Abstract
Exposures to particulate matter with a diameter of 2.5 μm or less (PM2.5) may influence the risk of birth defects and make you allergic, which causes serious harm to human health. Bamboo charcoal can adsorb harmful substances,that was of benefitto people’s health. In order to figure out the optimal adsorbtion condition and the intrinsic change of bamboo charcoal, five chemicals were adsorbed by bamboo charcoal and were analyzed by FT-IR. The optimal blast time was 80 min of Na2SO3, 100 min of Na2S2O8, 20 min of Na2SO4, 120 min of Fe2(SO4)3 and 60 min or 100 min of S. FT-IR spectra showed that bamboo charcoal had five characteristic peaks of S—S stretch, H2O stretch, O—H stretch, C O stretch or C C stretch, and NO2 stretch at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1, respectively. For Na2SO3, the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum at 20 min. For Na2S2O8, the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1 and 1530 cm−1 achieved the maximum at 40 min. For Na2SO4, the peaks at 3850 cm−1, 3740 cm−1 and 1530 cm−1 achieved the maximum at 40 min. For Fe2(SO4)3, the peaks at 3850 cm−1, 3740 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum at 120 min. For S, the peaks at 3850 cm−1 and 3740 cm−1 achieved the maximum at 40 min, the peaks at 1630 cm−1 and 1530 cm−1 achieved the maximum at 40 min. It proved that bamboo charcoal could remove sulfur powder from air to restrain sulfur allergies.
Keywords: Bamboo charcoal, Desulfuration, Na2SO3, Na2S2O8, Na2SO4, Fe2(SO4)3, S
1. Introduction
With the rapid development of global economy, the development and utilization of coal have brought serious pollution to the environment, especially as the coal-fired power plant boiler emits large amounts of sulfur dioxide and nitrogen oxides (accounts for about 35%∼40% of the total SO2 and NOx emissions) to further aggravate the deterioration of the environment. For example, Shanghai and Beijing are two of the largest cities in China. Both cities have populations of over 10 million. These two urban areas have experienced a rapid increase in the use of vehicles, concurrent with large increases in energy consumption. Particulate pollution has become a major problem (Yao et al., 2002). NOx and hydrocarbon with photochemical smog formation, cause serious harm to human health. In hazy weather during sports the human respiratory system changes direction, damaging the human respiratory system seriously (Li, 2014). Exposures to particulate matter with a diameter of 2.5um or less (PM2.5) may influence the risk of birth defects and make you allergic (Girguis et al., 2015).
Bamboo planting in China is very large, it is a kind of short growth cycle and timber fast biomass resource. Therefore, bamboo charcoal is a natural, renewable environmental protection material and functional material. Bamboo charcoal was created by heating bamboo at temperatures of 600–900 °C and then the charcoal itself was processed and mixed in with fabrics as part of the growing field of nanotechnology (Girguis et al., 2015, Yang et al., 2005, Ignatova et al., 2003, Abe et al., 2001, Kawashita et al., 2003, Mizuta et al., 1994, Wang et al., 2006, Xue et al., 2014, Cui et al., 2014, Le et al., 2015, Peng et al., 2014a, Peng et al., 2014b, Peng et al., 2014c, Peng et al., 2012a). Bamboo charcoal had many positive qualities (Girguis et al., 2015, Yang et al., 2005, Ignatova et al., 2003, Abe et al., 2001). The fabric inhibited bacterial metabolism causing fewer allergic skin reactions than other fibers sterilized with antimicrobial agents. Because the trait was due to the highly porous structure of the bamboo fabric, it could absorb sulfur-based compounds, nitrogen-based compounds and so on (Ignatova et al., 2003, Abe et al., 2001, Kawashita et al., 2003, Mizuta et al., 1994, Wang et al., 2006, Xue et al., 2014, Cui et al., 2014). What’s more, bamboo charcoal, which contained potassium, calcium and other minerals, could cause adsorption and filtration of extractives, oil, and other substances (Peng et al., 2013a, Xiao et al., 2013, Peng et al., 2013b, Wang et al., 2013, Peng et al., 2013c, Peng et al., 2012b, Peng and Le, 2012, Peng et al., 2011, Zhang et al., 2008, Qi et al., 2012), that was beneficial for people’s health. But so far, the bamboo charcoal in coal-fired flue gas pollution (sulfide, etc.) control in the field of study is less reported. In order to figure out the optimal adsorbtion condition and the intrinsic change of the bamboo charcoal, five chemicals were adsorbed by bamboo charcoal and were analyzed by FT-IR.
2. Materials and methods
2.1. Materials
Bamboo charcoal, Na2SO3, Na2S2O8, Na2SO4, Fe2(SO4)3 and S were purchased from the market.
2.2. Methods
Five kinds of pharmaceutical powder were weighed in amounts of 25 g. These powders and 4 g bamboo charcoal were put into the closed vessel. It was blasted in a closed vessel for 20 min, 40 min, 60 min, 80 min, 100 min and 120 min. Each bamboo charcoal was removed, dried, and weighed.
FT-IR spectra. FT-IR spectra of the above samples were obtained using a Thermo Scientific Nicolet iN10 FT-IR microscope as previously (Lin et al., 2015, Peng et al., 2014a, Peng et al., 2014b, Peng et al., 2014c, Sun et al., 2014).
3. Result and analysis
Based on the above test, the results of adsorption were obtained and listed in Table 1.
Table 1.
Adsorption results.
| Blast time [min] | 20 | 40 | 60 | 80 | 100 | 120 |
|---|---|---|---|---|---|---|
| Na2SO3 | 1.48 | 0.25 | 0.5 | 1.52 | 1.24 | 1.5 |
| Na2S2O8 | 1.75 | 0.75 | 0.74 | 2 | 2.02 | 1.73 |
| Na2SO4 | 2.25 | 2.02 | 2.02 | 0.74 | 1.49 | 0.5 |
| Fe2(SO4)3 | 1.49 | 0.49 | 1.01 | 1.26 | 1.24 | 1.74 |
| S | 2.76 | 3.25 | 4.47 | 2.24 | 4.47 | 2.98 |
3.1. SC Effect
Based on Table 1, Na2SO3’s adsorption capacity was 1.48 g/100 g, 0.25 g/100 g, 0.5 g/100 g, 1.52 g/100 g, 1.24 g/100 g, 1.5 g/100 g; Na2S2O8’s adsorption capacity was 1.75 g/100 g, 0.75 g/100 g, 0.74 g/100 g, 2 g/100 g, 2.02 g/100 g, 1.73 g/100 g; Na2SO4’s adsorption capacity was 2.25 g/100 g, 2.02 g/100 g, 2.02 g/100 g, 0.74 g/100 g, 1.49 g/100 g, 0.5 g/100 g; Fe2(SO4)3’s adsorption capacity was 1.49 g/100 g, 0.49 g/100 g, 1.01 g/100 g, 1.26 g/100 g, 1.24 g/100 g, 1.74 g/100 g; S’s adsorption capacity was 2.76 g/100 g, 3.25 g/100 g, 4.47 g/100 g, 2.24 g/100 g, 4.47 g/100 g, 2.98 g/100 g for a blast time of 20 min, 40 min, 60 min, 80 min, 100 min and 120 min, respectively. It showed that adsorption capacity changed the regularity difference. It might be because rapid stirring lead to a small amount of five kinds of pharmaceutical powders on the surface of bamboo charcoal. The optimal blast time was 80 min of Na2SO3, 100 min of Na2S2O8, 20 min of Na2SO4, 120 min of Fe2(SO4)3 and 60 min or 100 min of S (see Table 2).
Table 2.
Groups of bamboo charcoal during adsorption of Na2SO3, Na2S2O8, Na2SO4, Fe2(SO4)3 and S (%).
| Kind | Peak (cm−1) | Adsorption time (min) |
Groups | |||||
|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | 120 | |||
| Na2SO3 | 1530 | 87.7 | 56.9 | 51.3 | 44.3 | 56.9 | 52.2 | NO2 |
| 1630 | 87.0 | 59.3 | 54.3 | 43.0 | 60.7 | 52.8 | C O or C C | |
| 3430 | 81.7 | 55.1 | 47.2 | 37.4 | 55.9 | 47.2 | O—H stretch | |
| 3740 | 88.7 | 57.9 | 48.5 | 43.7 | 57.8 | 52.1 | H2O | |
| 3850 | 89.1 | 59.4 | 49.6 | 43.7 | 59.5 | 52.7 | S—S stretch | |
| Na2S2O8 | 1530 | 84.7 | 87.3 | 82.5 | 88.5 | 82.9 | 83.7 | NO2 |
| 1630 | 86.9 | 86.9 | 86.8 | 87.2 | 87.4 | 88.1 | C O or C C | |
| 3430 | 80.5 | 83.0 | 82.6 | 78.5 | 78.0 | 80.7 | O—H stretch | |
| 3740 | 81.3 | 88.7 | 81.8 | 87.1 | 81.5 | 82.0 | H2O | |
| 3850 | 84.4 | 89.0 | 84.0 | 88.1 | 83.0 | 84.0 | S—S stretch | |
| Na2SO4 | 1530 | 85.5 | 88.3 | 85.6 | 83.4 | 82.0 | 82.5 | NO2 |
| 1630 | 87.4 | 87.9 | 88.1 | 86.0 | 84.8 | 86.4 | C O or C C | |
| 3430 | 79.0 | 79.2 | 80.5 | 78.8 | 77.1 | 80.0 | O—H stretch | |
| 3740 | 83.2 | 86.5 | 82.6 | 82.3 | 81.4 | 81.1 | H2O | |
| 3850 | 85.0 | 87.5 | 84.6 | 84.0 | 83.4 | 83.0 | S—S stretch | |
| Fe2(SO4)3 | 1530 | 81.2 | 82.0 | 89.1 | 82.0 | 84.2 | 90.4 | NO2 |
| 1630 | 86.0 | 87.3 | 86.5 | 87.3 | 86.6 | 88.3 | C O or C C | |
| 3430 | 78.5 | 81.2 | 78.4 | 81.2 | 82.8 | 78.8 | O—H stretch | |
| 3740 | 79.3 | 81.2 | 88.1 | 81.2 | 84.3 | 88.9 | H2O | |
| 3850 | 81.5 | 83.4 | 88.8 | 83.4 | 85.8 | 89.2 | S—S stretch | |
| S | 1530 | 90.5 | 90.1 | 79.2 | 85.1 | 85.2 | 80.3 | NO2 |
| 1630 | 88.8 | 88.3 | 84.0 | 86.4 | 88.1 | 83.7 | C O or C C | |
| 3430 | 78.2 | 80.4 | 76.1 | 78.3 | 83.4 | 73.8 | O—H stretch | |
| 3740 | 88.4 | 89.2 | 78.9 | 84.3 | 83.5 | 79.5 | H2O | |
| 3850 | 88.7 | 89.4 | 81.3 | 85.5 | 85.2 | 81.7 | S—S stretch | |
3.2. FT-IR analysis
FT-IR spectra were recorded to investigate the functional groups of bamboo charcoal during adsorption of Na2SO3, Na2S2O8, Na2SO4, Fe2(SO4)3 and S. Spectra of the samples were shown in supporting information Figure 1, Figure 2, Figure 3, Figure 4, Figure 5. In the spectrum of adsorption, the S—S stretch, H2O stretch, O—H stretch, C O stretch or C C stretch, NO2 stretch were observed at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1, respectively [28–32].
Figure 1.
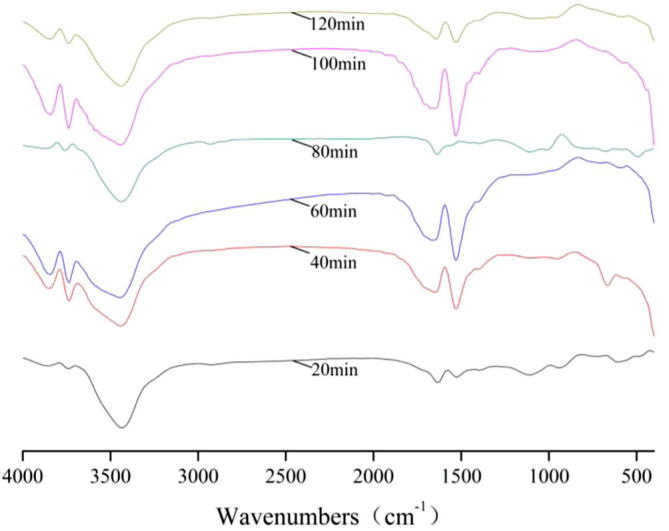
FT-IR spectra of bamboo charcoal during adsorption of Na2SO3.
Figure 2.
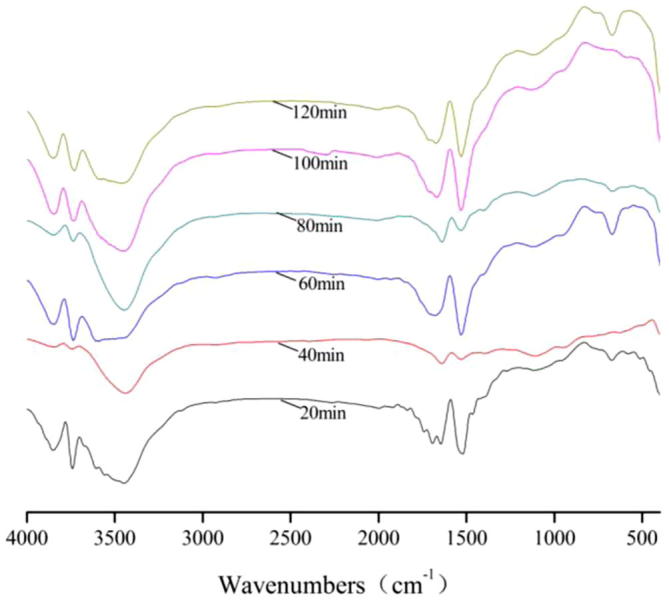
FT-IR spectra of bamboo charcoal during adsorption of Na2S2O8.
Figure 3.
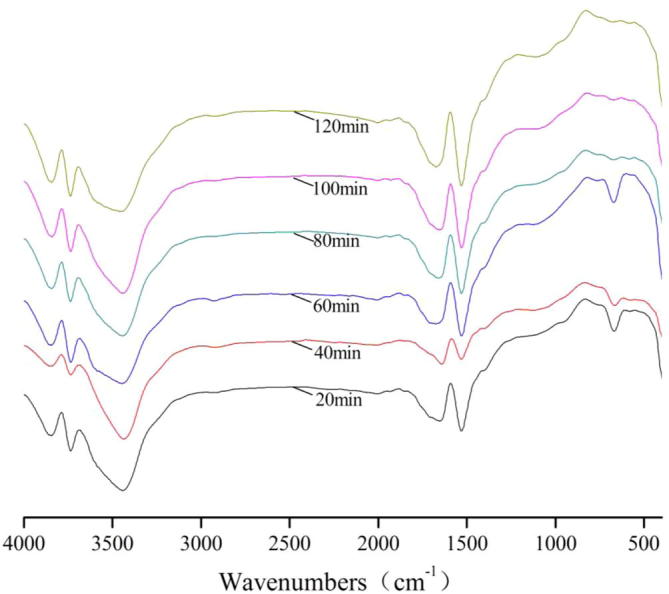
FT-IR spectra of bamboo charcoal during adsorption of Na2SO4.
Figure 4.
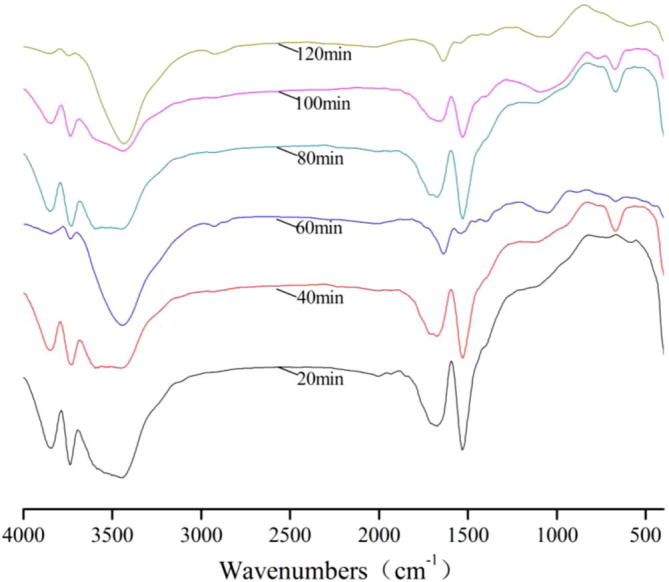
FT-IR spectra of bamboo charcoal during adsorption of Fe2(SO4)3.
Figure 5.
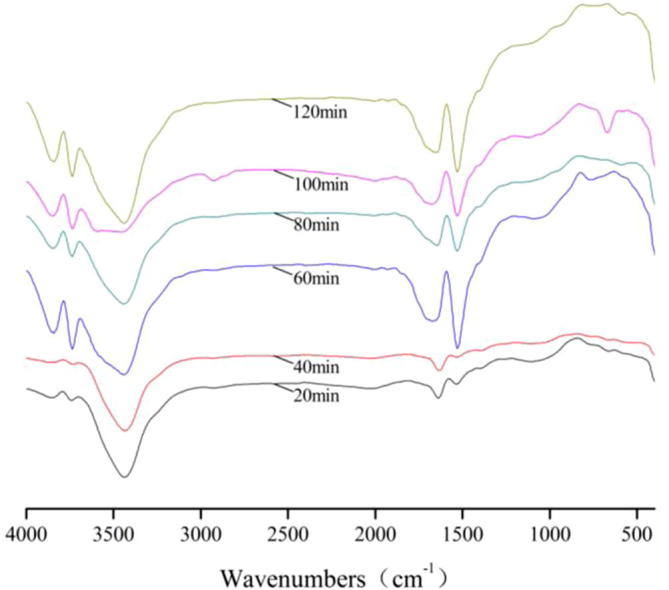
FT-IR spectra of bamboo charcoal during adsorption of S.
For FT-IR spectra of Na2SO3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum for 20 min.
For FT-IR spectra of Na2S2O8, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1 and 1530 cm−1 achieved the maximum for 40 min, the transmissivity of the peaks at 1630 cm−1 achieved the maximum for 120 min.
For FT-IR spectra of Na2SO4, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1 and 1530 cm−1 achieved the maximum for 40 min, the transmissivity of the peaks at 3430 cm−1 and 1630 cm−1 achieved the maximum for 60 min.
For FT-IR spectra of Fe2(SO4)3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum for 120 min, the transmissivity of the peaks at 3430 cm−1 achieved the maximum for 100 min.
For FT-IR spectra of S, the transmissivity of the peaks at 3850 cm−1 and 3740 cm−1 achieved the maximum for 40 min, the transmissivity of the peaks at 3430 cm−1 achieved the maximum for 100 min, the transmissivity of the peaks at 1630 cm−1 and 1530 cm−1 achieved the maximum for 40 min.
4. Conclusion
Na2SO3’s, Na2S2O8’s, Na2SO4’s, Fe2(SO4)3’s and S’s adsorption capacity were different for blast times of 20 min, 40 min, 60 min, 80 min, 100 min and 120 min, respectively. The optimal blast time was 80 min of Na2SO3, 100 min of Na2S2O8, 20 min of Na2SO4, 120 min of Fe2(SO4)3 and 60 min or 100 min of S.
FT-IR spectra showed that bamboo charcoal had the eight characteristic absorption band. And the S—S stretch, H2O stretch, O—H stretch, C O stretch or C C stretch, NO2 stretch were observed at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1, respectively. For FT-IR spectra of Na2SO3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum for 20 min. For FT-IR spectra of Na2S2O8, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 3430 cm−1 and 1530 cm−1 achieved the maximum for 40 min. For FT-IR spectra of Na2SO4, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1 and 1530 cm−1 achieved the maximum for 40 min. For FT-IR spectra of Fe2(SO4)3, the transmissivity of the peaks at 3850 cm−1, 3740 cm−1, 1630 cm−1 and 1530 cm−1 achieved the maximum for 120 min. For FT-IR spectra of S, the transmissivity of the peaks at 3850 cm−1 and 3740 cm−1 achieved the maximum for 40 min, the transmissivity of the peaks at 1630 cm−1 and 1530 cm−1 achieved the maximum for 40 min. In these states, the number of the transmissivity of the maximum peaks is the largest.
Acknowledgment
This work was financially supported by the National 948 Plan (2014-4-38).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abe I., Fukuhara T., Maruyama J., et al. Preparation of carbonaceous adsorbents for removal of chloroform from drinking water. Carbon. 2001;39:1069–1073. [Google Scholar]
- Cui L., Peng W.X., Sun Z.J., Shang L.L., Chen G.N. Weibull statistical analysis of tensile strength of vascular bundle in inner layer of moso bamboo culm in molecular parasitology and vector biology. Pak. J. Pharm. Sci. 2014;27(4):1083–1087. [PubMed] [Google Scholar]
- Girguis M.S., Strickland M.J., Hu X., Liu Y., Bartell S.M., Vieira V.M. Maternal exposure to traffic-related air pollution and birth defects in Massachusetts. Environ. Res. 2015;146:1–9. doi: 10.1016/j.envres.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova M., Labaye D., Lenoir S., et al. Jerome immobilization of silver in polypyrrole/polyanion composite coatings: preparation, characterization, and antibacterial activity. Langmuir. 2003;19:8971–8979. [Google Scholar]
- Kawashita M., Toda S., Kim H.M., Kokubo T., Masuda N. Preparation of antibacterial silver-doped silica glass microspheres. J. Biomed. Mater. Res. 2003;66:266. doi: 10.1002/jbm.a.10547. [DOI] [PubMed] [Google Scholar]
- Le C., Peng W.X., Sun Z.J., et al. Variability of macroscopic dimensions of Moso bamboo. Pak. J. Pharm. Sci. 2015;28:675–679. [PubMed] [Google Scholar]
- Li C. Haze weather sports damage analysis of the respiratory system. Bull. Sci. Technol. 2014;30(1):62–65. [Google Scholar]
- Lin Z., Ge S.B., Li D.L., Peng W.X. Structure characteristics of acidic pretreated fiber and self-bind bio-boards for public health. J. Pure Appl. Microbiol. 2015;9:221–226. [Google Scholar]
- Mizuta K., Matsumoto T., Hatate Y., Nishihara K., Nakanishi T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 1994;95:255. doi: 10.1016/j.biortech.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Peng W.X., Le C. Crystal structure of 3-(3-bromophenyl)-4-(3 5-dichloro-phenylamino)furan-2(5H)-one C16H10BrCl2NO2. Z. Kristallogr. New Cryst. Struct. 2012;227(2):267–268. [Google Scholar]
- Peng W.X., Wang L.S., Wu F.J., Xu Q. 3-(4-Bromophenyl)-4-(4-hydroxyanilino)furan-2(5H)-one. Acta Crystallogr. Sect. E Struct. Rep. Online. 2011;67 doi: 10.1107/S1600536811031849. O2329-U206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.X., Wang L.S., Xu Q., Wu Q.D., Xiang S.L. TD-GC-MS analysis on thermal release behavior of poplar composite biomaterial under high temperature. J. Comput. Theor. Nanosci. 2012;9(9):1431–1433. [Google Scholar]
- Peng W.X., Wu F.J., Wang L.S., Xu Q. Crystal structure of 3-(4-bromophenyl)-4-(4-chlorophenylamino)furan-2(5H)-one C16H11BrClNO2. Z. Kristallogr. New Cryst. Struct. 2012;227(1):61–62. [Google Scholar]
- Peng W.X., Lin Z., Chang J.B., Gu F.L., Zhu X.W. Biomedical molecular characteristics of YBSJ extractives from illicium verum fruit. Biotechnol. Biotechnol. Equip. 2013;27(6):4311–4316. [Google Scholar]
- Peng W.X., Wang L.S., Lin Z., Zhang M.L. Identification and chemical bond characterization of wood extractives in three species of eucalyptus biomass. J. Pure Appl. Microbiol. 2013;7:67–73. [Google Scholar]
- Peng W.X., Wang L.S., Zhang M.L., Lin Z. Molecule characteristics of eucalyptus hemicelluloses for medical microbiology. J. Pure Appl. Microbiol. 2013;7(2):1345–1349. [Google Scholar]
- Peng W.X., Ge S.B., Li D.L., Mo B., Daochun Q., Ohkoshi M. Molecular basis of antibacterial activities in extracts of Eucommia ulmoides wood. Pak. J. Pharm. Sci. 2014;27(6):2133–2138. [PubMed] [Google Scholar]
- Peng W.X., Wang L.S., Zhang M.L., Lin Z. Separation characteristics of lignin from Eucalyptus camaldulensis lignin celluloses for biomedical cellulose. Pak. J. Pharm. Sci. 2014;27:723–728. [PubMed] [Google Scholar]
- Peng W.X., Xue Q., Ohkoshi M. Immune effects of extractives on bamboo biomass self-plasticization. Pak. J. Pharm. Sci. 2014;27:991–999. [PubMed] [Google Scholar]
- Qi H.C., Peng W.X., Wu Y.Q., Wu S.B., Xu G.J. Effects of alkaline extraction on micro/nano particles of eucalyptus camaldulensis biology. J. Comput. Theor. Nanosci. 2012;9(9):1525–1528. [Google Scholar]
- Sun Y.C., Lin Z., Peng W.X., Yuan T.Q., Xu F., Wu Y.Q., Yang J., Wang Y.S., Sun R.C. Chemical changes of raw materials and manufactured binderless boards during hot pressing: lignin isolation and characterization. Bioresources. 2014;9(1):1055–1071. [Google Scholar]
- Wang J.X., Wen L.X., Wang Z.H., Chen J.F. Immobilization of silver on hollow silica nanospheres and nanotubes and their antibacterial effects. Mater. Chem. Phys. 2006;96(1):90–97. [Google Scholar]
- Wang L.S., Peng W.X., Zhang M.L., Lin Z. Separation characteristics of lignin from eucalyptus lignin cellulose for medicinal biocellulose preparation. J. Pure Appl. Microbiol. 2013;7:59–66. [Google Scholar]
- Xiao Z.P., Peng Z.Y., Dong J.J., et al. Synthesis molecular docking and kinetic properties of beta-hydroxy-beta-phenylpropionyl-hydroxamic acids as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2013;68:212–221. doi: 10.1016/j.ejmech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Xue Q., Peng W.X., Ohkoshi M. Molecular bonding characteristics of self-plasticized bamboo composites. Pak. J. Pharm. Sci. 2014;27:975–982. [PubMed] [Google Scholar]
- Yang F.C., Wu K.H., Liu M.J., Lin W.P., Hu M.K. Evaluation of the antibacterial efficacy of bamboo charcoal/silver biological protective material. Mater. Chem. Phys. 2005;113:474–479. [Google Scholar]
- Yao X.H., Chan C.K., Fang M., et al. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing. China Atmos. Environ. 2002;36:4223–4234. [Google Scholar]
- Zhang D.Q., Chen S.M., Peng W.X., Liu Q.M., Gu Z.J., Fan S.G., Deng S.Y. Rheology study of supercritically extracted tea-oil. J. Cent. South Univ. Technol. 2008;15:506–508. [Google Scholar]


