Abstract
Background
Sequential addition of tenofovir disoproxil fumarate (TDF) is often needed for patients coinfected with HIV and hepatitis B virus (HBV) who develop HBV resistance to lamivudine after combination antiretroviral therapy (cART) containing only lamivudine for HBV. We aimed to assess the virological response of HBV to add-on TDF in patients coinfected with lamivudine-resistant HBV.
Methods
Between November 2010 and December 2014, 33 HIV/HBV-coinfected patients with lamivudine-resistant HBV and 56 with lamivudine-susceptible HBV were prospectively included. TDF plus lamivudine was used to substitute zidovudine or abacavir plus lamivudine contained in cART in patients with lamivudine-resistant HBV infection, while patients with lamivudine-susceptible HBV infection received TDF plus lamivudine as backbone of cART. Serial determinations of plasma HBV DNA load, HBV serologic markers, and liver and renal functions were performed after initiation of TDF-containing cART.
Results
Of 89 patients included, 38.6% tested positive for HBV envelope antigen (HBeAg) at baseline. The plasma HBV DNA level at enrollment of lamivudine-resistant and lamivudine-susceptible group were 6.1 ± 2.2 log10 and 6.0 ± 2.2 log10 copies/mL, respectively (p = 0.895). The cumulative percentage of HBV viral suppression in lamivudine-resistant and lamivudine-susceptible group was 81.8% and 91.1% at 48 weeks, respectively (p = 0.317), which increased to 86.7% and 96.2% at 96 weeks, respectively (p = 0.185). At 48 weeks, 11 patients testing HBeAg-positive at baseline failed to achieve viral suppression. In multivariate analysis, the only factor associated with failure to achieve viral suppression at 48 weeks was higher HBV DNA load at baseline (odds ratio, per 1-log10 copies/mL increase, 1.861; 95% CI, 1.204–2.878). At 48 weeks, HBeAg seroconversion was observed in 5 patients (1 in the lamivudine-resistant group and 4 in the lamivudine-susceptible group; p = 0.166). During the study period, HBsAg levels decreased over time, regardless of lamivudine resistance. Loss of HBsAg was observed in 3 (3.4%) patients in the lamivudine-susceptible group.
Conclusions
Add-on TDF-containing cART in patients coinfected with lamivudine-resistant HBV achieved a similar rate of HBV viral suppression compared to TDF-containing cART as initial regimen in patients coinfected with lamivudine-susceptible HBV. A higher baseline HBV DNA load and HBeAg positivity were associated with failure to achieve HBV viral suppression.
Introduction
Hepatitis B virus (HBV) coinfection is common in HIV-positive patients [1]. In Taiwan, 19.8% of HIV-positive patients have concurrent chronic HBV infection [2], though the prevalence of HBsAg positivity has gradually declined after the implementation of nationwide neonatal HBV vaccination program in 1986 [3]. Individuals with both diseases are at greater risks to develop hepatitis and liver decompensation, and their course of chronic HBV infection is more aggressive than those with HBV mono-infection [4–6]. HBV DNA levels have also been shown to predict overall mortality in HIV/HBV-coinfected patients, especially prior to developing acquired immunodeficiency syndrome (AIDS) [7]. To prevent HBV-related liver damage and late complications, it is essential for patients with HBV infection to achieve durable viral suppression before strategies for functional and durable cure of chronic HBV infection are available [8].
Lamivudine that is contained in combination antiretroviral therapy (cART) for HIV used to be the only active antiviral agent against both HIV and HBV. However, the genetic barrier to development of lamivudine resistance is low, as mutations in tyrosine-methionine-aspartate-aspartate (YMDD) motif of HBV emerge frequently. When HIV/HBV-coinfected patients receive lamivudine as the only active drug for HBV, the resistance rates to lamivudine may reach 40% after 2 years and 90% after 4 years in these patients [9–11]. Sequential addition of another anti-HBV agent is often inevitable for HIV/HBV-coinfected patients who started cART in early days before the availability of other anti-HBV agents with greater activity against both HBV and HIV [1].
Among the antiretroviral agents that are active against HIV and HBV, tenofovir disoproxil fumarate (TDF) has potent antiviral effect on both wild-type and lamivudine-resistant HBV [12–14]. TDF-containing cART may lead to high rates of HBV envelope antigen (HBeAg) seroconversion and suppression of HBV replication compared with HBV monotherapy with lamivudine in HIV/HBV-coinfected patients [15]. In HIV/HBV-coinfected patients failing lamivudine, TDF is frequently used as rescue therapy [16, 17]. A previous study reported the association of prior lamivudine exposure with delayed HBV suppression in HIV/HBV-coinfected patients on TDF treatment [18], but this finding was not confirmed in the meta-analysis [19]. To date, data are still limited regarding the impact of prior lamivudine exposure with emergence of lamivudine resistance on the effectiveness of subsequent TDF/lamivudine- or TDF/emtricitabine-containing cART.
In this study, we aimed to assess the virological responses of HBV to TDF-containing cART in HIV/HBV-coinfected patients who had HBV resistance to lamivudine, and to identify factors associated with failure to achieve HBV viral suppression after 48 weeks of treatment with TDF-containing cART.
Methods
Patient population and study design
This prospective observational study was conducted at the National Taiwan University Hospital (NTUH), a tertiary care center and the largest designated hospital for HIV care in Taiwan. After TDF and TDF/emtricitabine became available in the clinical care in Taiwan in November 2010 and November 2014, respectively, all adult patients with HBV and HIV coinfection who regularly sought HIV care at NTUH between November 2010 and December 2014 were consecutively included and two groups of HIV/HBV-coinfected patients were defined: add-on TDF to replace one nucleoside reverse-transcriptase inhibitor (NRTI) other than lamivudine in patients who had been taking lamivudine-containing cART with emergence of lamivudine-resistant HBV with (lamivudine-resistant group); and initiation of TDF/lamivudine or TDF/emtricitabine-containing cART in patients who were antiretroviral-naïve or who resumed cART after interruption in the absence of HBV resistance to lamivudine (lamivudine-susceptible group). Patients were excluded if they were aged less than 20 years, their plasma HBV DNA levels were less than 1000 copies/mL within 3 months of enrollment, or they had received or were receiving anti-HBV therapy other than lamivudine such as interferon, telbivudine, entecavir or adefovir. After enrollment, all patients switched to or starting TDF-containing (TDF/lamivudine or TDF/emtricitabine) cART were followed for at least 48 weeks to evaluate the virological response of HBV to TDF-containing cART.
We performed on-treatment analysis to estimate the proportion of patients who achieved undetectable plasma HBV DNA load at each time point. Our primary end-point was the proportion of patients who achieved undetectable plasma HBV DNA load at week 48, and the secondary end-point was the proportion of patients who had undetectable plasma HBV DNA load at week 96. The study was approved by the Research Ethics Committee of NTUH (registration number NTUH-201201028RIB). All patients gave written informed consent before enrollment to provide their blood samples and clinical and laboratory data for research.
Data collection and definitions
We used a standardized case record form to collect the information on the patients’ demographics, comorbidity, treatment history of cART, abdominal ultrasonography, and laboratory data. The baseline values of plasma HBV DNA load, resistance mutations of HBV, serologies of HBV and HCV, aminotransferase levels, serum creatinine, serum alpha-fetoprotein, CD4 lymphocyte count, and plasma HIV RNA load were obtained within 3 months before initiation of TDF-containing cART. After starting TDF-containing cART, serial blood samples were collected from patients at weeks 4, 8, 12, 24, 36, and 48 and then every 48 weeks subsequently. Serial determinations were performed for plasma HBV DNA load, HBV serologic markers, alpha-fetoprotein, and liver and renal functions. The numbers of patients with HBeAg seroconversion, loss of HBV surface antigen (HBsAg), and HBsAg seroconversion were documented. The estimated glomerular filtration rate (eGFR) was calculated by the modification of diet in renal disease (MDRD) equation.
Undetectable plasma HBV DNA load was defined as <128 copies/mL. Chronic HBV coinfection was defined as the persistence of HBsAg for >6 months, and hepatitis C coinfection was defined by positive anti-HCV antibody. Hepatitis flare was defined as >5-fold elevation of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (upper limit of normal for AST and ALT, 36 and 41 IU/L, respectively); and hyperbilirubinemia as a total bilirubin >2.0 mg/dL without evidence of hemolysis or taking atazanavir-containing cART. We used AST-to-platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. The APRI was determined as follows: [(AST/upper limit of normal of AST)/platelet count (109/L)] x 100. Parenchymal liver disease or cirrhosis was documented by the presence of coarse echogenicity and irregular liver surface as demonstrated by abdominal ultrasonography.
Laboratory investigations
Determinations of plasma HBV DNA load and HBV serologic markers
Plasma HBV DNA load was quantified using the Abbott Real Time HBV assay (Abbott Laboratories, Abbott Park, IL, U.S.A) with a lower detection limits of 15 IU/mL after 2.5-fold dilution of serum samples, and the results were stated as 1 IU/mL = 3.41 copies/mL. HBeAg, anti-HBe antibody, HBsAg, anti-HBs antibody, and antibodies to HCV were determined using enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, U.S.A). Quantification of HBsAg levels was determined by ARCHITECT il000 chemiluminescence microparticle immunoassay (Abbott Laboratories, Abbott Park, IL, U.S.A).
HBV genotyping and detection of lamivudine-resistant mutations
A nested PCR was performed to amplify part of the polymerase gene containing the tyrosine-methionine-aspartate-aspartate (YMDD) motif from patients with detectable HBV DNA. The expected size of PCR product is 1.2kb. The first and second primer pairs used are 1821F (5'-TTT TTC CCC TCT GCC TAA TCA-3')/1825R (5'-AAA AAG TTG CAT GGT GCT GG-3') and 3106F (5'- ACA CTG CCA GCA GCA CCT CCT CC -3')/1088R (5'-AGC CTG CTT AGA TTG TAT ACA TGC-3'). The amplification condition was 35 cycles of 94°C for 30s, 56°C for 30s, 72°C for 2 min, and a final extension at 72°C for 7 min. A 5-μL aliquot of the first round PCR product was used for the second round PCR, whose condition was the same as the first round. Sequencing analysis was performed with an automatic ABI-DNA sequencer (Model 377 A; Applied Biosystems). The HBV genotype was determined by constructing the phylogenetic trees using the neighbor-joining method and the Kimura 2-parameter distance matrix listed in the MEGA (molecular evolutionary genetics analysis) analytical package [20]. The presence of the YMDD mutant (rt pol gene mutations rtM204V plus rtM204I) and/or rtL180M was confirmed by sequencing readout.
Determinations of plasma HIV RNA load CD4 lymphocyte count
Plasma HIV RNA load was quantified using the Cobas AmpliPrep/Cobas TaqMan HIV-1 test (version 2.0, Roche Molecular Systems, Inc.). CD4 lymphocyte count was determined using flow cytometry (BD FACS Calibur, Becton Dickinson and Coulter Epics XL, Beckman Coulter, CA, USA).
Statistical analysis
Patients’ demographics and basic characteristics were evaluated by descriptive statistics. Categorical variables were compared using chi-square test or Fisher’s exact test. Continuous variables were compared using the Kruskal-Wallis one-way analysis of variance or Mann-Whitney U test. For data from correlated-samples, variables were compared using Wilcoxon signed rank test. A two-tailed p value <0.05 was considered statistically significant. Univariate and multivariate logistic regression model was used to assess factors associated with failure to achieve viral suppression after 48 weeks of TDF-containing cART treatment. Stepwise model selection with Akaike Information Criterion (AIC) was performed, and variables were entered into the model with p value <0.25 as a requirement for acceptance. All statistical analyses were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of patients
During the 4-year study period, a total of 89 HIV/HBV-coinfected patients were included for analysis. Among them, 33 patients had coinfection with lamivudine-resistant HBV, and 56 patients had lamivudine-susceptible HBV. The baseline characteristics of the patients are shown in Table 1. The majority of patients were middle-aged men who have sex with men. Genotype B was the dominate HBV subtype in both groups, followed by genotype C. Patients with lamivudine-resistant HBV had been exposed to cART containing only lamivudine for HBV for an average of 6.5 years before enrollment, and all patients with lamivudine-susceptible HBV were cART-naïve, except one who had interrupted cART (15 months of abacavir/lamivudine plus atazanavir) for 4 years before enrollment.
Table 1. Clinical characteristics of 89 HIV-positive patients coinfected with lamivudine-resistant or lamivudine-susceptible hepatitis B virus.
| Patients with LAM-R HBV (n = 33) | Patients with LAM-S HBV (n = 56) | P value | |
|---|---|---|---|
| Age, years | 42 ± 8 | 36 ± 8 | 0.001 |
| Male sex | 33 (100) | 55 (98.2) | 0.999 |
| Years since HIV diagnosis | 12.8 ± 4.7 | 5.2 ± 2.9 | <0.001 |
| HBV genotype | |||
| B | 28/33 (84.8) | 37/43 (86.0) | 0.999 |
| C | 5/33 (15.2) | 6/43 (14.0) | |
| No data | 0 | 13 | |
| Previous LAM use, years | 6.5 ± 3.9 | NA | |
| Positive HBeAg at baseline | 16/33 (48.5) | 18/55 (32.7) | 0.142 |
| HBsAg level at baseline, log10 IU/mL | 5.3 ± 2.1 (n = 33) | 3.5 ± 1.0 (n = 38) | <0.001 |
| Plasma HBV DNA level at enrollment, log10 copies/mL | 6.1 ± 2.2 | 6.0 ± 2.2 | 0.895 |
| 3–5 log10 copies/mL | 14 (42.4) | 23 (41.1) | |
| >5 log10 copies/mL | 19 (57.6) | 33 (58.9) | |
| Hepatitis flares within the preceding one year of enrollment | 7 (21.2) | NA | |
| ALT at baseline, IU/L | 56 ± 55 | 52 ± 48 | 0.383 |
| APRI score at baseline | 0.5 ± 0.4 | 0.9 ± 1.4 | 0.099 |
| Cirrhosis or parenchymal liver disease at baseline | 10/31 (32.2) | 10/39 (25.6) | 0.543 |
| Chronic HCV infection at baseline | 0 (0) | 2 (3.6) | |
| CD4 cell count at baseline, cells/μl | 552 ± 382 | 249 ± 220 | <0.001 |
| Plasma HIV RNA load at baseline, log10 copies/mL, | 1.7 ± 0.6 | 4.9 ± 0.6 | <0.001 |
| Plasma HIV RNA load <200 copies/mL at baseline | 31 (93.9) | 0 (0) | |
| NRTI backbone before tenofovir and lamivudine | |||
| Zidovudine/lamivudine | 10 (30.3) | NA | |
| Abacavir/lamivudine | 22 (66.7) | NA | |
| Didanosine and lamivudine | 1 (3) | NA | |
| NNRTI-based cART | 15 (45.5) | 39 (69.6) | 0.024 |
| PI-based cART | 18 (54.5) | 14 (25) | 0.005 |
| II-based cART | 0 (0) | 3 (5.4) | |
| Follow-up duration, weeks, | 202 ± 58 | 147 ± 54 | <0.001 |
Results are n (%), or mean ± standard deviation.
Abbreviations: ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; cART, combination antiretroviral therapy; HBV, hepatitis B virus; HBeAg, HBV envelope antigen; HBsAg, HBV surface antigen; HCV, hepatitis C virus; II, integrase inhibitor; LAM, lamivudine; LAM-R, LAM-resistant; LAM-S, LAM-susceptible; NA, not applicable; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleos(t)ide reverse transcriptase inhibitors; PI, protease inhibitor.
While the two groups of patients had similar mean HBV DNA loads (6.1 ± 2.2 vs. 6.0 ± 2.2 log10 copies/mL, p = 0.895) and HBeAg-positive rates (48.5% vs. 32.7% in patients with available data, p = 0.142) at baseline, the two groups differed significantly in many clinical characteristics because the great majority of patients in the lamivudine-susceptible group were antiretroviral-naïve. Compared with patients in the lamivudine-susceptible group, patients in the lamivudine-resistant group had a higher mean age (p = 0.001), HBsAg titer (p<0.001), and CD4 cell count (p<0.001), a lower mean plasma HIV RNA load (p<0.001) and higher proportion of patients with baseline HIV RNA load <200 copies/mL (lamivudine-resistant vs. lamivudine-susceptible, 93.9% vs. 0%). Patients with HBeAg positivity had higher baseline and serial HBV DNA compared with those with HBeAg negativity (S1 Table).
Virological response of HBV to tenofovir-containing antiretroviral therapy
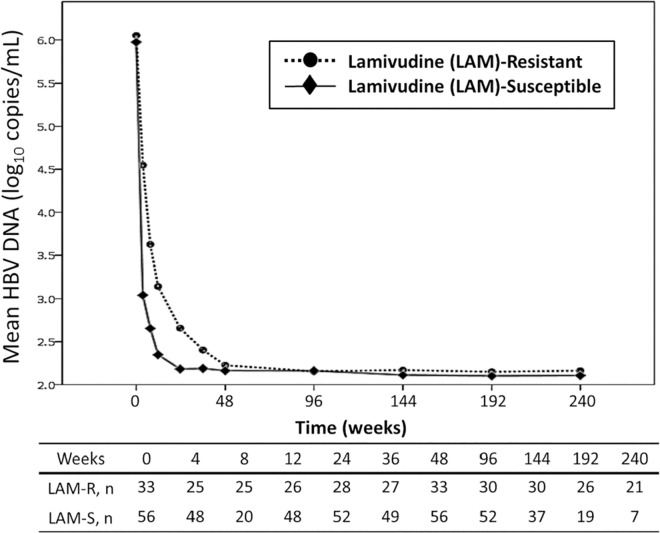
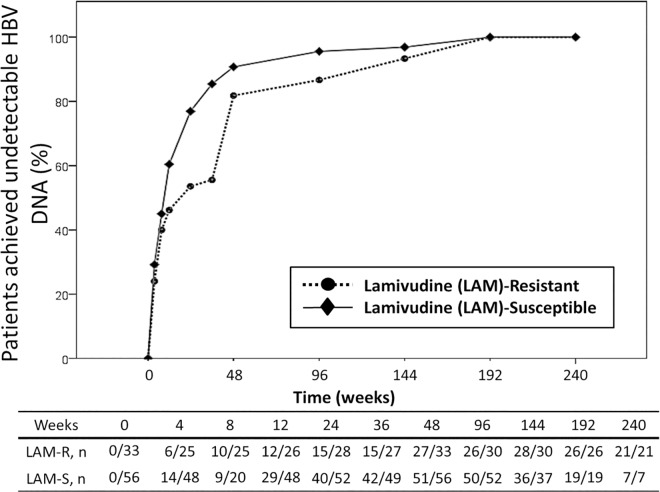
The average follow-up duration of the two groups of patients was 167 weeks. Fig 1 shows the mean changes of plasma HBV DNA load from baseline over time. The mean changes of plasma HBV DNA load from baseline were -2.2, -2.9, -2.8, -3.7, -4.0, -3.8, and -4.0 log10 copies/mL in patients with lamivudine-resistant HBV, and -2.5, -3.4, -3.2, -3.6, -3.9, -3.8, and -3.9 log10 copies/mL in patients with lamivudine-susceptible HBV at weeks 4, 8, 12, 24, 36, 48 and 96, respectively. The mean plasma HBV DNA loads of the two groups were similar at 48 weeks and 96 weeks (p = 0.169 and p = 0.351, respectively) (S2 Table). The cumulative percentage of patients who achieved undetectable plasma HBV viral load (<128 copies/mL) in patients with lamivudine-resistant HBV and those with lamivudine-susceptible HBV was 81.8% (27 of 33 patients) and 91.1% (51 of 56 patients) at 48 weeks, respectively (p = 0.317), which increased to 86.7% (26 of 30 patients) and 96.2% (50 of 52 patients) at 96 weeks, respectively (p = 0.185) (Fig 2). At 48 weeks, 11 patients did not achieve undetectable HBV DNA load. No resistance mutations to TDF were found in these patients, however. The mean CD4 count of all patients was 499 ± 503 cells/μl at week 48 and 521 ± 269 cells/μl at week 96. The mean plasma HIV RNA load of all patients was 1.6 ± 0.3 log10 copies/mL at week 48 and 1.5 ± 0.1 log10 copies/mL at week 96.
Fig 1. Changes of plasma HBV DNA load in HIV/HBV-coinfected patients with lamivudine-resistant HBV (n = 33) or lamivudine-susceptible HBV (n = 56) who were on tenofovir-containing combination antiretroviral therapy.
Fig 2. Cumulative percentage of HIV/HBV-coinfected patients with lamivudine-resistant HBV (n = 33) or lamivudine-susceptible HBV (n = 56) who had achieved undetectable HBV DNA (<128 copies/mL) during the follow-up period of tenofovir-containing combination antiretroviral therapy.
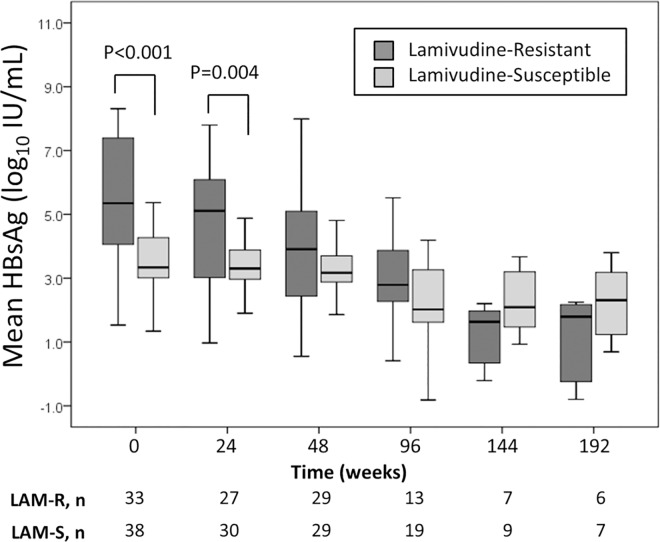
The changes of HBsAg levels with time are presented in Fig 3. Declines of mean HBsAg levels were observed in both groups of patients during the first 48 weeks of TDF-containing cART. Compared with patients in the lamivudine-resistant group, patients in the lamivudine-susceptible group had lower HBsAg levels at baseline (p<0.001) and at 48 weeks (p = 0.070), though the difference at week 48 did not reach statistical significance.
Fig 3. Changes of HBsAg levels in HIV/HBV-coinfected patients with lamivudine-resistant HBV or lamivudine-susceptible HBV on tenofovir-containing combination antiretroviral therapy.
The changes of plasma HBV DNA load and HBsAg levels with treatment with TDF plus emtricitabine or lamivudine in patients with HBeAg positivity and those with HBeAg negativity are shown in S3 Table and S1–S3 Figs.
Loss of HBsAg was observed in 3 (3.4%) patients at 96 weeks (n = 2) and 144 weeks (n = 1), and one of them had HBsAg seroconversion. All of these 3 patients were in the lamivudine-susceptible group. Another one patient in the lamivudine-susceptible group was found to have both positive HBsAg and anti-HBs at 144 weeks. At 48 weeks, HBeAg seroconversion was observed in 1 of 16 (6.3%) patients in the lamivudine-resistant group, and 4 of 16 (25%) patents in the lamivudine-susceptible group (p = 0.166).
Factors associated with failure to achieve viral suppression at 48 weeks
Overall, 78 (87.6%) patients had achieved undetectable HBV DNA load before 48 weeks. All 11 patients who failed to achieve undetectable HBV DNA load at 48 weeks had positive HBeAg at baseline, compared with only 23 (29.9%) in 77 patients that had successful HBV viral suppression (1 patients did not have HBeAg data at baseline). In univariate analysis, the factors associated with failure to achieve viral suppression at 48 weeks were higher HBV DNA and HBsAg levels at baseline (p = 0.004 and p = 0.009, respectively; Table 2). In multivariate analysis, the only independent factor associated with failure to achieve viral suppression at 48 weeks was higher plasma HBV DNA load at baseline (adjusted odds ratio, per 1-log10 copies/mL increase, 1.861; 95% CI, 1.204–2.878; p = 0.005).
Table 2. Multivariate logistic analysis to identify the factors associated with failure to achieve HBV viral suppression (<128 copies/ mL) after 48 weeks of tenofovir-containing antiretroviral therapy.
| Variablea | Achieve viral suppression (n = 78) | Failure to achieve viral suppression (n = 11) | Logistic Regression | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | Univariate p = | OR (95% CI) | Multivariate p = | |||
| Age, years | 39 ± 8 | 34 ± 7 | 0.931 (0.853–1.016) | 0.107 | 0.900 (0.806–1.003) | 0.058 |
| HBV genotype B | 56/65 (86.2) | 9/11 (81.8) | 0.723 (0.134–3.904) | 0.706 | ||
| Lamivudine resistance of HBV | 27 (34.6) | 6 (54.5) | 2.267 (0.633–8.113) | 0.208 | 4.429 (0.894–21.946) | 0.068 |
| CD4 count at baseline, cells/μl | 360 ± 334 | 375 ± 251 | 1.000 (0.998–1.002) | 0.884 | ||
| Plasma HIV RNA load at baseline, log10 copies/mL | 3.8 ± 1.6 | 3.1 ± 1.6 | 0.770 (0.521–1.139) | 0.191 | ||
| Plasma HBV DNA load at baseline, log10 copies/mL | 5.7 ± 2.1 | 8.1 ± 1.3 | 1.875 (1.223–2.874) | 0.004 | 1.861 (1.204–2.878) | 0.005 |
| HBsAg level at baseline, log10 IU/mL | 4.1 ± 1.8 (n = 61) | 5.8 ± 1.5 (n = 10) | 1.680 (1.141–2.474) | 0.009 | ||
| Positive HBeAg at baselineb | 23/77 (29.9) | 11/11 (100) | ||||
Results are n (%), or mean ± standard deviation.
Abbreviations: HBV, hepatitis B virus; HBeAg, HBV envelope antigen; HBsAg, HBV surface antigen; CI, confidence interval; OR, odds ratio.
aVariables considered for entry into multivariate logistic regression model included variables with P values <0.25 in univariate analysis.
b All patients failing to achieve viral suppression had positive HBeAg at baseline and, therefore, the factor was not included in the logistic regression.
Adverse events on TDF-containing treatment and patient outcomes
The mean baseline eGFR of patients with lamivudine-resistant HBV and those with lamivudine-susceptible HBV were 97 ml/min/1.73m2 and 110 ml/min/1.73m2, respectively, and 98 ml/min/1.73m2 and 102 ml/min/1.73m2 at week 48, respectively. The serial data of eGFR are shown in S4 Table. During the study period, one patient in the lamivudine-resistant group discontinued TDF because her eGFR decreased from 37 ml/min/1.73m2 at baseline to 12 ml/min/1.73m2 at week 36. After TDF was discontinued, renal function improved and the patient did not have hepatitis flares until study ended. No other TDF-related severe adverse events were documented. Two patients experienced acute hepatitis resulting from acute HCV infection and trimethoprim/sulfamethoxazole-associated hepatotoxicity, respectively. Seven patients were lost to follow-up after one year, one patient died from lung cancer, and the remaining patients continued their HIV care regularly. With TDF-containing cART, the ALT levels and APRI scores were significantly lower at week 48 (ALT, p<0.001; APRI, p = 0.007) and week 96 (ALT, p = 0.001; APRI, p<0.001) compared with the baseline data (S5 Table).
Discussion
This study demonstrates that virological response of HBV to subsequent add-on TDF to replace one NRTI of current cART in HIV/HBV-coinfected patients with emergence of lamivudine-resistant HBV was similar to that of initiation of TDF-containing cART as the first regimen in patients without lamivudine-resistant HBV. Among HIV/HBV-coinfected patients, the factors associated with failure to achieve HBV viral suppression at 48 weeks were a higher plasma HBV DNA load and HBeAg positivity prior to TDF-containing cART. During study period, HBsAg levels decreased over time, regardless of the presence or absence of lamivudine resistance.
To prevent complications of chronic HBV infection, the current goal of HBV therapy is to achieve sustained suppression of viral replication to the lowest detectable level. In antiretroviral-naïve HIV/HBV-coinfected patients, TDF/lamivudine- or TDF/emtricitabine-based regimens are recommended as first-line cART [21]. Dual therapy with TDF/lamivudine or TDF/emtricitabine against HBV not only prevents the emergence of lamivudine resistance [22], but also suppresses HBV replication more effectively than lamivudine monotherapy [15, 23, 24]. However, studies are limited that explore the activity of add-on TDF on HBV replication in HIV/HBV-coinfected patients who fail previous lamivudine monotherapy for HBV [16, 17, 25, 26], including those with advanced liver disease [27]. In 2006, Schmutz et al. published a 1:2 matched pair analysis comparing the antiviral efficacy of first-line treatment with TDF and lamivudine to sequential TDF after the development of HBV-DNA relapse (>105 copies/mL) while on lamivudine monotherapy [16]. They concluded that the proportions of patients with undetectable plasma HBV DNA load at 12, 48 or 96 weeks and loss of HBeAg and HBsAg were similar between the two study arms. However, not every patient in the study was assessed for lamivudine resistance before switching to add-on TDF. A later study investigating the long-term efficacy of TDF showed no difference of virological response (HBV DNA <20 IU/mL) up to 60 months between HIV/HBV-coinfected patients with or without lamivudine resistance at baseline [28].
In line with those previous reports, we found that similar proportions of patients who achieved undetectable HBV DNA load at 48 weeks and 96 weeks of treatment between the patients with and those without lamivudine-resistant HBV. While more studies of long-term clinical and virological outcomes of the two groups of patients are warranted, the findings support the use of TDF/lamivudine- or TDF/emtricitabine-based therapy in HIV/HBV-coinfected patients who develop lamivudine resistance of HBV. For HIV/HBV-coinfected patients currently on lamivudine monotherapy for HBV with good suppression of HBV replication, whether it is more beneficial to initiate TDF-based cART immediately or to continue lamivudine monotherapy for HBV with careful HBV load monitoring remains uncertain.
In our study, a higher HBV DNA load and HBeAg positivity at baseline were associated with failure to achieve HBV suppression at 48 weeks. These two factors have also been shown to influence time to accomplish virological response in TDF-experienced, HBV mono-infected patients with lamivudine failure [29]. As in the meta-analysis [19], we did not find the association between lamivudine exposure and delayed HBV suppression. Another study from Kosi et al [15] that compared the effect of different anti-HBV regimens contained in cART (lamivudine monotherapy, simultaneous TDF/lamivudine, and lamivudine followed by TDF/lamivudine) revealed no correlation between anti-HBV treatment options and HBV viral suppression. Kosi et al described that HBV genotype non-A, detectable HIV viremia at 1 year, lower CD4 count, and reporting <95% adherence were significant risk factors for HBV virological non-response [15]. Our patients in this study were only infected with HBV genotypes B and C, which were the dominant genotypes among Taiwanese patients [30], and no difference in HBV suppression was found between patients with either genotype. In contrast, plasma HIV RNA load and CD4 count prior to TDF therapy had no impact on HBV viral suppression in our study.
Along with HBV virological suppression, HBeAg seroconversion and HBsAg loss are also important therapeutic goals in HBV treatment. In therapeutic trials of lamivudine- or TDF-containing cART, HBeAg seroconversion rates among HIV/HBV-coinfected patients ranged from 17 to 46% after 2 to 5 years of cART [28, 31–33]. In our study, 5 of 32 (15.6%) patients with available data had HBeAg seroconversion at week 48. Although no statistical significance was demonstrated due the small sample size, a higher HBeAg seroconversion rate was observed in the lamivudine-susceptible group than the lamivudine-resistant group (25% vs. 6.3%, p = 0.166).
HBsAg loss was generally uncommon in HIV/HBV-coinfected patients. An annual HBsAg seroconversion rate of 2.6% in HIV/HBV-coinfected patients has been reported [34]. In our cohort, we found that 3.4% of patients experienced HBsAg loss. The study by Matthews et al demonstrated a higher rate (13%) of HBsAg loss over a median follow-up of 108 weeks [35]. The author suggested that the higher rate of HBsAg loss might result from immune restoration, represented by marked CD4 count elevation and sustained HIV suppression in their study. In HIV/HBV-coinfected populations, studies exploring quantitative HBsAg kinetics were still limited. Declines in quantitative HBsAg had been shown to predict HBsAg seroclearance [32, 36]. Our study demonstrated decreasing trends of quantitative HBsAg up to 96 weeks in response to TDF-containing cART. However, the number of patients with HBsAg loss is too small in our cohort for further investigation into the correlation between HBsAg titer and HBsAg seroclearance.
Our study has several limitations. First, the sample size is small and, therefore, we were not able to demonstrate a statistically significant difference of virological response rates of lamivudine-resistant and lamivudine-susceptible HBV to TDF-containing cART between the two groups. With introduction of TDF for HBV or HIV infection, HIV/HBV-coinfected patients start cART containing TDF/lamivudine or TDF/emtricitabine according to the HIV treatment guidelines [21]; it is therefore that resistance of HBV to lamivudine have become rare for such comparisons to conduct. Second, the study was not a randomized clinical trial and the two groups of patients with significant differences in several baseline characteristics were included in routine clinical care in different time periods when cART has evolved significantly. Third, we did not evaluate the adherence of patients. The failure of HBV viral suppression may result from non-adherence to cART. However, all 11 patients who failed to achieve HBV suppression at 48 weeks in our study had plasma HIV RNA loads ranging from undetectable to 90 copies/mL, suggesting good adherence to cART.
In conclusion, add-on TDF-containing cART in HIV/HBV-coinfected patients with lamivudine-resistant HBV achieved a similar rate of HBV viral suppression compared to initiation of TDF-containing cART in HIV/HBV-coinfected patients with lamivudine-susceptible HBV as the frontline regimen for both HIV and HBV. A higher plasma HBV DNA load and HBeAg positivity at baseline were associated with failure to achieve HBV viral suppression at week 48 of TDF-containing cART. For HIV/HBV-coinfected patients failing lamivudine, TDF combined with lamivudine or emtricitabine could serve as an effective and well-tolerated therapy against HBV.
Supporting Information
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We would like to thank the patients for participation in this study and Taiwan Centers for Disease Control for the research grant support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World journal of gastroenterology. 2014;20(40):14598–614. 10.3748/wjg.v20.i40.14598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun HY, Ko WC, Tsai JJ, Lee HC, Liu CE, Wong WW, et al. Seroprevalence of chronic hepatitis B virus infection among taiwanese human immunodeficiency virus type 1-positive persons in the era of nationwide hepatitis B vaccination. The American journal of gastroenterology. 2009;104(4):877–84. 10.1038/ajg.2008.159 [DOI] [PubMed] [Google Scholar]
- 3.Sun HY, Cheng CY, Lee NY, Yang CJ, Liang SH, Tsai MS, et al. Seroprevalence of hepatitis B virus among adults at high risk for HIV transmission two decades after implementation of nationwide hepatitis B virus vaccination program in Taiwan. PloS one. 2014;9(2):e90194 10.1371/journal.pone.0090194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thio CL, Seaberg EC, Skolasky R Jr., Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921–6. [DOI] [PubMed] [Google Scholar]
- 5.Sheng WH, Chen MY, Hsieh SM, Hsiao CF, Wang JT, Hung CC, et al. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;38(10):1471–7. [DOI] [PubMed] [Google Scholar]
- 6.van Griensven J, Phirum L, Choun K, Thai S, De Weggheleire A, Lynen L. Hepatitis B and C co-infection among HIV-infected adults while on antiretroviral treatment: long-term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PloS one. 2014;9(2):e88552 10.1371/journal.pone.0088552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolopoulos GK, Paraskevis D, Psichogiou M, Hatzakis A. HBV-DNA levels predict overall mortality in HIV/HBV coinfected individuals. Journal of medical virology. 2015. [DOI] [PubMed] [Google Scholar]
- 8.Brahmania M, Feld J, Arif A, Janssen HL. New therapeutic agents for chronic hepatitis B. The Lancet Infectious diseases. 2016;16(2):e10–21. 10.1016/S1473-3099(15)00436-3 [DOI] [PubMed] [Google Scholar]
- 9.Wolters LM, Niesters HG, Hansen BE, van der Ende ME, Kroon FP, Richter C, et al. Development of hepatitis B virus resistance for lamivudine in chronic hepatitis B patients co-infected with the human immunodeficiency virus in a Dutch cohort. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2002;24(3):173–81. [DOI] [PubMed] [Google Scholar]
- 10.Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. Journal of gastroenterology and hepatology. 2004;19(11):1276–82. 10.1111/j.1440-1746.2004.03428.x [DOI] [PubMed] [Google Scholar]
- 11.Gu L, Han Y, Li Y, Zhu T, Song X, Huang Y, et al. Emergence of Lamivudine-Resistant HBV during Antiretroviral Therapy Including Lamivudine for Patients Coinfected with HIV and HBV in China. PloS one. 2015;10(8):e0134539 10.1371/journal.pone.0134539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146(4):980–8. 10.1053/j.gastro.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Fung S, Gane EJ, Dinh P, Flaherty JF, Svarovskaia ES, et al. Evaluation of HBV DNA decay kinetics in patients containing both rtM204V/I mutant and wild-type HBV subpopulations during tenofovir DF (TDF) monotherapy or combination therapy with emtricitabine (FTC)/TDF. Journal of medical virology. 2014;86(9):1473–81. 10.1002/jmv.23982 [DOI] [PubMed] [Google Scholar]
- 14.Wang HL, Lu X, Yang X, Ning Q. Efficacy of tenofovir-based rescue therapy in patients with lamivudine-resistant hepatitis B virus: A systematic review and meta-analysis. Clinics and research in hepatology and gastroenterology. 2015. [DOI] [PubMed] [Google Scholar]
- 15.Kosi L, Reiberger T, Payer BA, Grabmeier-Pfistershammer K, Strassl R, Rieger A, et al. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. Journal of viral hepatitis. 2012;19(11):801–10. 10.1111/j.1365-2893.2012.01601.x [DOI] [PubMed] [Google Scholar]
- 16.Schmutz G, Nelson M, Lutz T, Sheldon J, Bruno R, von Boemmel F, et al. Combination of tenofovir and lamivudine versus tenofovir after lamivudine failure for therapy of hepatitis B in HIV-coinfection. Aids. 2006;20(15):1951–4. 10.1097/01.aids.0000247116.89455.5d [DOI] [PubMed] [Google Scholar]
- 17.Plaza Z, Aguilera A, Mena A, Vispo E, Sierra-Enguita R, Tome S, et al. Influence of HIV infection on response to tenofovir in patients with chronic hepatitis B. Aids. 2013;27(14):2219–24. 10.1097/QAD.0b013e328362fe42 [DOI] [PubMed] [Google Scholar]
- 18.Kim HN, Rodriguez CV, Van Rompaey S, Eron JJ, Thio CL, Crane HM, et al. Factors associated with delayed hepatitis B viral suppression on tenofovir among patients coinfected with HBV-HIV in the CNICS cohort. Journal of acquired immune deficiency syndromes. 2014;66(1):96–101. 10.1097/QAI.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, et al. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PloS one. 2013;8(7):e68152 10.1371/journal.pone.0068152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17(12):1244–5. [DOI] [PubMed] [Google Scholar]
- 21.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed September 14, 2016. [Google Scholar]
- 22.Hamers RL, Zaaijer HL, Wallis CL, Siwale M, Ive P, Botes ME, et al. HIV-HBV coinfection in Southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. Journal of acquired immune deficiency syndromes. 2013;64(2):174–82. 10.1097/QAI.0b013e3182a60f7d [DOI] [PubMed] [Google Scholar]
- 23.Khamduang W, Gaudy-Graffin C, Ngo-Giang-Huong N, Jourdain G, Moreau A, Luekamlung N, et al. Long-term hepatitis B virus (HBV) response to lamivudine-containing highly active antiretroviral therapy in HIV-HBV co-infected patients in Thailand. PloS one. 2012;7(7):e42184 10.1371/journal.pone.0042184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews GV, Seaberg EC, Avihingsanon A, Bowden S, Dore GJ, Lewin SR, et al. Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56(9):e87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez M, Perez-Olmeda M, Diaz B, Rios P, Gonzalez-Lahoz J, Soriano V. Activity of tenofovir on hepatitis B virus replication in HIV-co-infected patients failing or partially responding to lamivudine. Aids. 2002;16(17):2352–4. [DOI] [PubMed] [Google Scholar]
- 26.Ristig MB, Crippin J, Aberg JA, Powderly WG, Lisker-Melman M, Kessels L, et al. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. The Journal of infectious diseases. 2002;186(12):1844–7. 10.1086/345770 [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez S, Guillemi S, Jahnke N, Montessori V, Harrigan PR, Montaner JS. Tenofovir-based rescue therapy for advanced liver disease in 6 patients coinfected with HIV and hepatitis B virus and receiving lamivudine. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46(3):e28–30. [DOI] [PubMed] [Google Scholar]
- 28.de Vries-Sluijs TE, Reijnders JG, Hansen BE, Zaaijer HL, Prins JM, Pas SD, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010;139(6):1934–41. 10.1053/j.gastro.2010.08.045 [DOI] [PubMed] [Google Scholar]
- 29.Baran B, Soyer OM, Ormeci AC, Gokturk S, Evirgen S, Bozbey HU, et al. Efficacy of tenofovir in patients with Lamivudine failure is not different from that in nucleoside/nucleotide analogue-naive patients with chronic hepatitis B. Antimicrobial agents and chemotherapy. 2013;57(4):1790–6. 10.1128/AAC.02600-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng WH, Hung CC, Chang SY, Liu CJ, Chen MY, Hsieh SM, et al. Differential clinical and virologic impact of hepatitis B virus genotypes B and C on HIV-coinfected patients receiving lamivudine-containing highly active antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(4):548–55. [DOI] [PubMed] [Google Scholar]
- 31.Miailhes P, Trabaud MA, Pradat P, Lebouche B, Chevallier M, Chevallier P, et al. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;45(5):624–32. [DOI] [PubMed] [Google Scholar]
- 32.Boyd A, Maylin S, Gozlan J, Delaugerre C, Simon F, Girard PM, et al. Use of hepatitis B surface and "e" antigen quantification during extensive treatment with tenofovir in patients co-infected with HIV-HBV. Liver international: official journal of the International Association for the Study of the Liver. 2015;35(3):795–804. [DOI] [PubMed] [Google Scholar]
- 33.Boyd A, Maylin S, Moh R, Mahjoub N, Gabillard D, Eholie SP, et al. Hepatitis B surface antigen quantification as a predictor of seroclearance during treatment in HIV-hepatitis B virus co-infected patients from Sub-Saharan Africa. Journal of gastroenterology and hepatology. 2015. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, Gonzalez-Lahoz J, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. Aids. 2011;25(1):73–9. 10.1097/QAD.0b013e328340fde2 [DOI] [PubMed] [Google Scholar]
- 35.Matthews GV, Ali RJ, Avihingsanon A, Amin J, Hammond R, Bowden S, et al. Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals. PloS one. 2013;8(4):e61297 10.1371/journal.pone.0061297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, Reijnders JG, Mulder JW, Kroon FP, et al. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. The Journal of infectious diseases. 2012;206(6):974–80. 10.1093/infdis/jis439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.