Abstract
Purpose
To observe the effect of cynomorium total flavone on the depression model of perimenopausal rat and to analyze the action characteristics of cynomorium total flavone on depression of rat with perimenopausal syndrome.
Method
Duplicate the model of rat with perimenopausal depression based on the combined method of incomplete castration and chronic stimulation, and keep drug administration for 35d. And then measure related behavior indicators and the change of biochemical index level in serum and brains; measure the estrogen/androgen receptor (ER/AR) in related tissues and the ERmRNA expression in hypothalamus.
Result
It can be seen that cynomorium total flavone can significantly improve the behavior indicators of rat with perimenopausal depression; obviously or significantly change the level of related biomedical indexes in serum and brains of perimenopausal depressed rat; obviously or significantly increase the expression of ER/AR in related tissues of perimenopausal depressed rat; obviously or significantly increase the ERmRNA expression in hypothalamus.
Conclusion
Cynomorium total flavone can adjust hypothalamic-pituitary-gonadal axis by increasing E2, and make related biomedical indexes and hormone receptors tend to be normal, so as to relieve perimenopausal syndrome and perimenopausal syndrome with depression.
Keywords: Cynomorium total flavone, Perimenopausal syndrome with depression, Estradiol, Estrogen receptor
1. Introduction
With the increase in age and decline of ovarian function, estrogen secretion is gradually decreased, which leads to the dysequilibrium of HPO and hypothalamic-hypophyscal-ovarian axis (HPOA) and impacts autonomic center function and the functions of various organs controlled by autonomic center, thus causing a series of symptoms called perimenopause syndrome (PMS) or climacteric syndrome (CS). Epidemiological investigation was performed for women’s perimenopausal syndrome in Shanghai, showing that those with depression tendency account for 77.29% of total respondents, while those with depression symptoms account for 8.36%, and both belong to common symptoms of patients with PMS. Studies believe that the incidence of risk in general as early as 40 years old will begin to appear (Judy, 2011). In addition, studies have indicated that PMS symptoms can decrease the quality of life of PMS patients and increase the risk of the disease. So seeking another safe and effective therapy is in urgent need. At the initial phase of this experiment, we have done pharmacodynamic study on total flavonoids applied in mouse model of perimenopause. The study proves that total flavonoids from Cynomorium songaricum can improve related indicators of mouse model of perimenopause; but it lacks the discussion about the action and mechanism of total flavonoids from Cynomorium songaricum on animal models of perimenopausal depression. Therefore, this article will mainly study the action and mechanism of Cynomorium songaricum on the mouse model of perimenopausal depression.
2. Materials and methods
2.1. Experimental animal
SD rat; female; SPF level body weight: 250–270 g; Supplier: Shandong Lukang Pharmaceutical Co., Ltd.; Certification number of the animal: 0021925; Certification number of the laboratory: SYXK (Henan Province) 2010-001.
2.2. Experimental drug
Cynomorium total flavone prepared in Office of Chemistry, Henan University of Traditional Chinese Medicine, purity: 50.87%, batch number ZL20140716. Gengnianan capsules: produced by Shanxi Tianxing Pharmaceutical Co., Ltd. Batch number: 140112. Soybean Isoflavones Vitamin E Soft Capsules: produced by Weihai Purple Light Biotechnology Development Co., Ltd. Batch number: 14040301.
2.3. Experiment method
From 100 female wistar rats with weight ranging from 250 to 270 g, randomly selecting out 12 as blank group for sham-operation, and the menopausal models of the remaining rats are established. After weighing, injecting 10% chloral hydrate (0.3 ml/100 g) for anesthesia before fixing their abdominal regions, and then completely extirpating the left ovary and extirpating 80% of right ovary before suturing well the muscle and skin. After operation, carefully feeding them and injecting penicillin 200,000 u/kg (0.1 ml per rat) one a day for consecutively 3 days. From the 5th after operation, vaginal smear examination is applied on every single rat once a day for consecutively 5d. Those showing emotional reactions are not used, and finally 72 completely castrated rats are randomly divided into 6 groups including Gengnianan capsule group (fed with Gengnianan capsule suspension of 0.45 mg/kg, which equals 10 times of the clinical dosage), Soybean Isoflavones Vitamin E Soft Capsules group (fed with Soybean Isoflavones suspension of 0.167 mg/kg, which equals 10 times of the clinical dosage), big dosage cynomorium total flavone group (fed with 0.2 g/kg cynomorium total flavone, which equals 20 times of the clinical dosage), medium dosage cynomorium total flavone group (fed with 0.1 g/kg cynomorium total flavone, which equals 10 times of the clinical dosage), and small dosage cynomorium total flavone group (fed with 0.05 g/kg cynomorium total flavone, which equals 5 times of the clinical dosage). In addition, both blank group and model group are fed with 0.5% CMC solution of the same volume. Drug administration is given by 1 ml/100 g once a day for consecutively 35 days.
After keeping drug administration for 5 days, rats in the blank group are kept by 6 rats per cage without any simulations; while for the 6 model groups, rats are kept by 1 rat per cage, which is randomly applied with one from 6 different simulations including damp bedding (bedding: g, water: ml), ice water swimming (4 °C, 5 min), heat stress (45 °C, 5 min), all-day illumination (24 h), water deprivation (24 h), food deprivation (24 h), wherein it is worth noting that in the 18 consecutive days of medication period, a random simulation is given per day, and no same simulation should be arranged in 2 consecutive days. From the 1st day after simulation, open field is tested to observe stand-up times and horizontal move distance of all groups of rats. From the second day after simulation, forced swimming test is given to measure the immobility time within 4 days. From the 3rd or 4th day from simulation, sugar consumption experiment, prior to which 12 h of water deprivation is conducted, is given to measure 1% sugar water intake within 24 h of food deprivation.
At 2 h after final gavage (food deprivation for 15 h), draw the blood from the eyeball of the rat and measure the content of estradiol (E2 and testosterone (T) according to the instruction of test kits. Extricate brain homogenate and measure the content of methylepinephrine (NE), dopamine (DA), 5-hydroxytryptamine (5-HT) according to related instruction of test kits; extirpate uterus and remaining 20% ovarian tissue, extricate hypothalamus and hypophysis, and then fix the left side of the hypothalamus, hypophysis, uterus, and ovary in 10% formaldehyde solution before being processed into paraffin embedding sections to measure the expression of estrogen receptor (ER) in hypothalamus, hypophysis, uterus, and ovary, as well as the expression of AR in hypothalamus and hypophysis using the immunohistochemical method. On the other hand, store the right side of the hypothalamus in −80 °C refrigerator and measure the expression of ERmRNA using the RT-PCR method.
2.4. Statistical processing method
Data are statistically processed and analyzed using SPSS17.0 medical statistical package, and measurement data are expressed by mean value ± standard deviation (). One-way analysis of variance is performed for each group, wherein those of equal variance are tested by the LSD method, while those of heterogeneous variances are tested by the Games-Howell method.
3. Results
3.1. Effect on the behaviors of perimenopausal rats with depression
Effect on open-field test of perimenopausal rats with depression, and the results are shown in Table 1.
Table 1.
Effect of synomorium total flavone on open-field test of perimenopausal rats with depression ().
| Group no. | Movement distance (cm) | Stand-up times (time) |
|---|---|---|
| Blank group | 1577.42 ± 94.33⁎⁎ | 24.00 ± 1.91⁎⁎ |
| Model group | 952.83 ± 89.56 | 13.36 ± 1.50 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 1313.424 ± 90.69⁎⁎ | 21.55 ± 2.02⁎⁎ |
| Gengnianan capsule group | 1337.69 ± 94.63⁎⁎ | 21.73 ± 1.42⁎⁎ |
| Big dosage cynomorium total flavone group | 1297.31 ± 99.49⁎⁎ | 20.91 ± 1.76⁎⁎ |
| Medium dosage cynomorium total flavone group | 1225.33 ± 100.24⁎⁎ | 18.60 ± 1.91⁎⁎ |
| Small dosage cynomorium total flavone group | 1179.95 ± 86.04⁎⁎ | 17.73 ± 1.74⁎⁎ |
Note: compared with model group.
∗P < 0.05.
P < 0.01.
According to Table 1, it can be seen that compared with rats in the blank group, rats in the model group show a significant decrease of movement distance and stand-up times (P < 0.01), indicating that the perimenopausal depressed rats suffer decreased curiosity of fresh environment. Compared with rats in the model group, it can be seen that the big/medium/small dosage of cynomorium total flavone, Gengnianan capsule, and Soybean Isoflavones Vitamin E Soft Capsule can all significantly increase the movement distance and stand-up times of perimenopausal rats with depression (P < 0.01).
The effect of sugar water intake and immobility time in the forced swimming test of perimenopausal rats with depression is shown in Table 2.
Table 2.
Effect of cynomorium total flavones on sugar water intake and immobility time in forced swimming test of perimenopausal rats with depression ().
| Group no. | Immobility time (s) | Sugar water intake (ml) |
|---|---|---|
| Blank group | 58.94 ± 7.29⁎⁎ | 104.08 ± 6.82⁎⁎ |
| Model group | 113.73 ± 8.16 | 61.36 ± 6.23 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 96.27 ± 9.09⁎⁎ | 90.27 ± 6.60⁎⁎ |
| Gengnianan capsule group | 89.64 ± 7.40⁎⁎ | 86.55 ± 7.53⁎⁎ |
| Big dosage cynomorium total flavone group | 83.62 ± 9.56⁎⁎ | 84.45 ± 8.77⁎⁎ |
| Medium dosage cynomorium total flavone group | 94.09 ± 8.95⁎⁎ | 80.00 ± 5.75⁎⁎ |
| Small dosage cynomorium total flavone group | 100.64 ± 9.32 | 76.64 ± 6.47⁎⁎ |
Note: compared with the model group.
∗P < 0.05.
P < 0.01.
According to Table 2, it can be seen that compared with rats in blank group, rats in the model group have an significantly extended immobility time (P < 0.01) in the forced swimming test and a significantly decreased sugar water intake (P < 0.01), indicating that the perimenopausal rats with depression have a higher degree of desperation upon adverse environment and a declined reaction upon reward. Compared with rats in the model group, Soybean Isoflavones Vitamin E Soft Capsule, Gengnianan capsule, and big/medium dosage of cynomorium total flavone can significantly decrease the immobility time (P < 0.01) in the forced swimming test and significantly increase the sugar water intake (P < 0.01); however, small dosage of cynomorium total flavone can significantly increase the sugar water intake (P < 0.01) of perimenopausal rats with depression while merely leading to decreasing tendency of the immobility time in the forced swimming test.
3.2. Effect on biomedical indexes in the serum of perimenopausal rats with depression
Contents of E2 and T in serum of rats in all groups are shown in Table 3.
Table 3.
Effect of synomorium total flavone on contents of E2 and T in the serum of perimenopausal rats with depression ().
| Group no. | E2 (pmol/L) | T (pg/ml) |
|---|---|---|
| Blank group | 36.25 ± 2.54⁎⁎ | 96.37 ± 7.68⁎⁎ |
| Model group | 25.84 ± 2.62 | 63.31 ± 8.42 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 32.84 ± 2.28⁎⁎ | 78.13 ± 6.95⁎ |
| Gengnianan capsule group | 31.93 ± 1.64⁎⁎ | 80.37 ± 7.93⁎⁎ |
| Big dosage cynomorium total flavone group | 31.08 ± 1.36⁎⁎ | 78.42 ± 9.41⁎ |
| Medium dosage cynomorium total flavone group | 29.96 ± 3.46 | 80.23 ± 8.28⁎⁎ |
| Small dosage cynomorium total flavone group | 28.42 ± 1.57 | 74.13 ± 6.39 |
Note: compared with model group.
P < 0.05.
P < 0.01.
According to Table 3, it can be seen that compared to rats in the blank group, those in the model group suffer a significantly decreased level of E2 and T in the serum, indicating that incomplete removal of ovary can lead to sex hormone disturbances of perimenopausal rats, and the model replications of perimenopausal rats are successful. Compared to rats in the model group, it can be seen that big dosage of cynomorium total flavone, Gengnianan capsule, and Soybean Isoflavones Vitamin E Soft Capsule can all significantly increase the E2 level of perimenopausal rats with depression (P < 0.01), while small/medium dosage of cynomorium total flavone can merely lead to the increasing tendency of E2 level of perimenopausal rats with depression; on the other hand, medium dosage of cynomorium total flavone and Soybean Isoflavones Vitamin E Soft Capsule can significantly increase the T level of perimenopausal rats with depression (P < 0.05), while small dosage of cynomorium total flavone can barely lead to increasing tendency of L level.
3.3. Effect on biomedical indexes in brain homogenate of perimenopausal rats with depression
Effect on the level of 5-HT and DA in brain homogenate of perimenopausal rats with depression, and the results are shown in Table 4.
Table 4.
Effect of cynomorium total flavones on the level of 5-HT and DA in brain homogenate of perimenopausal rats with depression ().
| Group no. | 5-HT (ng/ml) | DA (pg/ml) |
|---|---|---|
| Blank group | 58.31 ± 6.45⁎⁎ | 1191.93 ± 70.13⁎⁎ |
| Model group | 37.95 ± 4.39 | 799.02 ± 50.49 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 50.27 ± 4.65⁎⁎ | 1042.82 ± 80.47⁎⁎ |
| Gengnianan capsule group | 52.63 ± 4.85⁎⁎ | 1082.08 ± 89.44⁎⁎ |
| Big dosage cynomorium total flavone group | 48.58 ± 4.79⁎⁎ | 998.72 ± 91.89⁎⁎ |
| Medium dosage cynomorium total flavone group | 51.54 ± 2.65⁎⁎ | 959.88 ± 128.49 |
| Small dosage cynomorium total flavone group | 44.92 ± 3.62⁎ | 891.35 ± 101.94 |
Note: compared with model group.
P < 0.05.
P < 0.01.
According to Table 4, it can be seen that compared with rats in the blank group, rats in model groups enjoy a significantly increased level of 5-HT and DA in brain homogenate, indicating that the chronic stress-induced depressive model is established successfully. In addition, as compared with rats in the model group, big/medium dosage of cynomorium total flavone, Gengnianan capsule, and Soybean Isoflavones Vitamin E Soft Capsule can significantly increase the 5-HT level in brain homogenate of rats (P < 0.01), while a small dosage of cynomorium total flavone can significantly increase the level of 5-HT (P < 0.05); on the other hand, Gengnianan capsule, Soybean Isoflavones Vitamin E Soft Capsule, and big dosage of cynomorium total flavone can all significantly increase the DA level in brain homogenate (P < 0.01), while medium/small dosage of cynomorium total flavone can merely lead to the increasing tendency of DA level in the brain homogenate.
3.4. Effect on immunohistochemical indexes of perimenopausal rats with depression
Effects on ER expression in uterus, ovary, hypophysis and hypothalamus as well as AR expression in hypophysis and hypothalamus. Results are shown in Table 5, Table 6 and Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6.
Table 5.
Effect of cynomorium total flavones on ER expression of perimenopausal rats with depression (mean optical density) ().
| Group no. | Hypophysis | Hypothalamus | Uterus | Ovary |
|---|---|---|---|---|
| Blank group | 0.097 ± 0.010⁎⁎ | 0.130 ± 0.015⁎⁎ | 0.153 ± 0.014⁎⁎ | 0.101 ± 0.009⁎⁎ |
| Model group | 0.048 ± 0.008 | 0.042 ± 0.006 | 0.063 ± 0.011 | 0.050 ± 0.007 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 0.084 ± 0.011⁎⁎ | 0.123 ± 0.023⁎⁎ | 0.129 ± 0.013⁎⁎ | 0.093 ± 0.014⁎⁎ |
| Gengnianan capsule group | 0.081 ± 0.011⁎⁎ | 0.103 ± 0.018⁎⁎ | 0.119 ± 0.017⁎⁎ | 0.079 ± 0.010⁎⁎ |
| Big dosage cynomorium total flavone group | 0.078 ± 0.009⁎⁎ | 0.095 ± 0.020⁎⁎ | 0.108 ± 0.013⁎⁎ | 0.084 ± 0.009⁎⁎ |
| Medium dosage cynomorium total flavone group | 0.072 ± 0.011⁎⁎ | 0.083 ± 0.015⁎⁎ | 0.101 ± 0.010⁎⁎ | 0.080 ± 0.011⁎⁎ |
| Small dosage cynomorium total flavone group | 0.067 ± 0.006⁎⁎ | 0.070 ± 0.013⁎⁎ | 0.099 ± 0.008⁎⁎ | 0.069 ± 0.011⁎⁎ |
Note: compared with the model group.
∗P < 0.05.
P < 0.01.
Table 6.
Effect of cynomorium total flavones on AR expression of perimenopausal rats with depression (mean optical density) ().
| Group no. | Hypophysis | Hypothalamus |
|---|---|---|
| Blank group | 0.133 ± 0.011⁎⁎ | 0.192 ± 0.024⁎⁎ |
| Model group | 0.056 ± 0.008 | 0.107 ± 0.019 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 0.094 ± 0.012⁎⁎ | 0.149 ± 0.021⁎⁎ |
| Gengnianan capsule group | 0.118 ± 0.010⁎⁎ | 0.156 ± 0.023⁎⁎ |
| Big dosage cynomorium total flavone group | 0.117 ± 0.010⁎⁎ | 0.146 ± 0.016⁎⁎ |
| Medium dosage cynomorium total flavone group | 0.102 ± 0.011⁎⁎ | 0.137 ± 0.024 |
| Small dosage cynomorium total flavone group | 0.089 ± 0.016⁎⁎ | 0.120 ± 0.017 |
Note: compared with the model group.
∗P < 0.05.
P < 0.01.
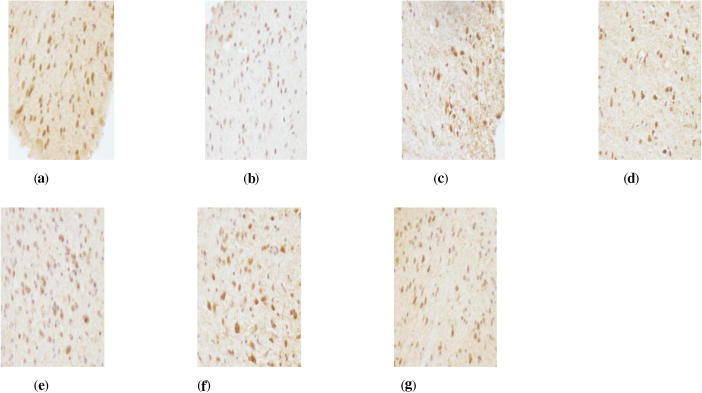
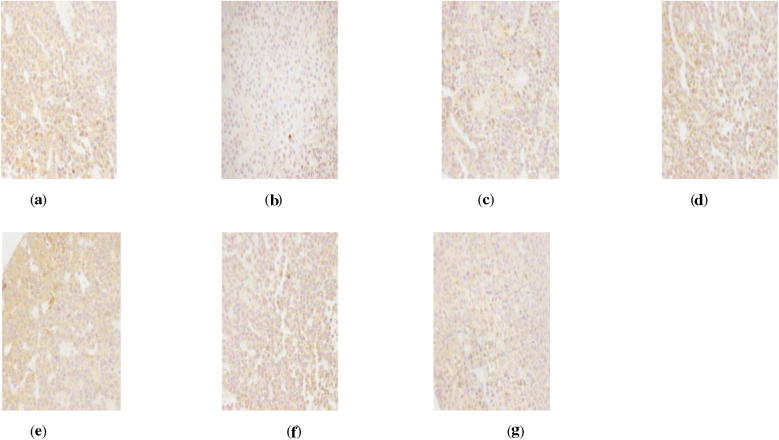
Figure 1.
AR immunohistochemistry in hypothalamus of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d) Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, (g) small dosage cynomorium total flavone group ×400.
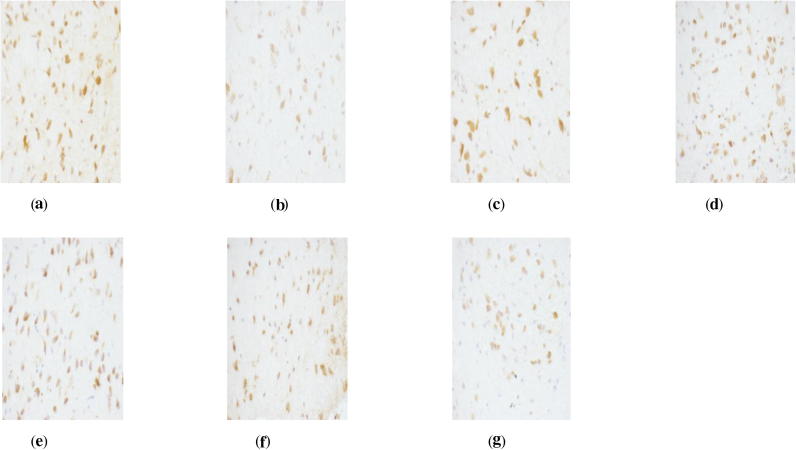
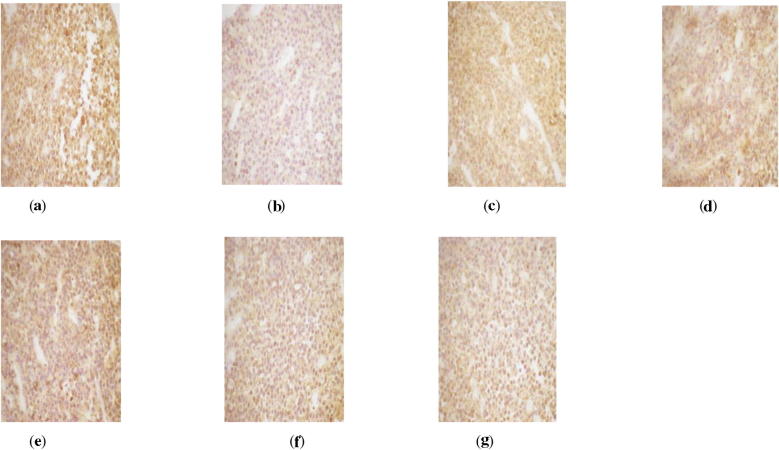
Figure 2.
ER Immunohistochemistry in hypothalamus of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d) Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, and (g) small dosage cynomorium total flavone group ×400.
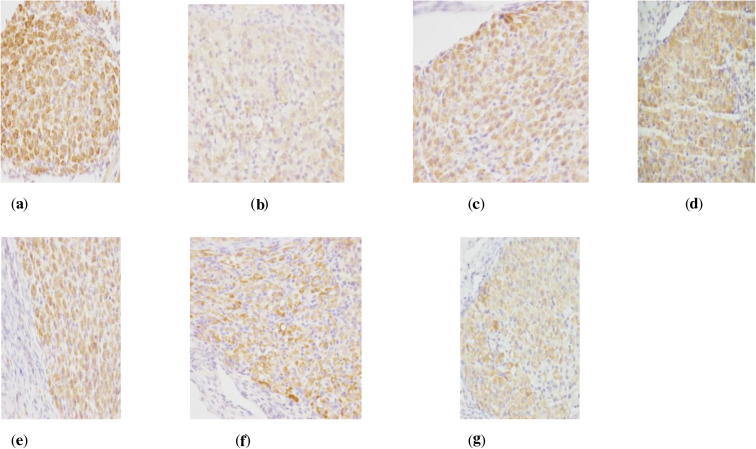
Figure 3.
ER Immunohistochemistry in ovary of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d): Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, and (g) small dosage cynomorium total flavone group ×400.
Figure 4.
ER Immunohistochemistry in uterus of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d) Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, and (g) small dosage cynomorium total flavone group ×400.
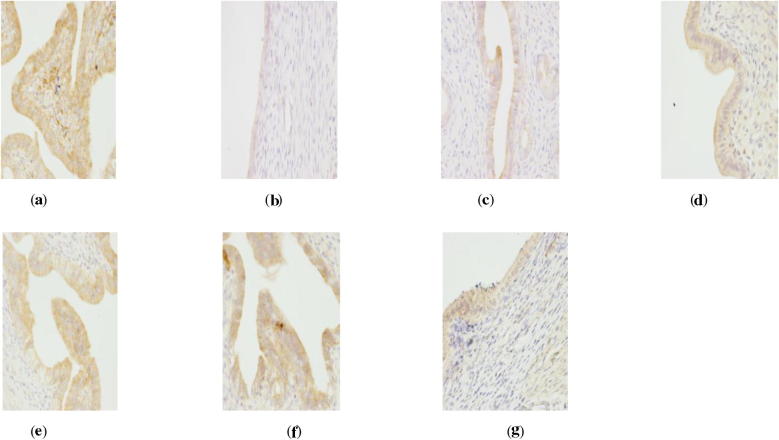
Figure 5.
ER Immunohistochemistry in hypophysis of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d) Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, and (g) small dosage cynomorium total flavone group ×400.
Figure 6.
AR Immunohistochemistry in hypophysis of perimenopausal rats with depression. (a) Blank group ×400, (b) model group ×400, (c) Soybean Isoflavones Vitamin E Soft Capsules group ×400, (d) Gengnianan capsule group ×400, (e) big dosage cynomorium total flavone group ×400, (f) medium dosage cynomorium total flavone group ×400, and (g) small dosage cynomorium total flavone group ×400.
According to Table 6, it can be seen that as compared with rats in the blank group, rats in the model group suffer a significant decrease of both ER and AR expression (P < 0.01), indicating that the distributions of sex hormone receptors in related tissues of perimenopausal depressed rats are decreased due to incomplete removal of ovary and chronic stimulations, and thus the biological effect of estrogen and androgen is reduced. On the other hand, as compared with rats in the model group, big dosage of cynomorium total flavone, Gengnianan capsule and Soybean Isoflavones Vitamin E Soft Capsule can all increase the ER expression in uterus, ovary, hypophysis and hypothalamus (P < 0.01), and significantly increase AR expression in hypophysis and hypothalamus (P < 0.01); while small/medium dosage of cynomorium total flavone can significantly the ER expression in the uterus, ovary, hypophysis and hypothalamus (P < 0.01), significantly increase AR expression in hypophysis (P < 0.01), while merely cause the increasing tendency of AR expression in the hypothalamus.
3.5. Effect on ERmRNA expression
Effect of cynomorium total flavone on ERmRNA expression in hypothalamus of perimenopausal rats with depression. See Table 7.
Table 7.
Effect of cynomorium total flavone on ERmRNA expression in hypothalamus of perimenopausal rats with depression.
| Group no. | ERmRNA |
|---|---|
| Blank group | 0.048 ± 0.008⁎⁎ |
| Model group | 0.010 ± 0.003 |
| Soybean Isoflavones Vitamin E Soft Capsules group | 0.030 ± 0.007⁎⁎ |
| Gengnianan capsule group | 0.039 ± 0.009⁎⁎ |
| Big dosage cynomorium total flavone group | 0.036 ± 0.011⁎⁎ |
| Medium dosage cynomorium total flavone group | 0.025 ± 0.010⁎ |
| Small dosage cynomorium total flavone group | 0.022 ± 0.004⁎ |
Note: compared with the model group.
P < 0.05.
P < 0.01.
According to Table 7, it can be seen that compared to rats in the blank group, those in the model group suffer a significant decrease of ERmRNA expression in the hypothalamus, indicating the ERmRNA expressions of perimenopausal depressed rats are limited due to the incomplete removal of ovary and chronic stimulations. On the other hand, compared with rats in the model group, it can be seen that big dosage of cynomorium total flavone, Gengnianan capsule, and Soybean Isoflavones Vitamin E Soft Capsule can all significantly increase ERmRNA expression in the hypothalamus (P < 0.01), while small/medium dosage of cynomorium total flavone can significantly improve ERmRNA expression in hypothalamus (P < 0.05).
4. Discussion
To treat CS, normally psychotherapy or sedative therapy is given to reduce uncomfortable symptoms. However, for those with more severe symptoms are basically given HRT, which is superior for its quick effect, highly targeted effect, and higher success rate. In clinical practice, it is also common to use Oryzaol Vitamin B1 and Vitamin B6 Tablets and Compound Methypregnone and Ethinylestradiol Capsules. However, estrogen increases the risk of getting endometrial cancer and breast cancer (Stevenson et al., 2011). In July 2002, the Heart, Lung, and Blood Institute of National Institutes of Health terminated early WHI’s clinical trial of using estrogen progesterone in healthy postmenopausal women. As of now, hormonotherapy is still a controversial issue, therefore seeking another safe and effective therapy is in urgent need.
Traditional Chinese medicine (TCM) recovers the “dying” follicle and slows aging ovaries by ovarian inner adjustment, the effectiveness of which is unrivalled by estrogen in replacement therapy (Love et al., 2010). TCM treatment of menopause has significantly fewer side effects. In the historical medical book Questions-Ancient Natural Integrity Theory, it was believed that the reproductive system exhaustion and imbalance lead to menstrual disorders, reproductive function decline and so on. Professor Guicheng (2010) pointed out the “Heart - Kidney - Uterine Axis” theory, noting kidneys dominate water in the lower part, the heart dominates fire in the upper part, and only when water from kidneys is elevated, and the fire from the heart is lowered, can the heart and kidneys meet, in order to balance yin and yang. Ye Yanping had analyzed 106 clinical cases of patients before and after menopause, and found that the percentage of patents suffering from liver depression is up to 79.2%. In TCM theories, kidneys (Zheng et al., 2011) are the root of personal health before birth, while stomach is the root of personal health after birth. Liu Xiaowei believed that all evidences of diseases before and after menopause are indicated by endogenous phlegm as the signal, while the loss of kidney essence is the root. It was recorded in “Compendium of Materia Medica” that: “Cynomorium songaricum is warm to kidneys, good to seminal fluid, moistening body, and curing impotence.” Cynomorium songaricum contains a variety of biologically active ingredients, including flavonoids, terpenes, steroids, organic acids, sugars and glycosides, tannins and volatile ingredients (Meng et al., 2013) commonly used in the treatment of kidney deficiency, seminal fluid deficiency, infertility, neurasthenia and other symptoms. Tests have showed that the flavonoids extracted from Cynomorium songaricum can enhance exercise endurance, and improve the body’s antioxidant (Jin and Yin, 2012) and anti-fatigue effect, which can significantly prolong swimming time of aged rats. The flavonoids from Cynomorium songaricum can improve the SOD activity of mice, and decrease serum MDA content, indicating its antioxidant function.
The application of Cynomorium songaricum in menopause treatment is based on analysis of its pathogenesis. It can speed the animal sexual maturity, protect the kidneys, replenish body’s androgen and stimulate the secretion of estrogen, finally balance the hormone levels, and cure menopausal syndrome. According to the high content of total flavonoids in Cynomorium songaricum and the coincidence of the former’s pharmacological activity with the latter’s main pharmacological effects, etc., we take the total flavonoids from Cynomorium songaricum as the main active site, and make in-depth study on its intervention in menopausal depression.
5. Conclusions
Neither existing emasculated perimenopausal animal model nor depression animal model can comprehensively reflect the change of reproductive endocrine hormone and neurotransmitter in perimenopausal depression, but only reflect one single aspect of the disease. In this paper, the model of perimenopausal depressive animal was established by a two-step method of incomplete emasculation and chronic unpredictable stress, so as to effectively simulate perimenopausal women’s clinical symptoms such as affective disorder and bad mood due to ovary aging. Results show that after incomplete removal of ovary, the estrous cycle of rat disappeared, indicating that the perimenopausal model has been successfully established. The experiment results indicate that the levels of E2 and T in the serum of model animal are significantly decreased, which further verifies that the sexual hormone disturbance of the model animal has emerged.
Through observing autonomic movement test, we can judge whether there are emerging changes of behavioral characteristics such as decline of athletic ability and social communication ability, explorative ability decline, defect of attack ability, and decline of sexual capacity. Through the sugar water intake test, we can measure whether the reaction intensity on rewards has been reduced. Through measuring the immobility time by forced swimming test, we can observe the desperation degree of rats. Through the open-field test, we can measure whether the curiosity on fresh environment has been reduced. Ovarian failure leads to the functional disorder of hypothalamic-pituitary-gonadal (HOP) axis, and thus causes the dysequilibrium of hypothalamic monoamine neurotransmitter (Shuo and Mingsan, 2014); while the sex hormone withdrawal induced by menopause can lead to the reduction of monoamine neurotransmitters such as DA and 5-HT (Mingsan et al., 2015). 5-HT is effective in adjusting mood, sleep, alertness, memory, appetite, sexual desire, hypothalamus – pituitary – endocrine 5-HT and body temperature (Carsote et al., 2013); a transient lack of 5-HT will lead to bad mood, depression psychosis and even perimenopausal depression symptoms. In this experiment, the contents of monoamine neurotransmitters such as 5-HT and DA in the brain homogenate of model rats are significantly reduced, which is consistent with the symptoms of depression psychosis. Therefore, we can conclude that the model replicating method in this paper is successful.
The experiment results show that cynomorium total flavone can significantly reduce the move distance and stand-up times, significantly increase sugar water intake, and significantly shorten immobility time in the forced swimming test, indicating cynomorium total flavone can increase the curiosity of perimenopausal rats on fresh environment, increase the reaction intensity on rewards, and decrease the degree of desperation on adverse environment, so as to improve depressive symptom. First, cynomorium total flavone can significantly increase the initially reduced level of E2 (Pachman et al., 2010) and T in the serum of perimenopausal rats with depression, indicating cynomorium total flavone can adjust the disorders of hormone levels and hypothalamic-pituitary-gonadal function of perimenopausal rats with depression (Wiacek et al., 2011). Secondly, cynomorium total flavone can significantly increase the contents of 5-HT and DA in the brain homogenate of perimenopausal rats with depression, indicating cynomorium total flavone is used in the prospect of treating perimenopausal depression, wherein the mechanism is to realize the adjustment of monoamine neurotransmitters through impacting hypothalamic-pituitary-gonadal axis. Thirdly, cynomorium total flavone can significantly increase the positive expressions of ER (Kammerer et al., 2013) and AR in hypothalamus, hypophysis, ovary, and uterus, indicating that cynomorium total flavone can basically enhance the biological effect of estrogen and androgen, and its function of increasing the positive expressions of ER and AR is probably related to the increased contents of estrogen and androgen.
Through experiment results, it can be seen that the adjustment function of cynomorium total flavone to perimenopausal syndrome is realized by mainly increasing the level of E2, however the further molecular mechanism still remains to be explored. The increased level of E2 adjusts the disorder of hypothalamic-pituitary-gonad axis, making related biomedical indexes such as 5-HT, DA, ER, AR, and ERmRNA tend to be normal, so as to relieve the symptoms of perimenopausal syndrome and perimenopausal syndrome with depression.
Currently, regarding the duplication of perimenopausal depression model, mostly refer to the duplication of single perimenopause model or depression model; however this paper successfully duplicated the model of perimenopause with depression, which is the first difference from that in previous researches. In addition, in the situation where few researches are conducted on the effect of cynomorium total flavone on perimenopausal depression, this experiment further explores pharmacological action of cynomorium total flavone, which is the second difference from that of previous researches. Moreover, as a common Chinese medicine for male, cynomorium was used to treat female menopause diseases, which is a novel research approach of “reverse balance of cure and tone”, providing a new treatment approach for body function imbalance diseases such as climacteric syndrome, uterine hypoplasia, hyperplasia of mammary glands, and male reproductive function diseases, which is the third difference from that in previous researches. Finally, with characteristics of high effectiveness, lower cost and convenience, cynomorium total flavone enjoys a wide planting area in China, therefore developing new drugs or health care products based on cynomorium total flavone can enhance the full use of Chinese material medicine resource. The experimental results of this paper show that cynomorium total flavone is of sound therapeutic effect to perimenopausal depression of rats, providing a scientific basis for regarding cynomorium total flavone as a preventive drug for perimenopausal depression and providing a new approach for developing new drugs based on cynomorium for perimenopausal depression.
Acknowledgement
Molecular mechanism of cynomorium total flavones for curing perimenopausal syndrome; foundation No.: 81274154.
Footnotes
Peer review under responsibility of King Saud University.
References
- Carsote M., Popescu M., Samoila R. The serum serotonin and 25-OH vitamin D levels: a study in 97 menopausal women. Bioscientifica. 2013 [Google Scholar]
- Guicheng X. first ed. Chinese Medicine Press; Beijing: 2010. pp. 318–326. (Practical Gynecology of Traditional Chinese Medicine). [Google Scholar]
- Jin S., Yin Y. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by D-galactose. Food Chem. Toxicol. 2012;50:3814–3818. doi: 10.1016/j.fct.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Judy R.S. The reciprocal relationship between menopausal symptoms and depressive symptoms: a 9-year longitudinal study of American women in midlife. Maturitas. 2011;70:302. doi: 10.1016/j.maturitas.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Kammerer M., Gutzwiller S., Stauffer D. Estrogen Recep-tor ɑ (ERɑ) and estrogen related receptor ɑ (ERRɑ) are both transcriptional regulators of the Runx2-I isoform. Mol. Cell. Endocrinol. 2013;369:150–160. doi: 10.1016/j.mce.2013.01.024. PMID: 23403054. [DOI] [PubMed] [Google Scholar]
- Love T., Smith Y.R., Persad C.C. Short-term hormone treatment modulates emotion response circuitry in postmenopausal women. Fertil. Steril. 2010;93:1929–1937. doi: 10.1016/j.fertnstert.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H.C., Wand S., Li Y. Chemical constituent and pharmacologic actions of Cynomorium plant. Chin. J. Nat. Med. 2013;11:321–329. doi: 10.1016/S1875-5364(13)60049-7. [DOI] [PubMed] [Google Scholar]
- Mingsan M., Lin G., Tan W., Ying Z. Effect of Cynomorium songaricum total flavonoids on perimenopausal syndrome mice model. Basic Clin. Pharmacol. Toxicol. 2015;117:9. [Google Scholar]
- Pachman D.R., Jones J.M., Loprinzi C.L. Management of menopause-associated vasomotor symptoms: current treatment options, challenges and future directions. Int. J. Women’s Health. 2010;2:123. doi: 10.2147/ijwh.s7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuo, T., Mingsan, M., 2014. Chemistry, pharmacologic action and clinical application feature of cynomorium 29, 245–251.
- Stevenson J.C., Hodis H.N., Pickar J.H. HRT and breast cancer risk: a realistic perspective. Climacteric. 2011;14:633–636. doi: 10.3109/13697137.2011.590618. [DOI] [PubMed] [Google Scholar]
- Wiacek M., Hagner W., Zubrzycki I. Measures of menopause driven differences in levels of blood lipids, follicle-stimulating hormone, and luteinizing hormone in women aged 35 to 60 years: National Health and Nutrition Examination Survey III and National Health and Nutrition Examination Survey 1999–2002 study. Menopause. 2011;18:60–66. doi: 10.1097/gme.0b013e3181e7060b. PMID: 20647956. [DOI] [PubMed] [Google Scholar]
- Zheng J., Li J., Song L.Y. Comprehensive traditional Chinese medicine intervention for perimenopausal syndrome in women: a community study. J. Chin. Integr. Med. 2011;9:287–291. PMID: 21419081. [PubMed] [Google Scholar]