Abstract
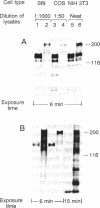
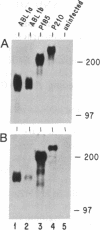
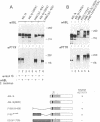
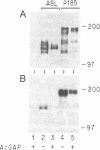
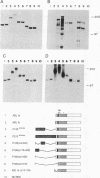
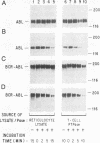
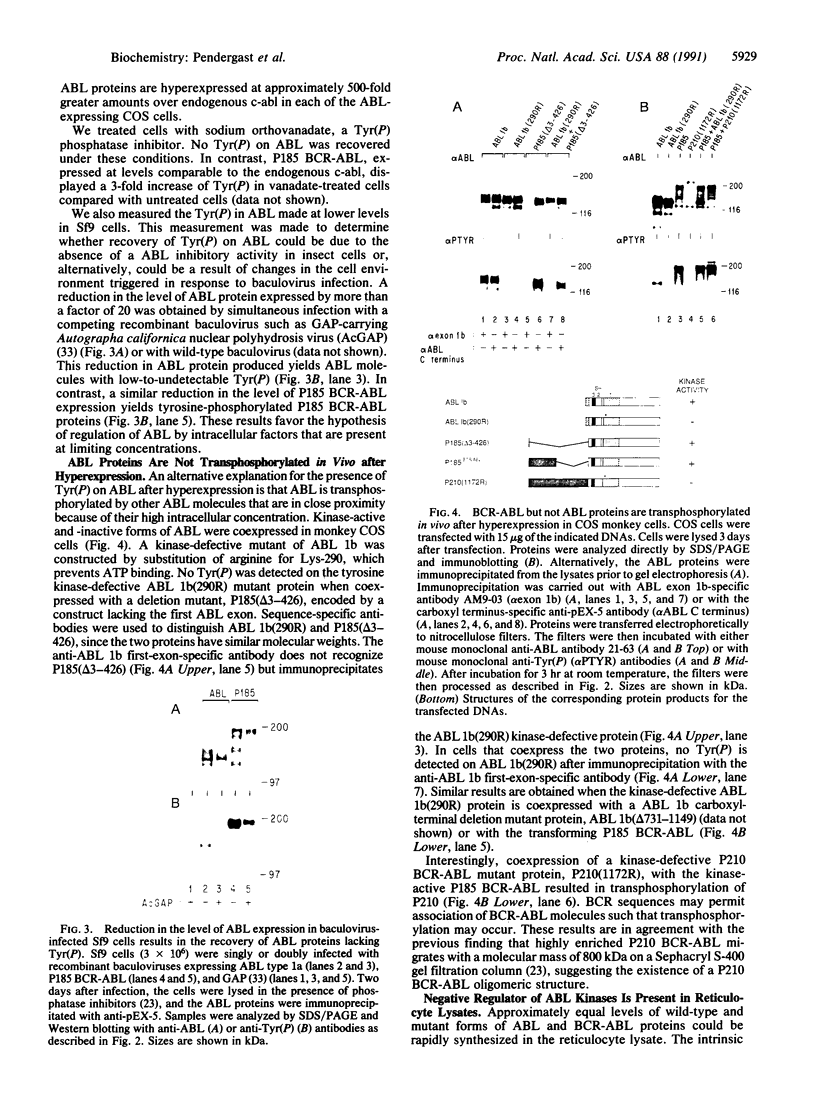
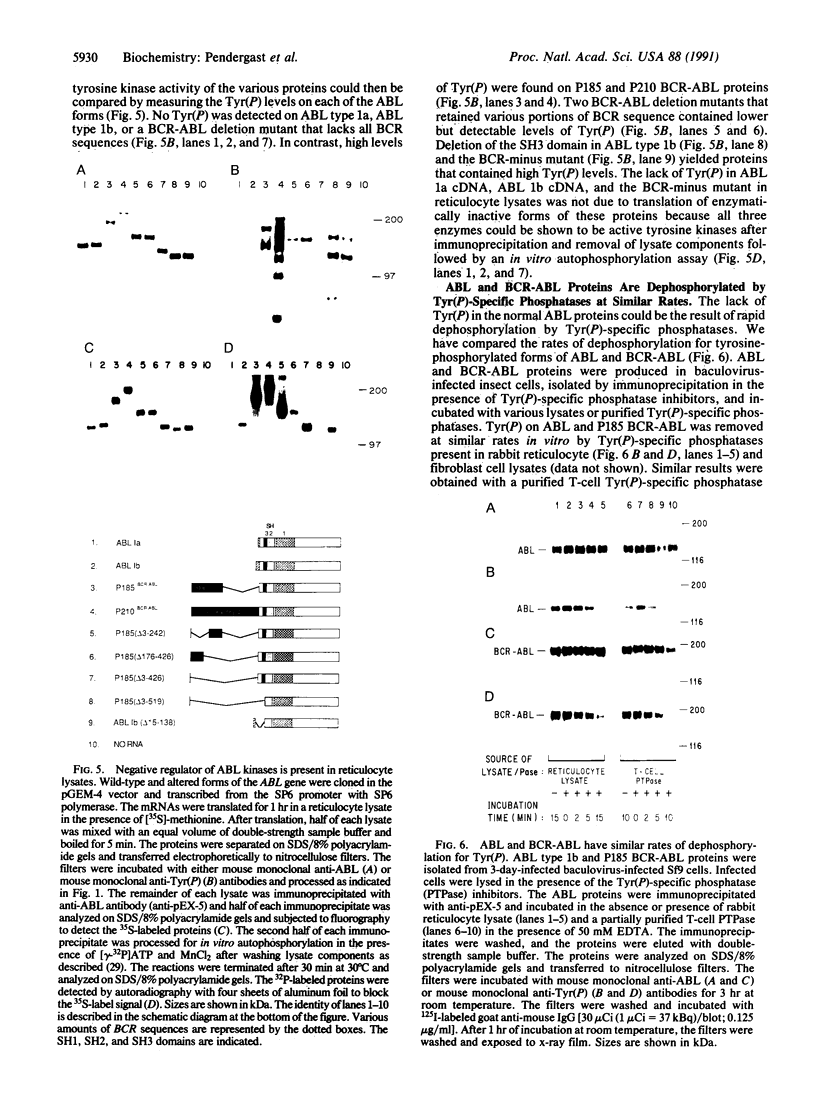
Phosphotyrosine cannot be detected on normal human ABL protein-tyrosine kinases, but activated oncogenic forms of the human ABL protein are phosphorylated on tyrosine in vivo. Activation of ABL can occur by substitution of the ABL first exon with breakpoint cluster region (BCR) sequences or by deletion of the noncatalytic SH3 (src homology region 3) domain. An alternative mode for the activation of the ABL kinases is hyperexpression at greater than 500-fold over endogenous levels. This is not a consequence of transphosphorylation of the hyperexpressed ABL molecules. ABL proteins translated in vitro lack phosphotyrosine, but tyrosine kinase activity is uncovered after immunoprecipitation and removal of lysate components. The rates of dephosphorylation of ABL and BCR-ABL fusion protein by phosphotyrosine-specific phosphatases are approximately the same. These combined results indicate that inhibition of ABL activity is reversible and suggest that a cellular component interacts noncovalently with ABL to inhibit its autophosphorylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Hardy W. D., Jr, Zuckerman E. E., Bergold P., Lederman L., Snyder H. W., Jr The Hardy-Zuckerman 2-FeSV, a new feline retrovirus with oncogene homology to Abelson-MuLV. Nature. 1983 Jun 30;303(5920):825–828. doi: 10.1038/303825a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Crist W. M., Champlin R., Witte O. N. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987 Jan 2;235(4784):85–88. doi: 10.1126/science.3541203. [DOI] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Walsh K. A., Fischer E. H., Krebs E. G. cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5257–5261. doi: 10.1073/pnas.86.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainstein E., Marcelle C., Rosner A., Canaani E., Gale R. P., Dreazen O., Smith S. D., Croce C. M. A new fused transcript in Philadelphia chromosome positive acute lymphocytic leukaemia. 1987 Nov 26-Dec 2Nature. 330(6146):386–388. doi: 10.1038/330386a0. [DOI] [PubMed] [Google Scholar]
- Franz W. M., Berger P., Wang J. Y. Deletion of an N-terminal regulatory domain of the c-abl tyrosine kinase activates its oncogenic potential. EMBO J. 1989 Jan;8(1):137–147. doi: 10.1002/j.1460-2075.1989.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Pendergast A. M., Muller A. J., Witte O. N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- Luo K. X., Sefton B. M. Cross-linking of T-cell surface molecules CD4 and CD8 stimulates phosphorylation of the lck tyrosine protein kinase at the autophosphorylation site. Mol Cell Biol. 1990 Oct;10(10):5305–5313. doi: 10.1128/mcb.10.10.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol Cell Biol. 1989 May;9(5):1866–1874. doi: 10.1128/mcb.9.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., McLaughlin J., Daley G. Q., Paskind M., Witte O. N. Overlapping cDNA clones define the complete coding region for the P210c-abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J., Witte O. N. The 5' noncoding region of the human leukemia-associated oncogene BCR/ABL is a potent inhibitor of in vitro translation. Mol Cell Biol. 1989 Nov;9(11):5234–5238. doi: 10.1128/mcb.9.11.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Wettenhall R. E., Means A. R., Hartshorne D. J., Kemp B. E. Autoregulation of enzymes by pseudosubstrate prototopes: myosin light chain kinase. Science. 1988 Aug 19;241(4868):970–973. doi: 10.1126/science.3406746. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Clark R., Kawasaki E. S., McCormick F. P., Witte O. N. Baculovirus expression of functional P210 BCR-ABL oncogene product. Oncogene. 1989 Jun;4(6):759–766. [PubMed] [Google Scholar]
- Ponticelli A. S., Whitlock C. A., Rosenberg N., Witte O. N. In vivo tyrosine phosphorylations of the Abelson virus transforming protein are absent in its normal cellular homolog. Cell. 1982 Jul;29(3):953–960. doi: 10.1016/0092-8674(82)90458-5. [DOI] [PubMed] [Google Scholar]
- Potts W. M., Reynolds A. B., Lansing T. J., Parsons J. T. Activation of pp60c-src transforming potential by mutations altering the structure of an amino terminal domain containing residues 90-95. Oncogene Res. 1988;3(4):343–355. [PubMed] [Google Scholar]
- Quilliam L. A., Der C. J., Clark R., O'Rourke E. C., Zhang K., McCormick F., Bokoch G. M. Biochemical characterization of baculovirus-expressed rap1A/Krev-1 and its regulation by GTPase-activating proteins. Mol Cell Biol. 1990 Jun;10(6):2901–2908. doi: 10.1128/mcb.10.6.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff-Maker L., Burns M. C., Konopka J. B., Clark S., Witte O. N., Rosenberg N. Monoclonal antibodies specific for v-abl- and c-abl-encoded molecules. J Virol. 1986 Mar;57(3):1182–1186. doi: 10.1128/jvi.57.3.1182-1186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Roe B. A., Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986 Oct 24;47(2):277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G. A., Ladner M., Long C. M., Crosier W. J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988 Dec 23;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Negative regulation of c-abl tyrosine kinase by its variable N-terminal amino acids. Oncogene Res. 1988;3(3):293–298. [PubMed] [Google Scholar]