Abstract
The presence of tumor‐infiltrating lymphocytes (TILs) is associated with favorable long‐term outcome in breast cancer. However, little is known about changes in TILs during metastatic progression. To confirm our hypothesis that malignant tumors escape from the host immune system during metastasis, we evaluated the percentage of TILs in paired samples of primary and metastatic breast tumors. We retrospectively identified 25 patients with human epidermal growth factor receptor‐2 (HER2+, n = 14) and triple negative (TN, n = 11) early breast cancer diagnosed between 1990 and 2009 at Tokai University Hospital (Isehara, Japan) and who subsequently experienced regional or distant recurrence confirmed by tumor biopsy/resection. Hematoxylin–eosin‐stained slides of these paired samples were evaluated for stromal TILs. Immunohistochemical staining was carried out using primary antibodies against CD4, CD8, Foxp3, programmed cell death ligand 1 (PD‐L1), PD‐L2, and HLA class I for characterizing the TILs and breast tumors. The percentage of TILs in the primary tumors was significantly higher (average 34.6%) than that in metastatic tumors (average 15.7%) (paired t‐test, P = 0.004) and that of CD8+ and CD4+ T cells significantly decreased from primary to metastatic tumors (paired t‐test, P = 0.008 and P = 0.026, respectively). The PD‐L1, PD‐L2, and HLA class I antibody expression changed from positive to negative and vice versa from the primary to the metastatic tumors. Tumors at first metastatic recurrence in HER2+ and TN breast cancers have a lower percentage of TILs and CD8+ and CD4+ T cells compared to primary tumors, which indicates that immune escape plays a role in tumor progression.
Keywords: Immune microenvironment, immunohistochemistry, metastatic breast tumor, primary breast tumor, tumor‐infiltrating lymphocytes
The presence of tumor‐infiltrating lymphocytes (TILs) is associated with favorable long‐term outcome in breast cancer.1, 2 Previous studies have reported that immune activation at the baseline, as assessed by pathology or gene expression arrays, is associated with a higher likelihood of pathological complete response after neoadjuvant chemotherapy (NAC), particularly in human epidermal growth factor receptor‐2 (HER2)‐positive and triple negative (TN) breast cancers.3, 4, 5, 6, 7, 8, 9, 10 Furthermore, trastuzumab has been predicted to have beneficial effects.11 Increased expression of a subset of immune function genes may provide a means of predicting the benefits of adjuvant trastuzumab treatment.12 Tumor‐infiltrating lymphocytes in breast tumors mainly comprise cytotoxic (CD8+) T cells, followed by helper (CD4+) T cells and natural killer cells.13 A high CD8+/Foxp3+ ratio in the TILs of biopsy specimens was found to be a strong predictor of pathological complete response after NAC in TN breast cancers.14 In addition, the presence of TILs in residual disease after NAC is associated with better prognosis in TN breast cancers patients. This suggests that chemotherapy could convert low‐TIL tumors into high‐TIL tumors. This finding supports the concept that chemotherapy could partly exert its antitumor effect through the immune system.15 Preclinical studies have also suggested that cytotoxic agents may partly exert their antitumor activity by inducing immune responses against tumor cells.16
However, little is known about the change in TILs during metastatic progression and the prognostic impact of TILs in metastatic sites.17, 18 The current concept of cancer immunoediting leading from immune surveillance to immune escape is proposed to comprise three essential phases: (i) elimination; (ii) equilibrium; and (iii) escape.19 In the elimination phase, tumor cells undergo angiogenesis and stromal remodeling, resulting in tumor cell variants with low immunogenicity and resistance to immune attack. These tumor cell variants then proceed to the equilibrium phase but the elimination phase continues through immune selection pressure. Tumor progression then leads to the release of tumor‐derived soluble factors that are involved in several mechanisms of immune evasion in the escape phase.20
We hypothesized that malignant tumors escape from the immune system of the host during the process of metastasis. We therefore aimed to study the immune escape by evaluating TILs in paired samples from primary and metastatic breast tumors. We also evaluated the prognostic impact of TILs in the metastatic sites.
Methods
Patients
This study was reviewed and approved by the Institutional Review Board for Clinical Research, Tokai University (Isehara, Japan). We retrospectively identified 25 patients with TN or HER2+ early breast cancer diagnosed between 1990 and 2009 at Tokai University Hospital and who subsequently experienced a regional or distant recurrence confirmed by tumor biopsy/resection. Patients who had only local events were excluded because it is difficult to determine whether the tumor has recurred or is a new primary tumor.21 The clinical characteristics of all the patients were obtained from their medical records.
Pathological assessment
All the tumor specimens were fixed in 10% formalin and embedded in paraffin, and 4‐μm‐thick sections were prepared for H&E staining and immunohistochemistry (IHC) and were reviewed by a pathologist. Immunohistochemistry was carried out using the following primary antibodies: anti‐estrogen receptor (ER) (–2009, clone 1D5; Dako, Carpinteria, CA, USA; 2010–, clone SP1; Roche Diagnostics, Basel, Switzerland), anti‐HER2 (–2009, polyclonal, HercepTest II, Dako; 2010–, clone 4B5, Roche Diagnostics), anti‐CD4 (clone SP35; Spring Bioscience, Pleasanton, CA, USA), anti‐CD8 (clone C8/144B; Nichirei, Tokyo, Japan), anti‐Foxp3 (clone 236A/E7; Abcam, Cambridge, MA, USA), anti‐programmed cell death ligand 1 (PD‐L1) (polyclonal, ab58810; Abcam), anti‐Pdcd‐1L2 (PD‐L2, clone XX19; Santa Cruz Biotechnology, Dallas, TX, USA), and anti‐HLA class I A, B, C (clone EMR8‐5; Hokudo, Sapporo, Japan). The specimens were considered positive for hormone receptor if ≥1% of the cancer cells expressed ER. For the patients determined as HER2+ by IHC, FISH (PathVysion kit; Abbott, Des Plaines, IL, USA) was used to confirm HER2+ disease. The breast cancer subtypes were classified using IHC as previously described:22 HER2‐positive or HER2‐overexpressing (HER2+) and TN (ER− and HER2−). Hematoxylin–eosin‐stained slides for the paired match cases were evaluated for stromal TILs using full sections in 10% increments (<10%, 10%–100%) by a pathologist (N.K.) blinded to the clinicopathological characteristics of the patients, as recommended.23 The specimens were classified into three groups: low TIL (<10%), intermediate TIL (10–<60%), and lymphocyte‐predominant breast cancer (LPBC) (≥60%).
To quantify the TILs in each antibody‐stained slide, we used a NanoZoomer 2.0 HT (Hamamatsu Photonics, Hamamatsu, Japan) at ×40 magnification. Three non‐overlapping fields with high numbers of TILs on the H&E‐stained slides were selected.14 CD4, CD8, and PD‐L1 positivity was determined by membranous lymphocyte staining, and Foxp3 and PD‐L2 positivity was determined by nuclear lymphocyte staining. The expression of CD4, CD8, Foxp3, PD‐L1, and PD‐L2 by TILs was recorded in 10% increments, and the score of three fields was averaged. The expression of PD‐L1, PD‐L2, and HLA class I in the tumors was scored as 0 (negative), 1 (weak), or 2 (strong).
Statistical analyses
Associations of the percentage of TILs with the positivity for each antibody between the primary and metastatic tumors were evaluated using Fisher's exact test for categorical variables and using the two‐sided t‐tests for continuous variables. Overall survival (OS) was defined as the time from date of the first biopsy of metastatic tissue to the date of death resulting from any cause. Patients who were alive and disease‐free were censored at the date of last contact. Post‐progression OS curves of the patients were drawn using the Kaplan–Meier method, and the statistical difference between two survival curves was calculated using the log–rank test. The correlation between the percentage of TILs and the expression of each of the antibodies was calculated using Spearman's rank correlation coefficient test. In all the analyses, the differences were considered significant at P < 0.05. Statistical analyses were carried out using SPSS, version 23 (Armonk, New York, USA).
Results
Comparison of TILs between primary and metastatic tumors
The characteristics of the 25 breast cancer patients (HER2+, n = 14; TN, n = 11) at the time of diagnosis of the primary breast cancer are presented in Table 1. Six primary tumors and one metastatic tumor were core needle biopsy specimens, and the rest were surgical specimens. We evaluated the core needle biopsy specimens before chemotherapy in the patients who received neoadjuvant therapy for excluding the possibility of alterations in the immune microenvironments of the tumors caused by the neoadjuvant therapy. The first biopsy sites of the metastatic tumors were the skin (n = 7), brain (n = 6), lymph node (n = 4), lung (n = 3), bone (n = 2), and bone marrow/liver/muscle (n = 1). The median follow‐up time after the first biopsy of recurrent tumors was 54 months (range, 2–176 months). Ten (40%) patients had died of metastatic disease at the last follow‐up.
Table 1.
Clinicopathological characteristics of primary surgical breast tumor specimens
| Characteristics | Total patients (n = 25) | HER2+ (n = 14) | TN (n = 11) |
|---|---|---|---|
| Age, years | |||
| Median (range) | 48 (28–64) | 46 (28–61) | 49 (40–63) |
| T | |||
| 1 | 7 (28) | 4 (29) | 3 (27) |
| 2 | 12 (48) | 8 (57) | 3 (27) |
| 3 | 2 (8) | 1 (7) | 1 (9) |
| 4 | 2 (8) | 0 (0) | 2 (18) |
| Unknown | 2 (8) | 1 (7) | 2 (18) |
| N | |||
| 0 | 14 (56) | 8 (57) | 6 (55) |
| 1 | 6 (24) | 5 (36) | 1 (9) |
| 2 | 0 (0) | 0 (0) | 0 (0) |
| 3 | 3 (12) | 1 (7) | 2 (18) |
| Unknown | 2 (8) | 0 (0) | 2 (18) |
| Stage | |||
| 1 | 5 (20) | 2 (14) | 3 (27) |
| 2 | 13 (52) | 10 (71) | 3 (27) |
| 3 | 4 (16) | 1 (7) | 3 (27) |
| 4 | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 3 (12) | 1 (7) | 2 (18) |
| Histological grade | |||
| 1 | 5 (20) | 2 (14) | 3 (27) |
| 2 | 13 (52) | 8 (57) | 5 (45) |
| 3 | 7 (28) | 4 (29) | 3 (27) |
| ER | |||
| Positive | 11 (44) | 11 (79) | 0 (0) |
| Negative | 14 (56) | 3 (21) | 11 (100) |
| Chemotherapy | |||
| No | 5 (20) | 4 (29) | 1 (9) |
| Neoadjuvant | 6 (24) | 3 (21) | 3 (27) |
| Adjuvant | 13 (52) | 7 (50) | 6 (55) |
| Unknown | 1 (4) | 0 (0) | 1 (9) |
| Trastuzumab | |||
| No | 21 (84) | 12 (86) | 9 (82) |
| Yes | 3 (12) | 2 (14) | 1 (9) |
| Unknown | 1 (4) | 0 (0) | 1 (9) |
| Endocrine therapy | |||
| No | 11 (44) | 4 (29) | 7 (64) |
| Yes | 13 (52) | 10 (71) | 3 (27) |
| Unknown | 1 (4) | 0 (0) | 1 (9) |
| Radiotherapy | |||
| No | 16 (64) | 10 (71) | 6 (55) |
| Adjuvant | 8 (32) | 4 (29) | 4 (36) |
| Unknown | 1 (4) | 0 (0) | 1 (9) |
| Alive at last follow‐up | |||
| No | 10 (40) | 6 (43) | 4 (36) |
| Yes | 15 (60) | 8 (57) | 7 (64) |
Data are shown as n (%) unless otherwise indicated. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; TN, triple negative.
The TILs of the primary and metastatic tumors are shown in Table 2. Of the primary tumors, 28% were LPBC, 52% were intermediate TIL tumors, and 20% were low TIL tumors. Among the corresponding first metastatic tumors, 44% were intermediate TIL tumors and 56% were low TIL tumors (Table 3). Overall, the percentage of TILs in the primary tumors was significantly higher (average, 34.6%) than that in the metastatic tumors (average, 15.7%) (paired t‐test, P = 0.004). This difference was similar in the HER2+ (P = 0.036) and TN (P = 0.06) breast cancer groups. The percentage of TILs decreased in 13 of the 25 cases (66%) and increased in 3 of the 25 cases (12%) from the primary tumors to the metastatic tumors (difference >10%). We next undertook an exploratory analysis of the post‐progression OS according to the percentage of TILs at a distant site of recurrence (n = 17). The group with low TILs had a significantly lower OS than that with intermediate TILs (hazard ratio = 3.77; 95% confidence interval, 0.99–14.9; log–rank test, P = 0.038) (Fig. S1).
Table 2.
Comparison of tumor‐infiltrating lymphocytes (TILs) between primary and metastatic breast cancer tumors for each subtype
| Subtype | First site of biopsy | Primary tumor TILs, % | Metastatic tumor TILs, % | Comparison |
|---|---|---|---|---|
| HER2+ | Bone marrow | 10 | <10 | → |
| Brain | 20 | <10 | ▼ | |
| Brain | <10 | <10 | → | |
| Brain | 80 | 10 | ▼ | |
| Brain | <10 | 10 | → | |
| Brain | 30 | 50 | ▵ | |
| Liver | 60 | 10 | ▼ | |
| LN (Ax) | 50 | 20 | ▼ | |
| LN (rotter) | 70 | 20 | ▼ | |
| LN (SCLN) | 70 | 10 | ▼ | |
| Lung | 10 | <10 | → | |
| Muscle (pectoralis) | <10 | 30 | ▵ | |
| Skin (thoracic wall) | 30 | <10 | ▼ | |
| Skin (thoracic wall) | <10 | <10 | → | |
| TN | Bone | 10 | 20 | → |
| Bone | 20 | 20 | → | |
| Brain | 50 | <10 | ▼ | |
| LN (rotter) | 70 | <10 | ▼ | |
| Lung | 10 | 50 | ▵ | |
| Lung | 30 | <10 | ▼ | |
| Skin (abdominal wall) | 80 | 30 | ▼ | |
| Skin (head) | 10 | <10 | → | |
| Skin (thoracic wall) | <10 | <10 | → | |
| Skin (thoracic wall) | 50 | 20 | ▼ | |
| Skin (thoracic wall) | 80 | 10 | ▼ |
The percentage of TILs in the primary tumors was significantly higher (average, 34.6%) than in the metastatic tumors (average, 15.7%) (paired t‐test, P = 0.004). This difference was similar in the human epidermal growth factor receptor 2 (HER2)+ (P = 0.036) and triple negative (TN) (P = 0.06) breast cancer groups. Ax, axillary lymph node; SCLN, supraclavicular fossa lymph node.
Table 3.
Comparison of tumor‐infiltrating lymphocytes (TILs) between primary and metastatic breast cancer tumors
| Primary | Rate, % | Metastatic | Rate, % | |
|---|---|---|---|---|
| Low TIL | 5 | 20 | 11 | 44 |
| Intermediate TIL | 13 | 52 | 14 | 56 |
| LPBC | 7 | 28 | 0 | 0 |
LPBC, lymphocyte‐predominant breast cancer.
Characteristics of TILs
Immunohistochemical evaluations could not be carried out in five primary tumors and two metastatic tumors because of the small quantity of the specimens. The results of the comparison of the expression of antibodies between the primary and metastatic tumors are shown in Table 4 and Figures S2–S4. Representative photographs of each antibody in the primary and metastatic tumors from the same patient are shown in Figure 1. The median percentage of CD8+ T cells was 15.8% (range, <10–37%) and 10.0% (range, <10–37%) and that of CD4+ T cells was 40.0% (range, <10–77%) and 25% (range, <10–83%) in the primary and metastatic tumors, respectively. The percentages of CD8+ and CD4+ T cells in the primary tumors were significantly higher than those in the metastatic tumors (paired t‐test, P = 0.008 and P = 0.026, respectively). Moreover, there was a strong correlation between the percentage of CD4+ T cells and CD8+ T cells in both the primary and the metastatic tumors (r = 0.607 and 0.656, P = 0.005 and 0.001, respectively). The percentage of Foxp3+ T cells was very low (approximately <10%) and stable in the primary and metastatic tumors.
Table 4.
Comparison of positivity rate between primary and metastatic breast cancer tumors for each antibody
| Primary tumor | Metastatic tumor | P‐value | |
|---|---|---|---|
| Total breast tumors, n (%) | 20 (100) | 23 (100) | |
| CNB specimens | 5 (25) | 1 (4) | |
| Surgical specimens | 15 (75) | 22 (96) | |
| TIL positivity rate, median % (range) | |||
| CD4 | 40 (<10–77) | 25 (<10–83) | 0.03 |
| CD8 | 16 (<10–37) | 10 (<10–37) | 0.01 |
| Foxp3 | <10 (<10–10) | <10 (<10) | 0.16 |
| PD‐L1 | <10 (<10–90) | <10 (<10–15) | 0.21 |
| PD‐L2 | 42 (<10–80) | 30 (<10–80) | 0.09 |
| Expression in tumor cells, n (%) | |||
| PD‐L1 | |||
| Strong: 2 | 8 (40) | 5 (25) | 0.46 |
| Weak: 1 | 10 (50) | 15 (75) | |
| Negative: 0 | 2 (10) | 3 (15) | |
| PD‐L2 | |||
| Strong: 2 | 6 (30) | 9 (45) | 0.78 |
| Weak: 1 | 10 (50) | 11 (55) | |
| Negative: 0 | 4 (20) | 3 (15) | |
| HLA | |||
| Strong: 2 | 4 (20) | 6 (30) | 0.89 |
| Weak: 1 | 14 (70) | 15 (75) | |
| Negative: 0 | 2 (10) | 2 (10) | |
CNB, core needle biopsy; PD‐L1/2, programmed cell death ligand 1/2; TIL, tumor‐infiltrating lymphocyte.
Figure 1.
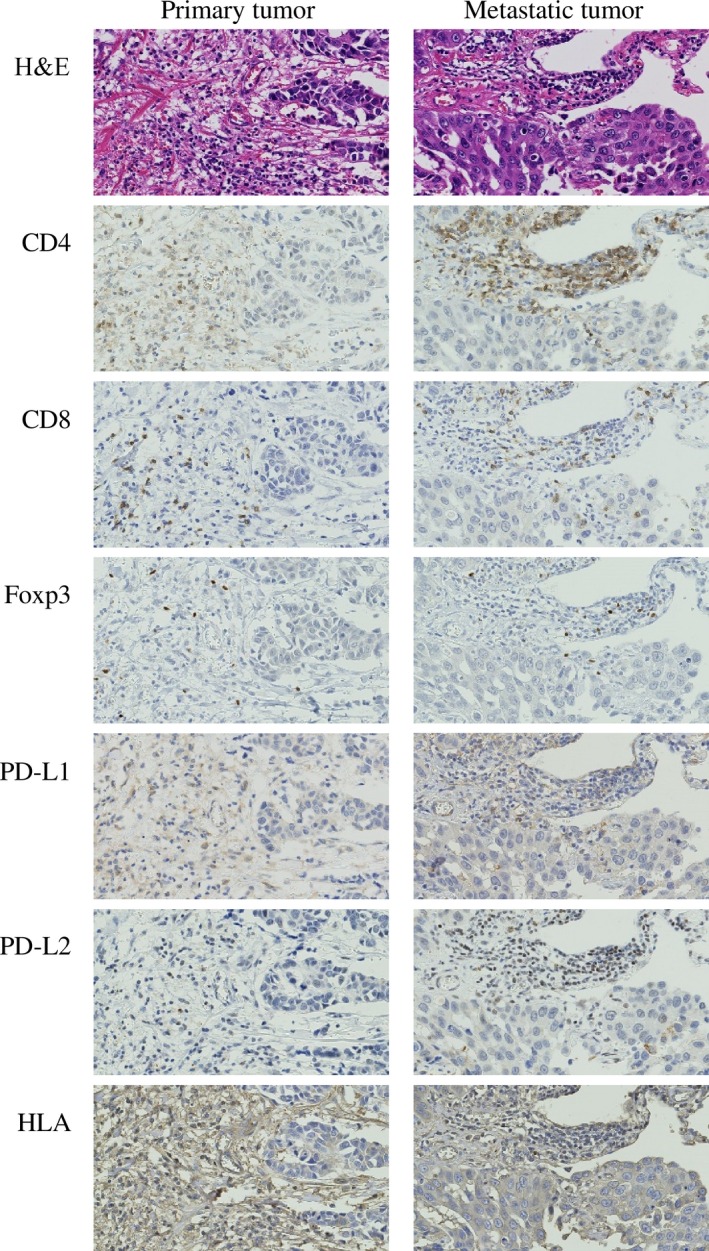
Immunohistochemical staining for primary antibodies against CD4, CD8, Foxp3, programmed cell death ligand 1 (PD‐L1), PD‐L2, and HLA class I to characterize tumor‐infiltrating lymphocytes. Representative photographs are shown from the same patient who had human epidermal growth factor receptor‐2‐positive primary breast tumor (left column) and lung metastasis (right column). Original magnification, ×400.
With regard to the TILs, the median percentage of PD‐L1+ TILs was <10% (range, <10–90%) and <10% (range, <10–15%) and that of PD‐L2+ TILs was 42% (range, <10–80%) and 30% (range, <10–80%) in the primary and metastatic tumors, respectively. There was no significant difference in the percentage of PD‐L1+ and PD‐L2+ TILs between the primary and metastatic tumors.
The expression of PD‐L1, PD‐L2, and HLA class I antibodies changed from strong or weakly positive to negative and vice versa from the primary to the metastatic tumor cells. There was a strong correlation between the expression of PD‐L1 and PD‐L2 (r = 0.602, P = 0.005) and between the percentage of PD‐L2+ TILs and the expression of PD‐L2 in the primary tumor cells (r = 0.788, P < 0.001). There was no strong correlation between the primary and metastatic tumors; the results of the correlation test for the antibody expression between the primary and metastatic tumors were: PD‐L1, r = 0.28; PD‐L2, r = −0.13; and HLA class I, r = 0.43. We next undertook an exploratory analysis of the post‐progression OS according to the expression score for PD‐L1, PD‐L2, and HLA class I of the tumors at a distant site of recurrence (n = 17), but no significance was observed (log–rank test: PD‐L1, P = 0.13; PD‐L2, P = 0.00012; and HLA class I, P = 0.35. The P‐value for PD‐L2 was <0.05, but the order of the survival curve was not theoretical (upper, score 1; middle, score 2; and lower, score 0).
Discussion
Previous studies have shown that a loss of concordance in the status of biomarkers such as ER, Progesterone receptor (PR), and HER2 can occur between primary and metastatic breast tumors.24, 25, 26, 27 According to the concept of cancer immunoediting, the possibility of discordance of immune microenvironments between primary and metastatic breast tumors should also be considered. In this study, we found that tumors at the first metastatic recurrence in HER2+ and TN breast cancers have a lower percentage of TILs and CD8+ and CD4+ T cells compared to primary tumors, suggesting that immune escape plays a role in tumor progression. To the best of our knowledge, this is one of the first studies to evaluate changes in the tumor microenvironment during the process of metastasis using pair‐matched specimens. Our study was similar to previous articles in reporting that TILs and the subset percentages of metastatic sites was lower than that of primary sites;17, 18 however, we focused on HER2+ and TN breast cancers, because TILs are a reliable predictive and prognostic biomarker in these subtypes. Furthermore, we found that the expression of PD‐L1, PD‐L2, and HLA class I were changeable between primary and metastatic breast cancer tumors.
The clinical utility of TILs in most patients is limited because the determination of the potential of TILs as a specific immune marker or their ability to facilitate the prediction of the use of T‐cell checkpoint inhibitors is a major challenge and because no methods to successfully modulate immunity to reduce mortality have been established so far.28 Antibodies targeting cytotoxic T‐lymphocyte‐associated antigen 4/programmed death 1/PD‐L1 have resulted in clinical responses in multiple tumor types including advanced melanomas, advanced non‐small‐cell lung cancers, and advanced renal cell carcinomas.29, 30, 31 These treatment methods are expected to slow cancer progression and significantly prolong the survival of patients with advanced cancer. Given that immune checkpoint therapy only benefits a fraction of patients, there are ongoing efforts being made to identify predictive biomarkers that could be used to select patients that will respond well to such treatment.32
There are many candidates for biomarkers, such as PD‐L1 expression in tumors, the percentage of TILs, the percentage of CD8+ T cells in the TILs, the level of cytokines and chemokines produced by lymphocytes in the peripheral blood, and myeloid‐derived suppressor cells in tumor lesions.9, 33 However, sometimes these biomarkers are also detected in primary tumors. These biomarkers could also be influenced by metastatic processes and cytotoxic chemotherapy.34 In our study, the expression of PD‐L1, PD‐L2, and HLA class I antigen was also found to change from primary to metastatic tumors. Therefore, the evaluation of targeted lesions just before the start of immunotherapy might be needed in future clinical trials.
Although many adjuvant and neoadjuvant studies have assessed infiltrating lymphocytes and stromal lymphocytic infiltration has been found to constitute a robust prognostic factor in primary HER2+ tumors or TN breast cancers,8, 9, 35 whether lymphocytic infiltration in metastatic tumors could be a prognostic factor has not yet been evaluated. In our study, the group with low TILs in metastatic tumors had a significantly lower OS than the group with intermediate TILs. Thus, our results indicate that a higher percentage of TILs could have a prognostic impact, even in metastatic tumors.
Previous studies showed that the results of evaluation of the stromal compartment were more reproducible than those of the evaluation of intratumoral TILs.23 We evaluated TILs within the borders of the invasive tumor and found that it was quite difficult to distinguish the invasive margin TILs clearly from stromal TILs. Although there are few studies involving the evaluation of the invasive edge, there is currently no evidence indicating that TILs at the invasive edge are functionally different from stromal TILs. We therefore evaluated stromal TILs of the breast tissue and other organs. Recommendations for TIL evaluation have been published previously,23 and guidelines for the same will be standardized in the years ahead. However, we encountered some difficulties in the evaluation of TILs from other tissues. In some cases, there was very little stromal area in the biopsy specimens, which was not the case in the surgical specimens. It was also difficult to precisely detect TILs among the background lymphocytes in the recurrent tumors in the lymph nodes or the bone marrow on the H&E‐stained slides. The TILs were differentiated from the background lymphocytes based on the structural patterns of infiltration in the case of bone marrow tumors, and in case of the tumors in the lymph nodes, the lymph node structure had been totally replaced by the tumor in our study.
One limitation of this study was the small number of patients; in particular, patients with LPBCs were few, which limited our ability to determine the prognostic value of lymphocyte predominance in breast cancer. The reason for the small number of cases is that metastatic biopsy samples were very rare. Previous articles that compared primary and metastatic breast tumors consisted of ER+/HER2− cases and the number of HER2+ and TN cases was approximately 30–40 in their cohorts.17, 18 Tumor‐infiltrating lymphocytes are associated with a better neoadjuvant chemotherapy response and prognosis in HER2+ and TN breast cancers. Therefore, we focused only on HER2+ and TN breast cancers, which resulted in a small number of cases.
In summary, we found that tumors at the first metastatic recurrence in HER2+ and TN breast cancer patients have a lower percentage of TILs and CD8+ and CD4+ T cells compared to primary tumors, suggesting a role for immune escape in tumor progression. These differences could occur in a time‐, site‐, and therapy‐ (chemotherapy, radiotherapy, and surgery) dependent manner; therefore, the evaluation of targeted lesions just before the start of immunotherapy might be needed in future clinical trials. Furthermore, a low percentage of TILs at the recurrence sites seemed to be associated with poor OS, suggesting a more aggressive phenotype. These findings warrant independent confirmation in future studies.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Overall survival after first recurrence. The group with low tumor‐infiltrating lymphocytes (TILs; <10%) had a significantly lower overall survival than the intermediate TIL group (≥10% TILs) (hazard ratio = 3.77; 95% confidence interval, 0.99–14.9; log–rank, P = 0.038).
Fig. S2. Comparison of positivity rate between primary and metastatic tumor‐infiltrating lymphocytes for each antibody. CD4, CD8, and programmed cell death ligand 1 (PD‐L1) positivity was defined by membranous lymphocyte staining, and FoxP3 and PD‐L2 positivity was defined by nuclear lymphocyte staining. CD4, CD8, Foxp3, PD‐L1, and PD‐L2 expression by the tumor‐infiltrating lymphocytes was recorded in 10% increments and the score of three fields was averaged.
Fig. S3. Comparison of expression score between primary and metastatic tumor cells for each antibody. The expression of programmed cell death ligand 1 (PD‐L1), PD‐L2, HLA class I A, B, and C in the tumor cells was scored as 0 (negative), 1 (weak), or 2 (strong). The number of cases is noted above the bars and each bar without annotation represents only one case.
Fig. S4. Representative photographs. The expression of programmed cell death ligand 1 (PD‐L1), PD‐L2, and HLA class I in the tumors was scored as 0 (negative), 1 (weak), or 2 (strong).
Acknowledgments
We would like to thank Toshiya Tanaka, Division of Diagnostic Pathology and Support Center for Medical Research and Education for technical assistance with staining. This research was supported in part by the Japanese Ministry of Education, Culture, Sports, Science and Technology's Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016. The authors thank Editage for providing editorial assistance.
Cancer Sci 107 (2016) 1730–1735
Funding Information
Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 2007; 9(4): 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt M, Bohm D, von Torne C et al The humoral immune system has a key prognostic impact in node‐negative breast cancer. Cancer Res 2008. (Jul 1); 68: 5405–13. [DOI] [PubMed] [Google Scholar]
- 3. Denkert C, Loibl S, Noske A et al Tumor‐associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010. (Jan 1); 28(1): 105–13. [DOI] [PubMed] [Google Scholar]
- 4. West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor‐infiltrating lymphocytes predict response to anthracycline‐based chemotherapy in estrogen receptor‐negative breast cancer. Breast Cancer Res 2011; 13(6): R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ono M, Tsuda H, Shimizu C et al Tumor‐infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple‐negative breast cancer. Breast Cancer Res Treat 2012. (Apr); 132: 793–805. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi R, Tanaka M, Yano A et al Tumor‐infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 2012. (Oct); 43: 1688–94. [DOI] [PubMed] [Google Scholar]
- 7. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor‐associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 2013. (Mar); 16(1): 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loi S, Sirtaine N, Piette F et al Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02‐98. J Clin Oncol 2013. (Mar 1); 31: 860–7. [DOI] [PubMed] [Google Scholar]
- 9. Adams S, Gray RJ, Demaria S et al Prognostic value of tumor‐infiltrating lymphocytes in triple‐negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014. (Jul 28); 32: 2959–66. PubMed PMID: 25071121. Epub 2014/07/30. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denkert C, von Minckwitz G, Brase JC et al Tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2‐positive and triple‐negative primary breast cancers. J Clin Oncol 2014. (Dec 22); 33: 983–91. PubMed PMID: 25534375. Epub 2014/12/24. Eng. [DOI] [PubMed] [Google Scholar]
- 11. Loi S, Michiels S, Salgado R et al Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014. (Aug); 25: 1544–50. [DOI] [PubMed] [Google Scholar]
- 12. Perez EA, Thompson EA, Ballman KV et al Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the north central cancer treatment group N9831 adjuvant trastuzumab trial. J Clin Oncol 2015. (Jan 20); 33: 701–8. PubMed PMID: 25605861. Epub 2015/01/22. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leong PP, Mohammad R, Ibrahim N et al Phenotyping of lymphocytes expressing regulatory and effector markers in infiltrating ductal carcinoma of the breast. Immunol Lett 2006. (Feb 15); 102: 229–36. [DOI] [PubMed] [Google Scholar]
- 14. Miyashita M, Sasano H, Tamaki K et al Tumor‐infiltrating CD8+ and FOXP3+ lymphocytes in triple‐negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2014. (Dec); 148: 525–34. [DOI] [PubMed] [Google Scholar]
- 15. Dieci MV, Criscitiello C, Goubar A et al Prognostic value of tumor‐infiltrating lymphocytes on residual disease after primary chemotherapy for triple‐negative breast cancer: a retrospective multicenter study. Ann Oncol 2014. (Mar); 25: 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burstein HJ, Lacchetti C, Anderson H et al Adjuvant endocrine therapy for women with hormone receptor‐positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016. (Feb 16); 34: 1689–701. PubMed PMID: 26884586. [DOI] [PubMed] [Google Scholar]
- 17. Cimino‐Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple‐negative breast cancers at first relapse have fewer tumor‐infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 2013. (Oct); 44: 2055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology 2016. (Jun); 5(6): e1153208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002. (Nov); 3: 991–8. [DOI] [PubMed] [Google Scholar]
- 20. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007. (May); 121(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moossdorff M, van Roozendaal LM, Strobbe LJ et al Maastricht Delphi consensus on event definitions for classification of recurrence in breast cancer research. J Natl Cancer Inst 2014. (Dec); 106(12): dju288. PubMed PMID: 25381395. Epub 2014/11/09. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011. (Aug); 22: 1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salgado R, Denkert C, Demaria S et al The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015. (Feb); 26: 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amir E, Miller N, Geddie W et al Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 2012. (Feb 20); 30: 587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simmons C, Miller N, Geddie W et al Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol 2009. (Sep); 20: 1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson AM, Jordan LB, Quinlan P et al Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res 2010; 12(6): R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niikura N, Liu J, Hayashi N et al Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2‐overexpressing primary breast tumors. J Clin Oncol 2012. (Feb 20); 30: 593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loi S, MacCallum P. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple‐negative breast cancer. J Clin Oncol 2014. (Sep 20); 32: 2935–7. [DOI] [PubMed] [Google Scholar]
- 29. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015. (Oct 22); 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber JS, D'Angelo SP, Minor D et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015. (Apr); 16: 375–84. [DOI] [PubMed] [Google Scholar]
- 31. Motzer RJ, Escudier B, McDermott DF et al Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015. (Nov 5); 373: 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015. (Apr 3); 348(6230): 56–61. [DOI] [PubMed] [Google Scholar]
- 33. Arato T, Daimon T, Heike Y et al Guidance on cancer immunotherapy development in early‐phase clinical studies. Cancer Sci 2015. (Dec);106(12):1761–71. PubMed PMID: 26767933. Pubmed Central PMCID: 4714668. Epub 2016/01/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Champiat S, Ileana E, Giaccone G et al Incorporating immune‐checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014; 9: 144–53. [DOI] [PubMed] [Google Scholar]
- 35. Salgado R, Denkert C, Campbell C et al Tumor‐infiltrating lymphocytes and associations with pathological complete response and event‐free survival in HER2‐positive early‐stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 2015. (Jul); 1: 448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Overall survival after first recurrence. The group with low tumor‐infiltrating lymphocytes (TILs; <10%) had a significantly lower overall survival than the intermediate TIL group (≥10% TILs) (hazard ratio = 3.77; 95% confidence interval, 0.99–14.9; log–rank, P = 0.038).
Fig. S2. Comparison of positivity rate between primary and metastatic tumor‐infiltrating lymphocytes for each antibody. CD4, CD8, and programmed cell death ligand 1 (PD‐L1) positivity was defined by membranous lymphocyte staining, and FoxP3 and PD‐L2 positivity was defined by nuclear lymphocyte staining. CD4, CD8, Foxp3, PD‐L1, and PD‐L2 expression by the tumor‐infiltrating lymphocytes was recorded in 10% increments and the score of three fields was averaged.
Fig. S3. Comparison of expression score between primary and metastatic tumor cells for each antibody. The expression of programmed cell death ligand 1 (PD‐L1), PD‐L2, HLA class I A, B, and C in the tumor cells was scored as 0 (negative), 1 (weak), or 2 (strong). The number of cases is noted above the bars and each bar without annotation represents only one case.
Fig. S4. Representative photographs. The expression of programmed cell death ligand 1 (PD‐L1), PD‐L2, and HLA class I in the tumors was scored as 0 (negative), 1 (weak), or 2 (strong).


