ABSTRACT
Bispecific antibodies have emerged as powerful therapeutic agents given their high specificity and ability to induce a potent immune response. Various bispecific antibody formats have been designed and studied regarding their applications in cancer therapy, though associated with issues of short half-life or manufacturing difficulties. Herein, a novel bispecific antibody, SS-Fc, was constructed by pairing 2 single-domain antibodies, anti-CD16 and anti-CEA, which were fused with CH3 “knobs into holes” mutations individually. SS-Fc was expressed and purified from E.coli. In vitro and in vivo experiments confirmed that SS-Fc can form a heterodimeric bispecific antibody when expressed and purified from E. coli. By engaging natural killer (NK) cells through an anti-CD16 single domain antibody, the SS-Fc bispecific antibody exhibited potent in vitro and in vivo cytotoxicity against cancer cells with carcinoembryonic antigen (CEA) expression. Thus, SS-Fc represents a novel bispecific antibody format that can be applied to a wide range of both discovery and clinical applications.
KEYWORDS: Bispecific antibody, CD16, CEA, single-domain antibody, SS-Fc
Background
Antibodies are widely used in clinical and discovery research given their unique properties. Typical immunoglobulin IgG antibodies are assembled from 2 identical heavy (H)-chain and 2 identical light (L)-chain polypeptides, for which the paired VH-VL domains constitute the variable fragments (Fv) that recognize antigens. The CH2-CH3 part (also known as the Fc fragment) allows the antibody to have a long half-life in vivo and links antibody-bound cells to immune cells via Fc receptors. Upon antigen binding, antibodies utilize various mechanisms to trigger downstream biological functions, such as inhibiting signaling, inducing apoptosis, triggering the classical complement pathway, and/or activating immune cells that express Fcγ receptors.
Recently, various non-conventional formats of antibodies have been studied and developed to perform unique biological functions for cancer therapy. Among them, bispecific antibody (BsAb) is actively being pursued for immunotherapy and as a diagnostic tool.1,2 In contrast to traditional antibodies, bispecific antibodies recognize 2 different antigens owing to 2 different antigen-binding domains.3 Given the increased potency of engaging immune cells and the high specificity conferred by a tumor-specific antibody, bispecific antibodies are actively pursued in cancer therapy. For example, the BiTE antibody (bispecific T cell Engager), which links 2 single chain Fv molecules, directly engages T cells and kills tumor cells.4-7
Herein, we exploited the combination of 2 single-domain antibodies via Fc pairing to produce bispecific antibodies, SS-Fc. The single-domain antibody VHH is derived from a natural camel heavy-chain antibody (HCAb),8 which lacks an L chain polypeptide and the first constant domain (CH1). At its N-terminal region, the H chain of HCAb contains one variable domain or single antibody domain, known as VHH. The VHH in HCAbs serves to recognize antigens and represents the functional equivalent of a Fab (antigen-binding) fragment of conventional antibodies. As building blocks for bispecific antibodies, VHH reduces the light chain pairing issue associated with bispecific antibodies in the human IgG format.
As a proof-of-concept, 2 different VHHs, anti-CEA and anti-CD16, which recognize tumor cells expressing carcinoembryonic antigen (CEA) and natural killer (NK) cells, respectively, were linked with knob-into-holes mutations of human IgG1 Fc.9,10 The SS-Fc antibody could be expressed and purified from E. coli. In vitro and in vivo experiments confirmed the potent tumor killing induced by SS-Fc. The anti-tumor activities of SS-Fc were dependent upon the engagement of NK cells and specific to CEA-expressing tumor cells. This study suggested that SS-Fc represents a novel bispecific antibody format with potential in immunotherapy and other applications.
Materials and methods
Plasmid construction
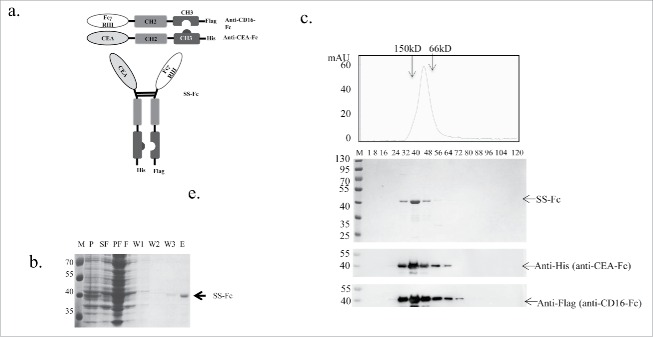
Previously published camel anti-CEA[11] and anti-CD1612 antibodies were synthesized (Genscript) and then linked to mutated human IgG1 Fc fragments, which the anti-CEA VHH was linked to the knob mutant (T366W), and the anti-CD16 VHH was linked to the hole mutant (T366S, L368A and Y407V).9,10 The anti-CEA-Fc and anti-CD16-Fc constructs were then cloned into the expression vectors pETDuet-1 and pRSF-duet-1 (Novagen), respectively. The signal peptide pelB sequence was inserted into the N terminal regions to facilitate periplasmic purification. His-tag and Flag-tag were added to the c-terminals to facilitate protein detection (Fig. 1a).
Figure 1.
Construction and purification of the SS-Fc bispecific antibody. (a) Anti-CEA was linked with a human IgG1 Fc fragment with “knob” mutation in the CH3 domain.10 Anti-CD16 was linked with a human IgG1 Fc fragment with “holes” mutations in the CH3 domain.10 The SS-Fc bispecific antibody was formed by heterodimerization of anti-CEA-Fc and anti-CD16-Fc mediated by a CH2–CH3 interaction. (b) Affinity purification of SS-Fc. The panel shows the coomassie blue staining of purification fractions (M, molecular weight ladder, unit: kDa; P, pellet; SF, sucrose fraction; PF, periplasmic fraction; F, flow through fraction of rProtein A affinity purification; W1, W2, W3, wash fraction of rProtein A affinity purification; E, elution of rProtein A affinity purification. (c) Gel filtration analysis of SS-Fc. Standard protein markers ranging from 12.4 to 150 kDa were used to estimate the size of the antibodies. Top panel displays fractions with coomassie blue staining, and bottom panels display the protein gel blots to detect anti-CEA-Fc using an anti-His6 antibody and anti-CD16-Fc using an anti-Flag antibody (arrows indicate the different proteins, respectively).
Protein purification and characterization
For protein expression, single or both expression plasmids were transformed into E. coli BL21 (DE3) and grown at 37°C in LB medium with appropriate antibiotics (100 µg/ml kanamycin and/or 100 µg/ml ampicillin). Protein expression was induced by 0.2 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 20 h at 16°C.
For protein purification, cells were pelleted by centrifugation at 4000 rpm for 30 min at 4°C. Periplasmic extraction was performed by resuspending the pellets 1:4 (m/v) in chilled sucrose solution (20 mM Tris–HCl, pH 8.0, 25% (w/v) sucrose, 1 mM EDTA, 1 mM PMSF). The suspension was centrifuged at 8500×g for 20 min and the supernatant was the sucrose fraction. The pellet was then resuspended in chilled periplasmic solution (5 mM MgCl2) and centrifuged at 8500 × g for 20 min; the supernatant contained the periplasmic fraction.
Anti-CEA-Fc or anti-CD16-Fc proteins were purified from the combined sucrose and periplasmic fractions by affinity purification using rProteinA Sepharose Fast Flow (GE Healthcare). For SS-Fc purification, the protein was firstly purified by using rProteinA Sepharose Fast Flow (GE Healthcare). In order to separate pure heterodimer of SS-Fc from homeodimer of anti-CEA-Fc or anti-CD16-Fc, the protein was further purified using Ni-NTA Superflow (Cat# 30410, Qiagen) and then ANTI-FLAG® M2 Affinity Agarose Gel(Cat#A2220, Sigma).
Gel filtration was performed using GE HiPrep 16/60 Sephacryl S-200 High Resolution, as described by the manufacturer (GE Healthcare). Gel filtration protein markers were obtained from Sigma (MWGF1000).
For LC-MS analysis, the SS-Fc no-reduced sample was prepared with 0.1% formic acid solution (1 mg / mL). The reduced sample was prepared by adding 1 μL 1M DTT to 50μL SS-Fc as prepared above (1 mg / mL). The mixture was incubated at 56°C for 30 min. For LC-MS analysis, 10μg of intact or reduced samples were first separated by UHPLC (Dionex UltiMate 3000 UHPLC, Thermo Scientific), and then detected by ESI-MS (Q Exactive MS, Thermo Scientific). Data were analyzed using Thermo Protein Deconvolution software.
Elisa was performed by coating recombinant CEA (R&D, Cat. # 2244-CM-050) or CD16a (R&D, Cat. # 4325-FC-050) on 96 well plates (100μL(0.1μg) per well). After blocking, primary antibody binding, and secondary antibody binding, chemi-luminescence was developed using TMB Reagent (GenScript Cat.# M00078). Data were analyzed using Prism software.
Cells and animals
SKOV3, HT29, and LS174T cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. SKOV3 and HT29 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (Gibco, Life Technologies, China), and LS174T cells were cultured in RPMI-1640 medium (Gibco, Life Technologies, China) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Life Technologies, USA) and 1% penicillin/streptomycin (Hyclone). All cells were cultured in a humidified 37°C incubator containing 5% CO2.
Human peripheral blood mononuclear cells (PBMCs) were isolated from fresh peripheral blood obtained from healthy donors. NK cells were isolated using a Human NK cell Enrichment Kit (Negative Selection, EasyStep, STEMCELL Technologies), following the manufacturer's protocol. Human blood collection and experimental protocols were approved by Sun Yat-Sen University.
Non-obese diabetic-severe combined immunodeficiency disease (NOD-SCID) mice were housed at the animal experiment center of Sun Yat-Sen University under sterile and standardized environmental conditions (20–26°C room temperature, 40–70% relative humidity, and a 12 h/12 h light/dark cycle). Animal care and experimental procedures were approved by Sun Yat-Sen University.
Western blot analysis
To detect CEA expression in tumor cells, tumor cells were collected and lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 1% Trioton-X-100 with protease inhibitor cocktails (Roche). To perform western blot, 40 μg of crude protein extract was used and diluted with SDS loading buffer (5×) (Tris-Cl 250 mM, pH 6.8, 1% β-Mercaptoethanol, 0.02% Bromophenol blue, 30% Glycerol, 10% Sodium dodecyl sulfate). For detecting SS-Fc, anti-CD16-Fc, or anti-CEA-Fc, 0.1μg (western blot), or 1μg (coomassie blue staining) of purified proteins were used. The samples were boiled at 95°C for 5 min and subjected to SDS-PAGE, then coomassie blue staining or protein gel blot analysis. The western blots were developed with chemiluminescent HRP substrate (Millipore Corporation, Billerica, USA) and detected using a Bio-Rad imager (ChemiDoc™ XRS+).
GADPH (14C10) Rb mAb (Cell signaling, Cat# 2118S), CEA/CD66e(CB30) mAb (Cell signaling, Cat#2383S. ), Anti-His tag-HRP (Abcam, Cat# ab1187), Anti-FLAG M2 HRP (Sigma, Cat # A8592), or goat- anti-mouse IgG, (Cell signaling, Cat#7076S) were used for the protein gel blots. Detection of Western blot was performed with Immobilon Western chemiluminescent HRP substrate (Merck Millipore, Cat# WBKLS#500).
Flow cytometry analysis
To assess the binding of antibodies to tumor cells, 1 × 106 of SKOV3, HT29, or LS174T cells were collected by centrifugation at 1000 rpm for 5 min, then washed with 1 ml ice-cold phosphate-buffered saline (PBS) + 0.1% BSA. Cell pellets were resuspended in 100 µl ice-cold PBS + 0.1% BSA with 50 µg/ml of SS-Fc. An aliquot of anti-flag-FITC (Sigma–Aldrich, F4049) was incubated with the cells on ice for 1 h. Flow cytometry analysis was performed after washing the cells twice. As positive controls, an anti-CEA antibody (Cell Signaling Technology, Cat# 2383S) and anti-mouse IgG Fluorescein Conjugated Goat F(ab’)2 (R&D systems, Cat# F01038,) antibodies were used.
Surface plasmon resonance (SPR) assay
To detect the binding of SS-Fc to CE and CD16 antigens, CEA protein (US Biological, Swampscott, MA), or CD16 protein (ACRO Biosystems, Cat#CDA-H5220), was immobilized on a CM5 sensor chip (GLC chip, Bio-Rad) according to a standard amine coupling procedure using an amine coupling kit (Biosense, Milan, Italy). After immobilization, an interaction analysis with different samples was performed at 25°C with PBST (10 mM Na3PO4, 150 mM NaCl, 0.01% Tween, pH = 7.4) as running buffer at a flow rate of 30 μl/min. Kinetic data were collected in duplicate for each antibody concentration, which had association and dissociation times of 120 and 180 sec, respectively. Data were collected using a ProteOnTM XPR 36 (Bio-Rad) and were analyzed to fit to an appropriate binding model using Protein On evaluation software (Bio-Rad). Positive control anti-CEA mouse mAb (#2383, Cell Signaling) was used to optimize this process.
Cytotoxicity assays
In vitro cytotoxicity assays were performed as described previously.13 In brief, SKOV3, HT29, and LS174T cells (5,000 cells per well in a 96-well plate) were used as target cells. Human PBMCs or isolated NK cells (50,000 cells per well) were used as effector cells with the indicated concentrations of added antibodies. After a 48-h incubation, the PBMC or NK cells were gently washed off with PBS 3 times, then live cells were quantified using the CCK8 reagent (Dojindo, CK04). Measurements were performed using TECAN Infinite F50 by Magellan software. The survival rate (%) of target cells was calculated using the following formula: [(living target cells (sample)–medium with NK cells only)/(living target cells (control)–medium with NK cells only)]×100.
In vivo efficacy studies
LS174T human colon carcinoma cells were harvested from cell culture, washed once with PBS, and then were resuspended in PBS. Cells were then mixed with freshly isolated human PBMCs to create cell suspensions (0.4 ml per mouse, a mixture of 1 × 106 LS174T cells and 5 × 106 PBMCs in PBS). Cells were then injected subcutaneously in the right flank of NOD-SCID mice. Proteins or vehicle control (PBS) were administered intraperitoneally (i.p.) 1 h after engraftment. Animals were then treated daily for 7 days, and the tumor volume was measured daily.
Results and discussion
SS-Fc bispecific antibody can be expressed and purified from E. coli as a dimer
A bispecific SS-Fc antibody was designed by linking an anti-CEA single domain VHH with the human IgG1 Fc fragment that contained the knob mutation (T366W) and an anti-CD16 single domain antibody with the human IgG1 Fc that contained the hole mutations (T366S, L368A and Y407V)9,10 (Fig. 1a). Anti-CEA VHH can be used to target cancer cells given that CEA is often overexpressed in several types of tumor of epithelial origin, and anti-CEA antibodies and their derivatives have been used in clinical radioimmunotherapy.14,15 Anti-CD16 VHH12 was used to engage immune cells, as FcγRIII (CD16) is a trans-membrane protein that is mainly expressed on NK cells and functions as one of the IgG receptors.16
To facilitate the expression and purification of SS-Fc, the 2 constructs, anti-CEA-Fc and anti-CD16-Fc, were cloned into 2 separate plasmids and then transformed into E.coli together. Periplasmic fractions of SS-Fc were purified using a rProteinA affinity chromatography (Fig. 1b). To determine whether SS-Fc folds correctly as a heterodimer, gel filtration was used to analyze the purified proteins. The majority of SS-Fc protein ran as a single peak at ∼80 kDa, which was the expected molecular weight of the heterodimerized protein of anti-CEA-Fc and anti-CD16-Fc (Fig. 1c). Western blots using anti-His or anti-Flag antibodies confirmed the presence of both anti-CEA-Fc and anti-CD16-Fc in the complex. Anti-CEA-Fc or anti-CD16-Fc alone were also expressed and purified using similar methods. The purified proteins are most homeodimers, suggesting that without the other pair of hole-Fc or knob-Fc, majority of Fc mutants still form a hoemodimer (data not shown).
The purified ss-Fc was also stable in vitro as no obvious degradation was observed even after 2 weeks incubation with human serum at 37°C in vitro (data not shown).
SS-Fc recognizes CEA and CD16 antigen
To determine whether SS-Fc binds to the tumor antigen CEA, western blotting was performed using purified anti-CEA-Fc, anti-CD16-Fc, or SS-Fc. Similar to a control anti-CEA antibody, both anti-CEA-Fc and SS-Fc specifically recognized CEA expression on LS174T, HT29, or SKOV3 cells transfected with CEA, but not anti-CD16-Fc (Fig. 2a). Furthermore, LS174T cells exhibited higher CEA expression than HT29 cells. No CEA expression was observed on SKOV3 cells, which is consistent with previous studies.13
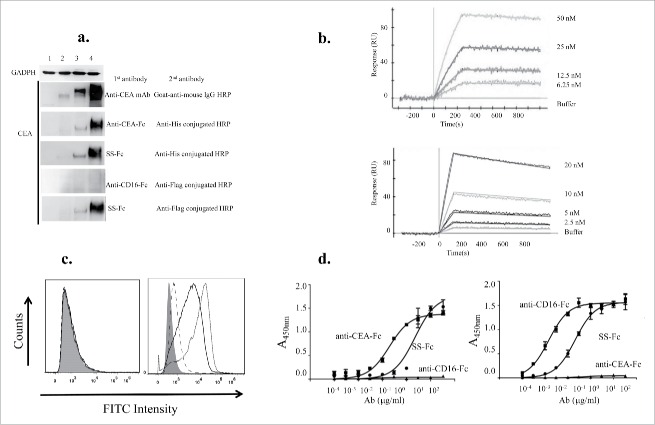
Figure 2.
Binding of the SS-Fc bispecific antibody to CEA and CD16. (a) Western blots were performed for different protein samples (1. SKOV3; 2, SKOV3 transfected with CEA; 3, HT29; and 4, LS174T) using control mouse anti-CEA antibody, anti-CEA-Fc, anti-CD16-Fc, or SS-Fc. Anti-His or anti-flag conjugated-HRP antibodies were used as secondary antibodies for detecting anti-CEA-Fc, anti-CD16-Fc, or SS-Fc. (b) SPR analysis of interactions between CEA (top panel), CD16 (lower panel), and SS-Fc was described as in the Materials and Methods. (c) Flow cytometry analysis of CEA expression on SKOV3 cells (left panel) and SKOV3 cells transfected with CEA (right panel). Filled gray: blank, no antibodies; dashed line: only anti-Flag-FITC; dotted line: primary antibody, anti-CEA-Fc, secondary antibody, anti-Flag-FITC; solid line: primary antibody, ss-Fc, secondary antibody, anti-Flag-FITC. (d) Elisa analysis of different proteins binding to either CEA (left) or CD16a (right). The data are the mean of triplicates with error bars representing the standard deviation.
To determine whether the SS-Fc construct compromises the affinity of anti-CEA VHH to CEA antigen. We also measured the interaction between the SS-Fc and CEA antigen using a SPR approach. Strong binding of the SS-Fc to CEA was observed with the association rate Ka (M−1s−1) at 1.77 × 105 and dissociation rates Kd (s−1) at 3.45 × 10−5 (Fig. 2b, top panel). The Kd value of 0.195nM is similar to previous studies using a Fab-based anti-CEA bispecific antibody.13 Furthermore, flow cytometry analysis also revealed that both SS-Fc and anti-CEA-Fc can bind to CEA positive cell line LS174T (Fig. 2c).
We also measured the interaction between the SS-Fc and CD16 using a SPR approach. Strong binding of the SS-Fc to CD16 was also observed with the association rate Ka (M−1s−1) at 5.58 × 104 and dissociation rates Kd (s−1) at 3.21 × 10−4 (Fig. 2b, lower panel). The Kd value of 5.75 nM is also similar to previous studies using a Fab-based anti-CD16 bispecific antibody.13
Interactions between the SS-Fc and CEA or CD16 were also measured using an Elisa method. Strong binding of the SS-Fc to CEA or CD16 was observed with association values at 28 nM and 2.59 nM respectively (Fig. 2d), though slightly weaker than the anti-CEA-Fc (left) or anti-CD16-Fc (right) alone with association values at 4.9 nM and 0.35 nM respectively. This is likely due to the single-valent binding of SS-Fc with CEA or CD16 binding sites comparing with bi-valent binding of anti-CEA-Fc to CEA, and anti-CD16-Fc to CD16. As a negative control, anti-CD16-Fc or anti-CEA-Fc has no binding on CEA or CD16 (Fig. 2d). These data confirmed that SS-Fc binds to both CEA and CD16 through its specific anti-CEA and anti-CD16 arm.
SS-Fc mediates NK cell and bispecific antibody dependent cytotoxicity
To determine whether SS-Fc can engage NK cells to kill tumor cells, in vitro cytotoxicity assays were performed using CEA positive cancer cells and freshly isolated NK cells at a 10:1 ratio of NK cells to cancer cells. For the CEA-negative cell line SKOV3, no cell killing was observed using anti-CEA-Fc, anti-CD16-Fc, or SS-Fc (Fig. 3a). Potent cytotoxic activities were observed for CEA-positive HT29 and LS174T cells when cancer cells were incubated with SS-Fc for at least 48hrs (Fig. 3a). With E:T = 1:1 or 5:1, ss-Fc showed much lower cytotoxicity assay (data not shown). With shortened incubation time, for example, 4 hours incubation, no cytotoxicity was observed (data not shown), suggesting that enough NK cells and longer incubation are necessary for tumor cell killing events.
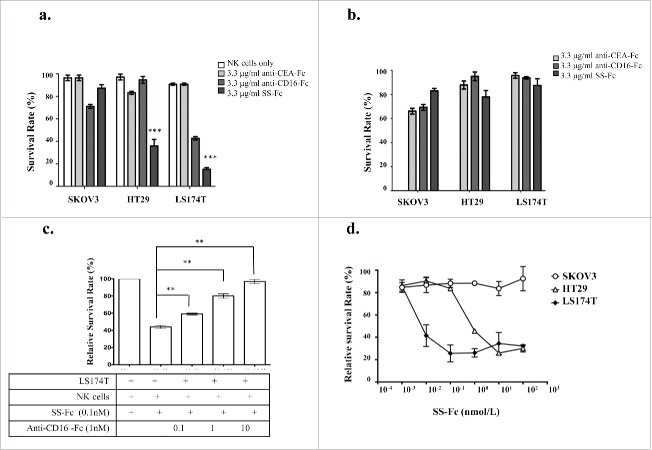
Figure 3.
SS-Fc mediates NK cell-dependent tumor cell killing. (a) The tumor cell lines SKOV3, HT29, and LS174T were incubated with 3.3 μg/ml anti-CEA-Fc, anti-CD16-Fc, or SS-Fc antibodies and freshly isolated human NK cells at a ratio of 10:1 (human NK cells 50,000 cells per well, tumor cells 5,000 cells per well on 96 well plates). The cytotoxicity assay was then performed as described in the Materials and Methods. The data are the mean of triplicates with error bars representing the standard deviation (*** stands for P < 0.001 by t-test, SS-Fc vs. control, anti-CEA-Fc, or anti-CD16-Fc). (b) The tumor cell lines SKOV3, HT29, and LS174T were incubated with 3.3 μg/ml anti-CEA-Fc, anti-CD16-Fc, or SS-Fc antibodies without human NK cells. The cytotoxicity assay was then performed as described in the Materials and Methods. (c) A competition assay was performed by incubating SS-Fc with LS174T cells and NK cells (5000 cells per well and 50,000 cells per well, respectively) with different amounts of anti-CD16-Fc. The cytotoxicity assay was performed as described in the Materials and Methods. The data are the mean of triplicates with error bars representing the standard deviation (** stands for P < 0.01 by t-test, no addition of anti-CD16-Fc vs. different concentrations of anti-CD16-Fc). (d) Dose-response analysis of SS-Fc with HT29 (open square), LS174T (open triangle), and SKOV3 (open diamond) cells. The survival rates at each SS-Fc concentration were normalized against tumor cells incubated with NK cells but without SS-Fc. Representative data from one of 4 different donors were shown here. The data are the mean of triplicates with error bars representing the standard deviation.
For HT29 and LS174T cells, anti-CEA-Fc alone does not affect cancer cell survival. Depending on the NK cells from different donors, there are variations in terms of tumor cell responding to anti-CD16-Fc. We have observed most no or partial effect of anti-CD16-Fc from different donors (data not shown). Even for NK cells that have partial cytotoxic effect by anti-CD16-Fc, the cytotoxicity activity of anti-CD16-Fc is much lower than the cytotoxicity of SS-Fc (Fig. 3a).
The cytotoxic activity of SS-Fc also depends on NK cells given that anti-CEA-Fc, anti-CD16-Fc, or SS-Fc had no effect on tumor cells in the absence of NK cells (Fig. 3b). Furthermore, when the amount of anti-CD16-Fc was increased in the SS-Fc mediated cytotoxicity assays, the cytotoxic activity of SS-Fc was reduced (Fig. 3c). This result suggests that anti-CD16-Fc competes with SS-Fc for NK cell binding. When increased anti-CD16-Fc protein decreases NK cell binding by SS-Fc, it reduces the cytotoxic activity of SS-Fc.
To further evaluate the activity of SS-Fc on tumor cells, dose-responses of SS-Fc on cancer cells were measured. SS-Fc triggered strong cytotoxicity against HT29 and LS174T cells in a dose-dependent manner, but had no effect on CEA-negative SKOV3 cells irrespective of the SS-Fc concentration (Fig. 3d). LS174T cells are more efficiently responding to SS-Fc than HT29, most likely due to increased CEA expression on LS174T cells (Fig. 2d), consistent with previous discovery which the cytotoxicity of a BITE antibody (anti-CEA and anti-CD3) correlated with the expression of CEA on tumor cells.17
SS-Fc inhibits tumor growth in xenograft model
To determine whether SS-Fc inhibits tumor growth in vivo, LS174T cells with or without PBMCs were transplanted into NOD/SCID mice. Mice were then treated with SS-Fc antibodies. No tumor growth inhibition was observed when tumors were treated with PBMC only. In mice treated with PBMCs and low-dose SS-Fc (5 µg per mouse), tumor developed in only 2 of 5 mice after a long latency period (Fig. 4a). No tumor growth was observed with a higher dosage (20 µg per mouse), even 41 d after transplantation. These data demonstrate that SS-Fc inhibits tumor growth in vivo using xenograft mouse models.
Figure 4.
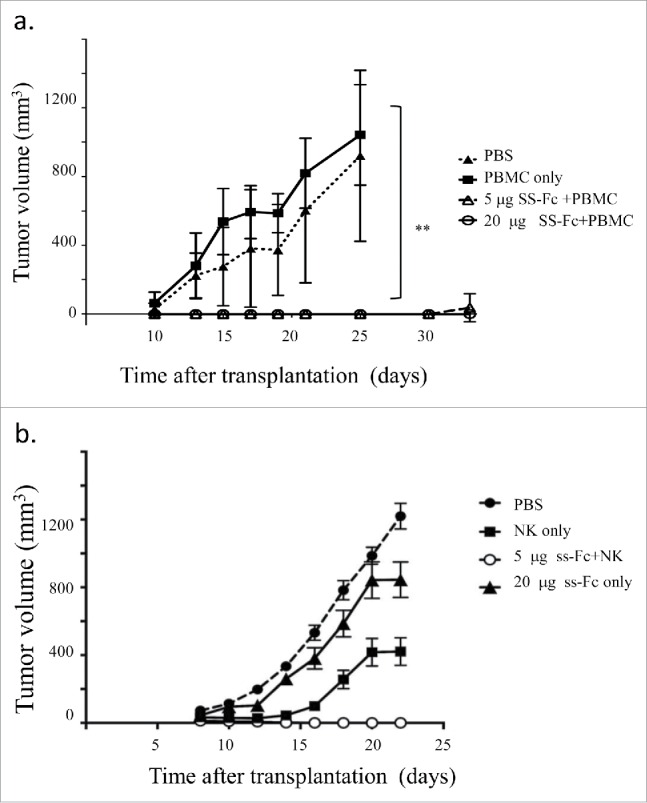
SS-Fc inhibits tumor growth in vivo. In vivo efficacy studies were performed as described in the Materials and Methods. NOD/SCID mice (n = 5 per group) were engrafted subcutaneously with LS174T cells with or without human PBMCs. (a) Mice were then treated with PBS (solid diamond, PBS treatment, no PBMC transplant); PBMC alone (solid square, PBS treatment, PBMC transplant); SS-Fc (5 μg per mouse, open circle, 20 μg per mouse, open square, with PBMC transplant). Data represent the average tumor volume of 5 mice for each treatment group with error bars representing the standard deviation (** stands for P < 0.01 by t-test, SS-Fc (either 5 μg or 20 per μg mouse) vs. control or PMBC only). (b) Mice were treated with PBS (solid circle, PBS treatment, no NK cell transplant); NK cells alone (solid square, PBS treatment, NK cell transplant); or ss-Fc with NK cells (5 μg per mouse, open circle, NK cell transplant); or ss-Fc only (20 μg per mouse, solid triangle, no NK cell transplant). Data represent the average tumor volume of 7 mice for each treatment group with error bars representing the standard deviation.
NK cells, instead of PBMCs, were then used for the xenograft studies. Similar to using PBMCs, SS-Fc antibody (5 µg per mouse) with NK cells can completely inhibit tumor growth (Fig. 4b). Complete tumor growth inhibition was also observed for SS-Fc antibody (20 µg per mouse) with NK cells (data not shown). Partial tumor inhibition was observed with NK cells treatment only. In contrast, minimal tumor inhibition was observed for ss-Fc without NK cells (Fig. 4b). Furthermore, anti-CD16-Fc (20 µg per mouse) or anti-CEA-Fc (20 µg per mouse) had no effect on tumor growth even with the co-transplantation of PBMCs or NK cells (data not shown). These data suggested that both ss-Fc and NK cells are required for the tumor inhibition in xenograft models.
Discussion
Herein, we present a novel bispecific antibody format, SS-Fc, for cancer immunotherapy. The SS-Fc bispecific antibody can be expressed and purified from E. coli. Through its 2 monovalent anti-CD16 VHH and anti-CEA VHH moieties, SS-Fc recruits NK cells and triggers cytotoxicity in CEA-positive cancer cells. In mice harboring CEA-positive cell LS174T xenografts, tumor growth is suppressed by SS-Fc in the presence of PBMCs.
The ability of bispecific antibodies to recruit immune cells, especially T cells and NK cells that can directly kill tumor cells has generated great interest in their use for cancer immunotherapy.1-3,18 Many bispecific antibody formats have been proposed and studied for their novel modes of function.1,3 Recently, blinatumomab, a bispecific antibody that targets CD19 and CD3, has been approved in the clinic for use against B cell leukemia.19 However, single-chain Fv fusions, used for blinatumomab and many other similar bispecific antibody formats, have very short half-lives in vivo given the lack of an Fc domain. To increase the half-life of bispecific antibodies, many formats have been proposed for building bispecific antibodies with an Fc by facilitating heterodimerization and minimizing the homodimerization of Fc fragments.9,20-23
In this study, we used the knob-into-holes strategy to promote heterodimerization.10 The overall structure of SS-Fc is similar to that of natural camel heavy chain IgG,8 which has 2 identical single-domain VHHs linked by homerdimerized Fc fragments. However, to create the bispecific antibody SS-Fc, 2 specific single-domain VHHs were linked with the knobs-into-holes mutations in the CH3 domain.9,10 The Fc heterodimerization leads to the bispecific antibody SS-Fc. Besides creating the bispecific antibody and extending the half-life of antibody, IgG1 Fc can also interact with high affinity Fc receptors, such as CD64 expressed by macrophages. However, 2 lines of evidence suggest that SS-Fc may not be able to induce cytotoxicity other than binding of CD16 on NK cells. First, both in vivo and in vitro experiments using anti-CD16-Fc (Fig. 3 and Fig. 4), or anti-CEA-Fc (Fig. 3) have not induced significant cell killing. Secondly, though previous studies suggested anti-CEA antibody with Fc can have potential clinical benefit,24 anti-CEA antibodies have not been able to kill tumor cells in vitro directly. Thirdly, the aglycosylated Fc in the SS-Fc suggests minimal binding or activation through Fc as aglycosylated Fc purified from E.coli are not being able to bind effector Fc Receptors.25
Another advantage of SS-Fc involves its simplicity. The correct light chain pairing based on human IgG structures is important for binding antigens. Various approaches to increase the correct pairing of light chains to heavy chains have been explored.11,26-28 However, it remains challenging to obtain correct light chain pairing with heavy chain to have optimal antigen binding. No light chain pairing issues were present in SS-Fc as single domain VHHs were used in SS-Fc.
In addition to the SS-Fc that was presented in this study, single-domain VHHs have been used as building blocks in the format of nanobodies13 because VHHs are stable and exhibit no light chain paring issues. One potential issue of VHHs in patients involves immunogenicity. However, the sequences of single-domain antibodies are very similar to those of human antibodies,8,11,28,29 and humanization of VHH can also help to eliminate immunogenicity,30 even if immunogenicity becomes a problem.
Compared with other bispecific formats, the manufacture of SS-Fc is considerably easier as most bispecific formats need to be expressed in mammalian cell culture, which could be potentially difficult. In this study, the SS-Fc was expressed and purified in E. coli at yields of 0.2 to 1 mg per liter without optimizing culture conditions. The two chains of anti-CEA-Fc and anti-CD16-Fc can be expressed in the same bacterial cells and were produced at reasonably equivalent amounts. Comparing to the bispecific antibodies with natural IgG1 format using knob-in-the-hole mutants,31 the purification process of SS-Fc is much easier without separated culture, refolding, and mixing processes. Although the exact percentage of SS-Fc heterodimerization was not clear in this study, our gel filtration (Fig. 1) and LC-MS studies (data not shown) suggested that majority of purified SS-Fc protein was in the form of heterodimer. Moreover, the potent bispecific antibody activity of SS-Fc (Fig. 3) suggests that majority of purified SS-Fc protein was in the form of heterodimer. However, caution should be taken because different VHH-CH2-CH3 proteins can behave differently.
In summary, the SS-Fc bispecific antibody maintains a natural Fc fragment to enhance its in vivo half-life. This antibody has good manufacturability and potent anti-tumor activity. In addition to its use as a bispecific antibody for immunotherapy, this format can also be universally applied to produce bispecific antibodies with other modes of action. With a variety of bispecific antibody formats proposed, it will be interesting to assess how different formats of bispecific antibodies perform in the clinic. Combinations of a variety of factors, such as types of diseases, antigen variation, available antibodies, and manufacturing feasibility, will likely influence the choice of bispecific antibody format.
Conclusion
A novel bispecific antibody, SS-Fc, was constructed and studied for its potential application in cancer immunotherapy. SS-Fc pairs 2 different single-domain antibodies, anti-CD16 VHH and anti-CEA VHH, by IgG1 Fc heterodimerization. The SS-Fc bispecific antibody can be soluble expressed in E. coli. SS-Fc exhibits potent cytotoxicity against cancer cells with carcinoembryonic antigen (CEA) expression by engaging NK cells. In xenograft models, SS-Fc also has potent anti-tumor effect. Thus SS-Fc represents a novel bispecific antibody format that can have broad applications in research and clinic.
Disclosure of potential conflicts of interest
The authors declare no conflict interests, including financial and non-financial interests.
Acknowledgment
We are grateful for Dr. Mengfeng Li and Dr. Jiang Li at Sun Yat-Sen University School of Medicine, Dr. Wei Xie at Sun Yat-Sen University School of Life Sciences, Cui Liu at Sun Yat-Sen University School of Pharmaceutical Sciences, and Dr. Lijian Hui at Institute of Biochemistry and Cell Biology Shanghai Institutes for Biological Sciences (SIBCB), Shanghai, China for their technical supports and valuable discussions.
Funding
The financial support for this work was from Introduced Innovative R&D Team Leadership of Guangdong Province (PR China) (2011Y038).
Authors contributions
JL, CZ, BD, ZH and JM carried out the experiments studies. JL, QL and ZW conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Reference
- 1.Byrne H, Conroy PJ, Whisstock JC, O'Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 2013; 31:621-32; PMID:24094861; http://dx.doi.org/ 10.1016/j.tibtech.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PC, Andrews SF. Tools to therapeutically harness the human antibody response. Nat Rev Immunol 2012; 12:709-19; PMID:23007571; http://dx.doi.org/ 10.1038/nri3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs 2012; 4:182-97; PMID:22453100; http://dx.doi.org/ 10.4161/mabs.4.2.19000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huston JS, Mudgett-Hunter M, Tai MS, McCartney J, Warren F, Haber E, Oppermann H. Protein engineering of single-chain Fv analogs and fusion proteins. Method Enzymol 1991; 203:46-88; PMID:1762568; http://dx.doi.org/ 10.1016/0076-6879(91)03005-2 [DOI] [PubMed] [Google Scholar]
- 5.Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today 2005; 10:1237-44; PMID:16213416; http://dx.doi.org/ 10.1016/S1359-6446(05)03554-3 [DOI] [PubMed] [Google Scholar]
- 6.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321:974-7; PMID:18703743; http://dx.doi.org/ 10.1126/science.1158545 [DOI] [PubMed] [Google Scholar]
- 7.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493-8; PMID:21576633; http://dx.doi.org/ 10.1200/JCO.2010.32.7270 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VK, Su C, Muyldermans S, van der Loo W. Heavy-chain antibodies in Camelidae; a case of evolutionary innovation. Immunogenetics 2002; 54:39-47; PMID:11976790; http://dx.doi.org/ 10.1007/s00251-002-0433-0 [DOI] [PubMed] [Google Scholar]
- 9.Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol 1998; 16:677-81; PMID:9661204; http://dx.doi.org/ 10.1038/nbt0798-677 [DOI] [PubMed] [Google Scholar]
- 10.Ridgway JB, Presta LG, Carter P. 'Knobs-into-holes' engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617-21; PMID:8844834; http://dx.doi.org/ 10.1093/protein/9.7.617 [DOI] [PubMed] [Google Scholar]
- 11.Behar G, Chames P, Teulon I, Cornillon A, Alshoukr F, Roquet F, Pugniere M, Teillaud J-L, Gruaz-Guyon A, Pelegrin A, Baty D. Llama single-domain antibodies directed against nonconventional epitopes of tumor-associated carcinoembryonic antigen absent from nonspecific cross-reacting antigen. FEBS J 2009; 276:3881-93; PMID:19531051; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07101.x [DOI] [PubMed] [Google Scholar]
- 12.Behar G, Siberil S, Groulet A, Chames P, Pugniere M, Boix C, Sautes-Fridman C, Teillaud JL, Baty D. Isolation and characterization of anti-Fc gamma RIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Eng Des Sel 2008; 21:1-10; PMID:18073223; http://dx.doi.org/23757164 10.1093/protein/gzm064 [DOI] [PubMed] [Google Scholar]
- 13.Rozan C, Cornillon A, Petiard C, Chartier M, Behar G, Boix C, Kerfelec B, Robert B, Pelegrin A, Chames P, et al. Single-domain antibody-based and linker-free bispecific antibodies targeting FcgammaRIII induce potent antitumor activity without recruiting regulatory T cells. Mol Cancer Ther 2013; 12:1481-91; PMID:23757164; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-1012 [DOI] [PubMed] [Google Scholar]
- 14.Pavoni E, Flego M, Dupuis ML, Barca S, Petronzelli F, Anastasi AM, D'Alessio V, Pelliccia A, Vaccaro P, Monteriu G, et al. Selection, affinity maturation, and characterization of a human scFv antibody against CEA protein. BMC Cancer 2006; 6:41; PMID:16504122; http://dx.doi.org/ 10.1186/1471-2407-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong H, Sun J, Cai W. Radionuclide-Based Cancer Imaging Targeting the Carcinoembryonic Antigen. Biomark Insights 2008; 3:435-51; PMID:19578524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siberil S, Dutertre CA, Boix C, Bonnin E, Menez R, Stura E, Jorieux S, Fridman WH, Teillaud JL. Molecular aspects of human FcgammaR interactions with IgG: functional and therapeutic consequences. Immunol Lett 2006; 106:111-8; PMID:16797726; http://dx.doi.org/ 10.1016/j.imlet.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Oberst MD, Fuhrmann S, Mulgrew K, Amann M, Cheng L, Lutterbuese P, Richman L, Coats S, Baeuerle PA, Hammond SA. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs 2014; 6:1571-84; PMID:25484061; http://dx.doi.org/ 10.4161/19420862.2014.975660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kontermann RE. Alternative antibody formats. Curr Opin Mol Ther 2010; 12:176-83; PMID:20373261 [PubMed] [Google Scholar]
- 19.Sanford M. Blinatumomab: first global approval. Drugs 2015; 75:321-7; PMID:25637301; http://dx.doi.org/ 10.1007/s40265-015-0356-3 [DOI] [PubMed] [Google Scholar]
- 20.Elliott JM, Ultsch M, Lee J, Tong R, Takeda K, Spiess C, Eigenbrot C, Scheer JM. Antiparallel Conformation of Knob and Hole Aglycosylated Half-Antibody Homodimers Is Mediated by a CH2-CH3 Hydrophobic Interaction. J Mol Biol 2014; 426:1947-57; PMID:24576605; http://dx.doi.org/ 10.1016/j.jmb.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 21.Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637-46; PMID:20400508; http://dx.doi.org/ 10.1074/jbc.M110.117382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, Huston JS. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23:195-202; PMID:20299542; http://dx.doi.org/21690412 10.1093/protein/gzp094 [DOI] [PubMed] [Google Scholar]
- 23.Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A 2011; 108:11187-92; PMID:21690412; http://dx.doi.org/ 10.1073/pnas.1019002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein R, Goldenberg DM. A humanized monoclonal antibody to carcinoembryonic antigen, labetuzumab, inhibits tumor growth and sensitizes human medullary thyroid cancer xenografts to dacarbazine chemotherapy. Mol Cancer Ther 2004; 3:1559-64; PMID:15634649 [PubMed] [Google Scholar]
- 25.Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, Georgiou G. Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A 2010; 107:604-9; PMID:20080725; http://dx.doi.org/ 10.1073/pnas.0908590107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A 2013; 110:5145-50; PMID:23479652; http://dx.doi.org/ 10.1073/pnas.1220145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol 1995; 155:219-25; PMID:7602098 [PubMed] [Google Scholar]
- 28.Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LGJ, Muyldermans S, Wyns L, Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci 2002; 11:500-515; PMID:11847273; http://dx.doi.org/ 10.1110/ps.34602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su C, Nguyen VK, Nei M. Adaptive evolution of variable region genes encoding an unusual type of immunoglobulin in camelids. Mol Biol Evolut 2002; 19:205-15; PMID:11861879; http://dx.doi.org/ 10.1093/oxfordjournals.molbev.a004073 [DOI] [PubMed] [Google Scholar]
- 30.Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem 2009; 284:3273-84; PMID:19010777; http://dx.doi.org/ 10.1074/jbc.M806889200 [DOI] [PubMed] [Google Scholar]
- 31.Spiess C, Merchant M, Huang A, Zheng Z, Yang N-Y, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, Scheer JM. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol 2013; 31:753-8; PMID:23831709; http://dx.doi.org/ 10.1038/nbt.2621 [DOI] [PubMed] [Google Scholar]