Abstract
We previously reported a phase II study of a cancer vaccine using five novel peptides recognized by HLA‐A*2402‐restricted CTL in combination with oxaliplatin‐containing chemotherapy (FXV study) as first‐line therapy for patients with metastatic colorectal cancer and demonstrated the safety and promising potential of our five‐peptide cocktail. The objective of this analysis was to identify predictive biomarkers for identifying patients who are likely to receive a clinical benefit from immunochemotherapy. Circulating cell‐free DNA (cfDNA) in plasma has been reported to be a candidate molecular biomarker for the efficacy of anticancer therapy. Unlike uniformly truncated small‐sized DNA released from apoptotic normal cells, DNA released from necrotic cancer cells varies in size. The integrity of plasma cfDNA (i.e. the ratio of longer fragments [400 bp] to shorter fragments [100 bp] of cfDNA), may be clinically useful for detecting colorectal cancer progression. We assessed plasma samples collected from 93 patients prior to receiving immunochemotherapy. The cfDNA levels and integrity were analyzed by semi‐quantitative real‐time PCR. Progression‐free survival was significantly better in patients with a low plasma cfDNA integrity value than in those with a high value (P = 0.0027). Surprisingly, in the HLA‐A*2402‐matched group, patients with a low plasma cfDNA integrity value had significantly better progression‐free survival than those with a high value (P = 0.0015). This difference was not observed in the HLA‐A*2402‐unmatched group. In conclusion, the integrity of plasma cfDNA may provide important clinical information and may be a useful predictive biomarker of the outcome of immunotherapy in metastatic colorectal cancer.
Keywords: Biomarker, cell‐free DNA, colorectal cancer, DNA integrity, immunotherapy
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer‐related deaths in industrialized countries.1 Despite the availability of several effective cytotoxic drugs and new biological agents, the prognosis of metastatic colorectal cancer (mCRC) remains poor. The majority of patients are offered several lines of palliative chemotherapy, but these can expose patients to further side effects; as such, careful selection of patients and monitoring during therapy are essential.2, 3, 4, 5, 6
In an attempt to validate a new treatment modality to overcome the limited disease control of mCRC, we conducted a phase I vaccine trial7 that confirmed the safety of a cocktail containing three peptides derived from oncoantigens (i.e. ring finger protein 43 [RNF43],8 34‐kDa translocase of the outer mitochondrial membrane [TOMM34]9 and IGF‐II mRNA binding protein 3 [KOC1, also known as IMP‐3],10 and two peptides targeting vascular endothelial growth factor receptor 1 (VEGFR1)11 and VEGFR212 for mCRC. We further conducted a phase II trial of a combination therapy with standard chemotherapy and five novel therapeutic peptides.13 However, because patients with very advanced‐stage disease and who have a very poor immune status are also usually allowed to enroll into clinical studies during the early phase of drug development, it is very difficult to evaluate the survival benefit of treatment.14 Hence, there is a desperate need for predictive biomarkers that allow the selection of suitable patients who are likely to respond well to the treatment. Better selection criteria in terms of predictive and prognostic markers to optimize treatment are needed.15
The level of circulating cell‐free DNA (cfDNA) and the integrity of the cfDNA, which is defined as the ratio of long‐base pair (bp) cfDNA/short‐bp cfDNA, have been shown to be promising diagnostic biomarkers of colorectal and breast cancers.16, 17, 18, 19 Circulating cfDNA in the blood has attracted a great deal of attention as an easy‐to‐use tool for the evaluation of the malignant potential of various cancers.20, 21, 22 Although circulating cfDNA is also present in healthy individuals, it has been implicated as a strong diagnostic and prognostic marker of malignancy. Its limitations are related to the contradictory findings reported regarding the proportion of tumor‐derived and non‐tumor‐derived cfDNA. Many different hypotheses concerning the origin of the circulating cfDNA have been considered, including active liberation by the tumor itself, the events of necrosis, apoptosis, mitotic catastrophe, autophagia, rupture of tumor cells, or circulation of micrometastases.23 Among the various concepts of DNA liberation, most studies seem to be in accord with the notion that it occurs as a result of apoptosis or necrosis.24 These two phenomena may be distinguished by the dimensions of the DNA fragments: the apoptotic death of cells causes the release of DNA fragments shorter than 200 bp into the circulation, whereas tumor necrosis is characterized by the presence of fragments that vary in size and are generally > 200 bp.25 Hence, high cfDNA integrity may indicate a high degree of tumor cell collapse. In contrast, it might be better to show the data of circulating tumor DNA which express the tumor‐related mutation. There are some major mutations in colorectal cancer, for example, APC, TP53, KRAS and BRAF. However, there is no common genetic mutation that is detected in all colorectal cancer.26 Hence, in the current study, we investigated cfDNA integrity to determine the proportion of cfDNA from the tumors.
The aim of this study was to investigate whether cfDNA integrity may be a biomarker for response to immunochemotherapy in patients with mCRC.
Materials and Methods
Study design
The detailed protocol of this study was described previously.13 Briefly, we conducted a phase II trial that was a non‐randomized, HLA‐A status double‐blinded study using five peptides recognized by HLA‐A*2402‐restricted CTL: RNF43, TOMM34, KOC1 (IMP‐3), VEGFR1 and VEGFR2. The therapy consisted of a cocktail of five therapeutic epitope peptides in addition to oxaliplatin‐containing chemotherapy. Although the peptides used in this study were peptides recognized by HLA‐A*2402‐restricted CTL, all enrolled patients, whose HLA‐A*2402 status was double‐blinded, were given the same regimen of peptide cocktail and oxaliplatin‐containing chemotherapy. Between January 2009 and November 2012, 96 patients received the peptide cocktail treatment in combination with chemotherapy for advanced CRC or mCRC. Plasma samples were collected from 93 of the 96 patients prior to treatment. This study was carried out in accordance with the Helsinki Declaration on experimentation involving human subjects, was approved by the institutional ethics review boards of Yamaguchi University (H20‐102) and each study site, and was registered in the UMIN Clinical Trials Registry (UMIN000001791).
Patients and plasma
Pretreatment plasma samples were available for cfDNA analysis from 93 patients (HLA‐A2402‐matched, n = 49; and HLA‐unmatched, n = 44) with mCRC who were treated in the phase II study. Peripheral blood from each patient was collected in EDTA tubes. The blood samples were centrifuged at 400 g for 15 min at 4°C. The plasma was then aliquoted and stored at −80°C until use.
DNA strand integrity analysis
DNA was purified from 500 μL of plasma with a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). The PicoGreen dsDNA Quantitation Kit (Molecular Probes, Eugene, OR, USA) was used to measure the DNA concentrations according to the manufacturer's instructions. DNA strand integrity was measured using semi‐quantitative real‐time PCR with LightCycler (Roche, Penzberg, Germany) to determine the integrity index, which was defined as the ratio of the relative abundances of 400‐bp versus 100‐bp PCR products of the β‐actine gene that are likely present in all normal and neoplastic cells. Both 100‐ and 400‐bp PCR fragments were amplified using the same forward primer: 5′‐GCACCACACCTTCTACAATGA‐3′. The nested reverse primers used were 5′‐GTCATCTTCTCGCGGTTGGC‐3′ and 5′‐TGTCACGCACGATTTCCC‐3′ for the 100‐bp and 400‐bp products, respectively. The PCR conditions were: denaturation for 3 min at 95°C followed by 45 cycles of denaturation at 95°C for 30 s, annealing at 57°C (100 bp) or 56°C (400 bp) for 30 s, and extension at 72°C for 10 s (100 bp) or 15 s (400 bp). The threshold (C T) value for each reaction was calculated by the iCycler software package. The Δ C T value for 400 bp was subtracted from that for 100 bp to obtain a ΔΔ C T value. The integrity index was calculated as the exponential of (−ΔΔC T × LN 2).
Statistical analysis
Overall survival (OS) and progression‐free survival (PFS) rates were analyzed using the Kaplan–Meier method, and survival was measured in days from the first vaccination to the day of patient death from any cause. P‐values were assessed using a log‐rank test. A Cox's proportional hazards model and a logistic regression model were used to estimate the hazard ratios (HR) for the treatment effect in relation to survival and biomarkers or prognostic clinical information. All statistical analyses were performed with SPSS Statistics 20.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The clinical characteristics of the patients are shown in Table 1. None of the baseline characteristics differed significantly between the HLA‐matched and HLA‐unmatched groups.
Table 1.
Patient characteristics
| Characteristic | HLA‐A*2402 | P‐value | |
|---|---|---|---|
| Matched (n = 49) | Unmatched (n = 44) | ||
| Age | 64.1 ± 1.5 | 63.4 ± 1.2 | NS |
| Sex | |||
| Male | 25 | 22 | NS |
| Female | 24 | 22 | |
| Unresectable metastatic organ | |||
| Liver | 27 | 34 | NS |
| Lung | 18 | 12 | |
| Peritoneum | 5 | 4 | |
| Lymph node | 12 | 12 | |
| Others | 5 | 1 | |
| Number of involved organs | |||
| 1 | 35 | 29 | NS |
| 2 | 9 | 10 | |
| 3 | 5 | 5 | |
| Resection of primary lesion | |||
| Yes | 40 | 41 | NS |
| No | 9 | 3 | |
| Tumor marker | |||
| CEA | 1220 ± 684 | 341 ± 86 | NS |
| CA19‐9 | 3609 ± 2157 | 4506 ± 3457 | |
Data are expressed as mean ± standard error. CA19‐9, carbohydrate antigen; CEA, carcinoembryonic antigen; HLA, human leukocyte antigen; NS, not significant.
cfDNA levels and cfDNA integrity
The pretreatment levels of cfDNA and the calculated cfDNA integrity of the 93 patients are shown in Table 2.
Table 2.
Results of cell‐free DNA levels before treatment
| Marker | HLA‐A*2402 | |
|---|---|---|
| Matched (n = 49) | Unmatched (n = 44) | |
| Cell‐free DNA amount (ng/mL plasma) | 25.10 ± 3.59 | 22.78 ± 3.49 |
| Integrity (400‐bp/100‐bp ratio) | 0.37 ± 0.03 | 0.28 ± 0.02 |
Data are expressed as mean ± standard error. HLA, human leukocyte antigen.
cfDNA levels and objective responses
In this study, we categorized the patients into two groups: those with a cfDNA level higher than the median and those with a value lower than the median. The objective response rate (ORR) was 63.2 and 61.3% in the HLA‐matched and HLA‐unmatched groups, respectively (P = 0.910). The proportion of patients with a complete response, partial response and stable disease was 2.0% (1/49), 61.2% (30/49) and 32.6% (16/49), respectively, in the HLA‐matched group, and 0% (0/44), 61.3% (27/44) and 38.6% (17/44), respectively, in the HLA‐unmatched group. There was no significant difference in ORR between patients with a high or low cfDNA level. Similarly, there was no significant difference in the integrity of cfDNA between patients with a high or low cfDNA level (data not shown).
cfDNA levels and survival
In this study, we categorized the patients into two groups according to the amount of cfDNA, as described above. There was no significant difference in PFS (Fig. 1a) or OS (Fig. 1b) between patients with a high or low cfDNA level.
Figure 1.
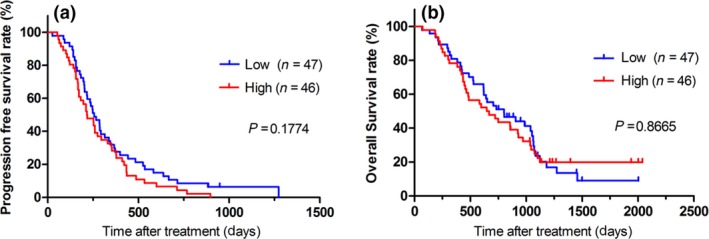
Kaplan–Meier survival plots of progression‐free survival (PFS) and overall survival (OS) in patients with a plasma cell‐free DNA level higher or lower than the median. (a) PFS. (b) OS.
cfDNA integrity and survival
Next, we also categorized the patients into two groups according to the integrity of cfDNA: those with a cfDNA integrity value higher than the median and those with a value lower than the median. We found that a low cfDNA integrity value was a prognostic marker for a longer PFS with peptide cocktail and oxaliplatin‐containing chemotherapy (Fig. 2a, P = 0.027). In contrast, there was no significant difference in OS between those with a high or low cfDNA integrity value, although the OS tended to be slightly better in the group with a low cfDNA integrity value (P = 0.0756, Fig. 2b).
Figure 2.
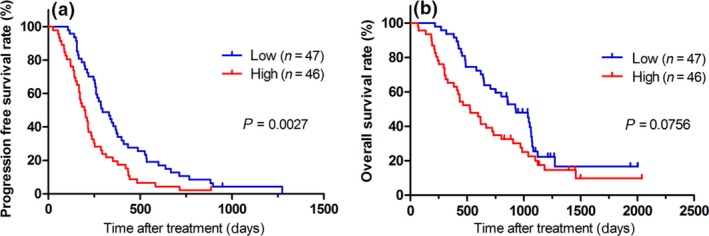
Kaplan–Meier survival plots of progression‐free survival (PFS) and overall survival (OS) in patients with a plasma cell‐free DNA integrity value higher or lower than the median. (a) PFS. (b) OS. The log‐rank test indicated that cell‐free DNA integrity might be a predictive biomarker for PFS (P = 0.0027).
Surprisingly, in the HLA‐A*2402‐matched group, patients with a low plasma cfDNA integrity value had a significantly better PFS than those with a high value (P = 0.0015; Fig. 3a). In contrast, in the HLA‐A*2402‐unmatched group, there was no difference between the patients with a high or low cfDNA integrity value (Fig. 3b). Hence, cfDNA integrity might be a predictive biomarker of PFS in patients treated with peptide vaccines. Multivariate analysis of the Cox regression model indicated that cfDNA integrity was the most significant predictor for PFS in the HLA‐A*2402‐matched group [P = 0.0037; HR = 2.66; 95% confidence interval (CI) = 1.34–5.44, Table 3]. In contrast, in both the HLA‐A*2402‐matched (Fig. 4a) and HLA‐A*2402‐unmatched (Fig. 4b) groups, there was no significant difference in OS between the patients with a high or low cfDNA integrity value.
Figure 3.
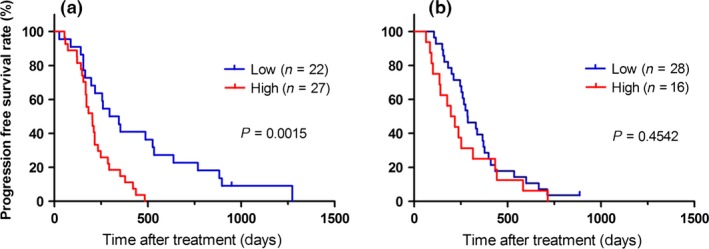
Kaplan–Meier survival plots of progression‐free survival (PFS) in patients with a plasma cell‐free DNA integrity value higher or lower than the median. (a) HLA‐A*2402‐matched group. (b) HLA‐A*2402‐unmatched group. In the HLA‐A*2402‐matched group, cell‐free DNA integrity might be a predictive biomarker for PFS (P = 0.0015).
Table 3.
Univariate and multivariate analysis of biomarkers for progression free survival using Cox regression model
| Factor | Cut‐off | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| CEA (ng/mL) | ≧2 × ULN | 1.21 | 0.63–2.23 | 0.5433 | |||
| CA19‐9 (U/mL) | ≧2 × ULN | 0.95 | 0.51–1.85 | 0.8885 | |||
| CRP (mg/dL) | ≧1 | 1.2 | 0.60–2.28 | 0.5799 | |||
| Number of involved organs | ≧2 | 1.32 | 0.66–2.46 | 0.4064 | |||
| cfDNA | ≧median | 1.24 | 0.69–2.22 | 0.4644 | |||
| cfDNA integrity | ≧median | 2.73 | 1.42–5.48 | 0.0023 | 2.64 | 1.34–5.44 | 0.0045 |
CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CI, confidence interval; cfDNA, cell free DNA; CRP, C‐reactive protein; HR, hazard ratio; ULN, upper normal of limit.
Figure 4.
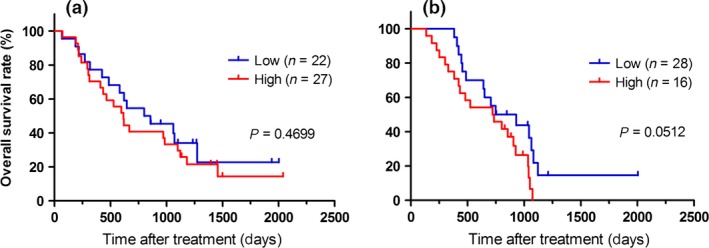
Kaplan–Meier survival plots of overall survival (OS) in patients with a plasma cell‐free DNA integrity value higher or lower than the median. (a) HLA‐A*2402‐matched group. (b) HLA‐A*2402‐unmatched group.
Discussion
Preservation of the host immune system is essential for active specific immunotherapy. Hence, it is crucially important to explore biomarkers for predicting the clinical responses for successful immunotherapy.27 We have investigated possible predictive biomarkers for the efficacy of immunochemotherapy based on clinical information and patient samples from our previous phase II study of a therapeutic peptide cocktail vaccine in combination with an oxaliplatin‐containing chemotherapy regimen against mCRC.7, 13, 15
To the best of our knowledge, this is the first study to report that cfDNA integrity might be a predictive biomarker for the efficacy of immunochemotherapy (Figs 2 and 3). In the present study, the PFS was significantly better in patients with a low cfDNA integrity value than in patients with a high value (P = 0.0027, Fig. 2a); surprisingly, this significance was observed clearly only in the HLA‐A*2402‐matched group (P = 0.0015, Fig. 3a), and not in the HLA‐A*2402‐unmatched group (P = 0.4542, Fig. 3b). These results indicated that the cfDNA integrity value is a predictive biomarker for peptide vaccines, but not for chemotherapy alone.
The immunogenic strength of apoptotic or necrotic tumor cells has been reported, controversially, to induce antitumor immunity.28, 29 In the tumor microenvironment, necrosis or necroptosis30 has been reported to result in the release of damage‐associated molecular pattern molecules, causing strong immunosuppression through the induction of regulatory T cells, myeloid‐derived suppressor cells and mesenchymal stromal cells.31 The plasma cfDNA integrity value may be determined from a liquid biopsy to remotely detect the amount of necrosis/necroptosis in tumors and the immunosuppressive microenvironment of the tumor site for predicting the efficacy of immunotherapy with peptide vaccines. A high cfDNA integrity value may indicate a high degree of tumor collapse and an excess of tumor necrosomes, which promote inducible immune suppression, leading to resistance to immunotherapy and a shorter PFS.32 Moreover, the half‐life period of cfDNA was reported as several minutes to several hours in the peripheral blood.33, 34, 35 Hence, the cfDNA integrity may reflect the process occurring within a living organism in real‐time.
In contrast, there was no significant difference in OS between the patients with a high or low cfDNA integrity value (Fig. 2b). In general, OS is a preferable endpoint to PFS due to the delayed effect of vaccine therapy.13 It was speculated that the tumor microenvironment might be altered after chemotherapy due to the large amount of necrosis in the tumor, which results in the release of damage‐associated molecular pattern molecules.31, 32 Most of the current treatment strategies for cancer (chemotherapy, radiation therapy and hormonal therapy) promote the release of damage‐associated molecular pattern molecules following therapy‐induced tumor death by necroptosis and necrosis. Regulatory T cells are strongly affected by pattern recognition receptor signaling in the tumor microenvironment and they limit immune reactivity in coordination with myeloid‐derived suppressor cells. To overcome the limitations of combination immunochemotherapy, some additional therapy may be needed to regulate the immunosuppressive tumor microenvironment. Several commonly used drugs, such as cyclophosphamide,36, 37 COX‐2 inhibitor,38 metformin39 and cimetidine,40 have been shown to modify the suppressive immune status in tumor microenvironments and might enhance the immune responses induced by peptide vaccines.
In conclusion, the plasma cfDNA integrity value might be a useful predictive biomarker for the outcome of immunotherapy, and an immunomodulating agent should be included in combination immunochemotherapy.
Disclosure Statement
Shoichi Hazama received research funding from NEC Corporation and Toyo Kohan Corporation. Shigefumi Yoshino received honoraria from MSD Corporation for lectures. The other authors have no conflict of interest to declare. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
This study was supported partially as part of a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P‐Direct), The Japan Agency for Medical Research and Development (AMED).
Cancer Sci 107 (2016) 1825–1829
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Cassidy J, Clarke S, Diaz‐Rubio E et al Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first‐line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26: 2006–12. [DOI] [PubMed] [Google Scholar]
- 3. Saltz LB, Clarke S, Diaz‐Rubio E et al Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–9. [DOI] [PubMed] [Google Scholar]
- 4. Douillard JY, Siena S, Cassidy J et al Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–705. [DOI] [PubMed] [Google Scholar]
- 5. Hegewisch‐Becker S, Graeven U, Lerchenmuller CA et al Maintenance strategies after first‐line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non‐inferiority, open‐label, phase 3 trial. Lancet Oncol 2015; 16: 1355–69. [DOI] [PubMed] [Google Scholar]
- 6. Tabernero J, Yoshino T, Cohn AL et al Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double‐blind, multicentre, phase 3 study. Lancet Oncol 2015; 16: 499–508. [DOI] [PubMed] [Google Scholar]
- 7. Hazama S, Nakamura Y, Takenouchi H et al A phase I study of combination vaccine treatment of five therapeutic epitope‐peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Trans Med 2014; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yagyu R, Furukawa Y, Lin YM, Shimokawa T, Yamamura T, Nakamura Y. A novel oncoprotein RNF43 functions in an autocrine manner in colorectal cancer. Int J Oncol 2004; 25: 1343–8. [PubMed] [Google Scholar]
- 9. Shimokawa T, Matsushima S, Tsunoda T, Tahara H, Nakamura Y, Furukawa Y. Identification of TOMM34, which shows elevated expression in the majority of human colon cancers, as a novel drug target. Int J Oncol 2006; 29: 381–6. [PubMed] [Google Scholar]
- 10. Kikuchi T, Daigo Y, Katagiri T et al Expression profiles of non‐small cell lung cancers on cDNA microarrays: identification of genes for prediction of lymph‐node metastasis and sensitivity to anti‐cancer drugs. Oncogene 2003; 22: 2192–205. [DOI] [PubMed] [Google Scholar]
- 11. Olofsson B, Korpelainen E, Pepper MS et al Vascular endothelial growth factor B (VEGF‐B) binds to VEGF receptor‐1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA 1998; 95: 11709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millauer B, Wizigmann‐Voos S, Schnurch H et al High affinity VEGF binding and developmental expression suggest Flk‐1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993; 72: 835–46. [DOI] [PubMed] [Google Scholar]
- 13. Hazama S, Nakamura Y, Tanaka H et al A phase II study of five peptides combination with oxaliplatin‐based chemotherapy as a first‐line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014; 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagorsen D, Thiel E. Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin Cancer Res 2006; 12: 3064–9. [DOI] [PubMed] [Google Scholar]
- 15. Hazama S, Takenouchi H, Tsunedomi R et al Predictive biomarkers for the outcome of vaccination of five therapeutic epitope peptides for colorectal cancer. Anticancer Res 2014; 34: 4201–5. [PubMed] [Google Scholar]
- 16. Umetani N, Kim J, Hiramatsu S et al Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin Chem 2006; 52: 1062–9. [DOI] [PubMed] [Google Scholar]
- 17. Umetani N, Giuliano AE, Hiramatsu SH et al Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol 2006; 24: 4270–6. [DOI] [PubMed] [Google Scholar]
- 18. Tsang JC, Lo YM. Circulating nucleic acids in plasma/serum. Pathology 2007; 39: 197–207. [DOI] [PubMed] [Google Scholar]
- 19. Kamat AA, Sood AK, Dang D, Gershenson DM, Simpson JL, Bischoff FZ. Quantification of total plasma cell‐free DNA in ovarian cancer using real‐time PCR. Ann N Y Acad Sci 2006; 1075: 230–4. [DOI] [PubMed] [Google Scholar]
- 20. Marzese DM, Hirose H, Hoon DS. Diagnostic and prognostic value of circulating tumor‐related DNA in cancer patients. Expert Rev Mol Diag 2013; 13: 827–44. [DOI] [PubMed] [Google Scholar]
- 21. Carcinoma Tokuhisa Y, Iizuka N, Sakaida I et al Circulating cell‐free DNA as a predictive marker for distant metastasis of hepatitis C virus‐related hepatocellular. Br J Cancer 2007; 97: 1399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomochika S, Iizuka N, Watanabe Y et al Increased serum cell‐free DNA levels in relation to inflammation are predictive of distant metastasis of esophageal squamous cell carcinoma. Exp Ther Med 2010; 1: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gormally E, Caboux E, Vineis P, Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutation Res 2007; 635: 105–17. [DOI] [PubMed] [Google Scholar]
- 24. Stroun M, Lyautey J, Lederrey C, Olson‐Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 2001; 313: 139–42. [DOI] [PubMed] [Google Scholar]
- 25. Giacona MB, Ruben GC, Iczkowski KA, Roos TB, Porter DM, Sorenson GD. Cell‐free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998; 17: 89–97. [DOI] [PubMed] [Google Scholar]
- 26. Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science 2015; 349: 1483–9. [DOI] [PubMed] [Google Scholar]
- 27. Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol 2013; 3: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt SV, Seibert S, Walch‐Ruckheim B et al RIPK3 expression in cervical cancer cells is required for PolyIC‐induced necroptosis, IL‐1alpha release, and efficient paracrine dendritic cell activation. Oncotarget 2015; 6: 8635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imamura R, Wang Y, Kinoshita T et al Anti‐inflammatory activity of PYNOD and its mechanism in humans and mice. J Immunol 2010; 184: 5874–84. [DOI] [PubMed] [Google Scholar]
- 30. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–14. [DOI] [PubMed] [Google Scholar]
- 31. Lotfi R, Kaltenmeier C, Lotze MT, Bergmann C. Until death do us part: necrosis and oxidation promote the tumor microenvironment. Transfus Med Hemother 2016; 43: 120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seifert L, Werba G, Tiwari S et al The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle‐induced immune suppression. Nature 2016; 532: 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischhacker M1, Schmidt B. Circulating nucleic acids (CNAs) and cancer–A survey. Biochim Biophys Acta 2007; 1775: 181–232. [DOI] [PubMed] [Google Scholar]
- 34. Diehl F, Schmidt K, Choti MA et al Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elshimali YI, Khaddour H, Sarkissyan M, Wu Y, Vadgama JV. The clinical utilization of circulating cell free DNA (CCFDNA) in blood of cancer patients. Int J Mol Sci 2013; 14: 18925–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noguchi M, Moriya F, Koga N et al A randomized phase II clinical trial of personalized peptide vaccination with metronomic low‐dose cyclophosphamide in patients with metastatic castration‐resistant prostate cancer. Cancer Immunol Immunother 2016; 65: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kan S, Hazama S, Maeda K et al Suppressive effects of cyclophosphamide and gemcitabine on regulatory T‐cell induction in vitro . Anticancer Res 2012; 32: 5363–9. [PubMed] [Google Scholar]
- 38. Gobel C, Breitenbuecher F, Kalkavan H et al Functional expression cloning identifies COX‐2 as a suppressor of antigen‐specific cancer immunity. Cell Death Dis 2014; 5: e1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune‐mediated antitumor effect by type 2 diabetes drug, metformin. Proc Nat Acad Sci USA 2015; 112: 1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lefranc F, Yeaton P, Brotchi J, Kiss R. Cimetidine, an unexpected anti‐tumor agent, and its potential for the treatment of glioblastoma (review). Int J Oncol 2006; 28: 1021–30. [PubMed] [Google Scholar]


