Abstract
Expressions of many microRNAs (miRNAs) in response to ionizing radiation (IR) have already been investigated and some of them seem to play an important role in the tumor radioresistance, normal tissue radiotoxicity or as predictive biomarkers to radiation. MiR-34a is an emerging miRNA in recent radiobiology studies. Here, we review this miR-34 family member by detailing its different roles in radiation response and we will discuss about the role that it can play in radiation treatment. Thus, we will show that IR regulates miR-34a by increasing its expression. We will also highlight different biological processes involved in cellular response to IR and regulated by miR-34a in order to demonstrate the role it can play in tumor radio-response or normal tissue radiotoxicity as a radiosensitizer or radioprotector. MiR-34a is poised to assert itself as an important player in radiobiology and should become more and more important in radiation therapy management.
Keywords: miR-34a, ionizing radiation, radiotherapy, biodosimetry, radiotoxicity, biomarkers, oncology
1.Introduction
MicroRNAs (miRNAs) are a family of 21–25-nucleotide small RNAs that initially were discovered as regulators for the timing of Caenorhabditis elegans development (Lee et al., 1993; Wightman et al., 1993; Reinhart et al., 2000; Pasquinelli et al., 2000). Since, discoveries showed that the functions of miRNAs are not limited to the regulation of developmentally timed events, but regulate many others aspects of biological processes in animals and plants (Bartel, 2009; He and Hannon, 2004) such as cell death, cell proliferation or hematopoiesis and patterning of the nervous system (Ambros, 2004). Consequently, these last few years, many studies have investigated the role of miRNAs in cancer and have shown that miRNAs can repress the expression of cancer-related genes, making them functioning as tumor suppressors or oncogenes (Esquela-Kerscher and Slack, 2006; Lin and Gregory, 2015). MiRNA-expression profiling of human tumors has identified signatures associated with diagnosis, staging, progression, prognosis and response to treatment (Calin and Croce, 2006). As such, miRNAs are promising therapeutic targets and might prove useful in the “war” against cancer.
With almost two-thirds of all cancer patients treated by any forms of radiation therapy during the course of treatment (Begg et al., 2011), radiation biology is a keystone for understanding radiation-related cellular events, and so improving radiotherapy efficiency while enabling more individualized treatment plans. Several methods can be used to improve the therapeutic index of radiotherapy such as complementary therapies by using radiosensitizing agents for creating a better tumor response, or selecting patients based on their intrinsic radiosensitivity or tumor radioresistance to tailor a personalized treatment (Lacombe et al., 2013). However, all these options need to study deeply the cellular response to ionizing radiation (IR). Hence, many studies have investigated the tumor radioresistance (Baumann et al., 2008) and normal tissue radiosensitivity (Barnett et al., 2009; Bentzen, 2006), usually focusing on genetic instability, microenvironment influence or protein alterations. With their large spectra of functions, miRNAs can regulate and interfere with all these processes. Some of them were already shown to be involved in many fields of radiation oncology (Czochor and Glazer, 2014; Korpela et al., 2015; Gandellini et al., 2014) such as potential predictive biomarkers of tumor radio-response, or as a target for treatments increasing radiosensitivity, thus offering interesting clinical perspectives in tumor radiotherapy (Zhao et al., 2012).
The 12 different let-7 family members (let-7a-1, 7a-2, 7a-3, 7b, 7c, 7d, 7e, 7f-1, 7f-2, 7g, 7i, and mir-98) have become the most studied miRNAs and they have attracted attention of researchers in various fields, such as developmental biology, stem cells biology, aging, and metabolism (Roush and Slack, 2008; Su et al., 2012). Consequently, this family has been also widely studied in radiation biology. Several studies showed the let-7 family of miRNAs is overrepresented in a class of miRNAs exhibiting altered expression in response to IR (Chaudhry, 2009; Simone et al., 2009; Weidhaas et al., 2007). Let-7g is particularly an important regulator since several studies have shown its expression could enhance the radiosensitivity (Wagner-Ecker et al., 2010) by suppression of KRAS (Jeong et al., 2009) and NFκB1 (Arora et al., 2011). Let-7a seems to play the same role as its overexpression also decreases expression of KRAS and it can radiosensitize lung cancer cells (Oh et al., 2010). As the let-7 family of miRNA is usually repressed following exposure to radiation in multiple cell lines, a recent study has investigated if this radiation-induced reduction in expression could be a part of the cellular response to oxidative stress (Saleh et al., 2011). Results showed a radiation-induced decrease in let-7a and let-7b expression in ATM and p53-positive cell lines, as well as in radiation-sensitive tissues in vivo. In contrast, this decreased expression is not observed in p53 knock-out mice suggesting that p53 directly repress let-7 expression. As the let-7 genes can also regulate the cellular response to stress, they could be good candidates for participating in radiation-induced bystander effect.
MiR-21 is another prevalent miRNAs and has emerged as the miRNA most frequently associated with cellular dysfunction, especially in many oncogenic pathways (Sicard et al., 2013). As it is also involved in apoptosis, cell cycle, DNA damage repair, autophagy or hypoxia, not unexpectedly, miR-21 is one of the most studied miRNA in radiation biology demonstrating its important role in the regulation of radiosensitivity and radioresistance (Liu et al., 2014). Indeed, miR-21 is presently known to promote the radioresistance of various cancers such as breast (Anastasov et al., 2012), glioma (Gwak et al., 2012), nasopharyngeal (Zhu et al., 2015) or lung cancers (Liu et al., 2013; J. Zhang et al., 2015), and its silencing is a promising alternative therapy for radiosensitizing tumors. Moreover, recent studies have shown that miR-21 is also involved in radiation-induced bystander effects (Xu et al., 2014), especially through the regulation of oxidative stress (Jiang et al., 2014; Tian et al., 2015). As bystanders effects play an important role in radiation-induced toxicity (Mothersill et al., 2004), miR-21 is also a promising target to decrease normal tissue radiosensitivity.
A few years ago, miR-34a has emerged in the field of radiobiology with many studies revealing its role in cellular response to IR, and its potential interest as a therapeutic target. In this paper, after a general introduction, we will emphasize miR-34a different roles in radiation response. First, we will review the effect of IR on its expression. Then, we will discuss about its influence on tumor radioresistance and its potential role as radiosensitizer. We will also highlight the different biological processes regulated by miR-34a and which of them may be involved in normal tissue radiation-induced toxicity in order to discuss the role of miR-34a as a therapeutic target or a radioprotector of radiation injury. Finally, we will conclude by discussing the future implication miR-34a could play in radiation therapy.
2. MiR-34a, a member of miR-34 family
MiR-34a is a member of the miR-34 family with two others members: miR-34b and miR-34c (Misso et al., 2014). MiR-34a is encoded by its own transcript on chromosome 1 (1p36.22) (Li et al., 2012) and is ubiquitously expressed at higher levels than miR-34b/c, in almost each tissue, with the exception of the lung tissue (Chen and Hu, 2012).
An important role of miR-34a consists on mediating p53 tumor suppressor function as p53 can induce expression of miR-34a in cultured cells (Chang et al., 2007) as well as in irradiated mice (Raver-Shapira et al., 2007). Therefore, miR-34a mainly induces p53-mediated apoptosis, cell-cycle arrest in the G1 phase and senescence (He et al., 2007a; Tarasov et al., 2007). However, it exists a positive feedback between p53 and miR-34a because miR-34a can also increases p53 protein levels and stability (Okada et al., 2014) while miR-34a knockdown diminishes p53 acetylation (Xiong et al., 2015). The complex positive and negative effects of miR-34 on the p53 network suggest that rather than simply promoting the p53 response, miR-34a might act at a systems level to stabilize the robustness of the p53 response to genotoxic stress (Navarro and Lieberman, 2015).
As described later in this paper, miR-34a also regulates a variety of target mRNAs involved in the cell cycle, cell proliferation, senescence, migration, and invasion, such as cyclin-dependent kinase 4/6 (CDK4/6), E2F transcription factor 3 (E2F3), Cyclin E2, hepatocyte growth factor receptor (MET), B-cell lymphoma 2 (Bcl-2), NAD-dependent deacetylase sirtuin-1 (SIRT1), Myc, Notch, and CD44 (Hermeking, 2009; Misso et al., 2014). As ectopic miR-34a induces tumor suppressive mechanisms linked to the inhibition of cancer regeneration, migration, and metastasis (i.e. G1-cell cycle arrest, senescence and apoptosis), not surprisingly, several studies have reported that miR-34a expression is silenced in several types of cancer (X. J. Li et al., 2014), especially due to aberrant CpG methylation of its promoter (Chim et al., 2010; Lodygin et al., 2008). Furthermore, miR-34a has also been associated with regulation of the cancer stem cells function in various cancer types such as prostate cancer, pancreatic cancer, medulloblastoma or glioblastoma (Misso et al., 2014).
3. Ionizing radiation increases miR-34a expression
In the recent era of big data several studies performed high-throughput screening analysis for investigating the effect of IR on miRNAs expression in several tissues and species. Some of these studies reveal a radiation-induced alteration of miRNA-34a expression (Table 1).
Table 1.
Overall screening analyses that showed an alteration of miRNA-34a expression after ionizing radiation.
| Species | Cell type | Cell lines | Irradiation dose (Gy) |
Collection time after radiation |
Screening method |
MiR-34a expression | Ref. |
|---|---|---|---|---|---|---|---|
| Human | Thyroid | Primary cells from biopsies |
1 & 10 | 4 & 24h | Microarray and qRT- PCR |
↑ at 4h after 1 & 10Gy ↓ at 24h after 1 & 10Gy |
(Nikiforova et al., 2011) |
| Human | Prostate | LNCaP PC3 |
1×5 & 1×10 10×0.5 & 10×1 |
24h after last dose |
Microarray and qRT- PCR |
↑ after 10×0.5 & 1Gy in LNCap ↑ after 10×1Gy in PC3 |
(John-Aryankalayil et al., 2012) |
| Human | Lung | A549 | 40 | 24h | Microarray | ↑ | (Shin et al., 2009) |
| Human | Lymphoblast | IM9 | 10 | 24h | Microarray | ↑ | (Cha et al., 2009) |
| Human | Coronary artery |
HCAEC | 1×10 & 5×2 | 6 & 24h after last dose |
Microarray | ↑ at 24h after 1×10Gy ↑ at 6 & 24h after 5×2Gy |
(Palayoor et al., 2014) |
| Human | Glioblastoma | A172 | 2×15 & 2×30 |
- | qRT-PCR | ↑ after 2×30Gy ↓ after 1×15Gy |
(Sasaki et al., 2012) |
| Human | Lymphoblast | TK6 | 2 | 4, 8, 12 & 24h | Sequencing | ↑ at 8 & 24h ↓ at 4 & 12h |
(Chaudhry et al., 2013) |
| Human | Lymphocyte | Whole blood |
0.2 & 2 | 4 & 24h | Microarray and qRT- PCR |
↑ at 24h after 0.2 & 2Gy ↓ at 4h after 0.2Gy |
(Girardi et al., 2012) |
| Human | T- lymphocyte |
Whole blood |
1, 2, 3, 4 & 5 |
15 & 30min, 1, 1.5, 2, 4, 5, 6 & 24 |
qRT-PCR | ↑ at 24h after 1, 2, 3, 4 & 5Gy ↑ at 3, 4, 5, 6 & 24h after 2Gy |
(Kabacik et al., 2015) |
| Mouse | Liver | Kunming | 4 (WBI) | 14 days | Sequencing and qRT- PCR |
↑ | (Lu et al., 2016) |
| Mouse | Skin Muscle Subcutaneous tissue |
CH3 | 35 (right hind leg) |
7, 14, 50, 90 & 120 days |
Microarray and qRT- PCR |
↑ at 7, 14 & 120 days | (Simone et al., 2014) |
| Mouse | Spleen | C57BL/6 | 2.5 (WBI) | 6h | Microarray and qRT- PCR |
↑ | (Ilnytskyy et al., 2008) |
| Rat | Retinal ganglion |
RGC-5 cells | 6 | 5 days | Microarray and qRT- PCR |
↑ | (Wang et al., 2015) |
| Rat | Lung | Wistar | 20 (right lung) |
12 weeks | Microarray | ↑ | (Xie et al., 2014) |
| Rat | Mammary gland |
Long-Evans | 0.1 & 2.5 (WBI) |
6 & 96h 4 & 24 weeks |
Microarray and qRT- PCR |
↑ at 96h & 24 weeks after 0.1 & 2.5Gy |
(Wang et al., 2013) |
| Rat | Mammary gland |
Long-Evans | 0.1 & 2.5 (WBI) |
96h | Microarray | ↑ after 0.1 & 2.5Gy | (Luzhna and Kovalchuk, 2014) |
WBI: Whole-Body Irradiation
Results from various experimental models comprising in vitro, in normal or cancer cell lines, or in vivo, independently of cells or tissue types (i.e. circulating cells or epithelial tissue), all show miR-34a expression level usually increased after IR. In particular, radiation-induced miR-34a expression seems to be delayed because its highest level is at 24 hours after IR while a decrease of its expression level may be observed only a few hours after IR. Only Nikifovora et al. showed the opposite trend of miR34a expression level increasing at 4 hours after IR and decreasing at 24 hours (Nikiforova et al., 2011). Moreover, the radiation dose has not any significant effect since irradiation doses ranging from 0.1Gy to 40Gy lead to an increase of miR-34a expression level. Finally, it is also important to note that other high-throughput screening studies assessing the changes in miRNAs expression after IR did not identify any perturbations in miR-34a level. As a majority of these studies used microarray technology, these observations could be explained by the common inherent problems encountered with miRNA microarray techniques, i.e a restricted linear range of quantification, imperfect specificity in some cases for miRNAs that are closely related in sequence, or lack of ability to perform absolute quantification of miRNA abundance easily (Pritchard et al., 2012). Studies which specifically focused on miR-34a also confirmed these observations. According to our bibliographic review, all data showed an increase of miR-34a expression at any doses, time points, and for any types of species (human or not) or tissues samples (Table 2). However, one study reported that miR-34 profiles were modified after similar conditions of IR exposure in HeLa cell culture but not in MCF-7 cell lines, suggesting that genotoxic stress may be cell-type specific (Mert et al., 2012). Moreover, the same study failed to find a modification of miR-34 expression under exposure to Bleomycin (a chemical agent often used to mimic IR effects due to its capacity to inducing DNA damages) suggesting the presence of a cell specific behavior in response to radiation injury (Cellini et al., 2014).
Table 2.
miRNA-candidate studies that showed an alteration of miRNA-34a expression after ionizing radiation.
| Species | Cell type | Cell lines | Irradiation dose (Gy) |
Collection time after radiation (h) |
Expression | Ref. |
|---|---|---|---|---|---|---|
| Human | Breast | HMEC | 0.1 & 2.5 | 6, 12, 24, 48 & 96 |
↑ at 96h after 0.1 & 2.5Gy | (Wang et al., 2013) |
| Human | Lymphoblast | TK6 NH32 WTK1 |
0.73, 1.5, 3 & 6 | 6, 12, 24, 48 & 72 |
↑ at 48h after all doses for TK6 ↑ at 48h after 3 & 6Gy for NH32 & WTK1 ↑ at 24 & 48h after 0.73Gy for TK6 |
(Chen et al., 2012) |
| Human | Nasopharynx | CNE1 CNE2 |
5×2 & 4×2.33 5×2 & 4×2.43 |
- | ↑ for all cell lines & all doses | (Long et al., 2014) |
| Human | Cervix Breast |
HeLa MCF-7 |
2 & 5 Gy | 24 | ↑ in HeLa after 2 & 5Gy | (Mert et al., 2012) |
| Human | Breast | MCF-7 MCF- 10A TD-47 |
0.003, 0.012, 0.048 & 5 |
4 & 24 | ↑ in MCF-10A at 4h after 4 all doses & at 24h after 0.012Gy ↑ in MCF-7 at 24h after 0.003Gy |
(Stankevicins et al., 2013) |
| Human | Lung | A549 | 4 | 6, 12, 18, 24 & 36 |
↑ at 18, 24 & 48h | (Salzman et al., 2016) |
| Human | Serum | In vivo | RT: 50Gy to the tumor (25×2Gy) |
24 after the end of RT |
↑ | (Halimi et al., 2016) |
| Mouse | Spleen | In vivo | 6 | 4 & 8 | ↑ | (He et al., 2007b) |
| Mouse | Spleen Thymus Liver Serum |
BALB/c C57BL/6 |
2, 7 & 12 (WBI) | 4, 8, 24 & 48 | ↑ in all 3 tissue at 8h after 7Gy ↑ in spleen & serum at 8h after 2, 7 & 12Gy ↑ in serum at 8h after 2, 7 & 12Gy ↑ in serum at 4, 8, 24 & 48h after 7Gy |
(Liu et al., 2011) |
| Human | Colon | HCT116 | 2, 4 & 8 | 4, 8, 24 & 48 | ↑ at 8h after 2, 4 & 8Gy ↑ at 4, 8 & 24h after 4Gy |
(Liu et al., 2011) |
| C. elegans | Wild-type N2 cep-1/p53 mutants |
In vivo | 200 (WBI) | 3, 6 & 9 | ↑ at 3h | (Kato et al., 2009) |
RT: Radiation treatment; WBI: Whole-Body Irradiation
Interestingly, increase of miR-34a expression could also be detected in biological fluids, e.g. in serum (Liu et al., 2011). Molecular 'liquid biopsies' are quickly moving into the clinic, creating a new palette of molecular diagnostics and becoming a central piece in the future of platform technology for precision medicine (Cai et al., 2015). Circulating biodosimetry markers collected from liquid biopsies are especially helpful to assess the dose of absorbed radiation in the case of an accidental exposure to IR requiring fast and efficient triage for guiding medical countermeasures. However, even if some recent studies have demonstrated that circulating miRNAs are potential biodosimetry markers in mouse model (Jacob et al., 2013) or human (Dinh et al., 2016; Summerer et al., 2013), any correlation between the delivered IR dose and miR-34a expression level could not be established so far..
MiR-34 induction post-radiation has been reported to depend on p53 induction. However, the totality of miR-34a studies, through different cell lines, show that miR-34a overexpression is induced by irradiation as in wild-type p53 cell lines (MCF-7, MCF-10A, HeLa, A549, HCT116, TK6) as in p53-mutated cell lines (WTK1, NH32, CNE1–2) (Table 2). These results are also supported by in vivo data in C. Elegans where miR-34 expression is radio-induced as in wild-type animals as in cep-1/p53 mutants (Kato et al., 2009). Hence, the miR-34a radio-induction appears more complex than expected and does not seem only p53-dependent. Nevertheless, it is interesting to note miR-34a is not radio-induced in totally p53-deficient mice highlighting the important role of p53 presence yet (He et al., 2007b). A recent report proposed a novel mechanism to explain how rapid miR-34 activation and a pool of mature miR-34 in cells that lacks a 5′-phosphate can be inactivated (Salzman et al., 2016). Following exposure to IR (4Gy), the inactive pool of miR-34 is rapidly activated through 5′-end phosphorylation in an ATM- and Clp1-dependent manner, enabling loading into Ago2. Importantly, this mechanism of miR-34 activation occurs faster than, and independently of, de novo p53-mediated transcription and processing. Note, although ultraviolet (UV) are non-ionizing radiations, and so out of the scope of this review, UVs seem to have the same effect as IR. Indeed, several publications which investigated miRNAs expression after UV exposure also highlighted an increase of miR-34a expression level in several cell lines (Table 3).
Table 3.
Studies that showed an alteration of miRNA-34a expression after non-ionizing ultraviolet radiation
| Species | Cell type | Cell lines |
Irradiation type |
Irradiation dose |
Collection time after radiation (h) |
MiR-34a expression | Ref. |
|---|---|---|---|---|---|---|---|
| Human | Keratinocytes | Primary | UVB | 30 mJ/cm2 | 4 & 24 | ↑ at 4 & 24h | (Zhou et al., 2012) |
| Human | Fibroblasts Cervix |
Primary HeLa |
UVC | 8 J/ cm2 | 4 & 24 | ↑ at 24h in fibroblasts | (Pothof et al., 2009) |
| Human | Liver | HepG2 | - | 50, 70 & 100 J/m2 |
2, 4 & 12 | ↑ after 50, 70 & 100 J/m2 ↑ at 2, 4 & 12h after 50 J/m2 |
(Liang et al., 2014) |
| Human | Cervix | HeLa | - | 20 J/ cm2 | 2, 4 & 8 | ↑ | (Pawlicki and Steitz, 2008) |
4. MiR-34a and tumor radiosensitivity
Reducing tumor radioresistance and improving tumor radiosensitivity are key challenges in clinical tumor radiotherapy. Many studies already showed that miRNAs levels are associated with cancer radioresistance (Metheetrairut and Slack, 2013).
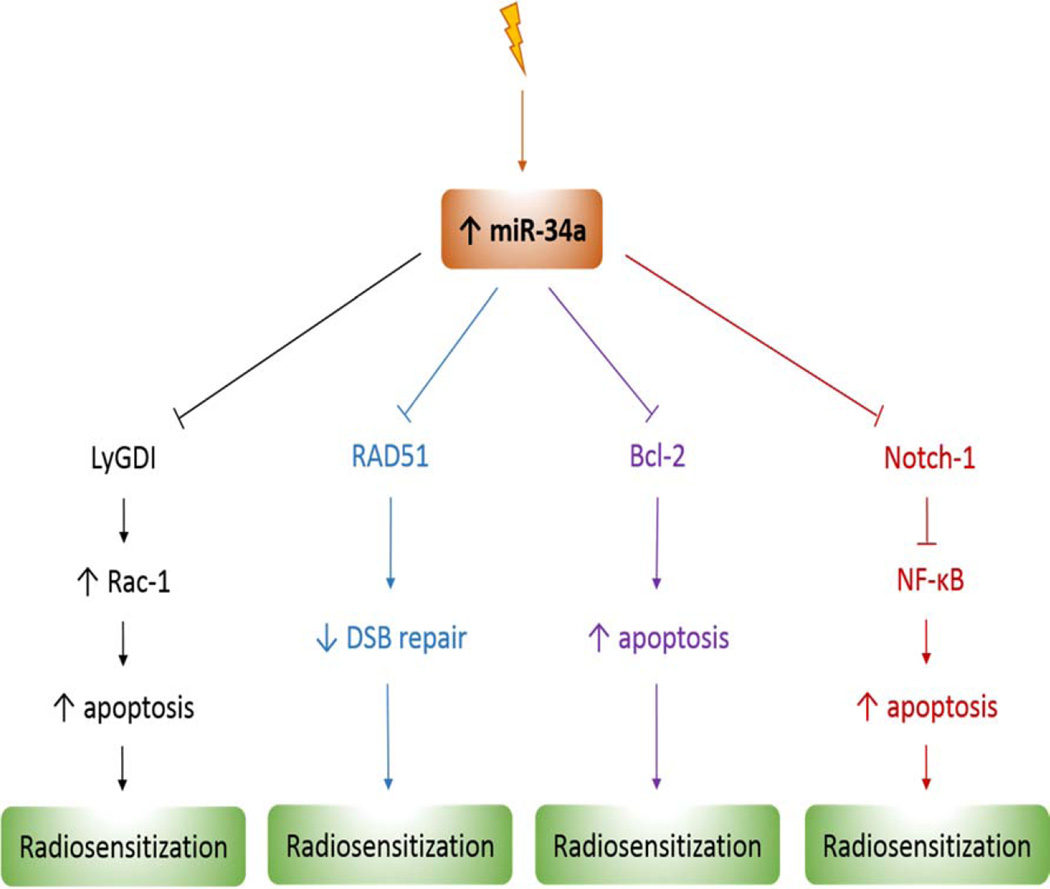
In the previous section we established that the level of expression of miR-34a is affected by irradiation, but this alone doesn’t demonstrate a causal role of miR-34a in the tumor radioresponse. However, other studies showed miR-34a could enhance radiosensitivity via several pathways (Figure 1).
Figure 1.
miR-34a targets which enhance tumor radiosensitivity. DSB: double-strand break
First, Liu et al. found that miR-34a played a critical role in radiosensitivity variations in different tissues by enhancing cell apoptosis and decreasing cell viability. These authors demonstrated that one possible downstream target of miR-34 leading to different radiosensitivity was Bcl-2 (Liu et al., 2011). When Bcl-2 expression is decreased by knockdown in brain glioblastoma and colon cancer cell lines, both the apoptosis is enhanced after IR and the radio-protective role of miR-34a inhibitor is attenuated.
A second study identified LyGDI as a target of miR-34a which could be involved in radiosensitivity regulation (Duan et al., 2013). In non-small cell lung cancer (NSCLC) cell lines with low miR-34a expression, it was shown that restoration of miR-34a expression inhibited cell growth, enhanced irradiation sensitivity, and downregulated LyGDI gene expression. Furthermore, this downregulation of LyGDI expression by miR-34a suppressed COX-2 expression, promoted Rac1 activation and membrane distribution resulting into IR-induced apoptosis enhancement and cellular radiosensitization.
There have also been many investigations related to the use of flavonoids as radiosensitizing agents. For example, Kang et al assessed the effect of two flavonoids drugs, rhamnetin or cirsiliol, in combination with radiation on NSCLC cells (Kang et al., 2013). It was shown that the drugs could reduce the cellular proliferation through the suppression of radiation-induced Notch-1 expression. Interestingly, rhamnetin or cirsiliol also increases the expression of miR-34a in a p53-dependent manner in NSCLC cells. Moreover, the expression of miR-34a mimics decreased Notch-1 protein levels and miR-34a, which could directly bind to the 3′-UTR of Notch-1, showing that miR-34a negatively regulate Notch-1 expression in NSCLC cells. As inhibition of Notch-1 expression increases apoptosis and radiosensitivity of NSCLC cells, taken together these results indicate miR-34a sensitizes NSCLC cells to radiation via Notch-1 pathway. Furthermore, the authors established miR-34a-mediated suppression of Notch-1 expression by rhamnetin and cirsiliol could also reduce epithelial-mesenchymal transition (EMT) in NSCLC cells. Finally, they confirmed the effect of the two drugs in vivo by showing correlated increase of radiosensitization and decrease of EMT in a xenograft mouse model.
Recently, a study identified RAD51 as a new direct target of miR-34a able to increase radiosensitivity in NSCLC cells (Cortez et al., 2015). The authors found that miR-34a overexpression promoted both the downregulation of RAD51 and the reduction in radiation-induced RAD51 foci formation. As overexpression of miR-34a also sensitized the cells to radiation by suppressing DNA repair, the authors confirmed that reintroduction of the RAD51 could rescue this phenotype. By examining the effect of miR-34a on radiosensitivity in two mouse models of human lung cancer, it was determined the delivery of miR-34a could be exploited therapeutically. Results indicated that the administration of miR-34a led to repression of RAD51 in lung tumors and consequently enhanced the effects of radiation on tumor growth inhibition.
All these results highlight the potential role of miR-34a to be a promising radiosensitizer. However, further studies are needed to investigate the role of miR-34a mimics or inhibitors by comparing the effects, in vitro, on a parental and radioresistant cell line after fractionnated irradiation or, in vivo, with mouse xenograft model that humanizes radioresistant/radiosensitive tumor cell lines.
5. MiR-34a and normal tissue radiation-induced toxicity
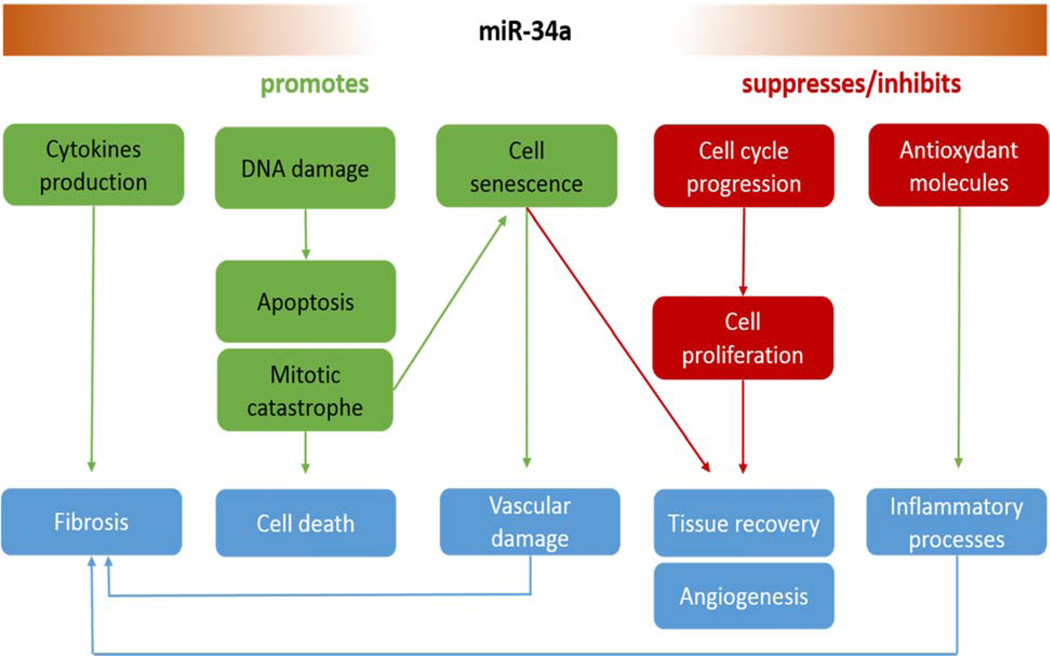
A key challenge in radiotherapy is to maximize radiation doses to cancer cells while minimizing damage to surrounding healthy tissue. As severe toxicity in a minority of patients limits the doses that can be safely given to the majority of the patients population, there is interest in better understanding the underlying mechanisms involved in the radiation-induced toxicity development in order to identify new molecules as therapeutic targets or to develop a test to measure an individual's radiosensitivity before treatment (Barnett et al., 2009). These underlying mechanisms involved in the pathogenesis of radiation-induced normal tissue injury have not been fully elucidated, but some evidences show that inflammation and chronic oxidative stress, depletion of tissue stem cells and progenitor cells or damage to microenvironment (i.e. vascular endothelial microvessels) could play an important role (Barker et al., 2015; Kim et al., 2014; Zhao and Robbins, 2009). Interestingly, several studies showed that miR-34a regulates some of these biological processes and so, could be involved in the establishment of radiotoxicity and be a potential therapeutic target (Figure 2).
Figure 2.
Biological processes regulated by miR-34a which might be involved in normal tissue radiation-induced toxicity
Late radiation damage in most tissues is characterized by the loss of parenchymal cells and the excessive formation of fibrous tissue. As such, radiation-induced fibrosis (RIF) is probably the most studied form of radiotoxicity. The normal phases of wound healing progress from the injurious stimulus to inflammation, proliferation and finally resolution. By contrast, radiation generates reactive oxygen species (ROS), DNA damage and inflammation as the early stimuli that mediate the activation of a dysregulated proliferative phase. Unlike wound healing, radiation exerts a field effect on the vascular compartment. Endothelial dysfunction leads to a progressive vasculopathy that is characterized by impaired gaseous exchange and the development of tissue hypoxia, which drive an uncontrolled proliferative stage that replaces the normal proliferative phase of wound healing. This may represent a poorly coordinated haemostatic response that aims to preserve tissue oxygenation. The progressive and perpetuating proliferative phase prevents tissue resolution and results in the development of RIF (Barker et al., 2015).
To our knowledge, only one report in the prior art investigated the direct role of miR-34a in RIF. By studying miRNA alterations driving acute and late stages of radiation-induced fibrosis in mice, Simone et al. identified miR-34a as a key player for skin fibrosis development (Simone et al., 2014). First, a large miRNA screening by microarray technique revealed an increase of miR-34a level in mice fibrotic tissue from 7 days to 120 days after 35Gy radiation dose. Then, the study demonstrated the radiation-induced upregulation of miR-34a in cell lines causes inhibition of c-Met production, a known effector of fibrosis. These results suggest miR-34a as a potential target to prevent or treat this devastating side effect of irradiation. However, numerous others studies demonstrated the role of miR-34a in fibrosis. Thus, miR-34a plays a critical role in the progression of cardiac tissue fibrosis especially by downregulating PNUTS (Boon et al., 2013) and Smad4 pathway (Huang et al., 2014). These studies show miR-34a inhibition reduces the severity of experimental cardiac fibrosis in mice whereas miR-34a overexpression results in greater tissue fibrosis (Yang et al., 2015). Moreover, overexpressing miR-34a levels increased the profibrogenic activity of TGF-β1 in cardiac fibroblast, whereas inhibition miR-34a levels weakened the activity. Another study showed TGF-β1-treated fibroblasts produce microvesicles with high miR-34a level which are able to induce tubular cell apoptosis in fibrotic kidney (Zhou et al., 2014). Furthermore, microRNA profiling also indicates that miR-34a is upregulated in bleomycin-induced pulmonary fibrosis suggesting it could be also play an important role in lung fibrosis (Honeyman et al., 2013; Xie et al., 2011). Although a causal link between chronic oxidative stress and radiation-induced late normal tissue injury remains to be established, a growing body of evidence appears to support the hypothesis that chronic oxidative stress might serve driving the progression of radiation-induced late effects (Azzam et al., 2012; Robbins and Zhao, 2004). Interestingly, several studies showed that miR-34a mimics increased, and antisense miR-34a inhibited, in vitro ROS production (Bai et al., 2011; Ferreira et al., 2014; S.-Z. Li et al., 2014; F. Zhang et al., 2015) in several cell lines, suggesting a direct role of miR-34a in oxidative stress. Moreover, a study showed miR-34a was able to inhibit intracellular pathways, such as those involving antioxidative enzymes. Thus, Bai et al. demonstrated that aging mesangial cells exhibited upregulation of miR-34a (in association with miR-335) while they marked downregulation of two mitochondrial antioxidative proteins: SOD2 and Txnrd2 (Bai et al., 2011). MiR-34a mimic inhibits Txnrd2 expression whereas antisense miR-34a increased its expression. Li et al. also showed that the upregulation of miR-34a (and miR-93) constitutes an inescapable repression of Sirt1 and Mgst1, as well as the transcription factors (Sp1 and Nrf2) controlling their activation and constituting a double dampening regulation at the post-transcriptional level (Li et al., 2011). MiR-34a also enhances ROS-generating enzymes production as NADPH oxidase 2 (NOX2) which produces superoxide ion (S.-Z. Li et al., 2014).
MiR-34a also changes the cytokines expression profile, molecules which play a vital role in the acute and chronic inflammatory responses that drive fibrosis in injured tissues (Borthwick et al., 2013). For example, overexpression of miR-34a mimics in blood monocytes induces tumor necrosis factor α (TNFα) production by downregulating AXL expression (Kurowska-Stolarska et al., 2010). Another study also showed that the expression levels of interleukin-6 (IL-6) and interleukin-8 (IL-8) are modulated by miR-34a in human adipose tissue-derived stem cells, as they are strongly increased by miR-34a (Park et al., 2015). However, these recent results are still unclear and need further investigations because they showed IL-6 treatment repressing miR-34a expression while miR-34a can also inhibit IL-6 receptor expression (H. Li et al., 2015; T. Li et al., 2015; Rokavec et al., 2014). It has also been experimentally confirmed that miR-34a directly regulates Interferon- β (IFN-β) production by targeting IFN-β 3′ UTR in macaque and human (Witwer et al., 2010; Zhou et al., 2011).
As hypoxia is involved in fibrosis development and can increase mitochondrial ROS generation, it is also interesting to note that hypoxia may downregulate the expression of miR-34a, promoting the epithelial–mesenchymal transition in renal tubular epithelial cells by targeting the Notch signaling pathway (Du et al., 2012). However, the role of miR-34a in hypoxia is still unclear as some studies showed contradictory results, for example hypoxia upregulating miR-34a expression (Doridot et al., 2014).
All these observations indicate that miR-34a has the potential to be a novel mediator and target of radiation injury, radiosensitivity and radioprotection.
6. Conclusion
It is now clear IR influences miR-34a expression in cancer and normal cells with miR-34a expression level being increased by IR. Unfortunately, these radiation-induced expression changes do not correlate with the absorbed dose. Indeed, all studies which investigated the miR-34a expression changes after several doses of radiation did not manage to establish a direct correlation between the increase of miR-34a level and the increase of radiation dose, especially for extreme conditions such as very low dose or dose > 10Gy. Moreover, although increase of miR-34a expression after IR is observed in numerous and different cell lines and tissue, these changes seem to be very cell-specific. Indeed, no expression modification are occurred in some cell lines and expression level can be very different among cell lines for the same dose of radiation. However, we cannot conclude if miR-34a could be a good biodosimetry marker or not so further studies are needed. To assess its real power as biodosimetry marker, we suggest to focus on miR-34a expression measurement on just one type of biospecimen, especially those easily collected with minimally invasive procedure (i.e. blood), and evaluate the expression changes for several doses at a determined time point. MiR-34a will not probably be the ideal dosimetry biomarker but it might help to determine the absorbed dose for a specific time after IR exposure. Moreover, it has also been known for a long time that individuals are not equal to their response to IR leading to the concept of individual radiosensitivity. This concept may be an important factor for the study of miR-34a response to IR, especially as a dosimetry biomarker. Studies which analyzed in vivo alteration of miR-34a expression after IR in humans have incidentally been confronted to this concept. For example, Kabacik et al. measured miR-34a dose response in T-lymphocytes from two healthy donors. If miR-34a showed an up-regulation for both donors compared to the non-irradiated control samples, it is interesting to note the two curves differ as the second donor displays a different response from radiation dose > 4Gy where miR-34a expression seems decrease. Halimi et al. measured serum miR-34a level before and after radiation treatment (RT) in 40 patients as well. They demonstrated a significant miR-34a overexpression after RT but results also showed a large standard deviation for miR-34a level value revealing an important difference in miR-34a response to IR between the 40 donors. This inter-individual variability does not seem to be sex-specific as demonstrated by Ilnytskyy et al. which investigated variability of miRNAs response to IR between male and female mice (Ilnytskyy et al., 2008). This study shows that whole body IR exposure results in a significant and sex-specific deregulation of microRNA expression in mouse spleen and thymus tissue. However, interestingly, miR-34a is one of the two miRNAs similarly regulated in male and female, revealing radiation-induced miR-34a expression would not be sex-specific.
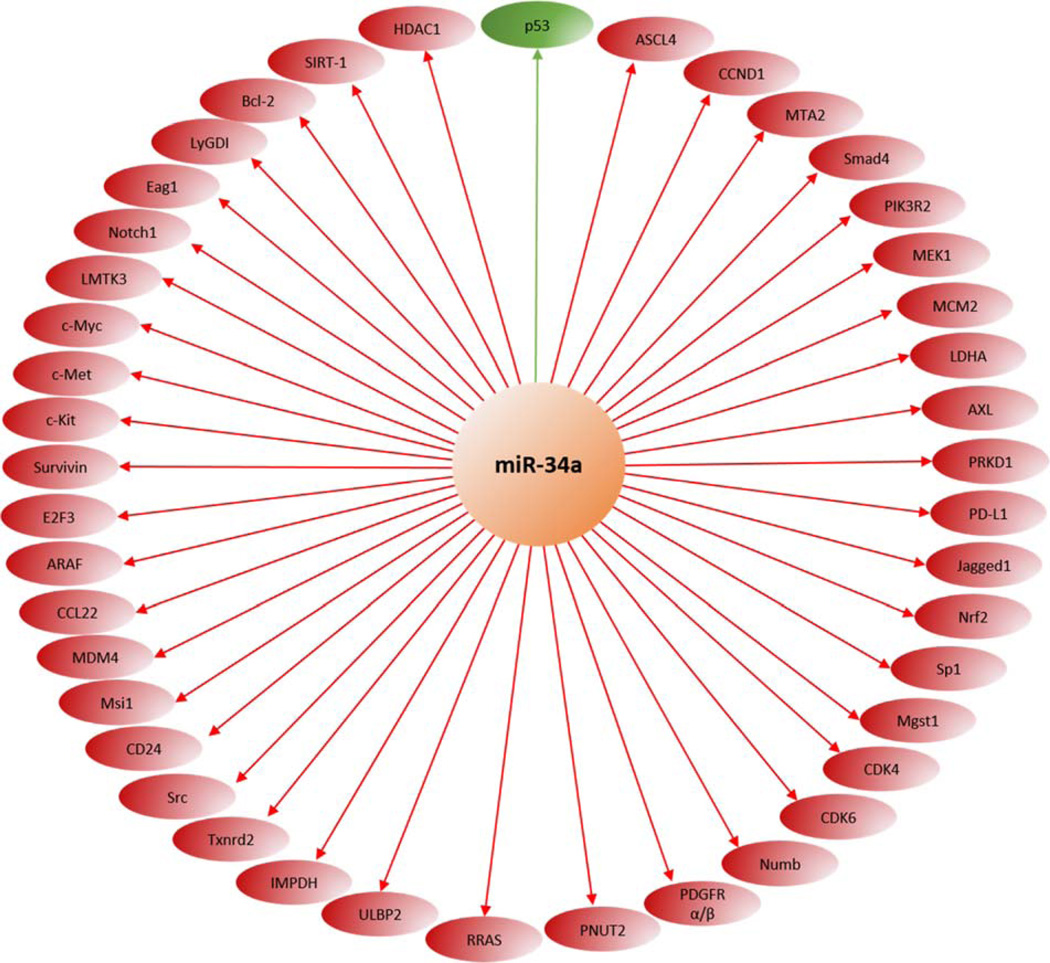
By binding the three prime untranslated region of many genes, miR-34a downregulates numerous important biological processes and is an important keystone for major cellular pathways, e.g. p53, SIRT-1, Notch, etc. (Figure 3).
Figure 3.
main miR-34a targets. MiR-34a downregulates many genes by binding their three prime untranslated region (red). MiR-34a is also known as an activator of the major p53 pathway (green).
As such, it may regulate several pathways involved in the cellular response to IR. First, miR-34a can act as a radiosensitizer in cancer cells; miR-34a increases apoptosis, decreases double-strand break repairs and so cell viability. As such, several studies already showed the use of miR-34a mimics can enhanced the tumor radiosensitivity. However, this effect has been only demonstrated in lung cancer, but miR-34a regulates numerous biological processes in numerous different tissue types, and so it might also be a good radiosensitizer candidate for many other tumors. Second, miR-34a can act as a radioprotector in normal cells; while delivery of miR-34a mimics enhances radiosensitivity, inhibition of miR-34a could also protect cells from radiation injury. However, with the exception of Liu et al. who performed a miR-34a knockdown on human embryonic kidney cells, demonstrating that radiation-induced apoptosis can be increased, only a few studies have directly investigated the effect of miR-34a inhibition in response to IR in normal cells. Therefore, there is a large field to be investigated in order to evaluate the miR-34a potential as a therapeutic target to prevent radiation injury in normal tissue. As miR-34a overexpression can enhance radiosensitivity and delivery of miR-34a inhibitor increases radioresistance, it might also be possible identifying if miR-34a could serve as predictive biomarker for radiotherapy response. Thus, measurement of miR-34a expression level prior to radiotherapy might predict normal tissue toxicity, or tumor radioresistance, suitable for tailoring a personalized treatment and improving its efficiency. Although it would be interesting to study this potential correlation between miR-34a expression and the clinical outcome, no studies have investigated this point so far.
Therefore, miR-34a could play an important role in radiation oncology and it could be exploited to improve cancer radiation therapy, either in cancer cells as radiosensitizer, or in normal cells as radioprotector or even serve as biomarkers of human radiation exposure. In this context, we predict an increase in the volume of future experimental work related to investigating the role of miR-34a in cellular response to IR whose outputs may lead to a significant impact in more precise radiation therapy treatments.
Highlights.
-
-
Ionizing radiation increases miR-34a expression
-
-
MiR-34a enhances tumor radiosensitivity by repressing several important cellular pathways
-
-
MiR-34a regulates numerous biochemical processes which could be involved in development of normal tissue radiation-induced toxicity
-
-
MiR-34a is a promising target for radiation therapy management
Acknowledgments
Funding
The authors were supported by funds from the University of Arizona’s Center for Applied Nanobiocience and Medicine and by some funds from a NIAID grant attributed to F.Z under contract # 2U19AI067773-11-revised. All authors commented on and approved the final version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anastasov N, Höfig I, Vasconcellos IG, Rappl K, Braselmann H, Ludyga N, Auer G, Aubele M, Atkinson MJ. Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat. Oncol. Lond. Engl. 2012;7:206. doi: 10.1186/1748-717X-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora H, Qureshi R, Jin S, Park A-K, Park W-Y. miR-9 and let-7g enhance the sensitivity to ionizing radiation by suppression of NFκB1. Exp. Mol. Med. 2011;43:298–304. doi: 10.3858/emm.2011.43.5.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, Jay-Gerin J-P, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X-Y, Ma Y, Ding R, Fu B, Shi S, Chen X-M. miR-335 and miR-34a Promote Renal Senescence by Suppressing Mitochondrial Antioxidative Enzymes. J. Am. Soc. Nephrol. JASN. 2011;22:1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett GC, West CML, Dunning AM, Elliott RM, Coles CE, Pharoah PDP, Burnet NG. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer. 2009;9:134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, Horrevoets AJG, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Müller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta BBA - Mol. Basis Dis., Fibrosis: Translation of basic research to human disease. 2013;1832:1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Janku F, Zhan Q, Fan J-B. Accessing Genetic Information with Liquid Biopsies. Trends Genet. TIG. 2015;31:564–575. doi: 10.1016/j.tig.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Cellini F, Morganti AG, Genovesi D, Silvestris N, Valentini V. Role of microRNA in response to ionizing radiations: evidences and potential impact on clinical practice for radiotherapy. Mol. Basel Switz. 2014;19:5379–5401. doi: 10.3390/molecules19045379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HJ, Shin S, Yoo H, Lee E-M, Bae S, Yang K-H, Lee S-J, Park I-C, Jin Y-W, An S. Identification of ionizing radiation-responsive microRNAs in the IM9 human B lymphoblastic cell line. Int. J. Oncol. 2009;34:1661–1668. doi: 10.3892/ijo_00000297. [DOI] [PubMed] [Google Scholar]
- Chang T-C, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry MA. Real-time PCR analysis of micro-RNA expression in ionizing radiation-treated cells. Cancer Biother. Radiopharm. 2009;24:49–56. [Google Scholar]
- Chaudhry MA, Omaruddin RA, Brumbaugh CD, Tariq MA, Pourmand N. Identification of radiation-induced microRNA transcriptome by next-generation massively parallel sequencing. J. Radiat. Res. (Tokyo) 2013;54:808–822. doi: 10.1093/jrr/rrt014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hu S-J. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J. Biochem. Mol. Toxicol. 2012;26:79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- Chen X, Yan J, Chen T. Expression level of miR-34a rather than P53 gene status correlates with mutability in related human lymphoblast cell lines. Mol. Carcinog. 2012;51:674–677. doi: 10.1002/mc.20830. [DOI] [PubMed] [Google Scholar]
- Chim CS, Wong KY, Qi Y, Loong F, Lam WL, Wong LG, Jin DY, Costello JF, Liang R. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis. 2010;31:745–750. doi: 10.1093/carcin/bgq033. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Valdecanas D, Niknam S, Peltier HJ, Diao L, Giri U, Komaki R, Calin GA, Gomez DR, Chang JY, Heymach JV, Bader AG, Welsh JW. In Vivo Delivery of miR-34a Sensitizes Lung Tumors to Radiation Through RAD51 Regulation. Mol. Ther. — Nucleic Acids. 2015;4:e270. doi: 10.1038/mtna.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czochor JR, Glazer PM. microRNAs in cancer cell response to ionizing radiation. Antioxid. Redox Signal. 2014;21:293–312. doi: 10.1089/ars.2013.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh T-KT, Fendler W, Chalubinska-Fendler J, Acharya SS, O’Leary C, Deraska PV, D’Andrea AD, Chowdhury D, Kozono D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat. Oncol. Lond. Engl. 2016;11:61. doi: 10.1186/s13014-016-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doridot L, Houry D, Gaillard H, Chelbi ST, Barbaux S, Vaiman D. miR-34a expression, epigenetic regulation, and function in human placental diseases. Epigenetics. 2014;9:142–151. doi: 10.4161/epi.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, Sun S. Hypoxia-Induced Down-Regulation of microRNA-34a Promotes EMT by Targeting the Notch Signaling Pathway in Tubular Epithelial Cells. PLOS ONE. 2012;7:e30771. doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Xu Y, Dong Y, Cao L, Tong J, Zhou X. Ectopic expression of miR-34a enhances radiosensitivity of non-small cell lung cancer cells, partly by suppressing the LyGDI signaling pathway. J. Radiat. Res. (Tokyo) 2013;54:611–619. doi: 10.1093/jrr/rrs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Ferreira DMS, Afonso MB, Rodrigues PM, Simão AL, Pereira DM, Borralho PM, Rodrigues CMP, Castro RE. c-Jun N-Terminal Kinase 1/c-Jun Activation of the p53/MicroRNA 34a/Sirtuin 1 Pathway Contributes to Apoptosis Induced by Deoxycholic Acid in Rat Liver. Mol. Cell. Biol. 2014;34:1100–1120. doi: 10.1128/MCB.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandellini P, Rancati T, Valdagni R, Zaffaroni N. miRNAs in tumor radiation response: bystanders or participants? Trends Mol. Med. 2014;20:529–539. doi: 10.1016/j.molmed.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Girardi C, De Pittà C, Casara S, Sales G, Lanfranchi G, Celotti L, Mognato M. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PloS One. 2012;7:e31293. doi: 10.1371/journal.pone.0031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak H-S, Kim TH, Jo GH, Kim Y-J, Kwak H-J, Kim JH, Yin J, Yoo H, Lee SH, Park JB. Silencing of MicroRNA-21 Confers Radio-Sensitivity through Inhibition of the PI3K/AKT Pathway and Enhancing Autophagy in Malignant Glioma Cell Lines. PLOS ONE. 2012;7:e47449. doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halimi M, Shahabi A, Moslemi D, Parsian H, Asghari SM, Sariri R, Yeganeh F, Zabihi E. Human serum miR-34a as an indicator of exposure to ionizing radiation. Radiat. Environ. Biophys. 2016;55:423–429. doi: 10.1007/s00411-016-0661-6. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007b;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2009;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- Honeyman L, Bazett M, Tomko TG, Haston CK. MicroRNA profiling implicates the insulin-like growth factor pathway in bleomycin-induced pulmonary fibrosis in mice. Fibrogenesis Tissue Repair. 2013;6:16. doi: 10.1186/1755-1536-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Qi Y, Du J-Q, Zhang D. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets. 2014;18:1355–1365. doi: 10.1517/14728222.2014.961424. [DOI] [PubMed] [Google Scholar]
- Ilnytskyy Y, Zemp FJ, Koturbash I, Kovalchuk O. Altered microRNA expression patterns in irradiated hematopoietic tissues suggest a sex-specific protective mechanism. Biochem. Biophys. Res. Commun. 2008;377:41–45. doi: 10.1016/j.bbrc.2008.09.080. [DOI] [PubMed] [Google Scholar]
- Jacob NK, Cooley JV, Yee TN, Jacob J, Alder H, Wickramasinghe P, Maclean KH, Chakravarti A. Identification of Sensitive Serum microRNA Biomarkers for Radiation Biodosimetry. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0057603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S-H, Wu H-G, Park W-Y. LIN28B confers radio-resistance through the posttranscriptional control of KRAS. Exp. Mol. Med. 2009;41:912–918. doi: 10.3858/emm.2009.41.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-β1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br. J. Cancer. 2014;111:772–780. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB, Falduto MT, Magnuson SR, Coleman CN. Fractionated Radiation Alters Oncomir and Tumor Suppressor miRNAs in Human Prostate Cancer Cells. Radiat. Res. 2012;178:105–117. doi: 10.1667/rr2703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacik S, Manning G, Raffy C, Bouffler S, Badie C. Time, dose and ataxia telangiectasia mutated (ATM) status dependency of coding and noncoding RNA expression after ionizing radiation exposure. Radiat. Res. 2015;183:325–337. doi: 10.1667/RR13876.1. [DOI] [PubMed] [Google Scholar]
- Kang J, Kim E, Kim W, Seong KM, Youn H, Kim JW, Kim J, Youn B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013;288:27343–27357. doi: 10.1074/jbc.M113.490482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Paranjape T, Ullrich R, Nallur S, Gillespie E, Keane K, Esquela-Kerscher A, Weidhaas JB, Slack FJ. The mir-34 microRNA is required for the DNA damage response in vivo in C. elegans and in vitro in human breast cancer cells. Oncogene. 2009;28:2419–2424. doi: 10.1038/onc.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014;32:103–115. doi: 10.3857/roj.2014.32.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela E, Vesprini D, Liu SK. MicroRNA in radiotherapy: miRage or miRador? Br. J. Cancer. 2015;112:777–782. doi: 10.1038/bjc.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Ballantine L, Stolarski B, Hunter J, Hueber A, Gracie JA, Liew FY, McInnes IB. miR-155 and miR-34a regulate proinflammatory cytokine production by human monocytes. Ann. Rheum. Dis. 2010;69:A30–A30. [Google Scholar]
- Lacombe J, Azria D, Mange A, Solassol J. Proteomic approaches to identify biomarkers predictive of radiotherapy outcomes. Expert Rev. Proteomics. 2013;10:33–42. doi: 10.1586/epr.12.68. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Li H, Rokavec M, Hermeking H, Li H, Rokavec M, Hermeking H. Soluble IL6R represents a miR-34a target: potential implications for the recently identified IL-6R/STAT3/miR-34a feed-back loop. Oncotarget. 2015;6:14026–14032. doi: 10.18632/oncotarget.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie X, Luo J, Liu M, Xi S, Guo J, Kong Y, Wu M, Gao J, Xie Z, Tang J, Wang X, Wei W, Yang M, Hung M-C, Xie X. Targeted Expression of miR-34a Using the T-VISA System Suppresses Breast Cancer Cell Growth and Invasion. Mol. Ther. 2012;20:2326–2334. doi: 10.1038/mt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Muthusamy S, Liang R, Sarojini H, Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech. Ageing Dev. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Li S-Z, Hu Y-Y, Zhao J, Zhao Y-B, Sun J-D, Yang Y, Ji C-C, Liu Z-B, Cao W-D, Qu Y, Liu W-P, Cheng G, Fei Z. MicroRNA-34a induces apoptosis in the human glioma cell line, A172, through enhanced ROS production and NOX2 expression. Biochem. Biophys. Res. Commun. 2014;444:6–12. doi: 10.1016/j.bbrc.2013.12.136. [DOI] [PubMed] [Google Scholar]
- Li T, Li L, Li D, Wang S, Sun J. MiR-34a inhibits oral cancer progression partially by repression of interleukin-6-receptor. Int. J. Clin. Exp. Pathol. 2015;8:1364–1373. [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Ren ZJ, Tang JH. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 2014;5:e1327. doi: 10.1038/cddis.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Li G, Wang Y, Lei W, Xiao Z. Aberrant miRNA expression response to UV irradiation in human liver cancer cells. Mol. Med. Rep. 2014;9:904–910. doi: 10.3892/mmr.2014.1901. [DOI] [PubMed] [Google Scholar]
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhou C, Gao F, Cai S, Zhang C, Zhao L, Zhao F, Cao F, Lin J, Yang Y, Ni J, Jia J, Wu W, Zhou L, Cui J, Zhang W, Li B, Cai J. MiR-34a in age and tissue related radio-sensitivity and serum miR-34a as a novel indicator of radiation injury. Int. J. Biol. Sci. 2011;7:221–233. doi: 10.7150/ijbs.7.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu H, Yang X, Ge Y, Zhang C, Qin Q, Lu J, Zhan L, Cheng H, Sun X. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014;35:3975–3979. doi: 10.1007/s13277-014-1623-8. [DOI] [PubMed] [Google Scholar]
- Liu Z-L, Wang H, Liu J, Wang Z-X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle Georget. Tex. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- Long Z, Wang B, Tao D, Huang Y, Tao Z. Hypofractionated radiotherapy induces miR-34a expression and enhances apoptosis in human nasopharyngeal carcinoma cells. Int. J. Mol. Med. 2014;34:1388–1394. doi: 10.3892/ijmm.2014.1937. [DOI] [PubMed] [Google Scholar]
- Lu J, Chen C, Hao L, Zheng Z, Zhang N, Wang Z. MiRNA expression profile of ionizing radiation-induced liver injury in mouse using deep sequencing. Cell Biol. Int. 2016 doi: 10.1002/cbin.10627. [DOI] [PubMed] [Google Scholar]
- Luzhna L, Kovalchuk O. Low dose irradiation profoundly affects transcriptome and microRNAme in rat mammary gland tissues. Oncoscience. 2014;1:751–762. doi: 10.18632/oncoscience.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert U, Ozgür E, Tiryakioglu D, Dalay N, Gezer U. Induction of p53-inducible microRNA miR-34 by gamma radiation and bleomycin are different. Front. Genet. 2012;3:220. doi: 10.3389/fgene.2012.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr. Opin. Genet. Dev. 2013;23:12–19. doi: 10.1016/j.gde.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, Caraglia M. Mir-34: A New Weapon Against Cancer? Mol. Ther. — Nucleic Acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill CE, Moriarty MJ, Seymour CB. Radiotherapy and the potential exploitation of bystander effects. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:575–579. doi: 10.1016/j.ijrobp.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Navarro F, Lieberman J. miR-34 and p53: New Insights into a Complex Functional Relationship. PLOS ONE. 2015;10:e0132767. doi: 10.1371/journal.pone.0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova MN, Gandhi M, Gandi M, Kelly L, Nikiforov YE. MicroRNA dysregulation in human thyroid cells following exposure to ionizing radiation. Thyroid Off. J. Am. Thyroid Assoc. 2011;21:261–266. doi: 10.1089/thy.2010.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J-S, Kim J-J, Byun J-Y, Kim I-A. Lin28-let7 modulates radiosensitivity of human cancer cells with activation of K-Ras. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:5–8. doi: 10.1016/j.ijrobp.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Okada N, Lin C-P, Ribeiro MC, Biton A, Lai G, He X, Bu P, Vogel H, Jablons DM, Keller AC, Wilkinson JE, He B, Speed TP, He L. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014;28:438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palayoor ST, John-Aryankalayil M, Makinde AY, Falduto MT, Magnuson SR, Coleman CN. Differential expression of stress and immune response pathway transcripts and miRNAs in normal human endothelial cells subjected to fractionated or single-dose radiation. Mol. Cancer Res. MCR. 2014;12:1002–1015. doi: 10.1158/1541-7786.MCR-13-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Park H, Pak H-J, Yang D-Y, Kim Y-H, Choi W-J, Park S-J, Cho J-A, Lee K-W. miR-34a inhibits differentiation of human adipose tissue-derived stem cells by regulating cell cycle and senescence induction. Differentiation. 2015;90:91–100. doi: 10.1016/j.diff.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Pawlicki JM, Steitz JA. Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. J. Cell Biol. 2008;182:61–76. doi: 10.1083/jcb.200803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothof J, Verkaik NS, van IJcken W, Wiemer EAC, Ta VTB, van der Horst GTJ, Jaspers NGJ, van Gent DC, Hoeijmakers JHJ, Persengiev SP. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009;28:2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat. Rev. Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Robbins MEC, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int. J. Radiat. Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, Greten FR, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Saleh AD, Savage JE, Cao L, Soule BP, Ly D, DeGraff W, Harris CC, Mitchell JB, Simone NL. Cellular Stress Induced Alterations in MicroRNA let-7a and let-7b Expression Are Dependent on p53. PLOS ONE. 2011;6:e24429. doi: 10.1371/journal.pone.0024429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman DW, Nakamura K, Nallur S, Dookwah MT, Metheetrairut C, Slack FJ, Weidhaas JB. miR-34 activity is modulated through 5'-end phosphorylation in response to DNA damage. Nat. Commun. 2016;7:10954. doi: 10.1038/ncomms10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Udaka Y, Tsunoda Y, Yamamoto G, Tsuji M, Oyamada H, Oguchi K, Mizutani T. Analysis of p53 and miRNA expression after irradiation of glioblastoma cell lines. Anticancer Res. 2012;32:4709–4713. [PubMed] [Google Scholar]
- Shin S, Cha HJ, Lee E-M, Lee S-J, Seo S-K, Jin H-O, Park I-C, Jin Y-W, An S. Alteration of miRNA profiles by ionizing radiation in A549 human non-small cell lung cancer cells. Int. J. Oncol. 2009;35:81–86. [PubMed] [Google Scholar]
- Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the Therapy of Pancreatic Cancer. Mol. Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone BA, Ly D, Savage JE, Hewitt SM, Dan TD, Ylaya K, Shankavaram U, Lim M, Jin L, Camphausen K, Mitchell JB, Simone NL. microRNA alterations driving acute and late stages of radiation-induced fibrosis in a murine skin model. Int. J. Radiat. Oncol. Biol. Phys. 2014;90:44–52. doi: 10.1016/j.ijrobp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, Cook J, Harris CC, Gius D, Mitchell JB. Ionizing radiation-induced oxidative stress alters miRNA expression. PloS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankevicins L, Almeida da Silva AP, Ventura Dos Passos F, Dos Santos Ferreira E, Menks Ribeiro MC, G David M, J Pires E, Ferreira-Machado SC, Vassetzky Y, de Almeida CE, de Moura Gallo CV. MiR-34a is up-regulated in response to low dose, low energy X-ray induced DNA damage in breast cells. Radiat. Oncol. Lond. Engl. 2013;8:231. doi: 10.1186/1748-717X-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J-L, Chen P-S, Johansson G, Kuo M-L. Function and regulation of let-7 family microRNAs. MicroRNA Shariqah United Arab Emir. 2012;1:34–39. doi: 10.2174/2211536611201010034. [DOI] [PubMed] [Google Scholar]
- Summerer I, Niyazi M, Unger K, Pitea A, Zangen V, Hess J, Atkinson MJ, Belka C, Moertl S, Zitzelsberger H. Changes in circulating microRNAs after radiochemotherapy in head and neck cancer patients. Radiat. Oncol. Lond. Engl. 2013;8:296. doi: 10.1186/1748-717X-8-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle Georget. Tex. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- Tian W, Yin X, Wang L, Wang J, Zhu W, Cao J, Yang H. The key role of miR-21-regulated SOD2 in the medium-mediated bystander responses in human fibroblasts induced by α-irradiated keratinocytes. Mutat. Res. 2015;780:77–85. doi: 10.1016/j.mrfmmm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Wagner-Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat. Oncol. 2010;5:25. doi: 10.1186/1748-717X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li D, Kovalchuk O. p53 Ser15 phosphorylation and histone modifications contribute to IR-induced miR-34a transcription in mammary epithelial cells. Cell Cycle Georget. Tex. 2013;12:2073–2083. doi: 10.4161/cc.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhu M, Ye P, Chen G, Wang W, Chen M. Ionizing radiation-induced microRNA expression changes in cultured RGC-5 cells. Mol. Med. Rep. 2015;12:4173–4178. doi: 10.3892/mmr.2015.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–11116. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Sisk JM, Gama L, Clements JE. MicroRNA Regulation of IFN-β Protein Expression: Rapid and Sensitive Modulation of the Innate Immune Response. J. Immunol. 2010;184:2369–2376. doi: 10.4049/jimmunol.0902712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Zhou J, Zhang S, Chen Q, Lai R, Ding W, Song C, Meng X, Wu J. Integrating microRNA and mRNA expression profiles in response to radiation-induced injury in rat lung. Radiat. Oncol. Lond. Engl. 2014;9:111. doi: 10.1186/1748-717X-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol. Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, Huang Q, Chen S, Zhang Z, Xu Y, Lai L, Zheng Y. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol. Aging. 2015;36:1692–1701. doi: 10.1016/j.neurobiolaging.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11:1161–1170. doi: 10.4161/rna.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng H-W, Qiu Y, Dupee D, Noonan M, Lin Y-D, Fisch S, Unno K, Sereti K-I, Liao R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015;117:450–459. doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z, Yu B. Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res. Ther. 2015;6 doi: 10.1186/s13287-015-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang S, Chen Y. Abnormal Expression of miR-21 and miR-95 in Cancer Stem-Like Cells is Associated with Radioresistance of Lung Cancer. Cancer Invest. 2015;33:165–171. doi: 10.3109/07357907.2015.1019676. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bode AM, Cao Y, Dong Z. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis. 2012;33:2220–2227. doi: 10.1093/carcin/bgs235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Robbins MEC. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr. Med. Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- Zhou B, Xu Y, Permatasari F, Liu W, Li W, Guo X, Huang Q, Guo Z, Luo D. Characterization of the miRNA profile in UVB-irradiated normal human keratinocytes. Exp. Dermatol. 2012;21:317–319. doi: 10.1111/j.1600-0625.2012.01465.x. [DOI] [PubMed] [Google Scholar]
- Zhou R, O’Hara SP, Chen X-M. MicroRNA regulation of innate immune responses in epithelial cells. Cell. Mol. Immunol. 2011;8:371–379. doi: 10.1038/cmi.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xiong M, Niu J, Sun Q, Su W, Zen K, Dai C, Yang J. Secreted fibroblast-derived miR-34a induces tubular cell apoptosis in fibrotic kidney. J. Cell Sci. 2014;127:4494–4506. doi: 10.1242/jcs.155523. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhu X, Cheng G, Zhou M, Lou W. Downregulation of microRNA-21 enhances radiosensitivity in nasopharyngeal carcinoma. Exp. Ther. Med. 2015;9:2185–2189. doi: 10.3892/etm.2015.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]