Highlights
-
•
Endometrial stromal sarcoma (ESS) may have sex cord differentiation, usually focal.
-
•
Diagnosis of ESS is difficult when variant morphology predominates.
-
•
Prognosis differs between ESS and UTROSCT; therefore, distinction is critical.
-
•
Extensive tumor sampling is mandatory to identify neoplastic endometrial stroma.
-
•
Sex cord differentiation may be associated with endometrial hyperplasia.
Keywords: Endometrial stromal tumor, Endometrial stromal sarcoma, Uterine tumor resembling ovarian sex cord tumor
1. Introduction
Uterine mesenchymal neoplasia is a broad category and includes smooth muscle tumors, endometrial stromal tumors (EST's), homologous sarcomas, heterologous sarcomas, sarcomas of uncertain histogenesis, and mixed epithelial and mesenchymal tumors, including uterine tumor resembling ovarian sex cord tumor (UTROSCT). EST's represent the second most common of these, but are still relatively rare, with malignant subtypes accounting for < 10% of uterine sarcomas and < 1% of primary uterine malignancies (Conklin and Longacre, 2014). The recently revised World Health Organization (2014) classification scheme of this entity defines four categories based on morphologic resemblance to proliferative-phase endometrial stroma, clinical behavior, and specific genetic translocations. Endometrial stromal nodule (ESN) and low-grade endometrial stromal sarcoma (LG-ESS) demonstrate sheets of cells with uniform oval nuclei, scant cytoplasm, minimal cytologic atypia, and variable mitotic activity. The neoplastic cells are only slightly larger than benign endometrial stromal cells. Permeative tongue-like growth into the myometrium and lymphovascular invasion set LG-ESS apart from ESN. High-grade ESS (HG-ESS) demonstrates cytologic atypia beyond that of LG-ESS but not so extreme as to abrogate its resemblance to benign proliferative endometrial stroma. HG-ESS is further characterized by the unique translocation t(10;17) not seen in other uterine mesenchymal tumors. Undifferentiated uterine sarcoma (UUS) demonstrates marked atypia without recognizable stromal differentiation. Destructive, rather than permeative, myometrial invasion is characteristic.
ESN and LG-ESS may focally demonstrate variant morphology including smooth muscle differentiation, fibromyxoid change, sex cord-like differentiation, and endometrioid-type glands. These understandably create diagnostic challenges, especially when the variant is extensive, as in the present case.
2. Case
A 56-year-old woman presented to her gynecologist with postmenopausal bleeding. Past medical history is insignificant. Family history included one sister with “uterine cancer” in her twenties without a history of obesity. The same sister received a diagnosis of breast cancer only several years later. The patient's BMI was 22.4 kg/m2 upon presentation. A transvaginal ultrasound revealed a thickened endometrial stripe (23 mm). Endometrial dilation and curettage revealed a fragmented mesenchymal lesion with mixed spindled and epithelioid cells suggestive of UTROSCT. Additional areas demonstrated focal complex atypical hyperplasia of endometrial glands.
The patient underwent exploratory laparotomy with total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymphadenectomy, omentectomy, and abdominopelvic washings. Operative findings included an enlarged uterus (15-weeks) with a retroverted fundus and a normal-appearing cervix. The bilateral adnexae were unremarkable.
Her post-operative course was uncomplicated, and she was followed at our institution for six months. No adjuvant treatment was given, and she remained disease-free during her follow-up period.
3. Pathologic findings
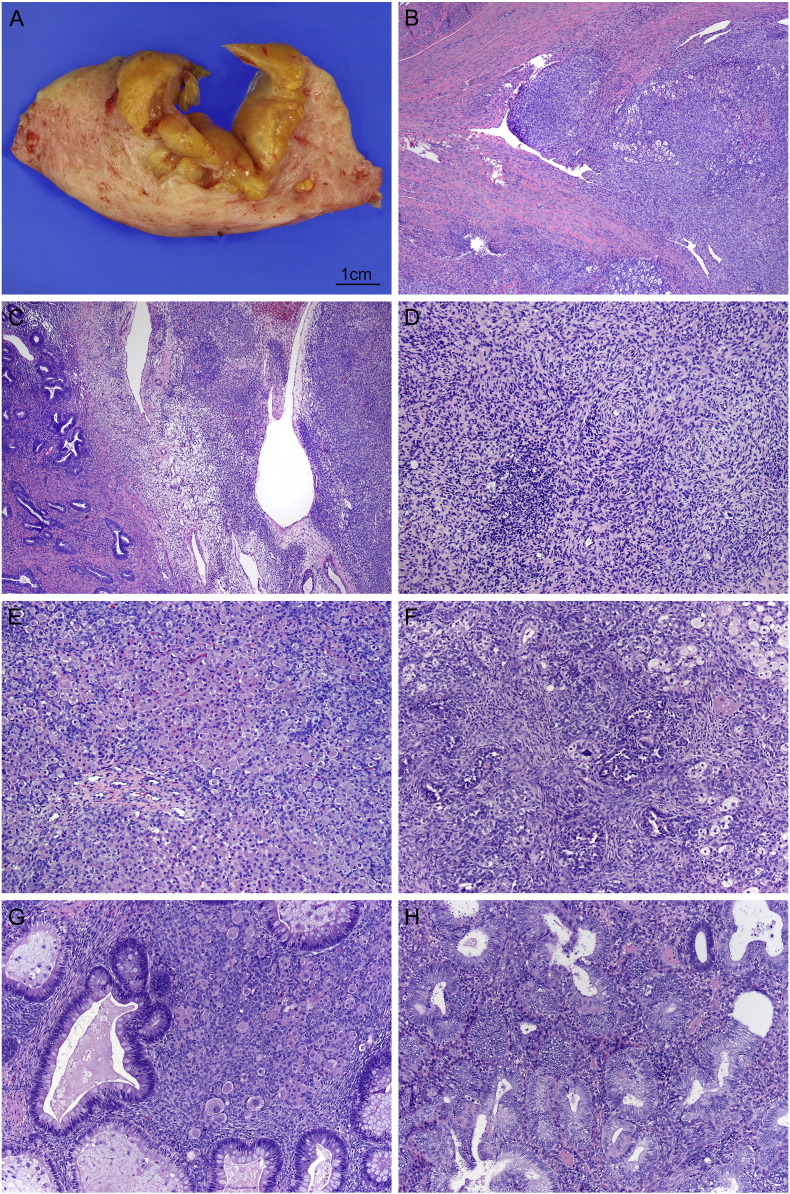
The hysterectomy specimen consisted of a 280 g uterus with a smooth serosal surface distorted anteriorly by a mass. Sectioning revealed a partially cystic tan to yellow lesion within the myometrium that was 5.1 × 4.6 × 4.5 cm (Fig. 1). While the mass appeared circumscribed, areas of myometrium distant from the main lesion contained infiltrative, worm-like extensions of tumor. The endometrium was uninvolved by tumor.
Fig. 1.
A–B – Gross and microscopic sections through tumor demonstrate permeative growth into surrounding myometrium. C – Tumor (right) adjacent to uninvolved endometrium (left). Note the resemblance between benign and malignant endometrial stroma. D – Tumor cells whorl around a delicate capillary network. E – Sheets and nodules of Leydig-like cells occupied the majority of the tumor. F – Sertoli-like elements were also present. G – Heterologous glands resemble colonic epithelium with columnar absorptive cells and goblet cells. Leydig-like cells are also seen (center). H – Complex atypical hyperplasia is present in the overlying endometrium. Note the differing cytology of neoplastic glands compared with a single entrapped benign gland. (b–h. hematoxylin and eosin; b–c. 40 ×, d. 100 ×, e. 20 ×, f–h. 100 ×).
Microscopic examination revealed heterogeneous morphology (Fig. 1). The most striking feature was overwhelming sex cord-like differentiation, namely sheets and nodules of large polygonal cells with abundant eosinophilic foamy cytoplasm, central round nuclei, and prominent nucleoli, suggestive of Leydig cell differentiation and interspersed cords and tubules resembling Sertoli cell tumor. Intimately associated with the sex cord-like areas were glands lined by colonic-type epithelium with columnar absorptive cells interspersed with goblet cells. A minor component of uniform round cells with scant cytoplasm and minimal cytologic atypia, mimicking proliferative endometrial stroma, was present in the background. Irregular permeative growth into surrounding myometrium was identified, and there was focal lymphovascular invasion that included both endometrial stromal and sex cord-like elements. Altogether, the sex cord-like areas and heterologous elements comprised > 60% of the tumor volume. The endometrium, which was grossly uninvolved by tumor, demonstrated complex atypical hyperplasia. The bilateral adnexa, all eighteen lymph nodes, and omentum were free of metastatic disease as were concurrent abdominopelvic washings.
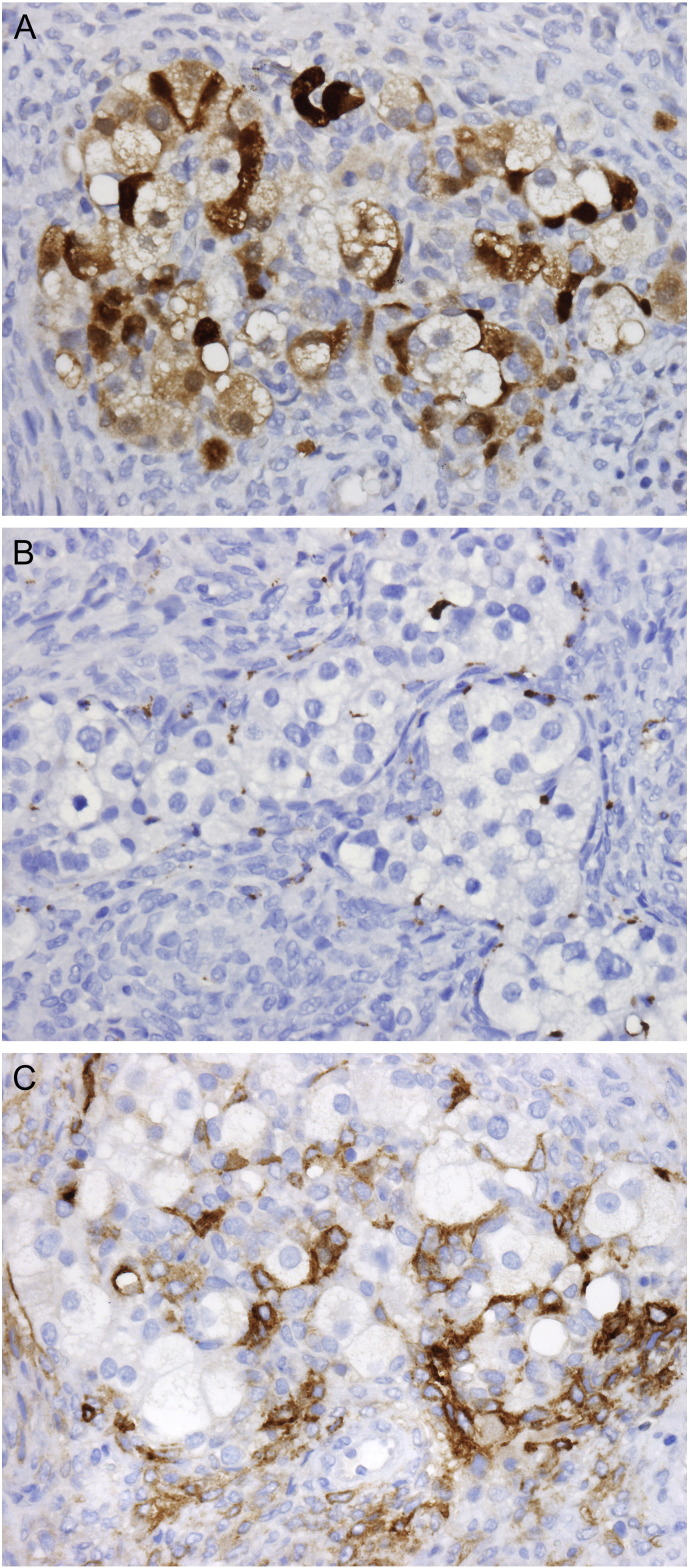
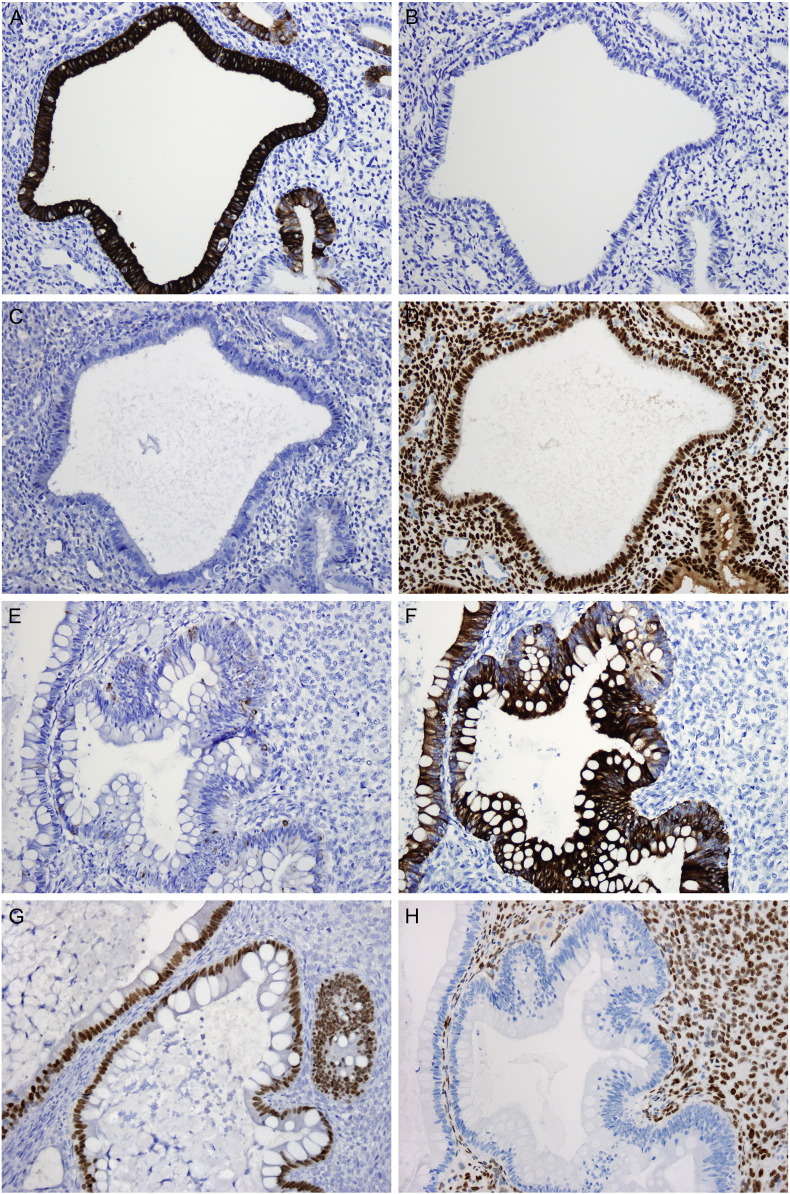
Immunohistochemical studies were performed on multiple sections of tumor and adjacent benign tissue. The LG-ESS was positive for ER, PR, CD10, Vimentin, CD56, and calretinin (focal, weak) and negative for inhibin, pan-cytokeratin (pan-CK), epithelial membrane antigen (EMA), and placental alkaline phosphatase (PLAP). The Sertoli-like tubules were positive for CD10, vimentin, CD56, calretinin, inhibin, and pan-CK (dot-like perinuclear pattern) and negative for ER, PR, EMA and PLAP. The foamy cells were positive for vimentin, CD56, calretinin, and inhibin and negative for CD68, ER, PR, EMA, and PLAP (Fig. 2). This immunoprofile supports the impression of an endometrial stromal neoplasm involved by Sertoli- and Leydig cell-like elements. The colonic-type glands were strongly and diffusely positive for CK20 and CDX2 and negative for ER. CK7 was focally positive. The endometrial glands were strongly and diffusely positive for CK7 and ER and negative for CK20. CDX2 was focally positive (Fig. 3). These findings support the impression of heterologous colonic elements.
Fig. 2.
Leydig-like cells are positive for calretinin (A) and negative for CD68 (B). Surrounding neoplastic endometrial stromal cells are positive for CD10 (C). (a–c. 400 ×).
Fig. 3.
Endometrial glands are positive for CK7 (A) and negative for CK20 (B). CDX2 is negative in the majority of endometrial glands (C) with only weak staining in rare glands, while ER is strongly and diffusely positive (D). Conversely, colonic-type epithelium is negative for CK7 (E) in the majority of glands and positive for CK20 (F). CDX2 is strongly and diffusely positive (G) in the colonic-type glands, while ER is uniformly negative (H). (a–h. 200 ×).
Fluorescent in-situ hybridization (FISH) analysis of the tumor did not demonstrate rearrangement of the JAZF1, PHF1, or YWHAE genes. The final diagnosis was that of LG-ESS with extensive sex cord differentiation and heterologous elements (FIGO Stage IB) with concurrent complex atypical hyperplasia of overlying endometrium.
4. Discussion
LG-ESS is a malignant mesenchymal tumor resembling proliferative-phase endometrial stroma. By definition, permeative invasion into the myometrium and/or lymphovascular invasion is present. It is typically characterized by an indolent course that is largely dictated by stage. Ten-year survival for early-stage disease approaches 90% whereas survival for stages III and IV is only 66%. However, late recurrences are common, even for early-stage disease (up to 56%), and an estimated 15–25% of patients die of recurrent disease (Rauh-Hain and del Carmen, 2013). LG-ESS typically presents in premenopausal women (mean age 52 years) as an intracavitary polypoid mass or intramural mass (WHO Classification of Tumours of Female Reproductive Organs, 2014). Cut surfaces are soft, yellow to tan, and may be cystic, hemorrhagic, or necrotic. Ill-defined borders with overt myometrial invasion are the norm, and macroscopically appreciable worm-like plugs of tumor within myometrial and parametrial veins are frequent. Microscopically, LG-ESS is composed of a dense monotonous proliferation of small oval cells with scant cytoplasm, round nuclei, inconspicuous nucleoli, and only mild cytologic atypia. Mitotic activity is usually low (< 5 mitotic figures per 10 high power fields), but higher mitotic counts do not preclude a diagnosis of LG-ESS. A delicate arteriolar network invests the tumor cells. Additional features include collagen bands, cholesterol clefts, and foamy histiocytes. Most but not all cases are marked by strong and diffuse immunoreactivity to CD10, vimentin, ER, and PR.
Sex cord-like differentiation within LG-ESS recapitulates ovarian granulosa cell or Sertoli cell tumors as nests, cords, trabeculae, or tubules of epithelioid cells with variable amounts of cytoplasm, round nuclei, small nucleoli, and minimal mitotic activity. Nuclear grooves or retiform growth are occasionally seen. The extent of sex cord-differentiation within a LG-ESS is variable and not well characterized in the literature. Clement and Scully originally described uterine tumors with sex cord-like elements in 1976. They reported six Group 1 tumors (EST with sex cord-like elements [ESTSCLE]) in which the sex cord-like elements occupied 10–40% of tumor volume. Subsequent studies have reported similar findings (Clement and Scully, 1976). In most cases, the stromal neoplasm predominates. Sex cord-like areas stain with typical sex cord-stromal markers, including inhibin and calretinin (Conklin and Longacre, 2014, WHO Classification of Tumours of Female Reproductive Organs,, Oliva, 2014).
Most LG-ESS's demonstrate recurrent genetic translocations by FISH and, of these, 80% harbor the JAZF1-SUZ12 fusion gene (t7;17). A rearranged PHF1 gene is identified in the majority of the remaining cases (Lee and Nucci, 2014). A subset of LG-ESS's without demonstrable genetic rearrangements have been reported, and these are thought to represent variant translocations. Data suggest these tumors behave similar to JAZF1-rearranged tumors.
UTROSCT represents the historical Group II tumors described in the original 1976 paper on the subject, and is defined as an endometrial or myometrial neoplasm composed entirely of sex cord-like elements; endometrial stromal neoplasia is necessarily lacking (Clement and Scully, 1976). Similar to ovarian Sertoli-Leydig cell tumors, UTROSCT's may rarely exhibit heterologous elements, usually enteric epithelium. While estrogen elaboration by ovarian Sertoli-Leydig cell tumors with consequent endometrial hyperplasia has been described, the co-occurrence of UTROSCT with endometrial hyperplasia is less well established.
The presentation of UTROSCT is similar to that of LG-ESS, but post-operative management differs as UTROSCT typically has a benign course with limited potential for recurrence (Conklin and Longacre, 2014, Blake et al., 2014). Therefore, the distinction between the two entities is important. In cases of UTROSCT, thorough sampling is required to rule out areas of endometrial stromal neoplasia. Furthermore, UTROSCT is almost always well-circumscribed and lacks the extensive myoinvasion typical of LG-ESS. Lymphovascular invasion is rare in UTROSCT, and the delicate arteriolar pattern of LG-ESS is not present. Immunohistochemistry is not of great utility in the differential, but molecular studies have shown UTROSCT lacks rearrangements of JAZF1 and PHF1 genes (Staats et al., 2009, Nucci et al., 2014).
To our knowledge, this case represents a previously unreported occurrence of extensive sex cord-like differentiation within a LG-ESS with concomitant heterologous elements and complex atypical endometrial hyperplasia. While the gross impression of tumor plugs throughout the myometrium strongly favored ESS over UTROSCT, thorough sampling was required to confirm areas of stromal neoplasia in a tumor predominated by sex cord-like and heterologous elements. The usual degree of sex cord differentiation within ESS is not well reported; however, a PubMed.gov search revealed only two prior cases wherein the sex cord elements represented a majority of the tumor volume (Fukunaga et al., 1997). An additional striking feature of this case was the complex atypical hyperplasia of overlying endometrium. While it is possible that the hyperplasia was an incidental synchronous finding, we believe it more likely that the extensive sex cord-like areas produced sufficient unopposed estrogen to locally influence the endometrium.
Disclosures
We have no conflicts of interest to disclose.
References
- Blake E.A. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;181:163–170. doi: 10.1016/j.ejogrb.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Clement P.B., Scully R.E. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am. J. Clin. Pathol. 1976;66(3):512. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- Conklin C.M., Longacre T.A. Endometrial stromal tumors: the new WHO classification. Adv. Anat. Pathol. 2014;21(6):383–393. doi: 10.1097/PAP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Miyazawa Y., Ushigome S. Endometrial low-grade stromal sarcoma with ovarian sex cord-like differentiation: report of two cases with an immunohistochemical and flow cytometric study. Pathol. Int. 1997;47(6):412–415. doi: 10.1111/j.1440-1827.1997.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Nucci M.R. Endometrial stromal sarcoma - the new genetic paradigm. Histopathology. 2014 doi: 10.1111/his.12594. [DOI] [PubMed] [Google Scholar]
- Nucci M.R., S.J., Sukov B., Oliva E. Uterine tumors resembling ovarian sex cord tumor (UTROSCT) lack rearrangement of PHF1 by FISH. Mod. Pathol. 2014;27:298A. [Google Scholar]
- Oliva E. Cellular mesenchymal tumors of the uterus: a review emphasizing recent observations. Int. J. Gynecol. Pathol. 2014;33(4):374–384. doi: 10.1097/PGP.0000000000000141. [DOI] [PubMed] [Google Scholar]
- Rauh-Hain J.A., del Carmen M.G. Endometrial stromal sarcoma: a systematic review. Obstet. Gynecol. 2013;122(3):676–683. doi: 10.1097/AOG.0b013e3182a189ac. [DOI] [PubMed] [Google Scholar]
- Staats P.N. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am. J. Surg. Pathol. 2009;33(8):1206–1212. doi: 10.1097/PAS.0b013e3181a7b9cf. [DOI] [PubMed] [Google Scholar]
- WHO Classification of Tumours of Female Reproductive Organs, Bosman F.T., Jaffe E.S., Lakhani S.R., Ohgaki H. In: World Health Organization Classification of Tumours. fourth ed. Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H., editors. IARC; Lyon: 2014. [Google Scholar]