Abstract
The contribution of a bee plant species to honey production depends on the plant’s nectar secretion quality and quantity, which is mainly governed by biotic and abiotic factors. The aim of the current study, was to investigate the nectar secretion dynamics and honey production potential of 14 major bee plant species of the target area. We examined the quantity and dynamics of nectar sugar per flower five times a day using a nectar sugar washing technique and direct measuring of nectar with calibrated capillary tubes. The average nectar sugar amount of the species varied from 0.41 mg/flower to 7.7 mg/flower (P < 0.0001). The honey sugar per flower was used to extrapolate the honey production potential per plant and per hectare of land. Accordingly the honey production potential of the species observed to vary from 14 kg/hectare in Otostegia fruticosa to 829 kg/hectare in Ziziphus spina-christi. The nectar secretion dynamics of the species generally showed an increasing trend early in the morning, peaking toward midday, followed by a decline but different species observed to have different peak nectar secretion times. Generally, the tree species secreted more nectar sugar/flower than the herbs. The nectar secretion amount of the species was positively correlated with the ambient temperature, indicating the adaptation of the species to hot climatic conditions. However, different species were observed to have a different optimum temperature for peak nectar secretion. Despite the limited rainfall and high temperature of the area, many plants were found to have good potential for honey production. The monetary value of honey per hectare of the studied honeybee plant species can be of equal or greater than the per-hectare monetary value of some cultivated crops that require numerous inputs. In addition, the information generated is believed to be useful in apiary site selection and to estimate the honey bee colony carrying capacity of an area.
Keywords: Bee plant, Floral phenology, Honey, Melliferous plants, Nectar secretion, Nectar sugar
1. Introduction
Honey bee plants are those plant species that provide bees with food sources in the form of nectar and/or pollen. According to Crane (1990), only 16% of the world’s flowering plant species contribute to honey bees as food sources. Moreover, not all bee plants are equally important to bees and honey production. Indeed, only 1.6% of the world’s honey bee plants are the sources of most of the world’s honey (Crane, 1990). This indicates that for every geographical region there are very few important honey source plants and it is of paramount importance to characterize them according to their degree of importance in honey production. Several studies have been performed on different plant species to quantify nectar secretion and to explore its dynamics, mainly in relation to pollination biology, floral phenology and biophysical environmental factors (e.g., Petanidou and Smets, 1996, Castellanos et al., 2002, Galetto and Bernardello, 2004). Moreover, quantitative studies on the nectar secretion of various melliferous plants have been conducted (Pesti, 1976, Mohr and Jay, 1990, Nepi et al., 2001, Farkas and Orosz-Kovács, 2003, Horváth and Orosz-Kovács, 2004, Zajácz et al., 2006). In addition, based on thorough studies of dynamics of nectar secretion and total soluble solids (TSS) concentration, it has been possible to estimate the honey production potentials of some major honey source plants such as Trifolium pratense L. (red clover) (883 kg of honey/ha/flowering season; Szabo and Najda, 1985); Asclepias syriaca L. (milkweed) (500–600 kg honey/ha/flowering season; Zsidei, 1993) and Phacelia tanacetifolia Benth (60–360 kg honey/ha/flowering season; Nagy, 2002). In addition, Crane et al. (1984) reported that the honey production potential of different Tilia (lime) species ranged from 90 to 1200 kg honey/ha. Moreover, Kim et al. (2011) estimated the amount of nectar secreted per flower and per tree for Crataegus pinnatifida Bunge (Chinese hawthorn).
Nonetheless, most of the studies have focused on melliferous plant species of temperate and subtemperate regions. Many important honey source plants of the tropics, subtropics and arid climatic zones, their nectar secretion potentials and their significance for honey production have not yet been well studied and documented. In Saudi Arabia, approximately 2200 flowering plants are reported to exist (Collenette, 1999, Chaudhary, 1999). The families Fabaceae, Lamiaceae and Rhamnaceae, which account for a significant share of the flowering plants of the country, are generally known as good sources of nectar for honey bees. Among these, some species from the genus Acacia, Lamaceae (lavandula), Ziziphus and others are known for being very good sources of honey. However, detailed characterization of the species, particularly the amount and dynamics of their nectar secretion are lacking.
The genus Acacia comprises more than 1200 species that are distributed in tropical and subtropical parts of the world, extending into the deserts of Africa and the Middle East and into large areas of the Arabian Peninsula (Wickens, 1995, Tandon and Shivanna, 2001, UNESCO., 1977, Walter and Breckle, 1986). The species are drought-tolerant and endures in the rainfall belts of 50–400 mm/annum (Wickens, 1995, Le-Houérou, 2012). Moreover, these species have multipurpose uses as important sources of firewood, timber, forage, gum, tannins, fiber, folk medicine, and food, and they are also useful for environmental protection and soil and water conservation (Boulos, 1983, Wickens, 1995, Midgely and Turnbul, 2003). They also contribute to the conservation of large numbers of herbivorous vertebrates and invertebrates (Krüger and McGavin, 1998) as well as many species of nectarivorous insects. Different species of Acacia have been reported as important honey bee forages in many semiarid regions of the tropics (Wickens, 1995, Stone et al., 1996, Stone et al., 1998). About 10 Acacia species, such as: Acacia origena Hunde, Acacia johnwoodii Boulos, Acacia tortilis Forssk., Acacia asak (Forssk.) Willd., Acacia ehrenbergiana Hayne, Acacia etbaica Schweinf., Acacia oerfota (Forssk.) Schweinf., Acacia gerrardii Benth. and others, have been reported to exist in Saudi Arabia, but their roles in honey production have not been quantified and documented.
The other important honey source plant family is Lamiaceae, which encompasses approximately 7200 species. This family is one of the most cosmopolitan in distribution, covering large areas in the world (Martin et al., 2013). According to recent studies, the Lamiaceae family is represented by 76 species in Saudi Arabia, most of which are useful for their medicinal values and antimicrobial properties (Abbasi et al., 2010, Dulger and Dulger, 2012, Raja, 2012, Venkateshappa and Sreenath, 2013, Saqib et al., 2014). Within Lamiaceae, the genus Lavandula is particularly important because it is naturally occurring and extensively cultivated in many parts of the world (Chu and Kemper, 2001, Boning, 2010, Lalande, 1984). The species grows well in arid and semiarid parts of the world and even in areas vulnerable to desertification (Azcón and Barea, 1997). Some of the species in genus Lavendula are used in cosmetics, food processing and aromatherapy (Welsh, 1995, Chu and Kemper, 2001, Lis-Balchin, 2003). Many species from Lamiaceae are known as good sources of high quality monofloral honey with a characteristic aroma and flavor (Tsigouri and Passaloglou-Katrali, 2000, Nicoleta, 2008, Nicoleta and Ion, 2007, Forler, 2013). Monofloral honeys from Lavandula sp. fetch premium prices ($50/kg) in specialty food stores (Forler, 2013).
Five Lavandula species (Lavandula atriplicifolia Benth, Lavandula citriodora, Lavandula coronopifolia Poir, Lavandula stricta Del., Lavandula dentata L. and Lavandula pubescens, Decne) grow naturally in Saudi Arabia (El-Karemy and Zayed, 1992, Rahman et al., 2003). The country is known as one of the main geographical area of Lavandula species diversity and endemism and has been suggested to be the center of origin of the genus (Miller, 1985). Lavandula species such as L. dentata and L. pubescens grow naturally and extensively in the southwestern mountain regions of Taif, Albaha and Asir and serve as a source of high quality Lavandula honeys, known locally as “Seyfi honey” and fetch premium prices of $50–120/kg. Most of the studies on Lavandula species generally have been limited to cultivated and commercial varieties in temperate regions. Despite the natural occurrence of different lavender species in the Arabian Peninsula, the nectar secretion dynamics and honey production potentials of these species in their natural habitats have not been studied.
Moreover, other plant species from the family Lamiaceae, such as Otostegia fruticosa (Forssk.) Schweinf. ex Penzig and Nepeta deflersiana Schweinf. ex Hedge, in addition to being used by humans for their medicinal properties (Aboutabl et al., 1995, Mothana, 2012), are frequently visited by honey bees for nectar collection.
The other important honey bee plant group belongs to the genus Ziziphus (family: Rhamnaceae) and consists of approximately 100 species that are distributed in the tropical and subtropical regions of the world (Cherry, 1985, Abalaka et al., 2010). Most of the species in this genus are drought and heat-tolerant and adapted to low rainfall conditions (Orwa et al., 2009). Some of the species, such as Ziziphus spina-christi (L.) Desf., grow in a wide range of habitats covering vast land areas of Africa, the Eastern Mediterranean, the Arabian Peninsula, and in the Tropical Asia (Scholte et al., 1991, Orwa et al., 2009). Some of the Ziziphus species produce a range of products that includes food, fodder, fuel, drink, timber, and medicine. Four Ziziphus species (Ziziphus glabrata Heyne, Ziziphus mucronata Willd., Ziziphus nummularia (Burm. f.) Wight & Arn., Z. spina-Christi var. divaricata Forssk., Z. spina-christi var. inermis Boiss., and Z. spina-christi var. spina-christi (L.) Willd.) are found in Saudi Arabia. Honey from Ziziphus trees is the most common and the most expensive honey in the region, selling for up to $60–100/kg (Nuru et al., 2014).
The contribution of a bee plant species to honey production not only relies on its flowering phenology and abundance but also on its nectar quality and quantity. Nectar is mainly produced by plants as a reward to flower visitors. Its production is a complex physiological process significantly influenced by flower species-specific characteristics (shape, size, and position) and is governed to a large extent by abiotic environmental conditions and the phenology of the flower (Mačukanović-Jocic et al., 2004). Nectar is the major raw material for honey production. However, not all flowering species produce nectar, and not all nectar produced by flowers is accessible to honey bees (Bastiaan, 1984). Even if accessible, the amount and concentration of nectar varies from plant to plant and over time (Roubik, 1991, Chalcoff et al., 2006).
The energy value of nectar in relation to its TSS concentration varies markedly among the different plant taxa and ranges from 10% to 80%. A wide range of nectar TSS concentrations from 12% (Rhodophiala mendocina) to 51.7% (Escallonia rubra) (Chalcoff et al., 2006) has been reported. The variation in concentration is not only due to plant species but also to the different times of the day (Roubik, 1991). Therefore, it is important to determine the value of bee plants in relation to the volume, dynamics and concentration of nectar secreted by the plant. In this regard, no adequate information exists on the apicultural values of major honey source plants of the country. The aim of the present study is to evaluate the nectar secretion dynamics and honey yield potential of some major honey bee plant species under Saudi Arabian environmental conditions. Accordingly, we investigated the nectar secretion dynamics of species and the amount of nectar TSS secreted per flower for major honey source plant species. We also extrapolated the honey production potential per tree and per hectare of land occupied with studied plant species.
2. Materials and methods
2.1. Studied species and sites
In this study, 14 important honey source plant species which are commonly visited by honey bees for nectar collection and also serve as source of honey in the region were considered. Eight of these belong to the genus Acacia (Fabaceae), four species belong to the family Lamiacae, and two species belong to the genus Ziziphus (Rhamnaceae). The species are widely grown and well adapted to the prevalent arid climatic conditions of Saudi Arabia and are frequently visited by honey bees for nectar and pollen collection. The list of species, their general features and distribution are indicated in Table. 1.
Table 1.
Some general features of the studied honey plant species.
| Family | Nomenclature | Habit | Common names |
Uses | Distribution | |
|---|---|---|---|---|---|---|
| English | Arabic | |||||
| Fabaceae | Acacia asak | Tree | Asak, Dhahia, Dhahian | LP, gum, FW | Af, Ar | |
| A. ehrenbergiana | Shrub | Salam | Salam, Hardha | LP, FW | Af, Ar | |
| A. etbaica | Tree | Savannah thorn | Arad, Qardh | LP, FW, TM, T | Af, Ar | |
| A. gerrardii | Tree | Red thorn, Gerrard’s acacia | Talh, Shaba,an | LP, FW, TM, T | Af, ME | |
| A. johnwoodii | Tree | Ar | ||||
| A. oerfota | Shrub | Green-barked acacia | Orfut, What | LP | Af, Ar | |
| A. origena | Tree | Kanahbal, Kulhab | Af, Ar | |||
| A. tortilis | Shrub | White thorn | Samar, Somra | LP, TM, FW | Af, Ar | |
| Lamiaceae | Lavandula dentate | Herb | Fringed lavender | Dhorm | TM, O | Af, Me |
| L. pubescens | Herb | French lavender | Thafra, Atan | Af, Me | ||
| Nepeta deflersiana | Herb | Sheah | TM, O | Ar | ||
| Otostegia fruticosa | Herb | Sharm | LP, TM, O | Af, Ar | ||
| Rhamnaceae | Ziziphus nummularia | Shrub | Wild jujube | Sidr | Fruit, LP, TM | Af, ME, SWA |
| Ziziphus spina-christi | Tree | Christ’s thorn jujube | Sidr | Fruit, TM, T | Af, SWA | |
∗Uses: LP, Livestock pasture; TM, Traditional medicine; FW, Firewood; T, Timber; O, Ornamental.
∗∗Distribution: Ar, Arabia; Af, Africa; ME, Middle East; Me, Mediterranean; SWA, Southern and Western Asia.
The study was carried out in five sites in two regions of Saudi Arabia. The first two sites (Rawdhat-Khoraim and the Educational Farm of King Saud University) are located in central Saudi Arabia in the heart of the Arabian desert at the southern edge of the Palearctic ecozone. The other three sites (Wadi-Alkhitan, Wadi-Berha and Baljurashi) are located in southwestern Saudi Arabia (Albaha), which belongs to the Asian division of the Afro-tropical ecosystem (Fig. 1). The studied species were investigated according to their spatiotemporal distribution (Table 2).
Figure 1.
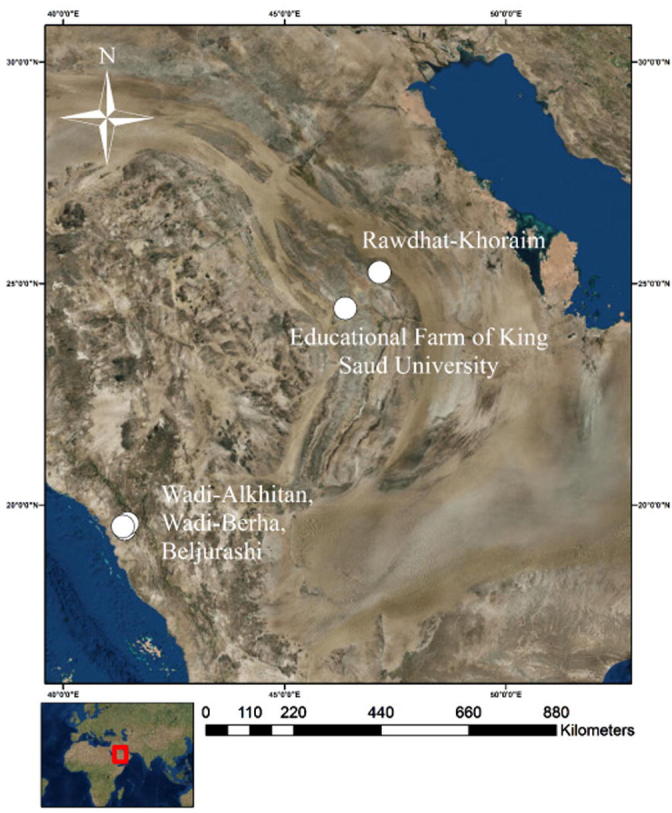
A physiographic map of Saudi Arabia with white points representing study sites.
Table 2.
Study sites, species and years of investigation.
| Regions | Study area and its description | Studied species | Year |
|---|---|---|---|
| Wadi-Alkhitan: 1100 masl∗, Wild forest | A. tortilis | 2012 | |
| A. ehrenbergiana | 2012 | ||
| A. asak | 2012 | ||
| A. johnwoodii | 2014 | ||
| A. oerfota | 2014 | ||
| Al Baha- | Wadi-Berha: 1750 masl, Wild forest | A. etbaica | 2013 |
| Beljurashi: 2200 masl, Wild forest | A. origena | 2013 | |
| L. dentate | 2013 | ||
| L. pubescens | 2013 | ||
| N. deflersiana | 2013 | ||
| O. fruticosa | 2013 | ||
| Riyadh | Rawdhat-Khoraim: 570 masl, subtropical oasis | A. gerrardii | 2012 |
| Educational Farm of KSU∗: 650 masl, irrigated farm | Z. nummularia | 2012 | |
| Z. spina-christi | 2011 | ||
Masl refers to elevation in meters above sea level.
KSU refers to King Saud University, Saudi Arabia.
2.2. Flowering period distribution
The distribution of the flowering period for each species was determined through continuous monitoring and recording of the plants’ flowering patterns, including the commencement, peak, and end of their flowering period. For each species, the peak flowering time was indicated when approximately 50% of the flower buds were in the blooming stages. In addition, species with wide ecological distribution (lowlands and midlands) and species with multiple flowering seasons were also considered and recorded.
2.3. Phenology
For flower phenology, observations were made on three individual plants per species, and from each plant, an average of five flower buds (total of 15/species) were labeled in late afternoon and monitored over the next few days. During marking, all previously opened flowers from the marked branch were removed to avoid confusion. On the next morning, the phenology of the flowers, including the time of flower opening, the wilting of the flower parts and the average duration of the flower remaining, was monitored and recorded through observations made on the flowers every two hours (from 0600 to 1800). For those flowers that remained for more than one day, the observations were continued until the flowers wilted.
2.4. Determination of nectar secretion dynamics and amount
The amount and dynamics of nectar TSS production were determined from an average of three individual plants per species. For this, branches of a plant with mature flower buds were randomly selected, labeled and bagged one day before the flowers opened using bridal-veil netting (Wyatt et al., 1992). For nectar volume estimation and nectar TSS determination, direct nectar removal and nectar sugar washing techniques were applied depending on the nature of the flower morphology and the amount and concentration of the nectar.
2.4.1. Nectar TSS determination using washing techniques
In Acacia species with both spherical and elongated types of inflorescences, the individual florets are very small, and the nectar was too viscous (because of low humidity and high temperature of the study areas) to be easily measured using capillary tubes. Therefore, in this study, for all Acacia spp., the nectar TSS amount was determined following the flower nectar sugar washing techniques of Mallick (2000). In this procedure, one flower head was used only for one time measurement in that each flower head was removed and kept in a small, narrow plastic vial and washed with 1 ml of distilled water except for A. tortilis flowers which was enough to use 0.5 ml because of its smaller size. The flower heads were then left for 5 min until the sugar was completely dissolved. The nectar TSS was measured from five flowers per plant and for each sampling time (five times a day at 0600, 0900, 1200, 1500, and 1800 h) which equals to a total of 25 flower heads/day/plant. The measurements were repeated for three consecutive days, with TSS being measured for 225 flowers for each species.
For flowers with elongated inflorescence (A. asak) because all florets do not open simultaneously, 20 opened florets were taken at one time, and their nectar TSS amount was determined using the same above-described nectar sugar washing technique and extrapolated for the whole inflorescence. From the pooled clear nectar solution washed sugar; a drop of solution was taken using micropipettes, and the concentration of this solution was measured using an automatic temperature-compensated, digital, hand-held refractometer (Reichert, Catalog number 13950000, USA). The mass of the TSS in the nectar solution for each measurement was calculated from the volume and concentration of the solution that was measured. The sucrose concentration readings (mass/total mass, g of TSS/100 g of solution) were converted to sucrose mass/volume using Weast’s (1986) conversion table. For each species, the mean nectar TSS values were computed for each sampling time and per flower.
In Ziziphus flowers, because of the rapid crystallization of the nectar sugar on surface of the flowers, a similar washing technique was applied. However, in Ziziphus, because the flowers remain for two days, the nectar secretion dynamics was studied by measuring the same labeled flowers repeatedly at four hour intervals (at 0600, 1000, 1400 and 1800) during the day for two consecutive days. During each washing, 10 μl of distilled water was deposited onto the crystallized nectar sugar on the surface of each flower using a calibrated micropipette (Eppendorf Research®), and the water was allowed to remain for one minute to dissolve the sugar on the surface of the flowers. Because the TSS present were dissolved in the 10 μl of water that was added, the concentration of the recovered solution reflects that of the 10 μl ‘pool’ in the flower and was used to estimate the TSS present assuming a volume of pool solution is 10 μl. From this pool, a drop of solution was taken to measure the concentration using the same above mentioned hand-held refractometer. In this repeated measuring procedure, to avoid re-measuring of sugars that might remain from the earlier measurements, the flowers were rinsed/washed three times with 10 μl of distilled water after each measurement, which was sufficient to lower the refractometer reading to approximately 0 or ⩽1%. The mean nectar TSS values were computed for each sampling time and flower.
2.4.2. Nectar volume and dynamics measurement through direct removal
For bee plants in the family Lamiaceae because their flower morphology is suitable to directly extract and measure the nectar volume; the volume of nectar was determined by directly removing the nectar from the flower using graduated capillary tubes. From each plant and for each sampling time, the nectar volume was measured from an average of 10 flowers, which was 50 flowers/day/plant. The nectar volume measurement was repeated for three consecutive days, totaling 450 flowers/species. The concentration of the nectar TSS was measured using the same device mentioned above, and the mass of TSS per volume was estimated following the above-described procedure. Then, the amount of nectar volume and nectar TSS concentration were computed per sampling time and flower and compared among plants, species and different hours.
2.5. Estimation of the honey production potentials of the species
The average amount of honey that can be obtained from a single plant was estimated from the average numbers of flowers per plant and the average mass of the nectar TSS per flower, following procedures similar to those of Masierowska (2003) and Kim et al. (2011).
The average number of flowers per tree was estimated by counting the number of flowers in three randomly sampled 1 m2 areas or 1 m3 volume per plant from three different plants. Surface area or volume was used depending on the distribution of flowers on the canopies of the studied species. The average number of flowers per tree was obtained by multiplying the average number of flower buds/m2 or /m3 by the average surface area or volume of the canopy of the species. The species canopy volume was calculated following Coder’s (2010) plant crown shape formula (shape value (0.375) × (crown diameter)2 × (crown height) × (0.2945) for fat cone canopies and (shape value (0.667) × (crown diameter)2 × (crown height) × (0.5236) for spheroid canopies, depending on the crown shapes of the species). For each plant species, the canopy volume was determined by measuring 10–20 individual tree canopies.
For perennial shrubs, (such as in Lavandula) the number of flowers per plant was determined by counting the average number of branches or flower spikes per plant and then multiplying by the average number of flowers per spike or branches through counting 10–20 individual plants per species. Then the honey production potential of the plants was estimated by multiplying the average number of flowers per plant by the average mass of the TSS per flower. The number of plants per hectare was estimated based on the average canopy area of each species plus the space required between plants. These data have been used to estimate the possible amount of honey that can be obtained per hectare of land occupied by the species.
2.6. Weather data
Along with the nectar secretion amount and dynamics studies, weather (temperature and humidity) data of the study area were also recorded and correlated with the nectar secretion amount and dynamics of the species.
2.7. Statistical analysis
An analysis of variance (ANOVA) was used to compare the mean amount of nectar TSS that was secreted per flower head per 3 h (and per 4 h for Ziziphus) period from the different trees within species and also among the different species. A pair wise correlation analysis was performed between the environmental factors (temperature and relative humidity of the area) and the amount of nectar TSS secreted per flower or inflorescence. The analysis was performed using JMP-5 statistical software (SAS, 2002).
3. Results
3.1. Flowering period distribution
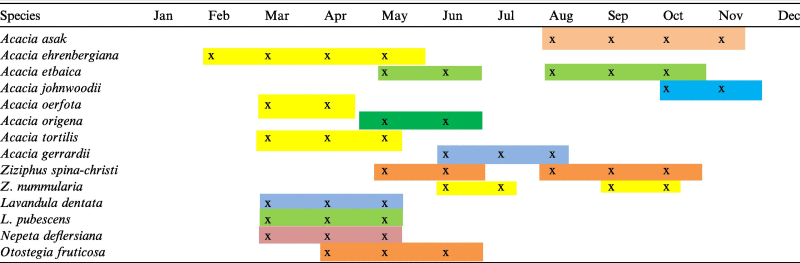
The flowering period distributions of the honey source plants varied from one species to another (Table 3). The majority of the species in this study flowered during spring with little extension into early summer. The remaining species flowered mostly in autumn. Generally, the species were characterized by a short flowering time, except for A. ehrenbergiana and A. asak, which have relatively longer flowering periods. Some species such as Ziziphus spp. and A. etbaica have multiple flowering periods, whereas A. asak and A. johnwoodii were observed to have intermittent flowering patterns. Moreover, species such as Z. spina-christi, A. tortilis, and A. ehernbergiana have a wide range of ecological distribution which varies from 200 to 1750 meters above sea level. Hence, their flowering periods varied accordingly within the same season. Moreover, species such as A. tortilis and A. ehernbergiana were observed to flower in the dry season during the leafless stage, but if rain occurred, the plants were observed to abort their flower buds and initiate new leaves.
Table 3.
Flowering periods distribution of the studied honey plant species. Bars with different colors indicate the lengths of the flowering duration of each species.
 |
3.2. Flower phenology
In this study, all Acacia spp. with spheroidal inflorescences; the flower heads commonly opened early in the morning at approximately 0500 h and stay for only a day, wilting at approximately 1500–1800 h. However, in A. oerefata, the opening of the flower heads was not restricted to a certain time of a day and observed to occur continuously throughout the day. In A. asak, which has an elongated inflorescence, half of the florets opened in one day, whereas the remaining half opened on the next day. In the genus Lavandula (L. dentata L. and L. pubescens Decne), the individual flowers from a spike were observed to open in a sequential manner during the day, and each flower lasted for an average of 12 h. In these species, when the flowers that had opened earlier were about to wilt, new flowers started to open so that flowering was continuous with some degree of overlap in the opening times of individual flowers. However, in O. fruticosa and Ziziphus species, the flowers opened early, between 0500 h and 0600 h, and stayed open for about two days.
3.3. Nectar secretion amount and dynamics
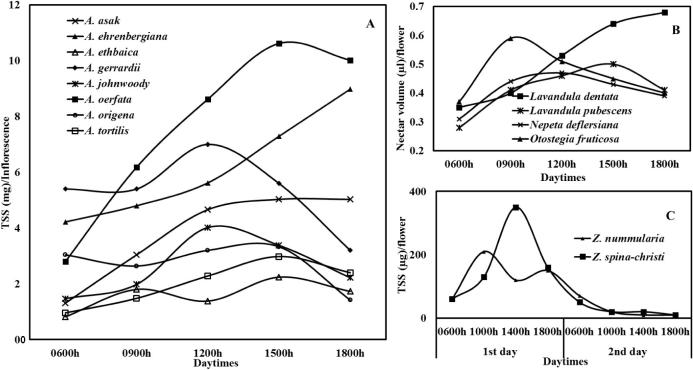
Within the genus Acacia, the highest average nectar TSS (7.7 ± 3.2 mg/inflorescence) was recorded for A. oerfata, whereas the lowest average of 1.6 ± 0.5 mg/inflorescence was recorded for A. etbaica (P < 0.0001; Table 4). In the case of species in the family Lamiaceae, the highest average nectar TSS (0.52 ± 0.22/flower) was recorded for L. dentata, whereas the lowest (0.41 ± 0.13 mg/flower) was recorded for L. pubescens and the variation was significant (P < 0.000) (Table 5). In the genus Ziziphus, a higher nectar TSS of 0.79 ± 0.10 mg/flower was recorded for Z. spina-christi compared to the 0.64 ± 0.04 mg/flower recorded for Z. nummularia (P < 0.000; Table 6).
Table 4.
Comparison of the mean nectar TSS (mg)/inflorescence of eight Acacia species growing in Saudi Arabia at different local times of the day.
| Species | 0600 h | 0900 h | 1200 h | 1500 h | 1800 h | Mean |
|---|---|---|---|---|---|---|
| A. asak | 1.3 ± 2.3c | 3.0 ± 2.8bc | 4.7 ± 2.2ab | 5.0 ± 4.2a | 5.0 ± 3.5a | 3.8 ± 1.6 |
| A. ehrenbergiana | 4.2 ± 2.4c | 4.8 ± 2.7c | 5.6 ± 2.8c | 7.3 ± 2.6b | 9.0 ± 2.3a | 6.2 ± 2.0 |
| A. ethbaica | 0.8 ± 0.9b | 1.8 ± 2.0a | 1.4 ± 1.3ab | 2.2 ± 1.7a | 1.7 ± 1.5a | 1.6 ± 0.5 |
| A. gerrardii | 5.4 ± 1.7a | 5.4 ± 2.3a | 7.0 ± 4.7a | 5.6 ± 3.9a | 3.2 ± 1.1b | 5.3 ± 1.4 |
| A. johnwoody | 1.5 ± 1.3b | 2.0 ± 1.6b | 4.0 ± 1.9a | 3.4 ± 2.1a | 2.2 ± 1.2b | 2.6 ± 1.0 |
| A. oerfata | 2.8 ± 1.6d | 6.2 ± 2.6c | 8.6 ± 2.7b | 10.6 ± 3.7a | 10.0 ± 4.0ab | 7.7 ± 3.2 |
| A. origena | 3.0 ± 2.0a | 2.6 ± 2.2a | 3.2 ± 2.6a | 3.3 ± 2.3a | 1.4 ± 0.9b | 2.7 ± 0.8 |
| A. tortilis | 1.0 ± 0.6c | 1.5 ± 1.0bc | 2.3 ± 1.8ab | 3.0 ± 2.4a | 2.4 ± 2.0ab | 2.0 ± 0.8 |
Values in the same row which are not connected by same letter are significantly (P < 0.0001) different; One inflorescence is used for one time measurement only; For each species: DF = 4, P < 0.0001, N = 45 (Except A. gerrardii N = 55).
Table 5.
The mean ± SD nectar volume amount secreted/flower in μl at different local times of the day for Lavandula (L.), Nepeta sp. (N.), and Otostegia sp. (O.) growing in Saudi Arabia.
| Species | 0600 h | 0900 h | 1200 h | 1500 h | 1800 h | Mean |
|---|---|---|---|---|---|---|
| L. dentata | 0.35 ± 0.15a | 0.40 ± 0.14a | 0.53 ± 0.19b | 0.64 ± 0.20c | 0.68 ± 0.19c | 0.52 ± 0.22 |
| L. pubescens | 0.28 ± 0.19a | 0.41 ± 0.25ab | 0.46 ± 0.23b | 0.50 ± 0.24b | 0.41 ± 0.21c | 0.41 ± 0.24 |
| N. deflersiana | 0.31 ± 0.10c | 0.44 ± 0.13a | 0.47 ± 0.13a | 0.43 ± 0.15ab | 0.39 ± 0.13b | 0.41 ± 0.13 |
| O. fruticosa | 0.37 ± 0.20c | 0.59 ± 0.21a | 0.51 ± 0.21ab | 0.45 ± 0.24bc | 0.40 ± 0.20c | 0.47 ± 0.21 |
Values in the same row which are not connected by the same letter are significantly (P < 0.0001) different; One flower is used for one time measurement only. For each species: N = 90, DF = 4, P < 0.0001.
Table 6.
The mean ± SD nectar TSS amount (mg/flower) measured at different local times of the day from a single flower of the two Ziziphus spp. growing in Saudi Arabia.
| Species | N | 1st day |
2nd day |
Total/flower | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0600 h | 1000 h | 1400 h | 1800 h | 0600 h | 1000 h | 1500 h | 1800 h | |||
| Z. nummularia | 80 | 0.06 ± 0.02e | 0.21 ± 0.08a | 0.12 ± 0.08c | 0.15 ± 0.07b | 0.07 ± 0.04d | 0.02 ± 0.01f | 0.01 ± 0.01g | 0.01 ± 0.01h | 0.64 ± 0.04 |
| Z. spina-christi | 90 | 0.06 ± 0.01c | 0.13 ± 0.11b | 0.35 ± 0.32a | 0.16 ± 0.17b | 0.05 ± 0.07d | 0.02 ± 0.03e | 0.02 ± 0.04e | 0.01 ± 0.02f | 0.79 ± 0.10 |
Values in the same raw which are not connected by same letter are significantly (P < 0.0001) different; One flower was used for repeated measurement. For each species: DF = 7, P < 0.0001.
The nectar TSS secretion dynamics of the studied species showed an increasing trend early in the morning, peaking toward midday, followed by a decline (Fig. 2A–C). However, different species were observed to have different peak nectar secretion times. Moreover, species such as A. ehrenbergiana and Lavandula dentata were observed to continue their nectar secretion even until late afternoon (1800 h).
Figure 2.
Nectar secretion dynamics of some honey plant species in Saudi Arabia at different times of a day (A = Acacia, B = Lamiacae, C = Ziziphus).
3.4. Honey production potentials
Nectar TSS and honey production potentials of the studied plants varied significantly at P < 0.000 among species (Table 7). Based on the number of flowers per unit area or volume and the amount of nectar TSS per flower or inflorescence, a minimum amount of nectar TSS of 0.0012 kg/plant was recorded for Nepeta deflersiana, whereas a maximum of 4.33 kg/plant was recorded for Z. spina-christi. Moreover, depending on the estimated number of plants per hectare of land, and also assuming that 18% of honey is water, a minimum of 14.49 kg and a maximum of 829 kg honey/hectare were estimated to be obtained from O. fruticosa and Z. spina-christi, respectively (Table 7). Within the genus Acacia, a maximum of 2.60 kg nectar TSS/tree was recorded for A. gerrardii, whereas a minimum of 0.15 kg nectar TSS/tree was recorded for A. etbaica. With the optimal plant densities of the Acacia species, the expected amount of honey per hectare of Acacia forestland was estimated at a maximum of 624.5 kg for A. johnwoodi and a minimum of 51.1 kg for A. etbaica (Table 7).
Table 7.
The expected nectar TSS amount per flower and per tree and honey production potential per hectare of land covered with the studied honey plant species in Saudi Arabia.
| Plant species | Max. average nectar TSS (mg)/flower | No. of flowers/m3 or per plant | Nectar TSS (mg)/m3 | Crown volume in m3 | Nectar TSS (kg) plant | Estimated plants/hectare | Expected nectar TSS (kg)/hectare | Expected honey yield (kg)/hectare |
|---|---|---|---|---|---|---|---|---|
| Acacia asak | 5.0 | 1954 | 9770 | 20.0 | 0.20 | 462 | 91 | 110 |
| A. ehrenbergiana | 9.0 | 2902 | 26,114 | 32.2 | 0.84 | 432 | 363 | 443 |
| A. etbaica | 1.8 | 2963 | 5333 | 27.6 | 0.15 | 284 | 42 | 51 |
| A. gerrardii | 7.0 | 4000 | 28,000 | 93.0 | 2.60 | 161 | 419 | 511 |
| A. johnwoodii | 4.0 | 11,560 | 46,240 | 46.3 | 2.14 | 239 | 512 | 625 |
| A. oerfota | 10.6 | 3000 | 31,800 | 1.9 | 0.06 | 1651 | 99 | 120 |
| A. origena | 3.3 | 4256 | 14,045 | 71.6 | 1.01 | 265 | 266 | 325 |
| A. tortilis | 3.0 | 6370 | 19,110 | 22.8 | 0.44 | 421 | 183 | 223 |
| Lavandula dentata | 0.2 | 18,537/plant | – | – | 0.004 | 10,454 | 43 | 51 |
| Lavandula pubescens | 0.2 | 17,750/plant | – | – | 0.003 | 6873 | 19 | 24 |
| Nepeta deflersiana | 0.3 | 56,099/plant | 16,830 | 0.1 | 0.001 | 12,548 | 16 | 18 |
| Otostegia fruticosa | 0.4 | 27,939/plant | 10,337 | 0.2 | 0.002 | 7708 | 12 | 14 |
| Ziziphus nummularia | 0.64 | 57,420 | 36,837 | 45 | 1.66 | 224 | 371 | 447 |
| Ziziphus spina-christi | 0.79 | 43,000 | 33,970 | 127.5 | 4.33 | 157 | 680 | 829 |
3.5. The effects of weather conditions on nectar TSS secretion
The average temperatures and RH recorded during the study period varied from 25–45 °C and 20–40% respectively. Generally, in all species, the amount of nectar TSS secreted has significant positive correlation with the ambient temperature and negatively correlated with RH, except in L. dentata, which was significantly positively correlated. However, the optimum temperatures and humidity recorded for peak nectar TSS secretion times differed among species. For example, the highest nectar TSS was recorded at an average temperature of 35.7 °C and 28.7% RH for L. pubescens and at 28.3 °C and 37.7% RH for L. dentata. However, in Z. spina-christi the peak nectar TSS was recorded at 45 °C.
4. Discussion
4.1. Flowering period distribution
The differences in the flowering periods of the species could be attributed to the variations in their ecological distribution and the climatic factors (temperature, rainfall and photoperiod). In this regard the effects of rainfall on the onset of green-up and growth and in defining flowering durations in some desert plants have been well reported (Fox, 1990, Borchert, 1994, Abd El-Ghani, 1997, Peñuelas et al., 2002). Moreover, photoperiod and temperatures have been stated as being the main factors in governing the flowering seasons of different plant species (Ausín et al., 2005). The variations in flowering periods within related and sympatric species (such as different Acacia species) could be considered as a mechanism to minimize competition for pollination. The temporal separation of flowering periods of sympatric species has been interpreted as their adaptation to avoid competition for pollination (Pleasants, 1983, Rathcke, 1983, Stone et al., 1998, Stone et al., 2003).
The aborting of flowers and the initiating of new leaves following the onset of rain in some studied Acacia species could be due to a shift in the resource allocation of the species from reproductive functions to vegetative growth. Such resource shifting patterns are known to be typical adaptations of plants to dry climatic conditions and considered as a strategy for partitioning of the use of resources between reproductive and vegetative purposes (Singh and Kushwaha, 2006). Moreover, general spatiotemporal phenological shifts in response to rainfall changes have been well documented (Peñuelas et al., 2004). With regard to this, some beekeepers have argued that when the rain occurs and the plants produce new green leaves while flowering, these plants will not be a good source of nectar (personal communication), which could indicate resource trade-offs by the species between vegetative and reproductive functions. The variation in the flowering periods among and within species allows beekeepers to harvest honey several times in a year by migrating their colonies to different localities (an average of 6 times/year) in search of better flowering plants (Nuru et al., 2013, Nuru et al., 2014). Moreover, the flowering seasons of Z. nummularia and A. gerrardii in the extremely harsh summer in central Saudi Arabia reported to be valuable for bees and beekeeping (Alqarni, 2015).
4.2. Phenology
The variations in phenology of the different species in this study could be attributed to the adaptations made to ensure maximum pollination through the partitioning of pollinators and the efficient distribution of resources. Variations in phenology and the timing in the release of floral rewards among sympatric species have been reported to be a selective response to competition for pollination and mechanisms of partitioning pollinators (Pleasants, 1983, Rathcke, 1983, Stone et al., 1998).
4.3. Nectar TSS amount and dynamics
Significant variations in the amount and patterns of nectar secreted by the different honey source plants could be due to the variations in biotic and abiotic factors associated with the different plant species in their respective environments. Variations in nectar concentration and production patterns as a result of variations in pollinator guilds have also been well documented (Baker and Baker, 1975, Cruden et al., 1983, Galetto and Bernardello, 1992). Moreover, separations in peak floral reward release times among different species have been interpreted as a means of partitioning of pollinators (Stone et al., 1998). One possible reason for the continuous increase and eventual peak in nectar at approximately 1800 h for species such as A. ehrenbergiana and L. dentata (Fig. 2 A and B) could be the absence of re-absorption of the nectar by the flower. In addition, it could also be an adaptation by the species to nocturnal flower visitors. These possibilities require further investigation.
4.4. Nectar TSS secretion amount and dynamics and its association with weather conditions
The significant positive correlations between the nectar secretion and the ambient temperature in all the studied species may indicate the adaptations of the species to higher temperatures. Similarly, the presence of a positive correlation between nectar values (volume/flower, TSS content and concentration) and temperature was recorded in the Mediterranean species Thymus capitatus up to 38 °C (Petanidou and Smets, 1996) and in Saudi Arabia up to 45 °C for Z. spina-christi (Adgaba et al., 2012) and Z. nummularia (Alqarni, 2015). In contrast, the negative correlation between the nectar values and relative humidity was expected because at midday when the flowers attained peak nectar TSS secretion, the humidity was generally low in the area. The secretion of more nectar TSS at high temperature and low humidity may indicate how well the species adapted to the prevalent weather conditions. However, high temperature and low humidity cause rapid crystallization of nectar TSS on the open surface of Ziziphus flowers, which makes it difficult for the bees to properly utilize the nectar. Similarly, Corbet et al. (1979) reported that low relative humidity and exposed nectaries enhance water evaporation and the concentration of the nectar, which ultimately leads to its crystallization.
4.5. Honey production potentials
Despite the arid and semiarid climatic conditions of the region, some of the studied plant species were observed to have high potential for honey production (Table 7); these results are comparable to the reports made for different annual plants and trees such as A. syriaca L. (milkweed) (500–600 kg honey/ha; Zsidei, 1993); T. pratense L. (red clover) honey yield of 883 kg/ha/flowering period (Szabo and Najda, 1985; various Tilia (lime) species (90–1200 kg honey/ha; Crane et al., 1984); and Brassica juncea and Sinapis alba crops (65.5 kg and 71.2 kg/hectare, respectively; Masierowska, 2003). In general, trees were more productive in nectar secretion than herbs due to their larger biomass, dense flowers, deep roots and resistance to moisture stress.
Moreover, in most trees, the flowers are not colorful and are expected to secrete more nectar to strongly attract sufficient pollinators. However, herbaceous plants have conspicuous colors and may not need to produce large amount of nectar (Schemske and Bradshaw, 1999). In line with this, Alqarni et al. (in press) described the mass flowering behavior A. gerrardi as evolutionary adaptation of the species to withstand pre and post fruiting obstacles and this may have contributed to copious gross nectar per tree.
In some species, the amount of nectar TSS per flower was high, but the amount of honey per tree or hectare of land was low because honey production potential also depends on the number of flowers per unit area or volume and the canopy of the plant. The actual honey production of the species is expected to be lower than the honey production potential estimated in this study because a significant amount of the nectar is utilized by the honey bees for brood rearing and for the energy required for the collection and processing of nectar and pollen.
The study indicated that despite the limited rainfall and high temperature in the region; the studied species secrete a significant amount of nectar sugar and are very potential for beekeeping. The contributions of the studied species as honey source plants are relatively better than other agricultural activities, which require sufficient water. Based on the estimated amount of TSS, the monetary value of honey that can be obtained per hectare for the species in this study can be equal or greater than the per-hectare monetary value of some cultivated crops that require many inputs. Therefore, rehabilitation and conservation of such multipurpose plants seems worthwhile, both for economic reasons and their environmental value. In addition, the information generated in this study is believed to be useful in apiary site selection and to estimate the honey bee colony carrying capacity of an area.
Acknowledgments
The authors are grateful to Deanship of Scientific Research and College of Food and Agricultural Science Research, King Saud University Riyadh, for providing research support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abalaka M.E., Daniyan S.Y., Mann A. Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana L. and Ziziphusspinachristi L.) on some microbial pathogens. Afr. J. Pharm. Pharmacol. 2010;4(4):135–139. (accessed Nov. 2014) [Google Scholar]
- Abbasi A.M., Khan M.A., Ahmad M., Zafar M., Jahan S., Sultana S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010;128:322–335. doi: 10.1016/j.jep.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Abd El-Ghani M.M. Phenology of ten common plant species in western Saudi Arabia. J. Arid Environ. 1997;35:673–683. [Google Scholar]
- Aboutabl E.A., Sokkar N.M., Megid R.M.A., de Pooter H.L., Masoud H. Composition and antimicrobial activity of Otostegia fruticosa Forssk. oil. J. Essent. Oil Res. 1995;7:299. [Google Scholar]
- Adgaba N., Awad M.A., Al-Ghamdi1 A.A., Alqarni A.S., Radloff S.E. Nectar of Ziziphus spina-christi (L.) Willd (Rhamnaceae): dynamics of secretion and potential for honey production. J. Apic. Sci. 2012;56(2):49–59. [Google Scholar]
- Alqarni A.S. Honeybee foraging, nectar secretion, and honey potential of wild jujube trees, Ziziphus nummularia. Neotrop. Entomol. 2015;44:232–241. doi: 10.1007/s13744-015-0279-4. [DOI] [PubMed] [Google Scholar]
- Alqarni, A.S., Awad M.A., Raweh, H.S.A., Owayss, A.A. in press. Pollination ecology of Acacia gerrardii Benth. (Fabaceae: Mimosoideae) under extremely hot-dry conditions. Saudi J. Biol. Sci. doi: http://dx.doi.org/10.1016/j.sjbs.2015.09.019. [DOI] [PMC free article] [PubMed]
- Ausín I., Alonso-Blanco C., Martínez-Zapter J. Environmental regulation of flowering. Int. J. Dev. Biol. 2005;49:689–705. doi: 10.1387/ijdb.052022ia. [DOI] [PubMed] [Google Scholar]
- Azcón R., Barea J. Mycorrhizal dependency of a representative plant species in Mediterranean shrublands (Lavandula spica L.) as a key factor to its use for revegetation strategies in desertification threatened areas. Appl. Soil Ecol. 1997;7(1):83–92. (accessed Nov. 2013) [Google Scholar]
- Baker H.G., Baker I. Studies of nectar constitution and pollinator plant coevolution. In: Gilbert L.E., Raven P.H., editors. Coevolution of Animals and Plants. University of Texas Press; Austin: 1975. pp. 100–140. [Google Scholar]
- Bastiaan M. Facts on File Publishers; New York: 1984. The Sex Life of Flowers; pp. 110–111. [Google Scholar]
- Boning C.R. Pineapple, PR INC; 2010. Florida’s Best Herbs and Spices: Native and Exotic Plants Grown for Scent and Flavor; p. 229. (accessed Dec. 2014) [Google Scholar]
- Borchert R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology. 1994;75:1437–1449. [Google Scholar]
- Boulos L. 1983. Medicinal Plants of North Africa; pp. 115–117. Algonac, Michigen. [Google Scholar]
- Castellanos M.C., Wilson P., Thomson J.D. Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae) Am. J. Bot. 2002;89:111–118. doi: 10.3732/ajb.89.1.111. [DOI] [PubMed] [Google Scholar]
- Chalcoff V.R., Aizen M.A., Galetto L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006;97(3):413–421. doi: 10.1093/aob/mcj043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S., editor. vols. 1–3. Ministry of Agriculture & Water; Riyadh: 1999. (Flora of the Kingdom of Saudi Arabia). [Google Scholar]
- Cherry M. The needs of the people. In: Wickens G.E., Goodin J.R., Field D.V., editors. Plants for Arid Lands. Unwin Hyman; London: 1985. pp. 48–49. [Google Scholar]
- Chu C.J., Kemper K.J. The Longwood Herbal Task Force and The Center for Holistic Pediatric Education and Research); 2001. Lavender (Lavandula spp.) p. 32. Available at: < http://www.longwoodherbal.org/lavender/lavender.pdf> (accessed Dec. 2014) [Google Scholar]
- Coder K.D. School of Forestry and Natural Resources, University of Georgia; February 2010. Assessing Soil Water Resource Space. Tree Water Availability Series, WSFNR10-11. [Google Scholar]
- Collenette I.S. National Commission for Wildlife Conservation; Riyadh: 1999. Wildflowers of Saudi Arabia. < http://www.plantdiversityofsaudiarabia.info/>. [Google Scholar]
- Corbet S.A., Willmer P.G., Beament J.W.L., Unwin D.M., Prys-Jones O.E. Post-secretory determinants of sugar concentration in nectar. Plant Cell Environ. 1979;2:293–308. [Google Scholar]
- Crane E. Heinemann Newnes; Oxford: 1990. Bees and Beekeeping: Science, Practice and World Resources. [Google Scholar]
- Crane E., Walker P., Day R. International Bee Research Association; London: 1984. Directory of Important World Honey Sources; p. pp. 384. [Google Scholar]
- Cruden R.W., Hermann S.M., Peterson S. Patterns of nectar production and plant-pollinator coevolution. In: Bentley B., Elias T.S., editors. The Biology of Nectaries. Columbia University Press; New York: 1983. pp. 80–125. [Google Scholar]
- Dulger B., Dulger G. Antimicrobial activity of the leaves of Ballota acetabulosa on microorganisms isolated from urinary tract infections. Turk. J. Pharm. Sci. 2012;9(3):257–262. [Google Scholar]
- El-Karemy Z.A., Zayed K.M. Distribution of plant communities Across Al Abna escarpment, SW Saudi Arabia. Phyton (Horn, Austria) 1992;32(1):79–101. [Google Scholar]
- Farkas Á., Orosz-Kovács Z.S. Nectar secretion dynamics of Hungarian local pear cultivars. Plant. Syst. Evol. 2003;238:57–67. [Google Scholar]
- Forler S. 2013. Lavender Honey. < http://www.honeytraveler.com/single-flower-honey/lavender-honey/> (accessed on Dec. 2014) [Google Scholar]
- Fox G.A. Drought and the evolution of flowering time in desert annuals. Am. J. Bot. 1990;77:1508–1518. [Google Scholar]
- Galetto L., Bernardello L. Nectar secretion pattern and removal effects in six Argentinean Pitcairnioideae (Bromeliaceae) Bot. Acta. 1992;105:292–299. [Google Scholar]
- Galetto L., Bernardello G. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Ann. Bot. 2004;94:269–280. doi: 10.1093/aob/mch137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth A., Orosz-Kovács Z.S. Individual variability of nectar secretion in the flowers of plum cv. ‘Reine-Claude d’Althan. Acta Hortic. 2004;636:357–363. [Google Scholar]
- Kim M.S., Kim S.H., Han J., Kang M.S., Park Y.K. Honeybee visit and nectar secretion characteristics of the Chinese Hawthorn Crataegus pinnatifida Bunge. J. Apic. 2011;26(1):11–14. The Apicultural Society of Korea. [Google Scholar]
- Krüger O., McGavin G.C. The insect fauna of Acacia species in Mkomazi game reserve, north east Tanzania. Ecol. Entomol. 1998;22:440–444. [Google Scholar]
- Lalande B. Lavender, lavandin and other French oils. Perfumer Flavorist. 1984;9:117–121. [Google Scholar]
- Le-Houérou H. FAO; 2012. Acacia ehrenbergiana Hayne. www.fao.org/ag/AGP/AGPC/doc/ (accessed on 10.12.2014) [Google Scholar]
- Lis-Balchin M. CRC Press, Science; UK: 1984. Lavendor: The Genus Lavandula; p. 296. < http://books.google.ca/books/about/Lavender> (accessed on 15.01.2015) [Google Scholar]
- Mačukanović-Jocic M., Duletić S., Jocić G. Nectar production in three melliferous species of Lamiaceae in natural and experimental conditions. Acta Vet. Beograd. 2004;54(5–6):475–487. [Google Scholar]
- Mallick S.A. Technique for washing nectar from the flowers of Tasmanian leatherwood (Eucryphia lucida Eucryphiaceae) Austral. Ecol. 2000;25:210–212. [Google Scholar]
- Martin E., Altinordu F., Özcan T., Dirmenci T. Karyomorphological Study in Nepeta viscida Boiss. (Lamiaceae) from Turkey. J. Appl. Biol. Sci. 2013;7(3):26–30. [Google Scholar]
- Masierowska M.L. Floral nectaries and nectar production in brown mustard (Brassica juncea) and white mustard (Sinapis alba) (Brassicaceae) Plant Syst. Evol. 2003;238:97–107. [Google Scholar]
- Midgely S.J., Turnbul J.W. Domestication and uses of Australian acacias: case studies of five important species. Austral. Syst. Bot. 2003;16:89–102. [Google Scholar]
- Miller A.G. The genus Lavandula in Arabia and Tropical NE Africa. Notes Royal Botany Garden. 1985;42(3):503–528. Edinburgh. [Google Scholar]
- Mohr N.A., Jay S.C. Nectar production of selected cultivars of Brassica campestris L. and Brassica napus L. J. Apic. Res. 1990;29(2):95–100. [Google Scholar]
- Mothana R.A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Nepeta deflersiana growing in Yemen. Rec. Nat. Prod. 2012;6(2):189–193. [Google Scholar]
- Nagy Z. Egyre népszerűbb növényünk a facélia III. Méhészet. 2002;50(4):22. [Google Scholar]; Nectar Production for the Hungarian Honey Industry. 2002. Reviewed by Farkas, A., Zajácz, E., 2007. Eur. J. Plant Sci. Biotech, Global Science Book, pp. 125–151. [Google Scholar]
- Nepi M., Guarnieri M., Pacini E. Nectar secretion, reabsorption, and sugar composition in male and female flowers of Cucurbita pepo. Int. J. Plant Sci. 2001;162:353–358. [Google Scholar]
- Nicoleta I. Research regarding the melliferous characteristics of Labiates from xerophile meadows from Danube Valley. Lucrări ştiinţifice Zootehnie şi Biotehnologii. 2008;41:6 pp. [Google Scholar]
- Nicoleta I., Ion V. Mlliferous characteristics of spontaneous Lamiaceae species, identified in the Danube Valley. Lucrări ştiinţifice Zootehnie şi Biotehnologii. 2007;40(2):71–79. [Google Scholar]
- Nuru A., Awraris S., Al-Ghamid A.A., Sammud R., Hegazy S., Tour A., Yilma T., Sharma D. Determining temporal and spatial availability of bee forages, based on ground inventory supported with GIS application and remote sensed satellite image processing. The Proceedings of 43rd International Apicultural Congress 29 Sep.–04 Oct., Kyiv, Ukraine; 2013. p. pp. 216. [Google Scholar]
- Nuru A., Awraris G.S., Al-Ghamdi A.A., Ismaiel S., Al-kahtani S., Yilma T., Mohammed J.A., Workneh A., Abdulaziz M.Q.A. Socio-economic analysis of beekeeping and determinants of box hive technology adoption in the Kingdom of Saudi Arabia. J. Anim. Plant Sci. 2014;24(6):2014. [Google Scholar]
- Orwa C., Mutua A., Kindt R., Jamnadass R., Simons A. 2009. Agroforestree Database: A Tree Reference and Selection Guide, Version 4.0. < http://www.worldagroforestry.org/treedb2/AFTPDFS/Zizyphus_spina-christi.pdf> (accessed Dec. 2014) [Google Scholar]
- Peñuelas J., Filella I., Comas P. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Change Biol. 2002;8:531–544. [Google Scholar]
- Peñuelas J., Filella I., Zhang X., Llorens L., Ogaya R., Lloret F., Comas P., Estiarte M., Terradas J. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol. 2004;161:837–846. doi: 10.1111/j.1469-8137.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- Pesti J. Daily fluctuations in the sugar content of nectar and periodicity of secretion in the Compositae. Acta Agron. Hung. 1976;25(1–2):5–17. [Google Scholar]
- Petanidou T., Smets E. Does temperature stress induce nectar secretion in Mediterranean plants? New Phytol. 1996;133:513–518. [Google Scholar]
- Pleasants J.M. Structure of plant and pollinator communities. In: Jones C.E., Little R.J., editors. Hand Book of Experimental Pollination Biology. Van Nostrand Reinhold; New York: 1983. pp. 375–393. [Google Scholar]
- Rahman M.A., Mossa J.S., Al-Said M.S., Al-Yahya M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2003;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Raja R.R. Medicinally potential plants of Labiate (Lamiaceae) family: an overview. Res. J. Med. Plants. 2012;6(3):203–213. [Google Scholar]
- Rathcke B. Competition and facilitation among plants for pollination. In: Real L., editor. Pollination Biology. Academic Press; New York: 1983. pp. 305–329. [Google Scholar]
- Roubik D.W. Aspects of Africanized honey bee ecology in tropical America. In: Spivak M., Fletcher D.J.C., Breed M.D., editors. The African Honey Bee. Westview Press; Boulder: 1991. pp. 259–281. [Google Scholar]
- Saqib Z., Mahmood A., Malik R.N., Mahmood A., Syed J.H., Ahmad T. Indigenous knowledge of medicinal plants in Kotli Sattian, Rawalpindi district, Pakistan. J. Ethnopharmacol. 2014;151:820–828. doi: 10.1016/j.jep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Institute Inc; Cary, NC, USA: 2002. JMP-5 Statistical Software, Version 5. [Google Scholar]
- Schemske D.W., Bradshaw H.D. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proc. Natl. Acad. Sci. USA. 1999;96(21):11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte P., Khuleidi A.W., Kessler J.J. Environmental Protection Council, Agriculture Research Authority, Range and Livestock Improvement Project, Dhamar; 1991. The Vegetation of the Republic of Yemen Western Part. [Google Scholar]
- Singh K.P., Kushwaha C.P. Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Ann. Bot. 2006;97(2):265–276. doi: 10.1093/aob/mcj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G.N., Pat W., Sean N. Daily partitioning of pollinators in an African acacia community. Proc. R. Soc. Biol. Sci. 1996;263(1375):1389–1393. [Google Scholar]
- Stone G.N., Willmer P.G., Rowe J.A. Partitioning of pollinators during flowering in an African Acacia community. Ecology. 1998;79:2808–2827. [Google Scholar]
- Stone G.N., Nigel E.R., Mathew P., Pat P.W. Pollination ecology of acacias (Fabaceae, Mimosoideae) Austral. Syst. Bot. 2003;16:113–118. [Google Scholar]
- Szabo T.I., Najda H.G. Flowering, nectar secretion and pollen production of some legumes in the Peace River Region of Alberta. Canada. J. Apic. Res. 1985;24(2):102–106. [Google Scholar]
- Tandon R., Shivanna K.R. Pollination biology and breeding system of Acacia senegal. Bot. J. Linn. Soc. 2001;135:251–262. [Google Scholar]
- Tsigouri A., Passaloglou-Katrali M. A scientific note on the characteristics of thyme honey from the Greek island of Kithira. Apidologie. 2000;31:457–458. [Google Scholar]
- UNESCO . UNESCO; Paris: 1977. Map of the World Distribution of Arid Regions. MAB Technical Note 7. [Google Scholar]
- Venkateshappa S.M., Sreenath K.P. Potential medicinal plants of Lamiaceae. Am. Int. J. Res. Formal, Appl. Nat. Sci. 2013;3(1):82–87. [Google Scholar]
- Walter H., Breckle S.W. vol. 2. Springer-Verglag; Berlin, Heidelburg: 1986. Ecological systems of the geobiosphere. (Tropical and Subtropical Zonobiomes). [Google Scholar]
- Weast R., editor. CRC Handbook of Chemistry and Physics. 67th ed. CRC Press Inc.; Boca Raton, Florida: 1986. [Google Scholar]
- Welsh C. Three essential oils for the medicine cabinet. Alternative Health Practitioner. 1995;3:11–15. [Google Scholar]
- Wickens G.E. FAO; 1995. Role of Acacia species in the rural economy of dry Africa and the Near East. FAO Conservation Guide 27; p. pp. 137. < http://www.fao.org/docrep/V5360E/V5360E00.htm> (accessed 25.05.2014) [Google Scholar]
- Wyatt R., Broyles S.B., Derda G.S. Environmental influences on nectar production in milkweeds (Ascelapias syriaca and A. exaltata) Am. J. Bot. 1992;79:636–642. [Google Scholar]
- Zajácz E., Zaják Á., Szalai-Mátray E., Szalai T. Nectar production of some sunflower hybrids. J. Apic. Sci. 2006;50(2):7–11. [Google Scholar]
- Zsidei B. Nectar production for the Hungarian Honey Industry. 1993. Méhészeti ismeretek. Fazekas és fiai nyomdája, Szarvas. Reviewed by Farkas, A., Zajácz, E., 2007. Eur. J. Plant Sci. Biotech. Global Science Book, pp. 125–151. [Google Scholar]