposite effects on the expression of IL‐1β or IFN‐α in the skin and spleen, in that these expressions were suppressed in the skin and upregulated in the spleen. The expression levels of the two genes in the spleen were respectively >10‐ and >100‐fold higher than those in the skin. Therefore, the upregulation of IL‐1β and IFN‐α in the spleen might be one of the main mechanisms of the augmentation of CTL induction. A similar tendency was observed in the expression of IL‐6. Downregulation of IL‐6 in the skin may positively affect antitumor immunity through antigen‐captured DC maturation, although upregulation of IL‐6 in the spleen negatively affects both the induction and effector phases of antitumor CTL response.17 Additionally, the suppression of TNF‐α, which is involved in tumor progression,6, 7 would also be of benefit in cancer immunotherapy. Tian et al.18 reported that the binding ability of HMGB1 to RAGE on plasmacytoid DCs was greatly improved after formation of the HMGB1‐DNA complex and induced subsequent IFN‐α production through DNA–TLR9 interaction. Therefore, the observed discrepancy in the cytokine production between the skin and spleen may have been due to the different levels of cell‐free DNA released by injection‐related tissue destruction in each site, and/or the fact that different cell types show the main responses to imiquimod in different organs, such as Langerhans cells in the skin and plasmacytoid DCs in the spleen.
The second possible mechanism of co‐adjuvant activity of glycyrrhizin is the inhibition of HMGB1‐mediated immune suppression. The immunosuppressive effect of HMGB1 has been reported, and one of the mechanisms of this suppression is considered to be the direct inhibition of Th1 cell function through TIM‐3.19 TIM‐3 is also expressed on DCs,19 macrophages,20 and natural killer cells.21, 22 Therefore, HMGB1 inhibits both the induction and effector phases of acquired immunity, particularly in the case of antitumor immunity.23 Another immunosuppressive mechanism of HMGB1 is acceleration of the development of myeloid‐derived suppressor cells.24 Myeloid‐derived suppressor cells in the tumor microenvironment inhibit antitumor immunity by secreting IL‐10, and glycyrrhizin suppresses the IL‐10 production of myeloid‐derived suppressor cells.24, 25
Many different types of adjuvants, including pathogen‐associated molecular patterns (PAMPs) and DAMP sensors, such as TLRs, have been developed, and some of them have very strong immunopotentiating activity when compared with classical adjuvants such as alum or incomplete Freund's adjuvant.15 However, relatively severe adverse events are also expected in these new adjuvants. The most frequently observed adverse event is an induction of inflammation at the injection site.3, 26 Inflammation involves tissue damage, resulting in the secretion of HMGB1. Activated macrophages at the inflammation site also secrete HMGB1.24 Therefore, HMGB1 is ubiquitously found in the inflammatory tissue. Glycyrrhizin exerts anti‐inflammation activity by blocking HMGB1, which is also known as an initiation factor of inflammation,20, 24 and glycyrrhizin is clinically used against various types of allergy and hepatitis.10, 27, 28 In this study, topical use of imiquimod induced skin redness, and this effect was completely inhibited by glycyrrhizin without inhibition of the subsequent induction of specific immunity. Another important adverse event of adjuvants is autoimmune disease. Associations of TLRs with rheumatoid arthritis,29 inflammatory bowel diseases and colitis,30 and systemic sclerosis31 have been reported. Systemic lupus erythematosus has been associated with various PAMP/DAMP sensors, such as the retinoic acid‐inducible gene‐I‐like receptors, TLR7, TLR8, TLR9, and RAGE.11, 15 Therefore, agonists to such sensors have the potential to induce autoimmune diseases. In addition, HMGB1 itself has the capacity to induce various autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and Sjogren's syndrome, through RAGE and/or TLR2/4, but also binds numerous inflammation‐related molecules such as bacterial lipopolysaccharide, IFN‐γ, IL‐1β, and chemokine (C‐X‐C motif) ligand 12 and induces synergistic effects.11, 15 Thus, glycyrrhizin may inhibit PAMP/DAMP sensor agonist‐induced autoimmune diseases as well as inflammation at the injection site.
In conclusion, we found that glycyrrhizin has a co‐adjuvant activity that enhances vaccination‐induced specific immunity and also facilitates a reduction in the adverse events of vaccination, most of which are caused by adjuvants. These results, together with the safety of glycyrrhizin, based on more than a half century of clinical experience, indicate that further clinical trials are warranted to confirm the usefulness of glycyrrhizin as a co‐adjuvant for vaccination against cancer.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgement
This study was supported by the Japan Society for the Promotion of Science KAKENHI Grant Number 26430176 (to A.Y.).
Cancer Sci 107 (2016) 1721–1729
Funding Information
Japan Society for the Promotion of Science (Grant/Award Number: JSPS KAKENHI Grant Number 26430176
References
- 1. Yamada A, Sasada T, Noguchi M, Itoh K. Next‐generation peptide vaccines for advanced cancer. Cancer Sci 2013; 104: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR‐based immune adjuvants. Vaccine 2011; 29: 3341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baxevanis CN, Voutsas IF, Tsitsilonis OE. Toll‐like receptor agonists: current status and future perspective on their utility as adjuvants in improving anticancer vaccination strategies. Immunotherapy 2013; 5: 497–511. [DOI] [PubMed] [Google Scholar]
- 4. Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004; 16: 3–9. [DOI] [PubMed] [Google Scholar]
- 5. Compagno M, Lim WK, Grunn A et al Mutations of multiple genes cause deregulation of NF‐kappaB in diffuse large B‐cell lymphoma. Nature 2009; 459: 717–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charles KA, Kulbe H, Soper R et al The tumor‐promoting actions of TNF‐alpha involve TNFR1 and IL‐17 in ovarian cancer in mice and humans. J Clin Invest 2009; 119: 3011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Zhou BP. TNF‐α/NF‐κB/Snail pathway in cancer cell migration and invasion. Br J Cancer 2010; 102: 639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basu GD, Tinder TL, Bradley JM et al Cyclooxygenase‐2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol 2006; 177: 2391–402. [DOI] [PubMed] [Google Scholar]
- 9. Inoue J, Aramaki Y. Cyclooxygenase‐2 inhibition promotes enhancement of antitumor responses by transcutaneous vaccination with cytosine‐phosphate‐guanosine‐oligodeoxynucleotides and model tumor antigen. J Invest Dermatol 2007; 127: 614–21. [DOI] [PubMed] [Google Scholar]
- 10. Mollica L, De Marchis F, Spitaleri A et al Glycyrrhizin binds to high‐mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 2007; 14: 431–41. [DOI] [PubMed] [Google Scholar]
- 11. Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1‐related pathologies. Pharmacol Ther 2014; 141: 347–57. [DOI] [PubMed] [Google Scholar]
- 12. Li G, Liang X, Lotze MT. HMGB1: the central cytokine for all lymphoid cells. Front Immunol 2013; 4: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JS, Svetkauskaite D, He Q et al Involvement of toll‐like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004; 279: 7370–7. [DOI] [PubMed] [Google Scholar]
- 14. Dumitriu IE, Baruah P, Valentinis B et al Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 2005; 174: 7506–15. [DOI] [PubMed] [Google Scholar]
- 15. Jounai N, Kobiyama K, Takeshita F, Ishii KJ. Recognition of damage‐associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol 2013; 2: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang D, Lotze MT. Tumor immunity times out: TIM‐3 and HMGB1. Nat Immunol 2012; 13: 808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher DT, Appenheimer MM, Evans SS. The two faces of IL‐6 in the tumor microenvironment. Semin Immunol 2014; 26: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian J, Avalos AM, Mao SY et al Toll‐like receptor 9‐dependent activation by DNA‐containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007; 8: 487–96. [DOI] [PubMed] [Google Scholar]
- 19. Chiba S, Baghdadi M, Akiba H et al Tumor‐infiltrating DCs suppress nucleic acid‐mediated innate immune responses through interactions between the receptor TIM‐3 and the alarmin HMGB1. Nat Immunol 2012; 13: 832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson AC, Anderson DE, Bregoli L et al Promotion of tissue inflammation by the immune receptor Tim‐3 expressed on innate immune cells. Science 2007; 318: 1141–3. [DOI] [PubMed] [Google Scholar]
- 21. Gleason MK, Lenvik TR, McCullar V et al Tim‐3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin‐9. Blood 2012; 119: 3064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ju Y, Hou N, Meng J et al T cell immunoglobulin‐ and mucin‐domain‐containing molecule‐3 (Tim‐3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010; 52: 322–9. [DOI] [PubMed] [Google Scholar]
- 23. Anderson AC. Tim‐3, a negative regulator of anti‐tumor immunity. Curr Opin Immunol 2012; 24: 213–6. [DOI] [PubMed] [Google Scholar]
- 24. Parker KH, Sinha P, Horn LA et al HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid‐derived suppressor cells. Cancer Res 2014; 74: 5723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand‐Rosenberg S. Cross‐talk between myeloid‐derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179: 977–83. [DOI] [PubMed] [Google Scholar]
- 26. Vasilakos JP, Tomai MA. The use of Toll‐like receptor 7/8 agonists as vaccine adjuvants. Expert Rev Vaccines 2013; 12: 809–19. [DOI] [PubMed] [Google Scholar]
- 27. Ghosh N, Ghosh R, Mandal V, Mandal SC. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol 2011; 49: 970–88. [DOI] [PubMed] [Google Scholar]
- 28. Coon JT, Ernst E. Complementary and alternative therapies in the treatment of chronic hepatitis C: a systematic review. J Hepatol 2004; 40: 491–500. [DOI] [PubMed] [Google Scholar]
- 29. Joosten LA, Abdollahi‐Roodsaz S, Dinarello CA, O'Neill L, Netea MG. Toll‐like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol 2016; 12: 344–57. doi: 10.1038/nrrheum.2016.61. [DOI] [PubMed] [Google Scholar]
- 30. Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among toll‐like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res 2015; 2015: 489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciechomska M, Cant R, Finnigan J, van Laar JM, O'Reilly S. Role of toll‐like receptors in systemic sclerosis. Expert Rev Mol Med 2013; 15: e9. [DOI] [PubMed] [Google Scholar]


