Despite the large number of papers that have been published on juglone and lawsone cytotoxicity, little is known about their redox cycling properties in plants. Here, the effects of these two naturally occurring naphthoquinones on H2O2 production and the activity of ROS scavenging enzymes (SOD, POX, CAT) in maize coleoptile segments were measured. It was found that lawsone was a more effective oxidative stress inducer in maize coleoptile cells than juglone. The results suggested that oxidative stress imposed by juglone and lawsone was one of the mechanisms of allelopathic action of the studied quinones in plants.
Keywords: Coleoptile segments, juglone, lawsone, oxidative stress, Zea mays
Abstract
Naphthoquinones are secondary metabolites widely distributed in nature and produced by bacteria, fungi and higher plants. Their biological activity may result from induction of oxidative stress, caused by redox cycling or direct interaction with cellular macromolecules, in which quinones act as electrophiles. The redox homeostasis is known as one of factors involved in auxin-mediated plant growth regulation. To date, however, little is known about the crosstalk between reactive oxygen species (ROS) produced by quinones and the plant growth hormone auxin (IAA). In this study, redox cycling properties of two naphthoquinones, juglone (5-hydroxy-1,4-naphthoquinone) and lawsone (2-hydroxy-1,4-naphthoquinone), were compared in experiments performed on maize coleoptile segments incubated with or without the addition of IAA. It was found that lawsone was much more effective than juglone in increasing both H2O2 production and the activity of antioxidative enzymes (SOD, POX and CAT) in coleoptile cells, regardless of the presence of IAA. An increase in the activity of Cu/Zn-SOD isoenzymes induced by both naphthoquinones suggests that juglone- and lawsone-generated H2O2 was primarily produced in the cytosolic and cell wall spaces. The cell potential to neutralize hydrogen peroxide, determined by POX and CAT activity, pointed to activity of catalase as the main enzymatic mechanism responsible for degradation of H2O2. Therefore, we assumed that generation of H2O2, induced more efficiently by LW than JG, was the major factor accounting for differences in the toxicity of naphthoquinones in maize coleoptiles. The role of auxin in the process appeared negligible. Moreover, the results suggested that oxidative stress imposed by JG and LW was one of mechanisms of allelopathic action of the studied quinones in plants.
Introduction
Juglone (JG) (5-hydroxy-1,4-naphthoquinone) and lawsone (LW) (2-hydroxy-1,4-naphthoquinone) are naturally occurring naphthoquinones that are widespread in nature. They have been identified as secondary metabolites that can be isolated from the leaves, roots, husks and bark of plants of the Juglandaceae family, particularly black walnut (Juglans nigra) in case of JG, and from the leaves of the henna plant (Lawsonia inermis) and water hyacinth (Eichhornia crassipes) in case of LW (Solar et al. 2006; Babula et al. 2009; Ashnagar and Shiri 2011; Nour et al. 2013). Juglone has varying effects on plants, including an inhibition of seed germination and plant growth (Hejl and Koster 2004; Böhm et al. 2006; Sytykiewicz 2011; Babula et al. 2014; Rudnicka et al. 2014), a reduction in the chlorophyll content (Terzi et al. 2003), a disruption of the root plasma membrane and a decrease in water uptake (Hejl and Koster 2004), as well as inhibition of photosynthesis (Hejl et al. 1993; Jose and Gillespie 1998), respiration (Jose and Gillespie 1998; Hejl and Koster 2004; Babula et al. 2009), transpiration (Jose and Gillespie 1998) and stomatal conductance (Jose and Gillespie 1998). In experiments performed on tobacco BY-2 cells, Babula et al. (2009) showed the ability of juglone to generate reactive oxygen species (ROS) and suggested that these substances play an important role in processes of programmed cell death. Additionally, in studies carried out on lettuce seedling roots, juglone caused enhanced production of H2O2, followed by a significant increase in the amount of free intercellular calcium ions in both cortical and peripheral cells of the root cap (Babula et al. 2014). JG and LW display a related chemical structure (Kumagai et al. 2012), however, knowledge about the effects of LW in plants is limited and the main area of LW research has been its interaction with animal and human tissues (Öllinger and Brunmark 1991; Kumbhar et al. 1996; Dasgupta et al. 2003).
One of the most important mechanisms underlying the phytotoxic influence of JG and LW is associated with their strong redox activity, which is involved in the peroxidation action within the tissues of targeted plants (El Hadrami et al. 2005; Chobot and Hadacek 2009; Ashnagar and Shiri 2011; Hao et al. 2012). On the other hand, some studies have demonstrated the protective role of juglone and lawsone, which has been observed as an abatement of oxidative stress and an inhibition of macromolecular oxidation (Chobot and Hadacek 2009; Chi et al. 2011; Cheniany et al. 2013).
Oxidative stress refers to the uncontrolled production of reactive oxygen species (ROS) such as superoxide anion (O2.−), hydrogen peroxide (H2O2), hydroxyl radical (.OH) and singlet oxygen (1O2). Every cell has various mechanisms, both non-enzymatic and enzymatic, to regulate the ROS level such as superoxide dismutases (SODs), catalases (CATs) and peroxidases (POXs) (recently reviewed in Kärkönen and Kuchitsu 2015). In plant cells, superoxide dismutases act as the first line of defence against ROS. SODs are classified into three groups according to their metal cofactor: copper-zinc (Cu/Zn-SOD), manganese (Mn-SOD) and iron (Fe-SOD) (Alscher et al. 2002). Unlike other organisms, plants have multiple SOD forms, i.e. one type of SOD can be present in several isoforms that have the same catalytic specificity, but have different kinetic proprieties and different migration rates on a gel (Kephart 1990). SODs catalyse the reaction of a disproportionation of two molecules of a superoxide radical ion to an oxygen molecule and H2O2 molecule, which is then further scavenged by catalases and peroxidases. Since biological membranes are impermeable to most of the ROS, SOD isoforms that occur at sites where O2.− is generated and play a role in the neutralisation of ROS in all cellular compartments, the cytosol and apoplastic spaces, mitochondria as well as in the water–water cycle and the ascorbate–glutathione cycle in chloroplasts (Asada 1999). Most of the H2O2 that is generated via SOD activity is then scavenged by peroxisomal and glyoxysomal catalases, while its molecules, which are unavailable for catalases, are degraded by peroxidases due to their high affinity to H2O2 and their presence in other cell compartments (Mittler 2002).
There is a close interdependence among the activity of SOD, CAT and POX because the product of the reaction that is catalysed by SOD becomes a substrate for POX and CAT. Moreover, the intermediates of the superoxide radical anion reduction to an oxygen molecule play a role in a number of regulatory and signalling processes. It is believed that H2O2 plays a key role in the regulation of the expression of antioxidative enzymes (SOD, POX, CAT) as well as other defence proteins such as pathogenesis-related proteins and heat shock proteins (HSPs) (Knight and Knight 2001).
The main aim of the present study was to compare the effects of two naturally occurring naphthoquinones, juglone (5-hydroxy-1,4-naphthoquinone) and lawsone (2-hydroxy-1,4-naphthoquinone), on H2O2 production and the activity of ROS scavenging enzymes (SOD, POX, CAT). In addition, the effect of auxin (IAA) on these processes was also studied. To the best of our knowledge, no research has been reported on the interdependence between oxidative stress induced by both naphthoquinones and the effects of plant growth hormone auxin (IAA). The experiments were performed with segments of etiolated maize coleoptiles, which are a classical model system for studies on the mechanism of auxin action on plant cell growth. In this system, the number of cells is constant and an organ grows only through elongation. Interestingly, most of the important evidence on the molecular mechanisms of auxin action was obtained in experiments performed with segments of maize coleoptiles (reviewed in Hager 2003). Despite the large number of papers that have been published on juglone and lawsone cytotoxicity, there is still a lack of a clear explanation of their redox cycling properties in plant cells. Moreover, our experiments may provide new data on the allelopathy of both naphthoquinones and the possible role of IAA in this process.
Methods
Plant material
Caryopses of maize (Zea mays cv. Cosmo) were soaked in tap water for 2 h, sown on wet wood wool in plastic boxes and were placed in a growth chamber for 4 days (Type MIR-553, Sanyo Electric Co., Japan) at 27 ± 1 °C. The experiments were performed with 10-mm-long coleoptile segments cut from etiolated seedlings. The coleoptile segments, with the first leaves removed, were excised 3 mm below the tip and incubated in a control medium of the following composition: 1 mM KCl, 0.1 mM NaCl, 0.1 mM CaCl2, initial pH 5.8–6.0.
Before chemical analysis, 20 intact coleoptile segments were placed in a measuring chamber and pre-incubated for 2 h in 6.0 cm3 (0.3 cm3 segment−1) of an intensively aerated control medium. After the pre- incubation of the coleoptile segments, juglone (5-hydroxy-1,4-naphthoquinone) (50 µM) or lawsone (2-hydroxy-1,4-naphthoquinone) (100 µM), with or without the addition of indole-3-acetic acid (IAA) (100 μM), were introduced into the incubation medium for 4 h. The concentrations of juglone and lawsone (50 and 100 µM, respectively) were selected in accordance with our previous studies, based on dose–response curves plotted for the effects of juglone and lawsone on endogenous and IAA-induced growth of maize coleoptile segments. In these experiments, the above-mentioned concentrations of juglone and lawsone inhibited both endogenous and IAA-induced growth of maize coleoptile segments by ca. 50 % (Rudnicka et al. 2014, 2015). Moreover, the experimental conditions such as number of coleoptiles per medium volume, composition of the incubation medium and initial pH of the medium were also adopted from our previous growth experiments (Karcz and Burdach 2002; Kurtyka et al. 2011, 2012; Burdach et al. 2014; Rudnicka et al. 2014). All experiments were performed in the time interval of 6 h, covering the time course of the model curve proposed by Becker and Hedrich (2002) for IAA-induced growth of maize coleoptile segments. On the other hand, our preliminary experiments also evidenced that the 6 h period is long enough to reveal differences between the action of both naphthoquinones. For the detection of H2O2 and the enzyme assay, samples were collected at 15, 60 and 120 min of the pre-incubation in the control medium and at 135, 180, 240, 300 and 360 min of the incubation in the mediums with IAA, JG and LW. All manipulations and experiments were conducted under a dim green light (0.04 W m−2), impinging omnilaterally on the coleoptiles.
Chemicals
Juglone (5-hydroxy-1,4-naphthoquinone) and lawsone (2-hydroxy-1,4-naphthoquinone) were obtained from Sigma Aldrich Inc. (St. Louis, MO, USA). The naphthoquinones, juglone and lawsone were dissolved in deionised water and used at a final concentration of 50 and 100 µM, respectively.
An aqueous stock solution (1 mM) of indole-3-acetic acid (IAA) (Serva, Heidelberg, Germany) was prepared with the potassium salt of IAA. IAA was used at a final concentration of 100 µM.
Hydrogen peroxide detection
The concentration of H2O2 was assayed using an Amplex®UltraRed fluorochrome (Molecular Probes). An aliquot of 100 µl of suspension that was withdrawn from the vessel in which the coleoptiles were incubated was immediately placed in a 96-well plate and 10 µl of a 100 µmol solution of Amplex®UltraRed in a HEPES buffer (pH 7.0, 0.05 M) was added. After incubating the plate for 20 min at 30 °C in darkness, the fluorescence was measured at Ex/Em wavelengths 490 nm/585 nm using a Varioskan® Flash Spectral Scanning Multimode Microplate Reader spectrofluorometer (ThermoScientific) running Varioskan Flash SkanIt Software (2006). The ‘blind’ probes, which contained a 110 µl of 10 µmol solution of Amplex®UltraRed in a HEPES buffer (pH 7.0, 0.05 M) or 100 µl of the coleoptile incubation buffer with 10 µl of a HEPES buffer, were measured to subtract autofluorescence of the fluorochrome or coleoptile incubation buffer, respectively. The standard curve was prepared using the stabilised H2O2 solution (Sigma Aldrich, Germany), which was diluted with a HEPES buffer (pH 7.0, 0.05 M) to a concentration range of 0.1–100 µmol. The concentration of H2O2 is presented as nmol of H2O2 per mg of the fresh weight of a coleoptile.
Enzyme isolation and assay
For protein isolation, maize coleoptiles (20 pcs.) were placed in 15 ml capacity test tubes with a portion of glass beads (∼0.5 g) and suspended in 0.5 ml of an extraction buffer. In order to isolate superoxide dismutase and catalase 0.05 M of a potassium phosphate buffer (pH 7.8) containing protease inhibitors (0.01 M EDTA, 0.01 M E-64 (transepoxysuccinyl-l-leucylamido(4-guanidino)butane), 0.01 M DTT (DL-dithiothreitol) were used and for peroxidase: 50 mM of a phosphate buffer 7.6, 0.86 mM AsA (ascorbic acid), 2.4 mM EDTA (ethylenediaminetetraacetic acid), 20 % glycerol and 2 % PVP (polyvinylpyrrolidone) were used. The coleoptiles were homogenised with glass beads in an MP Bio homogeniser (45 s, 5000 rps). The homogenate was transferred into an Eppendorf tube and the glass beads were washed three times with 0.5 ml of the appropriate buffer to a final volume of 2 ml. Debris was removed by centrifugation (20 min, 20 000 g, 4 °C). The supernatant that was obtained was used for the enzyme assays. The protein content in the supernatant was evaluated according to the method of Bradford (1976).
Total SOD activity was assayed following Beauchamp and Fridovich (1971). The reaction mixture contained 2.4 × 10−6 M riboflavin, 0.01 M methionine and 1.67 × 10−6 M nitroblue tetrazolium (NBT) in a 0.05 M potassium phosphate buffer, pH 7.8. Afterwards, 2.5 ml of the reaction mixture and 0.5 ml of the crude extract was illuminated in glass tubes for 10 min with white fluorescent light at an intensity of 60 Wm−2. The absorbance was measured at 560 nm with an illuminated 2.5 ml of the reaction mixture and 0.5 ml of a potassium phosphate buffer as a control. An SOD activity unit was defined as the amount that caused a 50 % inhibition of NBT reduction. Results are given as units of SOD activity per mg of protein (U mg−1).
The activity of SOD isoforms was determined using an indirect method that involved the inhibition of NBT as described by Tukaj and Pokora (2006). Briefly, non-denaturating PAGE was performed according to Davies (1971) with the modification that both the stacking (4.5 %) and resolving (12.5 %) gels were polymerised with riboflavin (2.8 × 10−5 M). Each sample contained 50 µg of the protein. SODs were located on the gels according to Beauchamp and Fridovich (1971). The gels were soaked in 2.45 × 10−3 M NBT for 20 min, followed by immersion (15 min) in a solution containing 0.028 M TEMED and 2.8 × 10−5 M riboflavin in a 0.036 M potassium phosphate buffer, pH 7.8. The gels were illuminated until they were uniformly blue and the sites where SOD was present were achromatic. SOD isoforms were identified according to their different sensitivities to H2O2 (5 mM) and KCN (2 mM). Cu/Zn-SOD was inhibited by both CN− and H2O2, Fe-SOD was resistant to CN- and sensitive to H2O2, while Mn-SOD was resistant to both chemicals. The activity of a separate SOD isoform band was calculated by comparing the relative band intensity mm−2 to the one obtained for the Cu/Zn-SOD reference pattern of the defined activity of 4.4 U mg−1 protein (Sigma-Aldrich). Densitometric analysis was performed using Quantity1D software (Bio-Rad).
Peroxidase activity was assayed according to Nakano and Asada (1981) with some modifications of the reaction mixture, which contained 50 mM of a phosphate buffer (pH 7.0), 18 mM pyrogallol and 0.1 mM H2O2. The reaction was started by adding 0.25 ml of the enzyme extract to 0.75 ml of the reaction mixture. The oxidation of pyrogallol was measured by the decrease in absorbance at 430 nm for 1 min at 25 °C (Hitachi U-2010 spectrophotometer, Japan). The enzyme activity was quantified using the molar extinction coefficient for pyrogallol (ε = 2.47 mM−1cm−1) and the results are expressed as mM pyrogallol oxidised × min−1 × mg−1 protein.
Catalase activity was determined spectrophotometrically (Hitachi U-2010, Japan). The reaction mixture contained 50 mM of a sodium phosphate buffer (pH 7.0) and 10 mM H2O2. CAT activity was estimated by monitoring the decrease in absorbance (λ = 240 nm) that was caused by H2O2 (ε = 36 M−1 cm−1) consumption (Aebi 1984). The results are expressed as μM of consumed H2O2 × min−1 × mg−1 protein.
Statistical analysis
Statistical analysis was performed using MS Excel 2010 (Microsoft) and Statistica 9.0 (StatSoft). All data were expressed as mean ± SD of at least four independent experiments, with two repeats for each measurement. In each sampling hour, effects of LW or JG in the presence or absence of IAA were analysed in four independent experiments, including two technical repeats of each measurement (n = 8), except for the interval of 0–2 h, in which only the control samples were analysed. SOD isoforms were examined in three independent experiments (isolation and PAGE analysis), including two technical repeats of each measurement (n = 6). Data were tested for normal distribution and variance homogeneity with the Levene's test. To compare results obtained for different variants, ANOVA and Tukey’s post hoc test were performed at P ≤ 0.05. Two-way ANOVA at P ≤ 0.05, with treatment variant and time as two predictor variables, was used to evaluate the time-dependent effects of chemicals applied.
Results
Hydrogen peroxide production
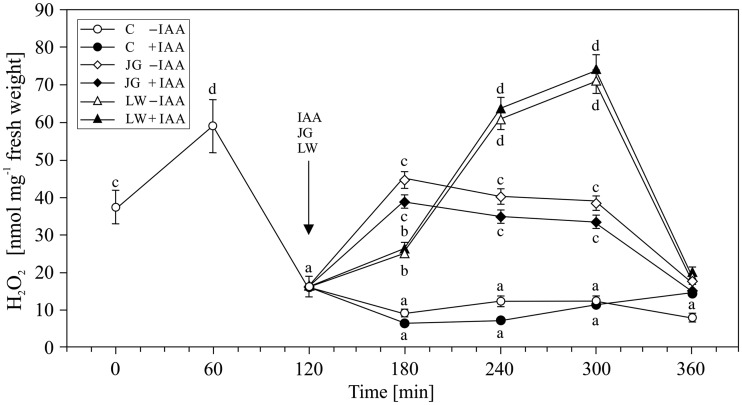
The first step in our study was to analyse the occurrence of H2O2 in the control medium during the pre-incubation (first 2 h) and incubation (next 4 h) of the maize coleoptiles. The highest level of H2O2 (58.99 ± 2.89 nmol mg−1 fresh weight) was recorded after 1 h of the pre-incubation of the segments, after which the H2O2 concentration decreased and was maintained at a constant level (9–16 nmol mg−1 fresh weight) (Fig. 1). The 2 h pre-incubation time appeared to be long enough to prevent oxidative stress associated with preparation (cutting) of maize coleoptile segments (F6,22 = 136, P = 0.0016). The addition of IAA at a final concentration of 100 µM to the incubation medium at 2 h did not significantly affect the level of H2O2 that was determined in each of the following hours of exposure (F12,44 = 1.85, P = 0.012), though it changed with the time of incubation [see Supporting Information—Table S1] (F12,44 = 241.5, P = 0.038). The application of either juglone or lawsone after 2 h of the pre-incubation of a segment in the control medium resulted in an increase in H2O2 production, which was significantly higher in the presence of lawsone (F10,42 = 115, P = 0.0023) than in juglone (F10,44 = 67, P = 0.0021), regardless of the time of incubation [see Supporting Information—Table S1] (F12,44 = 3.496, P = 0.03 and F12,44 = 5.928, P = 0.008, respectively). The highest levels of H2O2 were detected 1 h after application for juglone (44.77 ± 2.69 nmol mg−1 fresh weight) and 2 h later for lawsone (71.00 ± 4.26 nmol mg−1 fresh weight). These maximal values declined gradually for JG and reached the control value within the next 3 h; however, they decreased rapidly for LW (Fig. 1). The addition of IAA together with juglone or lawsone did not significantly change the level of H2O2 production that was observed for JG (F4,25 = 2.23, P = 0.015) or LW (F4,25 = 2.13, P = 0.012) alone.
Figure 1.
Generation of hydrogen peroxide in maize coleoptile segments exposed to juglone (JG) (50 µM) and lawsone (LW) (100 µM). Control, JG- and LW-treated coleoptiles were incubated for 4 h with or without indole-3-acetic acid at a concentration of 100 µM. The arrow indicates the moment of the application of IAA, JG and LW to the coleoptile incubation medium. The amount of hydrogen peroxide detected in the coleoptile incubation medium was expressed per fresh weight unit. Values are the means of at least four independent experiments. Bars indicate ± SDs. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test.
Antioxidative enzyme activity
Superoxide dismutase
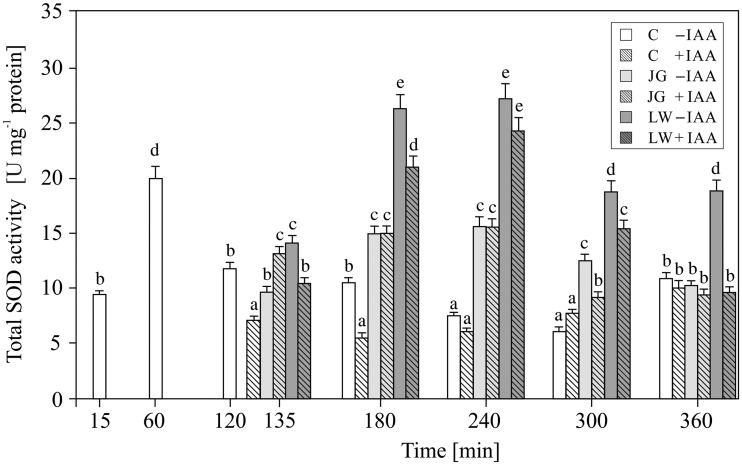
In the control maize coleoptile segments, total SOD activity ranged from 6 to 20 U mg−1 protein and depended on the duration of the experiment (Fig. 2). The highest enzymatic activity, 19.35 ± 0.97 U mg−1 protein, was detected 1 h after the preparation of the coleoptile segments; however, it decreased to 6–10 U mg−1 protein in the subsequent hours of incubation. The addition of IAA after 2 h of segment pre-incubation resulted in a decrease in SOD activity in the control samples at 3–4 h of the experiment (F6,22 = 56, P = 0.0045). Afterwards, the enzymatic activity remained at the same level (F6,22 = 56, P = 0.0045), regardless of the presence of auxin and time of incubation [see Supporting Information—Table S1] (F6,22 = 460, P = 0.86). After 1 and 2 h of the exposure of the coleoptile segments to JG and LW (3 and 4 h of the experiment), the activity of SOD showed a significant increase (F10,42 = 136, P = 0.0018), nearly two-fold greater in the presence of LW (F4,25 = 96, P = 0.0026) than JG (F4,25 = 74, P = 0.0035). In most cases (except for the fourth hour of the experiment), the addition of IAA together with LW caused a decrease in SOD activity (Fig. 2; F4,25 = 23, P = 0.013; see Supporting Information—Table S1; F4,25 = 1.964, P = 0.02), not observed if IAA was applied with JG (Fig. 2; F4,25 = 1.56, P = 0.016; see Supporting Information—Table S1; F4,25 = 3.599, P = 0.09).
Figure 2.
Effect of juglone (JG) and lawsone (LW) treatment on the enzymatic activity of total superoxide dismutase (SOD) in maize coleoptiles. Coleoptiles were incubated with the naphthoquinones (added after 2 h of segment pre-incubation) for 4 h, with or without auxin (100 µM). Values are the means of four independent experiments. Bars indicate ± SDs. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test.
Five SOD isoenzymes with different migration rates in the polyacrylamide gel were identified in the maize coleoptile segments [see Supporting Information—Table S1]. Using the KCN and H2O2 inhibition assay, two isoforms of Mn-SOD, which are referred to as Mn-SOD 1 and Mn-SOD 2, were found. We also identified three isoforms of Cu/Zn-SOD, namely Cu/Zn-SOD 1, 2 and 3. However, Fe-SOD was not found in the maize coleoptile tissue.
In the control coleoptiles, the activity of each identified isoform of Cu/Zn-SOD fell within a range of 1.05 ± 0.12 to 2.95 ± 0.18 U mg−1 protein (Table 1). As for total SOD, the highest activity of SOD isoforms was detected at 1 h of segment incubation in the control medium (F18,165 = 235, P = 0.0042 for Cu/Zn-SOD; F12,112 = 35, P = 0.0042 for Mn-SOD) and also at 4 and 6 h of incubation in the case of the Cu/Zn-SOD 3 isoform (F18,165 = 235, P = 0.0042). Both JG and LW treatment resulted in an increase in the activity of all of the Cu/Zn-SOD isoforms (F42,357 = 112, P = 0.012) (Table 1). The activity of the Cu/Zn-SOD 1 and 3 isoforms increased within 1 h of JG or LW treatment; however, the decreased in LW-treated tissues after the third hour (F12,117 = 35, P = 0.0045 and F12,117 = 28, P = 0.0036, respectively). The Cu/Zn-SOD 2 isoform exhibited an elevated although rather constant activity level in the JG-treated tissues (F42,357 = 112, P = 0.012). The highest stimulation of Cu/Zn-SOD activity was recorded for the Cu/Zn-SOD 3 isoform at 3 h exposure (F6,22 = 32, P = 0.0031). At the same time, all of the Cu/Zn-SOD isoforms displayed the greatest activity in the tissues that had been exposed to LW at 1 and 2 h (F12,117 = 35, P = 0.0045) (Table 1).
Table 1.
Effect of juglone (JG) and lawsone (LW) treatment on the enzymatic activity of three Cu/Zn-SOD isoforms detected in 4-day-old maize coleoptile segments.
| Treatment | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 60 | 120 | 135 | 180 | 240 | 300 | 360 | ||
| Control | Cu/Zn-SOD 1 | 1.05 a | 1.71 b | 1.33 a | 1.39 a | 1.40 a | 1.83 b | 1.91 b | |
| Cu/Zn-SOD 2 | 1.28 a | 2.95 c | 1.77 b | 1.86 b | 1.65 b | 1.98 b | 1.05 a | ||
| Cu/Zn-SOD 3 | 1.27 a | 2.36 c | 1.66 b | 1.81 b | 2.60 c | 1.20 a | 2.50 c | ||
| Juglone | Cu/Zn-SOD 1 | 2.44 c | 4.07 d | 3.28 d | 2.99 c | 3.47 d | |||
| Cu/Zn-SOD 2 | 3.17 d | 4.72 e | 3.73 d | 3.51 d | 3.32 d | ||||
| Cu/Zn-SOD 3 | 2.62 c | 3.38 d | 3.01 c | 4.29 e | 2.74 c | ||||
| Lawsone | Cu/Zn-SOD 1 | 2.59 c | 4.81 e | 4.60 e | 1.52 a | 2.64 c | |||
| Cu/Zn-SOD 2 | 3.13 d | 5.51 e | 5.92 e | 2.85 c | 2.77 c | ||||
| Cu/Zn-SOD 3 | 3.63 d | 4.65 e | 4.64 e | 2.84 c | 3.56 d | ||||
The activity of a separate SOD isoform band was calculated by comparing the relative band intensity to the one obtained for the Cu/Zn-SOD standard of the defined activity of 4408 U mg−1 protein. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test. Each value stands for the mean (n = 4).
The average activity of Mn-SODs in the control coleoptile tissues was similar to that of the Cu/Zn-SOD isoform and attained a level of 1.02 ± 0.08 to 2.87 ± 0.12 U mg−1 protein (Table 2). As in the case of the Cu/Zn-SOD isoforms, both LW and JG treatment stimulated the activity of the Mn-SOD isoforms (F16,156 = 67, P = 0.0075). In the JG-exposed coleoptiles, the Mn-SOD 1 isoform showed an increased (F8,36 = 22, P = 0.0024), though constant level, while the activity of Mn-SOD 2 fluctuated in the subsequent hours of exposure. Nevertheless, the activity of both Mn-SOD isoforms was much higher than in control tissues at 3 h of LW treatment (F27,234 = 144, P = 0.019) (Table 2).
Table 2.
Effect of juglone (JG) and lawsone (LW) treatment on the enzymatic activity of two Mn-SOD isoforms detected in 4-day-old maize coleoptile segments.
| Treatment | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 60 | 120 | 135 | 180 | 240 | 300 | 360 | ||
| Control | Mn-SOD 1 | 2.87 d | 2.81 d | 1.34 a | 1.02 a | 1.38 a | 1.72 b | 1.22 a | |
| Mn-SOD 2 | 1.59 b | 1.80 b | 1.48 a | 1.09 a | 1.83 b | 1.87 b | 1.18 a | ||
| Juglone | Mn-SOD 1 | 2.24 c | 2.61 c | 2.02 c | 2.21 c | 2.26 c | |||
| Mn-SOD 2 | 2.14 c | 1.54 b | 1.60 b | 2.19 c | 1.59 b | ||||
| Lawsone | Mn-SOD 1 | 1.83 b | 2.31 c | 1.61 b | 3.13 e | 2.68 d | |||
| Mn-SOD 2 | 1.90 b | 2.33 c | 2.68 d | 3.18 e | 1.99 b | ||||
The activity of a separate SOD isoform band was calculated by comparing the relative band intensity to the one obtained for the Cu/Zn-SOD standard of the defined activity of 4408 U mg−1 protein. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test. Each value stands for the mean (n = 4).
Peroxidase and catalase activity
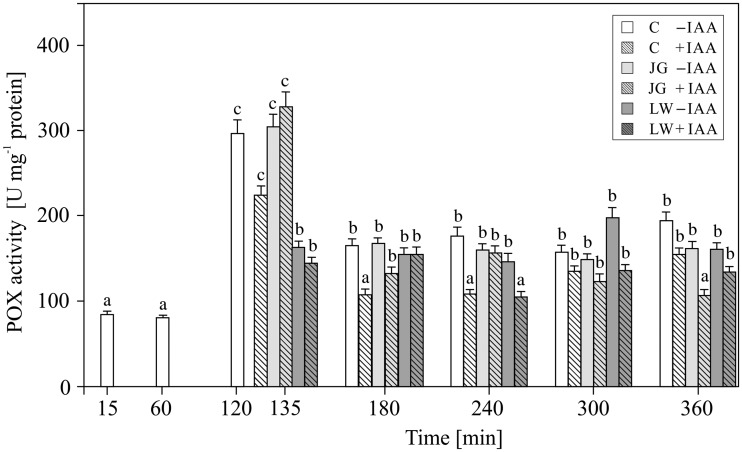
Peroxidases (POX) activity in the control maize coleoptile tissue increased until 2 h (F6,22 = 133, P = 0.0025) when it attained peak value of 298.91 ± 20.92 12 U mg−1 protein. As shown in Fig. 3, the application of auxin resulted in a significant decrease in the POX activity (F6,22 = 29, P = 0.0014); however, POX activity was not affected neither by JG nor LW (Fig. 3; F10,42 = 1.63, P = 0.0016), regardless of the presence of IAA in the incubation medium (see Supporting Information—Table S1; F10,42 = 85.5, P = 0.54 and F10,42 = 21.8, P = 0.23, respectively).
Figure 3.
Effect of juglone (JG) and lawsone (LW) treatment on the total peroxidase (POX) activity in maize coleoptiles. Coleoptiles were incubated with the naphthoquinones (added after 2 h of segment pre-incubation) for 4 h, with or without auxin (100 µM). Values are the means of four independent experiments. Bars indicate ± SDs. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test.
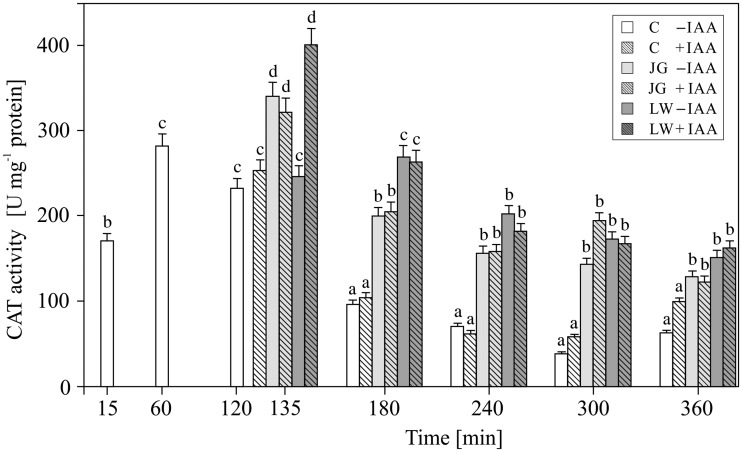
In the untreated maize coleoptiles, the highest CAT activity of ca. 300 U mg−1 protein was detected within the first 2 h, while in the subsequent hours of incubation, it decreased below 100 U mg−1 protein (F6,22 = 53, P = 0.0017) (Fig. 4). IAA applied to the incubation medium at 2 h did not significantly change CAT activity (F6,22 = 1.65, P = 0.023). The addition of both juglone and lawsone after 2 h of segment pre-incubation in the control medium resulted in an increase in CAT activity, regardless of the presence of auxin (F10,42 = 2.04, P = 0.021).
Figure 4.
Effect of juglone (JG) and lawsone (LW) treatment on the enzymatic activity of catalase (CAT) in maize coleoptiles. Coleoptiles were incubated with the naphthoquinones (added after 2 h of segment pre-incubation) for 4 h, with or without auxin (100 µM). Values are the means of four independent experiments. Bars indicate ± SDs. Means followed by the same letters are not significantly different from each other (P ≤ 0.05) according to the ANOVA and Tukey’s post hoc test.
Discussion
The obtained results indicate that both juglone and lawsone, in the presence or absence of IAA, contribute to H2O2 generation and activity of ROS scavenging enzymes (SOD, POX and CAT) in maize coleoptile segments. Previous reports evidenced both pro- and antioxidative action of quinones, depending on their chemical structure, particularly the number and arrangement of hydroxyl substituents (Liszkay et al. 2004; Murakami et al. 2010; Klotz et al. 2014), as well as on the dose (Babula et al. 2014), time of exposure (Kot et al. 2010) and co-presence of other factors that modulate redox homeostasis, including plant hormones, e.g. IAA (Liszkay et al. 2004; for a review see Tognetti et al. 2012). Our results indicate that the concentrations of JG and LW used here induce oxidative stress in cells of coleoptiles. This can be clearly seen in the amount of H2O2 that was generated (Fig. 1) and the elevated activity of the antioxidative enzymes (Figs 2 and 4), particularly the superoxide dismutase isoforms (Tables 1 and 2). The highest level of H2O2 recorded after 1 h of the pre-incubation of the segments in control medium probably is associated with preparation of maize coleoptile segments and is in good agreement with the growth rate kinetics of maize coleoptile segments after their excision from maize seedlings (Karcz and Burdach 2002; Karcz and Kurtyka 2007; Burdach et al. 2014). The total amount of H2O2 produced and the increased level of superoxide dismutase activity indicate that LW is a more efficient oxidative stress than JG. In comparison with JG, LW-treated tissues displayed a 2 h delayed and at least two-fold higher maximum H2O2 production (Fig. 1). In contrast, the studies with jack bean urease that were carried out by Kot et al. (2010) showed that the effectiveness of urease inhibition by juglone with reference to the quantity of generated H2O2 was 2.5 times higher than that of lawsone. Although the time required for the induction of SOD activity was similar for both quinones, the stimulation of SOD activity was much more pronounced in the LW-exposed coleoptiles. Since H2O2 is a molecule that is involved in the regulation of the activity and expression of ROS scavenging enzymes as a product of SOD activity (Alscher et al. 2002), and is a substrate for peroxidases and catalases (Mittler 2002), we considered the differences in the induction of SOD isoform activity as the main factor that determined the level of hydrogen peroxide. Our results indicate that the activity of catalase in maize coleoptiles is the main enzymatic mechanism that is responsible for the degradation of the H2O2 that is generated under the oxidative stress induced by JG or LW (Fig. 4). We found that exposure to both quinones resulted in at least ca. a two-fold stimulation of catalase activity, regardless of the time of exposure or the type of quinone applied. We also observed that, except for the moment of the application of JG and LW, peroxidase activity was not involved in the neutralisation of H2O2 (Fig. 3). It can either be assumed that the enzyme did not participate in H2O2 scavenging or that it was inhibited by an elevated H2O2 concentration (Hema et al. 2007). We would rather explain the lack of an effect of LW or JG on the peroxidase activity with its H2O2-mediated inhibition rather than with direct LW or JG action. Some experimental data indicate a greater resistance of catalase to high H2O2 concentrations (Asada 1999; Alscher et al. 2002). In the present study, we identified five SOD isoforms (Cu/Zn-SOD 1, 2 and 3 as well as Mn-SOD 1 and 2) in maize coleoptile segments. However, we did not determine any Fe-SOD isoforms, typically assigned to chloroplasts (Scandalios 1993; Allen et al. 2007) [see Supporting Information—Figure S1]. Such an observation probably results from the fact that coleoptile segments were excised from etiolated maize seedlings. All five of the SOD isoforms that were identified in the examined tissue responded to JG- and LW-induced stress. Generally, the Cu/Zn-SOD isoforms attained the highest enzymatic activity within the first 2 h of exposure to quinones; afterwards, the activity gradually decreased to a level only slightly higher than in the control cells. Cu/Zn-SOD is primarily considered to be a cytosolic enzyme (Allen et al. 2007); however, because it is also present in the chloroplasts and cell wall space, we believe it is reasonable to assume that both JG and LW induce oxidative stress in the cytosolic or cell wall spaces of maize coleoptiles. In contrast to Cu/Zn-SOD, the highest recorded activity of mitochondrial Mn-SOD isoforms was observed at 2 and 3 h of coleoptile exposure to JG and LW with effects of LW appearing to be more pronounced in comparison with JG. Since mitochondrial activity is assumed to be the only source of metabolic energy in the tissue of an etiolated coleoptile (Hager 2003), the elevated activity of Mn-SOD isoforms, which are associated with the mitochondrial space, can be expected. Among all of the SODs, Mn-SOD displayed the highest resistance to the increased concentrations of H2O2 (Allen et al. 2007). Moreover, due to the fact that the cytosolic space may be the site of the modification of JG and LW (Sharma et al. 2009) or their cross-reaction with glutathione (Sytykiewicz 2011), the mitochondrial space might be less susceptible to JG- or LW-mediated oxidative stress (Hejl et al. 1993). Therefore, it is not surprising that the induction of this isoform activity is delayed in comparison to the cytosol. On the other hand, since both quinones may potentially interact with the compounds of the mitochondrial electron transport chain (particularly substitute other quinones), a mitochondrion is likely to be expected as the main target action site of naphthoquinones.
According to Schopfer et al. (2002), the coleoptile growth of maize seedlings is accompanied by the release of reactive oxygen intermediates in the cell wall. These authors found that auxin promoted the release of O2− and the subsequent generation of ˙OH when elongation growth is induced by IAA. Moreover, their experimental data indicated that the generation of ˙OH in the cell wall causes an increase in the wall extensibility in vitro and replaces auxin in the induction of growth. It was also proposed that auxin not only induces cell elongation but is also involved in the tolerance to oxidative stress (for a review see Tognetti et al. 2012). On the one hand, auxin can modulate ROS homeostasis by regulating H2O2 levels (Iglesias et al. 2010), inducing ROS detoxification enzymes (Laskowski et al. 2002) and interacting with other hormonal signalling networks (Sun et al. 2009; De Tullio et al. 2010). On the other hand, the response and sensitivity to auxin are affected by the redox state (Bashandy et al. 2010). For example, an increase in ROS levels can affect auxin biosynthesis (Woodward and Bartel 2005), transport (Grunewald and Friml 2010) and distribution (Pasternak et al. 2005; Santelia et al. 2008). Here, in most cases, presence of auxin did neither affect the activity of antioxidative enzymes nor production of hydrogen peroxide. In the presented experimental model, we also did not establish any relationships between the effects of JG or LW and presence of the applied auxin. Such an observation may be associated with the ability of JG (probably of LW as well) to inhibit the auxin-dependent interaction between Aux/IAA proteins (family of transcriptional regulators) and the SCF-TIR1 complex, which in turn blocks auxin activity (Dharmasiri et al. 2003).
Conclusions
To conclude, we found that LW was a more effective oxidative stress inducer in maize coleoptile segments than JG. It was found that JG- and LW-mediated changes in the activity of Cu/Zn-SOD isoenzymes are the main factors that determine the amount of H2O2 that is generated in tissues that are exposed to both quinones. The cell potential to neutralise hydrogen peroxide, which is determined by the POX and CAT activity, points to the activity of catalase as the main enzymatic mechanism responsible for the degradation of the H2O2 that is generated under the oxidative stress that is induced by JG or LW in maize coleoptiles. The results presented here also indicate that IAA does not play any role in JG- and LW-induced oxidative stress in maize coleoptiles.
Supplementary Material
Acknowledgements
The authors thank the reviewers and editors for constructive remarks that helped to improve the quality of the manuscript.
Sources of Funding
Funding was provided by the University of Silesia.
Contributions by the Authors
W.K. and Z.T. conceived and designed the experiments. R.K. and W.P. performed the experiments, analyzed the data, contributed reagents/materials/analysis tools and wrote the paper.
Conflict of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article —
Figure S1. SOD isoenzyme profile obtained for control 4-day-old etiolated maize (Zea mays L.) coleoptiles incubated with or without IAA (0.1 mM) for 4 h.
Table S1. A set of results of two-way ANOVA analysiswith treatment variant and time of exposition as the two predictor variables, performed to evaluate the time dependent effects of chemicals applied on the hydrogen peroxide production and antioxidative enzymes activity in themaize coleoptiles.
Literature Cited
- Aebi H. 1984. Catalase in vitro. Methods in Enzymology 105:121–126. [DOI] [PubMed] [Google Scholar]
- Allen MD, Kropat J, Tottey S, Del Campo JA, Merchant SS. 2007. Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides and secondary phosphorus and iron deficiency. Plant Physiology 143:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher RG, Ertruk N, Heath LS. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany 53:1331–1334. [PubMed] [Google Scholar]
- Asada K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50:601–639. [DOI] [PubMed] [Google Scholar]
- Ashnagar A, Shiri A. 2011. Isolation and characterization of 2-hydroxy-1,4-naphthoquinone (lawsone) from the powdered leaves of henna plant marketed in Ahwaz city of Iran. International Journal of ChemTech Research 3:1941–1944. [Google Scholar]
- Babula P, Adam V, Havel L, Kizek R. 2009. Noteworthy secondary metabolites naphthoquinones—their occurrence, pharmacological properties and analysis. Current Pharmaceutical Analysis 5:47–68. [Google Scholar]
- Babula P, Vaverkova V, Poborilova Z, Ballova L, Masarik M, Provaznik I. 2014. Phytotoxic action of naphthoquinone juglone demonstrated on lettuce seedling roots. Plant Physiology and Biochemistry 84:78–86. [DOI] [PubMed] [Google Scholar]
- Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld J-P. 2010. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. The Plant Cell 22:376–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assays applicable to acrylamide gels. Analytical Biochemistry 44:276–287. [DOI] [PubMed] [Google Scholar]
- Becker D, Hedrich R. 2002. Channelling auxin action: modulation of ion transport by indole-3-acetic acid. Plant Molecular Biology 49:349–356. [PubMed] [Google Scholar]
- Böhm PAF, Zanardo FML, Ferrarese MLL, Ferrarese-Filho O. 2006. Peroxidase activity and lignification in soybean root growth inhibition by juglone. Biologia Plantarum 50:315–317. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 2:248–254. [DOI] [PubMed] [Google Scholar]
- Burdach Z, Kurtyka R, Siemieniuk A, Karcz W. 2014. Role of chloride ions in the promotion of auxin-induced growth of maize coleoptile segments. Annals of Botany 114:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniany M, Ebrahimzadeh H, Vahdati K, Preece JE, Masoudinejad A, Mirmasoumi M. 2013. Content of different groups of phenolic compounds in microshoots of Juglans regia cultivars and studies on antioxidant activity. Acta Physiologiae Plantarum 35:443–450. [Google Scholar]
- Chi W-C, Fu S-F, Huang T-L, Chen Y-A, Chen Ch C, Huang H-J. 2011. Identification of transcriptome profiles and signaling pathways for the allelochemical juglone in rice roots. Plant Molecular Biology 77:591–607. [DOI] [PubMed] [Google Scholar]
- Chobot V, Hadacek F. 2009. Milieu-dependent pro- and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. Journal of Chemical Ecology 35:383–390. [DOI] [PubMed] [Google Scholar]
- Dasgupta T, Rao AR, Yadava PK. 2003. Modulatory effect of henna leaf (Lawsonia inermis) on drug metabolising phase I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Molecular and Cellular Biochemistry 245:11–22. [DOI] [PubMed] [Google Scholar]
- Davies BJ. 1971. Disc electrophoresis. II. Method and application to human serum protein. Annals of the New York Academy of Sciences 121:404–427. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M. 2003. Auxin action in a cell-free system. Current Biology 13:1418–1422. [DOI] [PubMed] [Google Scholar]
- De Tullio MC, Jiang K, Feldman LJ. 2010. Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiology and Biochemistry 48:328–336. [DOI] [PubMed] [Google Scholar]
- El Hadrami A, Kone D, Lepoivre P. 2005. Effect of juglone on active oxygen species and antioxidant enzymes in susceptible and partially resistant banana cultivars to Black Leaf Streak Disease. European Journal of Plant Pathology 113:241–254. [Google Scholar]
- Grunewald W, Friml J. 2010. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. The EMBO Journal 29:2700–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. 2003. Role of the plasma membrane H+-ATPase in auxin induced elongation growth: historical and new aspects. Journal of Plant Research 116:483–505. [DOI] [PubMed] [Google Scholar]
- Hao F, Zhai M-Z, Wang Y, Yan T. 2012. Allelopathic effects of juglone on the growth of wheat seedlings and seed germination. Xibei Zhiwu Xuebao 32:518–524. [Google Scholar]
- Hejl AM, Einhellig FA, Rasmussen JA. 1993. Effects of juglone on growth, photosynthesis and respiration. Journal of Chemical Ecology 19:559–568. [DOI] [PubMed] [Google Scholar]
- Hejl AM, Koster KL. 2004. Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays). Journal of Chemical Ecology 30:453–471. [DOI] [PubMed] [Google Scholar]
- Hema R, Senthil-Kumar M, Shivakumar S, Reddy PC, Udayakumar M. 2007. Chlamydomonas reinhardtii, a model system for functional validation of abiotic stress responsive genes. Planta 226:655–670. [DOI] [PubMed] [Google Scholar]
- Iglesias MJ, Terrile MC, Bartoli CG, D’Ippólito S, Casalongué CA. 2010. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Molecular Biology 74:215–222. [DOI] [PubMed] [Google Scholar]
- Jose S, Gillespie AR. 1998. Allelopathy in black walnut (Juglans nigra L.) alley cropping. II. Effects of juglone on hydroponically grown corn (Zea mays L.) and soybean (Glycine max L. Merr) growth and physiology. Plant and Soil 203:199–205. [Google Scholar]
- Karcz W, Burdach Z. 2002. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. Journal of Experimental Botany 53:1089–1098. [DOI] [PubMed] [Google Scholar]
- Karcz W, Kurtyka R. 2007. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biologia Plantarum 51:713–719. [Google Scholar]
- Kärkönen A, Kuchitsu K. 2015. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112:22–23. [DOI] [PubMed] [Google Scholar]
- Kephart SR. 1990. Starch gel electrophoresis of plant isoenzymes: a comparative analysis of techniques. American Journal of Botany 77:693–712. [Google Scholar]
- Klotz LO, Hou X, Jacob C. 2014. 1,4-Naphthoquinones: from oxidative damage to cellular and inter-cellular signaling. Molecules 19:14902–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Knight MR. 2001. Abiotic stress signalling pathways: specificity and cross-talk. Trends in Plant Science 6:262–267. [DOI] [PubMed] [Google Scholar]
- Kot M, Karcz W, Zaborska W. 2010. 5-Hydroxy-1,4-naphthoquinone (juglone) and 2-hydroxy-1,4-naphthoquinone (lawsone) influence on jack bean urease activity: elucidation of the difference in inhibition activity. Bioorganic Chemistry 38:132–137. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Shinkai Y, Miura T, Cho AK. 2012. The chemical biology of naphthoquinones and its environmental implications. Annual Review of Pharmacology and Toxicology 52:221–247. [DOI] [PubMed] [Google Scholar]
- Kumbhar A, Padhye S, Ross D. 1996. Cytotoxic properties of iron-hydroxynaphthoquinone complexes in rat hepatocytes. Biometals 9:235–240. [DOI] [PubMed] [Google Scholar]
- Kurtyka R, Kita A, Karcz W. 2011. Fusicoccin counteracts the toxic effect of cadmium on the growth of maize coleoptile segments. Archives of Environmental Contamination and Toxicology 61:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtyka R, Małkowski E, Burdach Z, Kita A, Karcz W. 2012. Interactive effects of temperature and heavy metals (Cd, Pb) on the elongation growth in maize coleoptiles. Comptes Rendus Biologies 335:292–299. [DOI] [PubMed] [Google Scholar]
- Laskowski MJ, Dreher KA, Gehring MA, Abel S, Gensler AL, Sussex IM. 2002. FQR1, a novel primary auxin-response gene, encodes a flavin mononucleotide-binding quinone reductase. Plant Physiology 128:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. 2004. Production of reactive oxygen intermediates (O2.−, H2O2, and .OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology 136:3114–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7:405–410. [DOI] [PubMed] [Google Scholar]
- Murakami K, Haneda M, Iwata S, Yoshino M. 2010. Effect of hydroxy substituent on the prooxidant action of naphthoquinone compounds. Toxicology in Vitro 24:905–909. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22:867–880. [Google Scholar]
- Nour V, Trandafir I, Cosmulescu S. 2013. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. Journal of Chromatographic Science 51:883–890. [DOI] [PubMed] [Google Scholar]
- Öllinger K, Brunmark A. 1991. Effect of hydroxyl substituent position on 1,4-naphthoquinone toxicity to rat hepatocytes. The Journal of Biological Chemistry 266:21496–21503. [PubMed] [Google Scholar]
- Pasternak T, Potters G, Caubergs R, Jansen MAK. 2005. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. Journal of Experimental Botany 56:1991–2001. [DOI] [PubMed] [Google Scholar]
- Rudnicka M, Ludynia M, Karcz W. 2015. The poison is in the dose: growth and electrophysiological responses of maize coleoptile cells to naphthoquinones. Joint 7th Conference of the Polish Society for Experimental Plant Biology and the Intercollegiate Faculty of Biotechnology UG & MUG. Abstract Book p. 191.
- Rudnicka M, Polak M, Karcz W. 2014. Cellular responses to naphthoquinones: juglone as a case study. Plant Growth Regulation 72:239–248. [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, et al. 2008. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. The Journal of Biological Chemistry 283:31218–31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. 1993. Oxygen stress and superoxide dismutase. Plant Physiology 101:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. 2002. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sharma BK, Prabhakar YS. 2009. Juglone derivatives as antitubercular agents: a rationale for the activity profile. European Journal of Medicinal Chemistry 44:2847–2853. [DOI] [PubMed] [Google Scholar]
- Solar A, Colaric M, Usenik V, Stampar F. 2006. Seasonal variation of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Science 170:461–543. [Google Scholar]
- Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, et al. 2009. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. The Plant Cell 21:1495–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytykiewicz H. 2011. Expression patterns of glutathione transferase gene (GstI) in maize seedlings under juglone-induced oxidative stress. International Journal of Molecular Sciences 12:7982–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi I, Kocaçalişkan I, Benlioğlu O, Solak K. 2003. Effects of juglone on growth of muskmelon seedlings with respect to physiological and anatomical parameters. Biologia Plantarum 47:317–319. [Google Scholar]
- Tognetti VB, Mühlenbock P, van Breusegem F. 2012. Stress homeostasis—the redox and auxin perspective. Plant, Cell and Environment 35:321–333. [DOI] [PubMed] [Google Scholar]
- Tukaj Z, Pokora W. 2006. Individual and combined effect of anthracene, cadmium, and chloridazone on growth and activity of SOD izoformes in three Scenedesmus species. Ecotoxicology and Environmental Safety 65:323–331. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95:707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.