ABSTRACT
Objective: To report 3 cases in which doses of bivalirudin higher than commonly used in clinical practice were required in order to achieve therapeutic anticoagulation as monitored by the activated partial thromboplastin time (aPTT).
Case Summary: The medical records of 3 patients who required large doses of bivalirudin to remain therapeutic were thoroughly reviewed. In all 3 patients, bivalirudin was initiated at a rate appropriate for the patients' renal function and titrated using a nurse-driven protocol with recommended dose adjustments based on aPTT. Indications for bivalirudin were anticoagulation in intra-aortic balloon pump, treatment of deep vein thrombosis, and heparin-induced thrombocytopenia with thrombosis. Target aPTT was achieved between 25.5 and 134 hours after initiation despite appropriate titration intervals per protocol.
Discussion: Bivalirudin is a direct thrombin inhibitor frequently used off-label for the medical management of heparin-induced thrombocytopenia. It typically exhibits predictable, dose-dependent anticoagulation. Heparin-induced thrombocytopenia was suspected in 2 of the 3 cases and confirmed in 1. In all 3 patients, target aPTT was initially achieved with doses between 0.456 and 1.0 mg/kg/h after a median of 30.7 hours; up to 1.8 mg/kg/h was required to maintain therapeutic aPTT. In 2 of the cases, the international normalized ratio also increased unexpectedly upon achievement of therapeutic aPTT values.
Conclusion: Direct thrombin inhibitors may be subject to resistance mechanisms similar to those previously described in patients receiving heparin. The anticoagulation status of these patients remains unknown.
Keywords: bivalirudin, direct thrombin inhibitor, resistance
Bivalirudin is an intravenous (IV) direct thrombin inhibitor (DTI) that directly and reversibly inhibits both circulating and clot-bound thrombin.1 Bivalirudin is US Food and Drug Administration (FDA)–approved for patients with, or at risk of, heparin-induced thrombocytopenia (HIT) undergoing percutaneous coronary intervention (PCI), patients undergoing PCI with a provisional GIIa/IIIb inhibitor, and in patients with unstable angina undergoing percutaneous transluminal coronary angioplasty (PTCA).2 It exhibits predictable and dose-dependent anticoagulation with a short half-life of 25 minutes. Although studies are limited, bivalirudin can be used off-label in the medical management of HIT and HIT with thrombosis syndrome (HITTS).1 Typically, the initial infusion rate is 0.06 to 0.15 mg/kg/h and is titrated to maintain an activated partial thromboplastin time (aPTT) of 1.5 to 2.5 times baseline.3–7 The initial infusion rate is adjusted in patients with renal insufficiency.3–5 In studies of bivalirudin in medically managed HIT, therapeutic aPTT is typically achieved within 5 to 12 hours.5–7 Often the first aPTT after initiation is within the therapeutic range.5 In addition to prolonging the aPTT, DTIs are known to prolong the international normalized ratio (INR), making the transition to warfarin challenging.2
Heparin resistance is a well-described occurrence in which patients require a dose of heparin higher than commonly used in clinical practice in order to achieve and maintain a therapeutic level of anticoagulation. Several causes of heparin resistance are known, including antithrombin deficiency, increased heparin clearance, increased heparin binding proteins, and apparent heparin resistance due to aPTT interference by Factor VIII or fibrinogen.8 Most of these mechanisms of resistance can be managed by increasing the dose of heparin, with the exception of apparent heparin resistance, which is best managed by monitoring a chromogenic heparin assay for anti-Xa activity.9 To our knowledge, resistance to bivalirudin has not been reported in the literature. The following cases support the suggestion that direct thrombin inhibitors may be subject to similar resistance mechanisms as heparin.
METHODS
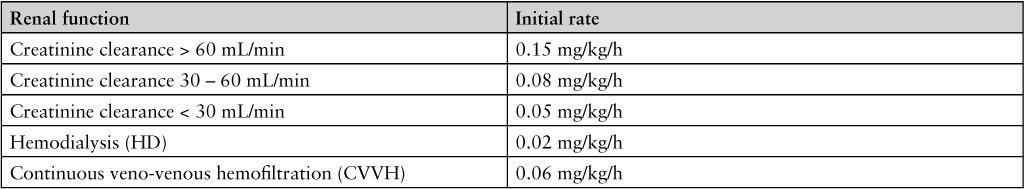
The medical records of 3 patients who required large doses of bivalirudin to remain therapeutic were thoroughly reviewed. Medical records were reviewed retrospectively. Patients were identified by the investigators through the course of their clinical responsibilities. According to our institutional guideline, bivalirudin is therapeutic at an aPTT between 60 and 80 seconds. The coagulation analyzer used by our institution is the Siemens BCS XP System, and the assay utilized the Dade Actin FSL aPTT reagent (Siemens Healthcare Diagnostics, Newark, DE).10 In all patients, bivalirudin was initiated at an infusion rate of 0.15 mg/kg/h in normal renal function or a reduced rate for a creatinine clearance (CrCl) less than 60 mL/min and titrated using a nurse-driven protocol with recommended dose adjustments depending on the current aPTT. The nurse-driven protocol utilized at our institution is included in Appendix 1. A summary of dosing requirements and corresponding aPTT for the 3 cases is shown in Figures 1, 2 and 3.
Figure 1.
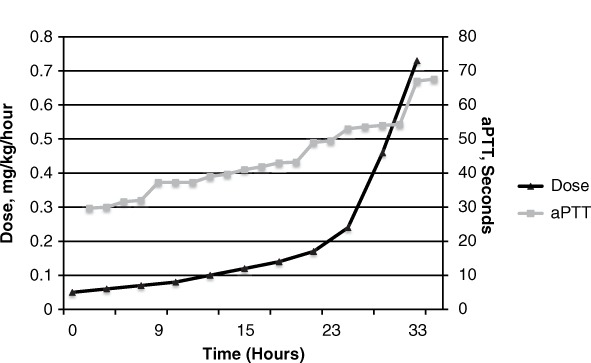
Bivalirudin dose and activated partial thromboplastin time (aPTT) over time, Patient 1.
Figure 2.
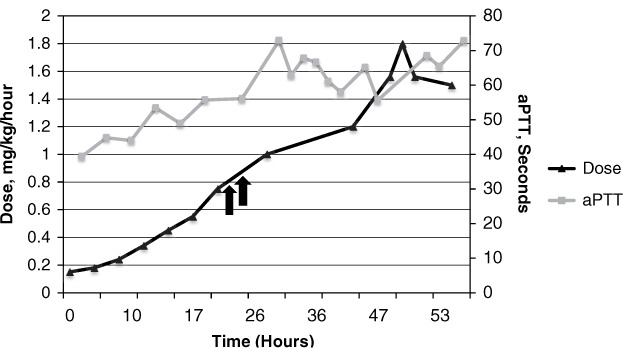
Bivalirudin dose and activated partial thromboplastin time (aPTT) over time, Patient 2. Arrows denote bolus doses given.
Figure 3.
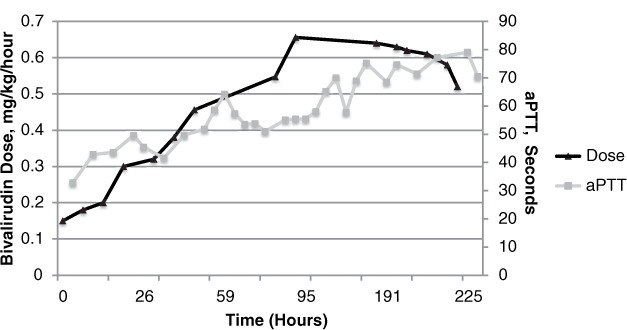
Bivalirudin dose and activated partial thromboplastin time (aPTT) over time, Patient 3.
RESULTS
Case 1
A 75-year-old woman with a past medical history significant for multivessel coronary artery disease and cirrhosis presented from home to the emergency department (ED) with chest pain. She was diagnosed with non-ST segment elevation myocardial infarction (NSTEMI) and critical aortic stenosis, and she required placement of an intra-aortic balloon pump (IABP) for blood pressure support. Her NSTEMI was managed with 2 bare metal stents as well as antiplatelet therapy on hospital day 0. While the IABP was in place, she received IV heparin for anticoagulation. Between hospital day 1 and 16, multiple attempts to wean the IABP were unsuccessful. Notably, throughout this time she had an elevated INR of 1.9, for which she was given 10 mg of IV phytonadione daily for 3 days and 2 units of fresh frozen plasma, which had no effect.
On hospital day 18, her platelets decreased from 176,000 to a nadir of 52,000, which prompted initiation of bivalirudin at 0.05 mg/kg/h based on a CrCl of 26 mL/min. Her INR at initiation was 2.17, and her liver function tests were unremarkable. The HIT-platelet factor 4 antibody test (HIT-PF4 Ab) and serotonin release assay (SRA) were drawn and eventually returned negative, signifying that she likely did not have HIT. The following day, her aPTT remained below target range, requiring postponement of IABP removal. Her dose of 0.24 mg/kg/h resulted in an aPTT of 53.6 seconds, which prompted an off-protocol doubling of her dose by the attending physician to 0.46 mg/kg/h. Two hours later, this resulted in an aPTT of 54.3 seconds. Her INR values during hospital day 19 were 2.02 and 2.57, and her renal function was stable from the day before.
After more than 33 hours of therapy, her dose was increased to 0.76 mg/kg/h, which resulted in an aPTT of 67.6 seconds and subsequent removal of the IABP on hospital day 20. An INR drawn with the therapeutic aPTT was 6.97, which was increased from her previous INR of 2.57. After the IABP was removed, bivalirudin was discontinued and a follow-up INR was found to be 1.7. During bivalirudin therapy, other coagulation tests revealed her Factor VIII activity was elevated at 438% and fibrinogen was 316 mg/dL (>740 at admission). Elevated Factor VIII activity in this patient may have been due to liver disease or inflammation. Her platelets recovered to 140,000 on hospital day 22. On hospital day 28, she underwent a CT angiogram for access mapping during transcatheter aortic valve replacement evaluation and was found to have extensive clots in the bilateral pulmonary arteries. She was restarted on heparin IV, which she tolerated, and was ultimately discharged home on warfarin.
Case 2
A 22-year-old woman who was 5 weeks and 6 days into her second pregnancy presented to an outside facility with acute onset right lower extremity swelling and pain with rapid progression. During her previous pregnancy, she was diagnosed with anti-thrombin III deficiency and started on subcutaneous heparin for deep vein thrombosis (DVT) prophylaxis. She developed urticaria on heparin therapy and was switched to enoxaparin. She was placed on rivaroxaban postpartum, which was discontinued 2 weeks prior to her current presentation.
Upon presentation to the outside hospital, ultrasound revealed extensive DVT of the right ileo-femoral system. The extremity was cool and discolored, and the patient was transferred to our institution for consideration for thrombolysis. After discussion with the patient and her family, an IVC filter was placed with plans for focal thrombolytic therapy due to the large clot burden and findings consistent with right phlegmasia cerulea dolens. Due to her heparin allergy and need for an anticoagulant with a relatively short half-life, the patient was started on bivalirudin 0.15 mg/kg/h based on her CrCl greater than 120 mL/min. Her baseline liver function tests and INR were unremarkable. During the first 24 hours of the infusion, the aPTT reached 56.1 seconds after rapid titration to 0.75 mg/kg/h, but remained below the target range. Within this time, the patient was noted to have dyspnea and cough, and a computed tomography angiogram revealed multisegmental bilateral pulmonary emboli without evidence of right ventricular strain on echocardiogram or hemodynamic instability. As the aPTT remained below the target range, 2 bolus doses of 0.3 mg/kg were given, and she achieved a therapeutic aPTT after 25.5 hours of therapy. She was ultimately titrated up to 1.8 mg/kg/h, which resulted in an aPTT of 68.5 seconds and an INR of 1.67. On her fourth day of bivalirudin therapy, she underwent catheter-directed thrombolysis with reduction of swelling and discoloration. After the procedure, she was transitioned back to enoxaparin daily and was ultimately discharged. Her INR returned to normal following discontinuation of bivalirudin.
Case 3
A 49-year-old man with multiple comorbidities was diagnosed with hypertrophic cardiomyopathy with septal hypertrophy and left ventricular outflow tract obstruction. He underwent a septal myectomy under cardiopulmonary bypass and was admitted to the cardiothoracic intensive care unit. His course was complicated by upper extremity DVT that was initially treated with heparin, but he was switched to bivalirudin when his platelets decreased from 136,000 to 85,000 on hospital day 6. He also developed distal ischemia and blue discoloration of his fingers and toes, consistent with HITTS. Both HIT-PF4 Ab and SRA returned positive.
Bivalirudin was initiated at 0.15 mg/kg/h, and his platelets recovered to 140,000 by hospital day 8. The patient's INR at the time of initiation was 1.16, but he had elevated aspartate transaminase (600) and alanine transaminase (1,290) results. He did not achieve a target aPTT until hospital day 9. His dose at that time was 0.456 mg/kg/h and his INR was 4.43. Despite worsening kidney function (CrCl decreased from 83.5 to 41.8 mL/min), he was not consistently at target aPTT until 134 hours of bivalirudin therapy, when his dose was increased to 0.656 mg/kg/h and his INR reached as high as 5.06. He received multiple doses of IV phytonadione due to the concern that the persistently elevated INR may be the result of either shock liver from a hypoxemic episode or vitamin K deficiency from prolonged inadequate tube feeding. During this period, Factor V and VII activities were low at 30.8% and 34.5%, respectively.
Bivalirudin was held on day 17 of therapy for pacemaker placement. Despite high dose requirements previously, it was restarted at 0.15 mg/kg/h and ultimately titrated in 27 hours to 0.95 mg/kg/h, when his aPTT reached 70.2 seconds. Three hours later, his INR was 6.28. Bivalirudin was continued and ultimately transitioned to warfarin after a prolonged course. The patient was eventually discharged following a complicated hospital admission.
DISCUSSION
These cases represent a surprising inability of the medical team to achieve therapeutic aPTT at commonly used doses of bivalirudin. Target aPTT was achieved between 28 and 134 hours after therapy initiation despite mostly appropriate titration intervals per protocol. In medically managed HIT, bivalirudin can be expected to achieve therapeutic anticoagulation within a few hours at doses much lower than those required by these 3 patients.5–7 Also noteworthy was the dramatic increase in INR that occurred upon attainment of a therapeutic aPTT in the first and third case. Although bivalirudin is expected to increase INR, this effect is typically less pronounced compared to other DTIs and the INR rarely exceeds 4.0 during warfarin cotherapy.11 It should be noted that although the doses required by these patients were high for their respective indications, higher doses of bivalirudin are used for other indications, such as during PCI or PTCA.2
Upon review of the literature, 2 cases were identified describing a similar phenomenon in which patients with confirmed HIT required unusually high doses of argatroban to achieve and maintain a therapeutic aPTT.12,13 In our first case, bivalirudin was initiated at a renally adjusted dose according to institutional protocol, but remained below target aPTT despite aggressive dose titrations. During this patient's hospital course, she was noted to have elevated Factor VIII and fibrinogen, 2 acute phase reactants that shorten the aPTT. Similarly to apparent heparin resistance, this interaction with the aPTT has been observed previously with argatroban and may be a potential explanation for the persistently short aPTT.13 In apparent heparin resistance, the aPTT is a poor marker of anticoagulation and the anti-Xa assay is preferred. Similarly, when Factor VIII or fibrinogen are elevated in patients receiving DTIs, the aPTT may be inaccurate and alternative assays should be considered.
In Cases 2 and 3, patients with tremendous clot burdens required higher than commonly seen doses of bivalirudin to achieve a therapeutic aPTT. Because bivalirudin inhibits both free and clot-bound thrombin, large clot burden may result in a larger number of binding sites that require saturation before a steady state can be achieved. This may represent a need for higher dosing due to more significant consumption.
Regarding the INR, Cases 1 and 3 demonstrated a sudden and unexpected increase in INR upon achievement of therapeutic aPTT levels. In both cases, vitamin K deficiency due to suspected liver dysfunction or prolonged malnourishment may have contributed to this observation. Because Factor VII has a very short half-life compared to the other vitamin K-dependent coagulation factors, fluctuations may play a role in sudden INR changes. Direct thrombin inhibitors can cause an increase in INR via dose-dependent inhibition of Factor II, the factor with the longest half-life.14 Therefore, in the setting of Factor VII deficiency, extremely high doses of bivalirudin may cause a sudden and unexpected increase in INR, especially if the dose was significantly increased, such as in Case 1. Indeed, Factor VII activity was decreased in Case 3, suggesting this may be a possible explanation.
The major limitation in the interpretation of these cases is the use of aPTT. It should be emphasized that the aPTT is inadequate for monitoring DTIs due to a nonlinear dose-response curve, especially at higher concentrations.12 In our 3 patients, the level of anticoagulation cannot be quantified completely by the aPTT.11,12,15 When monitoring patients receiving DTIs, a more sensitive marker, such as the chromogenic Anti-Xa level for heparin, would be very valuable. Dilute thrombin time, ecarin thrombin time, and chromogenic substrate-based assays specific for DTIs have been studied and may prove to be promising alternatives.15–17 Although potentially useful, the clinical applicability of these tests is currently limited due to a lack of widely available commercial products, leaving clinicians without objective data to guide decision making.
In conclusion, bivalirudin appears to be subject to resistance mechanisms similar to those previously described in patients receiving heparin as well as argatroban, and the aPTT may be a poor indicator for anticoagulation in certain patients on bivalirudin.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
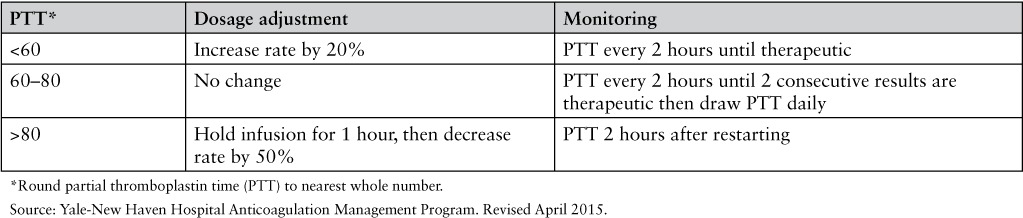
APPENDIX 1
Yale-New Haven Hospital Nurse-Driven Bivalirudin Dose Adjustment Protocol
Bivalirudin renal dose adjustment for treatment of heparin-induced thrombocytopenia (HIT)
Monitoring and dose adjustments
REFERENCES
- 1. Seybert AL, Coons JC, Zerumsky K.. Treatment of heparin-induced thrombocytopenia: Is there a role for bivalrudin? Pharmacotherapy. 2006; 26( 2): 229– 241. [DOI] [PubMed] [Google Scholar]
- 2. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; May 2013. [Google Scholar]
- 3. Wisler JW, Washam JB, Becker RC.. Evaluation of dose requirements for prolonged bivalirudin administration in patients with renal insufficiency and suspected heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2012; 33: 287– 295. [DOI] [PubMed] [Google Scholar]
- 4. Kiser TH, Burch JC, Klem PM, . et al. Safety, efficacy, and dosing requirements of bivalirudin in patients with heparin-induced thrombocytopenia. Pharmacotherapy. 2008; 28( 9): 1115– 1124. [DOI] [PubMed] [Google Scholar]
- 5. Skrupky LP, Smith JR, Deal EN, . et al. Comparison of bivalirudin and argatroban for the management of heparin-induced thrombocytopenia. Pharmacotherapy. 2010; 30( 12): 1229– 1238. [DOI] [PubMed] [Google Scholar]
- 6. Joseph L, Casanegra AI, Dhariwal M, . et al. Bivalirudin for the treatment of patients with confirmed or suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2014; 12: 1044– 1053. [DOI] [PubMed] [Google Scholar]
- 7. Burcham PK, Abel EE, Gerlach AT, . et al. Development and implementation of a nurse-driven, sliding-scale nomogram for bivalirudin in the management of heparin-induced thrombocytopenia. Am J Health Syst Pharm. 2013; 70: 980– 987. [DOI] [PubMed] [Google Scholar]
- 8. Garcia DA, Baglin TP, Witz JI, . et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141; e24S– e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine MN, Hirsh J, Gent M, . et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med. 1994; 154( 1): 49– 56. [PubMed] [Google Scholar]
- 10. Dade® Actin® FSL Activated PTT Reagent [package insert]. Newark, DE: Siemens Healthcare Diagnostics; 2006. [Google Scholar]
- 11. Gosselin RC, Dager WE, King JH, . et al. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban on prothrombin time and INR values. Am J Clin Pathol. 2004; 121( 4): 593– 599. [DOI] [PubMed] [Google Scholar]
- 12. Hellwig TR, Peitz GJ, Gulseth MP.. High-dose argatroban for treatment of heparin-induced thrombocytopenia with thrombosis: A case report and review of laboratory considerations. Am J Health Syst Pharm. 2012; 69: 490– 495. [DOI] [PubMed] [Google Scholar]
- 13. Kennedy DM, Alaniz C.. Apparent argatroban resistance in a patient with elevated factor VIII levels. Ann Pharmacother. 2013; 47: e29. [DOI] [PubMed] [Google Scholar]
- 14. McAlister RK, Ito S.. Minimal prolongation of prothrombin time with extended exposure to argatroban. Pharmacotherapy. 2015. 35( 7): e122– 126. [DOI] [PubMed] [Google Scholar]
- 15. Hafner G, Roser M, Nauck M.. Methods for the monitoring of direct thrombin inhibitors. Semin Thromb Hemost. 2002; 28( 5): 425– 430. [DOI] [PubMed] [Google Scholar]
- 16. Curvers J, van de Kerkhof D, Stroobants AK, . et al. Measuring direct thrombin inhibitors with routine and dedicated coagulation assays: Which assay is helpful? Am J Clin Pathol. 2012; 138( 4): 551– 558. [DOI] [PubMed] [Google Scholar]
- 17. Carrol RC, Chavez JJ, Simmons JW, . et al. Measurement of patients' bivalirudin plasma levels by a Thrombelastograph ecarin clotting time assay: A comparison to a standard activated clotting time. Anesth Analg. 2006; 102( 5): 1316– 1319. [DOI] [PubMed] [Google Scholar]


