Background
Soybean oligosaccharides (SBOSs) are potential prebiotics that may be used to improve immune function. Here, we investigated the effects of intragastric administration of SBOSs in mice to determine the effects on autochthonous intestinal microbial communities and immunological parameters. Results E: After 22-day administration, 4.0 g kg body weight (BW)−1 SBOSs significantly enhanced the proliferation of bifidobacteria and lactic acid bacteria (LAB) as compared to the control. This dose of SBOSs also significantly increased numbers of enterococci and decreased numbers of Clostridium perfringens. Treatment with 4.0 g kg BW−1 SBOSs also significantly increased the percentage of T-lymphocytes and lymphocyte proliferation as compared to the control, suggesting that SBOSs promoted cellular immunity in mice. Additionally, 4.0 g kg BW−1 SBOSs induced significant differences in hemolysin production, natural killer (NK) cell activity, phagocytic activity, cytokine production, and immunoglobulin levels compared to the control. Conclusion: Our data demonstrated that intragastric administration of SBOSs at a dose of 4.0 g kg BW−1 improved the numbers of beneficial intestinal microbes and enhanced immunological function of mice. Therefore, these data supported that SBOSs may have applications as a prebiotic to improve immune responses in humans. Further studies are warranted.
Keywords: Soybean oligosaccharides, Prebiotic, Intestinal microbial communities, Immune modulation
1. Introduction
Prebiotics are nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health (Gibson and Roberfroid, 1995). Intake of prebiotics increases the number of specific bacteria in the colonic microbiota, thus changing the composition of the microbiota (Gibson and Roberfroid, 1995). Flickinger and Fahey (2002) is usually short-chain oligosaccharides with 2–60 polymers and are not digested by human or animal digestive enzymes of the upper gastrointestinal tract, but are instead selectively fermented by some types of intestinal bacteria in the large intestine (Patterson and Burkholder, 2003). Among the bacteria present in the gastrointestinal tract, bifidobacteria and lactobacilli are those that most often utilize prebiotic oligosaccharides and are therefore the only microorganisms able to beneficially affect the health of the host in this context (Mikkelsen and Jensen, 2004, Vernazza et al., 2005).
Soybean oligosaccharides (SBOSs), which are isolated from soybean seeds, are well-established prebiotics approved by the United States Food and Drug Administration as a generally recognized as safe (GRAS) ingredient (Chen et al., 2010, Kim et al., 2003). SBOSs are a group of soluble oligosaccharides found in soy or other legumes and consist primarily of raffinose, stachyose, and sucrose, common components formed by various linkages of mono- and oligosaccharides (Zhou et al., 2012, Jianxian and Liping, 2003).
In the intestine, SBOSs can be fermented by certain bacteria, such as lactobacilli and bifidobacteria. Dietary inclusion of SBOSs will thus selectively support the growth and survival of these bacteria in the gastrointestinal tract of animals. Indeed, one study has shown that feeding of wheat bran oligosaccharides and SBOSs results in significantly higher concentrations of bifidobacteria and Clostridium perfringens relative to that of fructooligosaccharide (FOS) feeding or the control, while FOS had no effect compared to the control (Martin et al., 1998). Furthermore, soybean meal oligosaccharides (SMOs) have been reported to potentially promote competitive exclusion of pathogens (Xu et al., 2009). Therefore, consumption of these prebiotics may be useful for maintaining populations of beneficial microbes in the gut.
In addition, SBOSs may benefit immune function by stimulating the growth and metabolism of protective commensal intestinal bacteria, part of the first line of defense in the gastrointestinal tract (Boehm et al., 2005). Indeed, prebiotics can enhance nonspecific defense mechanisms and increase resistance to infectious diseases by enhancing innate humoral and cellular defense mechanisms (Safarpour et al., 2011). Some studies have shown that SBOSs may influence hematological and immunological parameters; for example, SBOSs have been shown to increase superoxide dismutase levels in the blood, raise IgG levels, alter the weights and indices of immunity organs (e.g., bursa of thymus, fabricius, and spleen), promote splenocyte proliferation, enhance the number of antibody forming cells (AFCs) in normal mice, and ameliorate harmful immune effects in SAM- and S180-treated mice (Xu et al., 2005). These studies have shown that SBOSs have dramatic effects on gut health and immune modulation. However, the details of mechanisms through which intestinal microbes and immune modulation are affected by SBOSs are not known. While the positive effects of some prebiotics, including FOS, arabinoxylan-oligosaccharides (AXOSs), and mannan oligosaccharides (MOSs), have been reported (e.g., growth performance, innate immunity, hematological and serum biochemical parameters, microbial fermentation, and autochthonous intestinal microbiota (Akrami et al., 2013, Geraylou et al., 2012, Huynh et al., 2009), the effects of SBOSs on intestinal microbial communities and immune modulation have not yet been studied in a mouse model.
In this study, we assessed prebiotic effects of SBOSs on the intestinal microbial communities, including bifidobacteria, lactic acid bacteria (LAB), Escherichia coli, enterococci, and C. perfringens, in order to evaluate immune responses of mice consuming different levels of oligosaccharides.
2. Material and methods
2.1. Reagents and animals
SBOSs were purchased from Baolingbao Biological Co. (Shandong, China). Chicken red blood cells (CRBCs) and lactate dehydrogenase were obtained from Applygen Technologies Inc. (Beijing, China). Methyl thiazolyl tetrazolium (MTT) and sodium dodecyl sulfate (SDS) were purchased from AMRESCO LLC. (Solon, USA). Concanavalin A (ConA) and erythrocyte lysates were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Mouse tumor necrosis factor-α (TNF-α), mouse interleukin-4 (IL-4), and mouse interferon-γ (IFN-γ) were purchased from R&D Systems, Inc. (San Diego, CA, USA). Mouse TNF-α, IL-4, and IFN-γ ELISA kits were purchased from Bogu Biological Technology Co. (Shanghai, China). Biological stains, including Giemsa dye, vice magenta, and malachite green were obtained from Aojia Chemical Co. (Nanjing, China).
Kunming mice (weighing 22.2 ± 0.6 g each) were obtained from Biotechnology Co., Ltd. (Liaoning, China) and maintained in specific pathogen-free conditions. They had free access to water and standard rodent chow and were weighed daily.
2.1.1. Experimental animal grouping
Mice were randomly assigned to four groups: 0 (control group), 0.5 (low-dose group), 1.5 (middle-dose group), and 4.0 (high-dose group) g kg body weight (BW)−1 (n = 20 mice per group, 10 males and 10 females). Before the experiment, mouse feces were placed in sterile containers, and the numbers of fecal bacteria (CFU/g) were determined by using logarithm statistics. Mice were then treated by intragastric administration of SBOSs, and the number of fecal bacteria was determined again at 24 h after the final treatment in weeks 1 and 2. At 24 h after treatment in week 3, 10 mice within each group (five males and five females) were randomly chosen for analysis of cellular immunity, natural killer (NK) cell numbers, and cytokine and immunoglobulin levels. The remaining 10 mice in each group were used for detection of humoral immunity and phagocytic activity following transfer of chicken erythrocytes into the mice. Feces production, body weights, and water and food intake were recorded daily throughout the experiment.
2.2. Analysis of intestinal microbial communities
Under sterile conditions, the intestinal microbial communities within mouse feces were counted after continuous intragastric administration of SBOSs for 8.15 or 22 days. Changes in bifidobacteria, lactobacilli, E. coli, enterococci, and C. perfringens were compared. The effects of SBOSs on the balance of intestinal microbial communities were determined in accordance with the Technical Standards for Testing and Assessment Health Food (2003 Edition, China). Media, culture conditions, and identification methods are shown in Table 1.
Table 1.
Media, culture conditions, and identification methods of intestinal microbes.
| Species name | Medium | Culture conditions | Identification method |
|---|---|---|---|
| Bifidobacteria | TYG (tryptone yeast glucose extract agar) | 37 °C, 48 h, anaerobic | Gram-positive staining of all non-bacillus |
| LAB | EMB (eosin methylene blue medium) | 37 °C, 24 h | Gram-negative staining of all bacilli |
| E. coli | MRS (Lactobacillus bacteria-selective medium) | 37 °C, 48 h, anaerobic | GB/T.4789.34-2003 |
| Enterococci | SSM (Streptococcus-selective medium) | 37 °C, 24 h | Gram-positive staining of all cocci and obvious brown circle |
| C. perfringens | TSC (tryptone-sulfite cycloserine medium) | 37 °C, 48 h, anaerobic | All fluorescent black colonies under UV |
2.3. Analysis of immune modulation parameters
The effects of SBOSs on immune modulation were determined in accordance with the Technical Standards for Testing and Assessment Health Food (2003 Edition, China). Cellular immune function, humoral immune function, macrophagic phagocytosis, NK cell activity, and cytokine and immunoglobulin levels were determined.
Cellular immunity was evaluated by determining the percentages of T-lymphocytes and lymphocytic transformation induced by ConA in mice using enzyme-labeled staining of lymphocytes in the peripheral blood. Additionally, MTT assays were performed to measure changes in cell proliferation using the differences in optical densities (ΔOD570).
Humoral immunity was assessed by the analysis of hemolysin using CRBCs as an immune source, and the absorbance was read spectrophotometrically (Hitachi) at 540 nm.
Phagocytic activity was assessed by incubating phagocytic cells from the head kidney with CRBCs overnight at 15 °C. Phagocytic activity was determined as the percentage and index of phagocytic cells quantified from 100 cells observed under a microscope. The phagocytic percentage and phagocytic index were calculated as follows:
The activity of NK cells was measured according to previously described methods using lactate dehydrogenase (LDH) activity (2003 Edition, China).
Cytokine levels were measured as previously described (2003 Edition, China), with slight modifications. At 24 h after the last administration, blood was sampled via retro-orbital collection. Serum was obtained after centrifugation at 2000 rpm for 10 min and was stored at −20 °C. TNF-α, INF-γ, and IL-4 levels in serum were determined by enzyme-linked immunosorbent assay. Standard wells and test sample wells were included in the microtiter plate. A set of serial dilutions of the standard, in a volume of 50 μL, were added into the standard wells. Ten microliters of each test sample was added to the appropriate wells, along with 40 μL of sample diluent. The blank wells were empty. The wells were then blocked with 100 μL of HRP-conjugated reagent at 37 °C for 1 h. After blocking, the plate was washed five times with 400 μL Wash Solution. Fifty microliters of chromogen solutions A and B was added, and the plates were incubated for 15 min at 37 °C. The reaction was stopped with 50 μL of Stop Solution, and the absorbance was measured at 450 nm.
Total immunoglobulin (Ig) was measured by the method described by Siwicki and Anderson (1993). Serum was separated from collected blood as described above, and IgA, IgG, and IgM contents were determined.
2.4. Statistical analysis
All data were compared using analysis of variance (ANOVA), and differences between means were evaluated by Duncan’s Multiple Range Test. The SPSS statistic program (Version 19.0) was used for data analysis.
3. Results
3.1. Effects of different doses of SBOSs on the intestinal microbial communities in mice
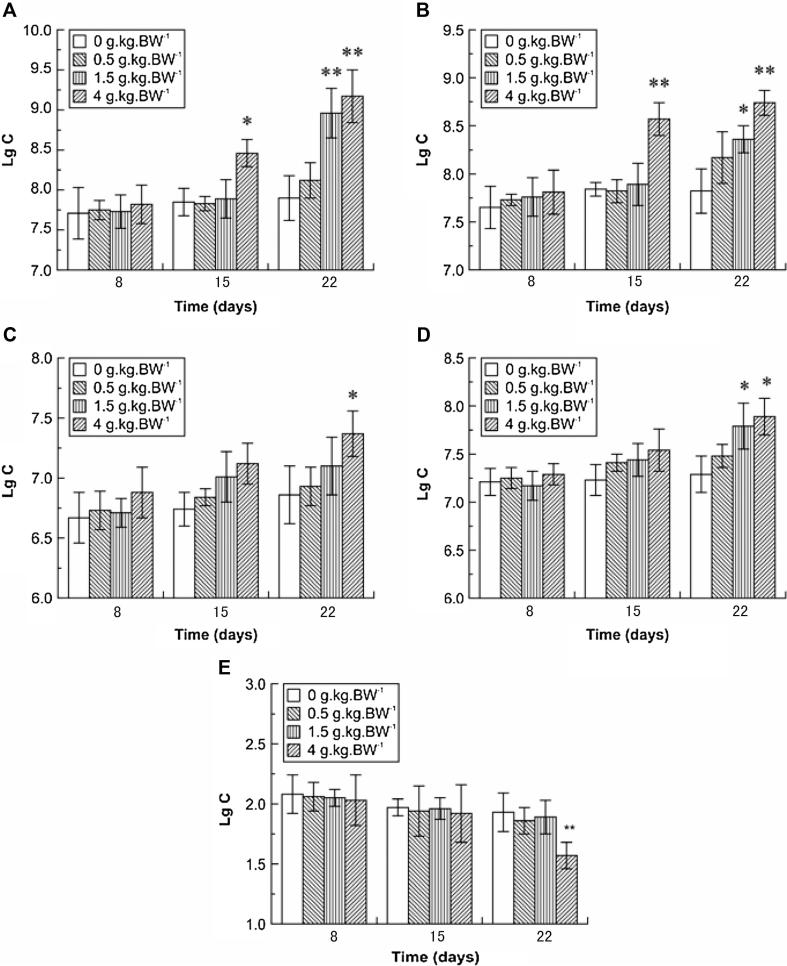
As shown in Fig. 1, the numbers of bifidobacteria, LABs, E. coli, enterococci, and C. perfringens in control mice did not change throughout the experiment (p > 0.05). Additionally, there were no significant differences between the low-dose and control groups for these five types of bacteria following treatment for 22 days. However, the middle- and high-dose groups exhibited significant increases in the number of intestinal bifidobacteria (increased by 13.42% and 16.08%, respectively; p < 0.01). Additionally, after administration of SBOSs for 8.15 or 22 days, the numbers of LAB in the high-dose group were significantly increased (by 9.31% and 11.76%, respectively; p < 0.01) when compared to the control group. Moreover, the number of E. coli was significantly increased by 7.43% in the high-dose group compared to that of the control (p < 0.05). Similarly, the numbers of enterococci in the high- and middle-dose groups were significantly increased (by 6.86% and 8.23%, respectively; p < 0.05) compared to the control.
Figure 1.
Effects of different doses of SBOSs on intestinal microbes. (Bifidobacteria (A), LAB (B), E. coli (C), enterococci (D), and C. perfringens (E) (mean ± SD, n = 20) were analyzed. Statistical significance is indicated versus the control group. ∗p < 0.05, ∗∗p < 0.01. C: colonies.).
In contrast, intestinal C. perfringens numbers were significantly reduced (by 18.65%; p < 0.01) following administration of high-dose SBOSs for 22 days; however, no differences were observed between the control group and the low- or middle-dose groups (p > 0.05). These findings indicated that SBOSs had differential effects on the populations of intestinal microbes and that certain oligosaccharides may inhibit the growth of specific types of intestinal bacteria.
3.2. Effects of SBOSs on nonspecific immunity in mice
The effects of SBOSs on nonspecific immunity in mice are shown in Fig. 2.
Figure 2.
The activation of SBOS on systemic immunity.
First, we measured phagocytosis, an immune process mediated by macrophages (Hume, 2006). Compared with the control group, high-dose SBOSs enhanced the percentage of macrophage phagocytosis (p < 0.01) and the phagocytic index (p < 0.01). However, low-dose SBOSs did not alter these parameters (p > 0.05). These data indicated that high-dose SBOSs promoted the phagocytic activity of peritoneal macrophages, improving nonspecific immunity.
Next, we analyzed the activity of NK cells, important indicators of the nonspecific immune response, to investigate the influence of SBOSs on immune function. As shown in Table 2, the activity of NK cells was dramatically increased in the high-dose group as compared to that of the control group (p < 0.05).
Table 2.
Effects of SBOSs on nonspecific immunity in mice.
| Groupc | Dose (g kg−1) | Phagocytosis of macrophages |
NK cells | Cytokine secretion |
|||
|---|---|---|---|---|---|---|---|
| Phagocytic percentage (%) | Phagocytic index | Activity of NK cells | INF-γ | TNF-α | IL-4 | ||
| (ng/mL) | (ng/mL) | (pg/mL) | |||||
| Control group | 0 | 22.27 ± 2.45 | 0.24 ± 0.04 | 20.21 ± 2.13 | 94.04 ± 7.29 | 50.16 ± 3.25 | 16.63 ± 1.78 |
| Low-dose group | 0.5 | 23.67 ± 3.10 | 0.27 ± 0.08 | 21.15 ± 2.57 | 101.19 ± 4.46 | 52.22 ± 3.65 | 17.88 ± 2.67 |
| Middle-dose group | 1.5 | 25.96 ± 1.54a | 0.36 ± 0.05a | 22.36 ± 2.98 | 109.88 ± 5.97a | 55.73 ± 3.28a | 19.24 ± 1.56a |
| High-dose group | 4 | 29.38 ± 2.78b | 0.47 ± 0.13b | 25.42 ± 2.43a | 123.46 ± 6.65b | 60.87 ± 2.83b | 23.2 ± 2.42b |
p < 0.05.
p < 0.01 compared to the control group.
Values shown are mean ± SD (n = 10).
Cytokines play essential roles in many immune-related processes. Therefore, we next analyzed changes in the expression levels of TNF-α, IFN-γ, and IL-4. As shown in Table 2, levels of TNF-α, INF-γ, and IL-4 were significantly increased in the high-dose group as compared to those in the control group (p < 0.01). In contrast, low-dose SBOSs did not affect the levels of these cytokines (p > 0.05).
Taken together, these data demonstrated that SBOSs promoted the activity of monocytes, macrophages, T cells, B cells, and NK cells and increased cytokine secretion, supporting the role of SBOSs in modulating immune function.
3.3. Effects of SBOSs on specific immunity in mice
The effects of SBOSs on specific immunity (i.e., cellular immunity and humoral immunity) in mice are shown in Table 3. Cellular immunity was measured by determining the numbers of T lymphocytes in the peripheral blood using an acid-α-naphthyl acetate esterase (ANAE) activity assay; this assay specifically targets T lymphocytes because B lymphocytes lack the ANEA protein, and hydrolysis of hydrolyze α-naphthyl acetate, which leads to production of an insoluble red brown precipitation, is only observed in T lymphocytes. Importantly, high-dose SBOSs significantly increased the percentage of T lymphocytes compared to that in control mice (p < 0.01), indicating that administration of SBOSs promoted cellular immunity in mice. Additionally, high-dose SBOSs improved ConA-induced lymphocytic transformation (p < 0.01), while low- and middle-dose SBOSs did not (p > 0.05). Because lymphocyte proliferation is an important indicator of lymphocyte activation and function, which govern many processes in cellular immunity (Cerqueira et al., 2004), these data indicated that high-dose SBOSs stimulated cellular immunity through promoting lymphocyte proliferation.
Table 3.
Effects of SBOSs on specific immunity in mice.
| Groupc | Dose (g kg−1) | Cellular immunity |
Humoral immunity | |
|---|---|---|---|---|
| Percentage of T-lymphocytes | Lymphocytic transformation | |||
| Percentage (%) | Lymphocyte proliferation | Hemolysin production(OD540) | ||
| (ΔOD570 D-value) | ||||
| Control group | 0 | 44.38 ± 2.86 | 0.161 ± 0.03 | 0.218 ± 0.028 |
| Low-dose group | 0.5 | 46.72 ± 3.25 | 0.173 ± 0.05 | 0.277 ± 0.037a |
| Middle-dose group | 1.5 | 48.68 ± 2.95a | 0.187 ± 0.06 | 0.322 ± 0.058b |
| High-dose group | 4 | 54.36 ± 3.12b | 0.276 ± 0.07b | 0.419 ± 0.069b |
p < 0.05.
p < 0.01 compared to the control group.
Values shown are mean ± SD (n = 10).
Hemoglobin content can reflect the level of serum hemolysin, which in turn reflects the state of immune function (Wang et al., 2002). Therefore, we analyzed the effects of SBOSs on hemolysin production (Table 3). Hemolysin levels were significantly higher in all SBOS-treatment groups than in the control group (p < 0.01 for high- and middle-dose groups and p < 0.05 for the low-dose group). These data demonstrated that SBOSs promoted humoral immune function.
3.4. Effects of SBOSs on immunoglobulin levels in mice
Immunoglobulins are essential components of the humoral immune system and have been shown to prevent bacterial adhesion to the surface of epithelial cells, thereby providing protection against infection. As shown in Table 4, compared with the control group, serum levels of IgA, IgG, and IgM were significantly increased in the high-dose group (p < 0.01). Additionally, serum IgA and IgG levels were also significantly increased in the middle-dose group (p < 0.01 and p < 0.05, respectively). Thus, these data demonstrated that SBOSs enhanced immunoglobulin secretion from the intestinal mucosa, thereby improving immune function.
Table 4.
Effects of SBOSs on immunoglobulin content in mouse serum.
| Groupc | Dose (g kg−1) | IgA (g/L) | IgG (g/L) | IgM (g/L) |
|---|---|---|---|---|
| Control group | 0 | 4.46 ± 1.29 | 1.33 ± 0.52 | 0.57 ± 0.17 |
| Low-dose group | 500 | 6.23 ± 2.32 | 2.03 ± 0.95 | 0.59 ± 0.15 |
| Middle-dose group | 1500 | 8.64 ± 1.77b | 2.32 ± 0.78a | 0.71 ± 0.26 |
| High-dose group | 4000 | 11.46 ± 2.73b | 2.77 ± 0.83b | 1.31 ± 0.33b |
p < 0.05.
p < 0.01 compared to the control group.
Values shown are mean ± SD (n = 10).
4. Discussion
In this study, we investigated the prebiotic functions of SBOSs in a mouse model. Our data showed that high-dose SBOSs (4.0 g kg BW−1) induced dramatic changes in all examined immune parameters, functioning to modulate cellular, humoral, and nonspecific immune pathways. Therefore, our data provide important insights into the role of SBOSs as prebiotics.
Intestinal epithelial cells are in direct contact with the microbiota in the gut lumen. Kagnoff and Eckmann (1997) are thought to participate in the onset and regulation of the mucosal immune response to bacteria, especially pathogens, by interacting with the immune cells of Peyer’s patches, lymphoid tissue in the lamina propria of the gut, and intraepithelial lymphocytes. Prebiotics can enhance innate immune functions, including phagocytic activity of neutrophils and cytotoxic activity of NK cells (Arnold et al., 2006). The activation of neutrophils and NK cells may be closely connected with the anti-infectious or anticancer activities of prebiotics. Moreover, the use of prebiotics may reinforce innate function. Indeed, studies have shown that dietary supplementation with Lactobacillus rhamnosus HN001 increases NK cell numbers in humans (Gill et al., 2001) and consumption of L. casei Shirota fermented milk enhances the cytotoxic activity of NK cells (Takeda et al., 2006).
In our study, high-dose SBOSs modulated the numbers of bifidobacteria and LABs and caused changes in immunological parameters in mice. Under established conditions of intestinal microbial community colonization, SBOSs enhanced the activation, proliferation, and differentiation of T cells into effective T cells that secreted increased levels of IFN-γ, TNF-α, and IL-4. Moreover, our data demonstrated that intestinal immunity was activated, as measured by analyzing T-lymphocyte percentages, lymphocytic transformation, and cytokine secretion. The increase in IgA, IgG, and IgM and evidence of enhanced humoral immunity indicated the occurrence of lymphoid follicular hyperplasia and increased B-cell production, both of which can lead to increases in immunoglobulin secretion. The enhanced phagocytic activity of macrophages and killing ability of NK cells promote the ability of T cells to identify targets and stimulate the immune response indirectly (Feng et al., 2010). In adaptive immunity, many prebiotic bacteria can stimulate IgA secretion by B cells and the activation of helper T lymphocytes and macrophages by increasing production of cytokines, which are involved in communication between lymphocytes, macrophages, and other cells involved in inflammatory reactions and immune responses (Arseneau et al., 2007). In addition, there is a wide variation in the response of cytokines induced by different strains or species of prebiotics (Flickinger and Fahey, 2002). Therefore, measurement of these varied representative immune system markers provided a broad view of the effects of SBOSs on immunity function.
The intestinal mucosal immune system is an important part of the local immune system and is considered the first barrier of the immune system (Guoping et al., 2000), playing an important role in resisting the invasion of bacteria, viruses, and toxins (Kwon et al., 2002, Blaschitz and Raffatellu, 2010). Many immunoreactive substances first contact the body via the gut after oral administration or consumption, thus resulting in systemic immune system induction (Challacombe, 1983). Intestinal lymphocytes are produced by the intestinal lymph tissue itself, especially the PP knot, which is the main location of induction of intestinal mucosal immunity; indeed, antigen uptake, immune response, and regulation of IgA generation and other effects occur in the intestinal lymph tissue. In vitro application of SBOSs had no significant effect on the proliferation of spleen cells and Peyer’s Patchs (Xu et al., 2005), indicating that the stimulatory effect of SBOSs on immune function was not due to its direct activation of immune cells. In the intestine, SBOSs can promote the proliferation of bifidobacteria and lactobacilli, which can be used as nonspecific regulatory factors to affect intestinal mucosal immune dysfunction (Huang et al., 2006) In addition, bifidobacteria and lactobacilli can produce large amounts of lactic acid and acetic acid, which can inhibit the growth of E. coli and enterococci, thereby increasing the probability of intestinal mucosal colonization and limiting the contact of the intestinal epithelium with pathogenic bacteria and their toxins.
In conclusion, our current study provided further support for the prebiotic functions of high-dose SBOSs; in mice, this dose positively affected the intestinal microbial communities and enhanced immunological parameters. With increasing concerns about prebiotic use in the food industry, we suggest that a combination of pro- and prebiotics as symbiotics may be needed to obtain beneficial effects in practice.
Acknowledgements
This work was supported by the welfare fund for scientific research projects of Liaoning province (2015005004).
Footnotes
Peer review under responsibility of King Saud University.
References
- Akrami R., Iri Y., Rostami H.K., Mansour M.R. Effect of dietary supplementation of fructooligosaccharide (FOS) on growth performance, survival, lactobacillus bacterial population and hemato-immunological parameters of stellate sturgeon (Acipenser stellatus) juvenile. Fish Shellfish Immunol. 2013;35:1235–1239. doi: 10.1016/j.fsi.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Arnold J.N., Dwek R.A., Rudd P.M., Sim R.B. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol. Lett. 2006;106:103–110. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Arseneau K.O., Tamagawa H., Pizarro T.T., Cominelli F. Innate and adaptive immune responses related to IBD pathogenesis. Curr. Gastroenterol. Rep. 2007;9:508–512. doi: 10.1007/s11894-007-0067-3. [DOI] [PubMed] [Google Scholar]
- Blaschitz C., Raffatellu M. Th-17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm G., Stahl B., Jelinek J., Knol J., Miniello V., Moro G.E. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr. Suppl. 2005;94:18–21. doi: 10.1111/j.1651-2227.2005.tb02149.x. [DOI] [PubMed] [Google Scholar]
- Cerqueira F., Cordeiro-Da-Silva A., Gaspar-Marques C., Simões F., Pinto M.M., Nascimento M.S. Effect of abietane diterpenes from Plectranthus grandidentatus on T- and B-lymphocyte proliferation. Bioorg. Med. Chem. 2004;12:217–223. doi: 10.1016/j.bmc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Challacombe S.J. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann. NY Acad Sci. 1983;409:177–193. doi: 10.1111/j.1749-6632.1983.tb26868.x. [DOI] [PubMed] [Google Scholar]
- Chen H., Jun L.L., Jun J.Z., Bo X., Rui L. Chemical composition analysis of soybean oligosaccharides and its effect on ATPase activities in hyperlipidemic rats. Int. J. Biol. Macromol. 2010;46:229–231. doi: 10.1016/j.ijbiomac.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Feng T., Wang L., Schoeb T.R., Elson C.O., Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger E.A., Fahey J.G.C. Pet food and feed applications of inulin, oligofructose and other oligosaccharides. Br. J. Nutr. 2002;87:S297–300. doi: 10.1079/BJNBJN/2002552. [DOI] [PubMed] [Google Scholar]
- Geraylou Z., Souffreau C., Rurangwa E., D’Hondt S., Callewaert L., Courtin C.M., Delcour J.A., Buyse J., Ollevier F. Effects of arabinoxylan-oligosaccharides (AXOS) on juvenile Siberian sturgeon (Acipenser baerii) performance, immune responses and gastrointestinal microbial community. Fish Shellfish Immunol. 2012;33:718–724. doi: 10.1016/j.fsi.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota, introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gill H.S., Rutherfurd K.J., Cross M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly, an investigation of age-related immunological changes. J. Clin. Immunol. 2001;21:264–271. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- Guoping A., Yongping S., Tianmin C. Structure and function of the intestinal mucosal immunity. J. Immunol. 2000;6:164–165. [Google Scholar]
- Health food inspection and evaluation of technical specifications Ministry of Health of the People’s Republic of China. People’s Medical Publishing House. 2003:63–108. [Google Scholar]
- Huang R.L., Zhang Z.H., Chen P.G., Ma K.W., Yang L. Effect of soybean oligosaccharides on antioxidation and the immunity of broilers. J. Agri. Univ. Hebei. 2006;29:87–94. [Google Scholar]
- Hume D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Huynh M.S., Le T.K., Ravi F. Dietary supplementation of mannan oligosaccharide improves the immune responses and survival of marron, Cherax tenuimanus (Smith, 1912) when challenged with different stressors. Fish Shellfish Immunol. 2009;27:341–348. doi: 10.1016/j.fsi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Jianxian Z., Liping G. An analysis of the functional oligosaccharides. Food Ferm. Indust. 2003;2:39–46. [Google Scholar]
- Kagnoff M.F., Eckmann L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim W., Hwang I.K. Optimization of the extraction and purification of oligosaccharides from defatted soybean meal. Int. J. Food Sci. Technol. 2003;38:337–342. [Google Scholar]
- Kwon K.H., Kim K.I., Jun W.J., Shin D.H., Cho H.Y., Hong B.S. In vitro and in vivo effects of macrophage-stimulato polysaccharide from leaves of Perilla frutescens var crispa. Biol. Pharm. Bull. 2002;25:367–371. doi: 10.1248/bpb.25.367. [DOI] [PubMed] [Google Scholar]
- Martin J.K., Jinmo K., Francis F.B., Daniel D.G., Linda J.B. Carbohydrate source and bifidobacteria influence the growth of clostridium perfringens in vivo and in vitro. Nutr. Res. 1998;18:1889–1897. [Google Scholar]
- Mikkelsen L.L., Jensen B.B. Effect of fructo-oligosaccharides and transgalacto-oligosaccharides on microbial populations and microbial activity in the gastrointestinal tract of piglets post-weaning. Anim. Feed Sci. Technol. 2004;117:107–119. [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Safarpour A.A., Falahatkar B., Sattari M., Tolouei G.M. Effect of dietary vitamin E on growth, muscle composition, hematological and immunological parameters of sub-yearling beluga Huso huso L. Fish Shellfish Immunol. 2011;30:807–814. doi: 10.1016/j.fsi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Siwicki A.K., Anderson D.P. Nonspecific defence mechanisms assay in fish II; potential killing activity of neutrophils and manocytes, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Siwicki A.K., Anderson D.P., Waluga J., editors. Disease Diagnosis and Prevention Methods, Olsztyn, Poland. 1993. pp. 105–111. [Google Scholar]
- Takeda K., Suzuki T., Shimada S.I., Shida K., Nanno M., Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin. Exp. Immunol. 2006;146:109–115. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernazza C.L., Gibson G.R., Rastall R.A. In vitro fermentation of chitosan derivatives by mixed cultures of human faecal bacteria. Carbohydr. Polym. 2005;60:539–545. [Google Scholar]
- Wang Y.P., Li X.Y., Song C.Q., Hu Z.B. Effect of astragaloside IV on T, B lymphocyte proliferation and peritoneal macrophage function in mice. Acta Pharmacol. Sin. 2002;23:263–266. [PubMed] [Google Scholar]
- Xu C.D., Liang A.J., Zhu Y., Tao S.Y. Immunopotentiating effect of soybean oligosaccharides. Pharm. J. Chin. People’s Lib. Army. 2005;21:37–39. [Google Scholar]
- Xu Q., Chao Y.L., Wan Q.B. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009;77:435–441. [Google Scholar]
- Zhou X.L., Kong X.F., Yang X.J., Yin Y.L. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 2012;90:37–39. doi: 10.2527/jas.50269. [DOI] [PubMed] [Google Scholar]