Abstract
Polyethylene glycol (PEG) addition can prolong the pharmacokinetic and pharmacodynamic actions of a bioactive peptide in vivo, in part by impeding rates of glomerular filtration. For parathyroid hormone (PTH) peptides, pegylation could help in exploring the actions of the hormone in the kidney; e.g., in dissecting the relative roles that filtered versus blood-borne PTH play in regulating phosphate transport. It could also lead to potential alternate forms of treatment for hypoparathyroidism. We thus synthesized the fluorescent pegylated PTH derivative [Lys13(tetramethyl rhodamine {TMR}), Cys35(PEG-20,000 Da)]PTH(1-35) (PEG-PTHTMR) and its non-pegylated counterpart [Lys13(TMR), Cys35]PTH(1-35) (PTHTMR) and assessed their properties in cells and in mice. In PTHR1-expressing HEK-293 cells, PEG-PTHTMR and PTHTMR exhibited similar potencies for inducing cAMP signaling, whereas when injected into mice, the pegylated analog persisted for much longer in the circulation (>24 hours versus ~1 hour) and induced markedly more prolonged calcemic and phosphaturic responses than did the non-pegylated control. Fluorescence microscopy analysis of kidney sections obtained from the injected mice revealed much less PEG-PTHTMR than PTHTMR on the luminal brush-border surfaces of renal proximal tubule cells (PTCs), on which PTH regulates phosphate transporter function, whereas immunostained phosphorylated PKA substrate, a marker of cAMP signaling, was increased to similar extents for the two ligands and for each, was localized to the basolateral portion of the PTCs. Pegylation of a bioactive PTH peptide thus led to prolonged pharmacokinetic/pharmacodynamic properties in vivo, as well as to new in vivo data that support a prominent role for PTH action at basolateral surfaces of renal proximal tubule cells.
Introduction
Parathyroid hormone (PTH) plays a critical role in maintaining constant levels of ionized calcium (Ca2+) and inorganic phosphate (Pi) in the blood and extracellular fluids. PTH mediates these biological actions via effects on bone and kidney cells, which express the PTH receptor (PTHR1). In bone, PTH acts on osteoblasts, which, in turn, activate, via the RANKL-RANK signaling system, osteoclasts, leading to increased bone resorption and mineral efflux.(1) In kidney, PTH acts on cells of the proximal and distal tubule and modulates in these cells the expression and function of proteins involved in Ca and Pi transport, as well as the synthesis of 1,25-dihydroxyvitamin D (1,25(OH)2D).(2) Impaired PTH production or PTH mutations define the condition of hypoparathyroidism (HP), which is characterized by chronic hypocalcemia/hyperphosphatemia and an array of associated neuromuscular symptoms.(3–5) Clinical studies have explored the use of PTH peptides, such as PTH(1-34), as potential therapies for HP,(6) and full-length recombinant human PTH(1-84), administered by once-daily injection, is now available as one such treatment option.(4) When administered by once-daily injection, PTH peptides can also result in an increased bone mass deposition and thus can be used in the treatment of osteoporosis.(7)
When injected intravenously into humans, unmodified PTH(1-34) disappears from the circulation rapidly, with a measured half-time (t1/2) of 10 ± 0.5 minutes,(8) whereas subcutaneous injection extends the half-time to about 1 hour.(9,10) As a means to overcome the relatively short PK profile exhibited by an injected PTH peptide, continuous infusion, via an implanted pump, of PTH(1-34) was evaluated in HP patients and was indeed found to be more effective at maintaining normal blood calcium levels than was repeated daily injection of the peptide.(6) Full-length PTH(1-84) when injected subcutaneously in humans exhibits an extended PK profile, with a serum half-time of about 2.5 hours as compared to 2 to 4 minutes for iv injection, which likely reflects in part a relatively slow rate of absorption from the subcutaneous compartment.(9,11,12)
PTH analogs that can control blood calcium levels in vivo more effectively than unmodified PTH peptides could help meet an important medical need. Extensive investigations into the structure-activity relationships underlying the binding of PTH analogs to the PTHR1 have yielded several types of PTH peptide analogs that exhibit potentially useful pharmacological profiles. For example, modified PTH(1-34) analogs have been identified that form highly stable complexes with the PTHR1 and thus induce markedly prolonged cAMP signaling responses in PTHR1-expressing cells, as well as significantly protracted calcemic and hypophosphatemic responses when injected subcutaneously into animals, even though the analogs disappear from the circulation more rapidly than does PTH(1-34).(13–16) Other, structurally distinct, PTH analogs have been developed that mediate prolonged actions in vivo due to extended pharmacokinetics, a property conferred to the peptides by the incorporation of several beta-amino acids, each of which introduces an extra methylene group into the peptide backbone, which enhances resistance to proteases in serum and tissue.(17)
The process of glomerular filtration can contribute importantly to the overall effectiveness in vivo of a bioactive molecule or peptide that is of small size, such as PTH(1-34) (~4,000 Daltons), given that the rate of solute passage through the glomerular capillary filtration system into the urine is inversely related to solute molecular weight.(18) Accordingly, strategies have been employed to increase the bulk size of various low-molecular-weight drug-like molecules so as to reduce their rates of glomerular filtration and to thus enhance their PK/PD properties. One such strategy involves attaching to the target molecule a polyethylene glycol (PEG) chain that is composed of a sufficient number of repeating ethylene glycol groups such that renal filtration is reduced.(19–21) In addition to sterically impeding filtration, such PEG moieties can potentially further enhance PK/PD properties of a target agent by increasing binding to serum carrier proteins and/or reducing susceptibility to metabolizing enzymes in serum or tissue.(19–21) Among the bioactive molecules that have been effectively pegylated are salmon calcitonin(22) and glucagon-like peptide-1,(23) each of which is a peptide ligand that is similar in size to PTH(1-34) and binds to a related family B GPCR—the CTR and GLP1R, respectively. An obvious requirement for this general strategy is that the PEG adduct does not interfere with action of the ligand at the receptor.
To explore potential avenues of enhancing the PK/PD properties of PTH peptide fragments in vivo, we synthesized a PTH(1-34) derivative in which a 20 kD PEG group was attached, via a thiol-linkage, to a C-terminal Cys35 residue, and we assessed the properties of the analog in PTHR1-expressing cells in culture, as well as in mice after subcutaneous injection. We further used the analog to help dissect the relative roles that PTH action at the serosal/basolateral surfaces of renal tubules versus at the luminal/apical surfaces of the tubules play in mediating the biological responses that the hormone induces in the kidney.(24)
Materials and Methods
Peptides and synthesis of pegylated PTH analog
PTH peptides were based on the human PTH(1-34) sequence and were synthesized by the Massachusetts General Hospital Biopolymer Core facility using solid-phase chemistry. The non-pegylated parent peptide [Lys13(TMR), Cys35]-PTH(1-35) (PTHTMR) was obtained by post-synthetically attaching a fluorescent tetramethylrhodamine (TMR) group to the epsilon amino function of Lys-13 of [Cys35]-PTH(1-35). The pegylated derivative, [Lys13(TMR), Cys35-PEG]-PTH(1-35) (PEG-PTHTMR), was then obtained by post-synthetically attaching a single PEG group (MW = 20,000) to the side chain thiol of the C-terminal cysteine at position 35. The 20-kDa thiol-reactive PEG reagent, α-[3-(3-Maleimido-1-oxopropyl) amino]propyl-ω-methoxy, polyoxyethylene; SUNBRIGHT ME-200MA0B, was obtained from NOF America Corp. (White Plains, NY, USA). The thiol conjugation reaction was performed overnight at room temperature in 100 mM sodium citrate buffer, pH 4.0 containing 1 mM EDTA and 10 mM TCEP reducing agent. Unconjugated PEG reagent was removed from the reaction by cation exchange chromatography using SP-sepharose resin and a linear salt gradient formed using 20 mM sodium acetate pH 4.0 as buffer A and the same buffer containing 1 M NaCl as buffer B. Elution of the PEG-PTHTMR peptide was monitored by measuring the fractions for TMR absorbance at 543 nm. The peak fractions were pooled and desalted using a C2tp-reverse-phase cartridge and 75% aceto-nitrile/0.1% TFA for peptide elution. The eluted sample was then lyophilized and reconstituted in 10 mM acetic at a final ligand concentration of 12.3 mg/mL (0.5 mM); aliquots of these stock solutions were stored at −80°C until needed for experiments. All other non-pegylated PTH peptides were purified by reverse phase HPLC and assessed for quality and purity by analytical HPLC and matrix-assisted laser desorption/ionization mass spectrometry; purity was at least 90%. The peptide TMR-NNGEDHSQITKV (NStmr) was synthesized and used to assess non-specific (non-PTH-dependent) TMR staining in the kidney. Peptide concentration of stock solutions were validated by acid hydrolysis and amino acid analysis.
cAMP signaling properties of PTH analogs in GP-2.3 cells
Ligand potency for cAMP signaling was assessed in the HEK293-derived cell line, GP2.3, in which is stably expressed the human PTHR1 and the luciferase-derived glosensor cAMP reporter.(25,26) The cells were seeded into 96-well white plates and assayed 24 to 48 hours after confluency. Assays were performed at room temperature in CO2-independent culture media (Life Technologies, Carlsbad, CA, USA) containing 0.1% BSA (CIDB). The cells were preloaded with luciferin (0.5 mM in CIDB) for 15 minutes, then PTH peptides were added at varying concentrations and cAMP-dependent luminescence was measured at 2-minute intervals using a PerkinElmer Envision plate reader (PerkinElmer, Waltham, MA, USA). For each well, the maximum luminescence (counts per second, cps) observed, which typically occurred 10 to 20 minutes after ligand addition, was used to generate ligand dose-response curves. The maximum cps values were thus plotted versus ligand concentration using GraphPad Prism 7.0 software (GraphPad, La Jolla, CA, USA) and a four-parameter logistics curve-fitting equation, which yielded parameters of potency (EC50) and maximal efficacy (Emax).
Assessment of PTH analogs in mice
Ten-week-old male C57BL/6J mice were purchased from the Charles River Laboratories (Wilmington, MA, USA). Mice were maintained in facilities operated by the Center for Comparative Research of the Massachusetts General Hospital and acclimated in the facilities for 7 days before being used for study. All experimental procedures were approved by the MGH Institutional Animal Care and Use Committee (IACUC). In each study, animals were assigned randomly to treatment groups. Power calculations established that the number of animals used per study group was sufficient to detect statistically significant differences in intended primary experimental outcomes (ie, changes in serum Ca and Pi).
Mice were injected intravenously (iv) via the tail vein with either PTHTMR or PEG-PTHTMR, each diluted in vehicle (0.05% Tween80; 10 mM citrate; 150 mM NaCl; pH 5.0) to give a final ligand dose of 50 nmol/kg body weight. At times immediately before (t = 0) and after injection, tail vein blood or spot urine samples were collected and analyzed, as follows. Blood ionized calcium (Ca2+) was measured with a RAPIDLAB 348 analyzer (Siemens Healthcare Diagnostics, Camberley, UK). Plasma and urine phosphorous (Pi) was determined using a UV spectroscopic assay kit (Wako Pure Chemical Industries, Osaka, Japan). Urine total calcium was determined using a Calcium LiquiColor Test kit (Stanbio Laboratory, Boerne, TX, USA). Urine creatinine was measured with a creatinine assay kit (R&D Systems, Minneapolis, MN, USA). The fractional excretion index for Pi (FEIPi) and Ca (FEICa) was calculated as follows: urine Pi or Ca/(urine creatinine × plasma Pi or blood Ca). Levels of urine cAMP were quantified by RIA and normalized to urine creatinine.(14)
Bioactive PTH peptide content of blood plasma was assessed by applying 5 μL of plasma (supplemented with protease inhibitors) to GP2.3 cells and measuring cAMP-dependent luminescence, as described above. Ligand-dependent TMR fluorescence in plasma and urine samples was assessed by adding 1 μL of sample to 100 μl phosphate-buffered saline in a 96-well black plate and measuring TMR fluorescence using the PerkinElmer Envision plate reader and an excitation filter of 510 nm and an emission filter of 580 nm; the resulting fluorescent signals were converted to nanomolar peptide amounts by interpolating from a standard curve of TMR fluorescence versus ligand concentration generated using the corresponding stock peptide ligand.
Potential effects of the PTH ligand on renal function were assessed by measuring the rate of clearance of fluorescein isothiocyanate (FITC)-conjugated inulin from the blood after co-injection of the FITC-inulin and either PEG-PTHTMR or PTHTMR.(14) A solution of FITC-inulin (Sigma, St. Louis, MO, USA; cat. no. F3272) was prepared at a concentration of 50 mg/mL in 150 mM NaCl and dialyzed (MW cut-off = 1,000) against 150 mM NaCl. Mice were then co-injected via the tail vein with FITC-inulin (100 μg/mouse) together with either PEG-PTHTMR or PTHTMR (each at 50 nmol/kg), and tail vein blood was collected and plasma FITC fluorescence was assessed using the PerkinElmer Envision plate reader and an excitation filter of 485 nm and an emission filter of 535 nm. The same samples were also assessed for TMR fluorescence as described above.
Confocal microscopy analysis of PTH-TMR fluorescence in GP-2.3 cells and mouse kidneys
Confocal fluorescence microscopy images were acquired using Olympus FV-1000 MPE confocal system (Center Valley, PA, USA), performed by the Photopathology Core facility of the Wellman Center for Photomedicine at the Massachusetts General Hospital (Boston, MA, USA). Fluorescent images were captured with either 60× (PLANAPO, 1.2NA, WD 0.28) or 40× (LUMPLFL 0.8NA WD 3.3 mm) water-immersion objectives, and with 405 nm and 559 nm excitation lasers, at a resolution of 1024 × 1024.
The internalization properties of PEG-PTHTMR and PTHTMR were assessed in GP-2.3 cells and non-specific binding was assessed in GS-22A cells (HEK-293 with stable transfection of glosensor; parental to GP-2.3 cells). The cells were cultured on glass cover-slips in 24-well plates to ~75% of confluency, and treated with PEG-PTHTMR or PTHTMR ligand (100 nM) in HBB for 15 minutes at 21°C, or for conditions of blocked internalization, for 30 minutes at 4°C. The cells were then rinsed thrice with HBB, fixed with 4% formalin for 5 minutes, mounted with vector-shield containing DAPI on a glass microscope slide, and viewed and digitally imaged as described above.
Ligand localization in the kidney was assessed by injecting mice iv via the tail vein with either PEG-PTHTMR or PTHTMR each at a dose of 50 nmol/kg, euthanizing the mice at 5 minutes, 60 minutes, or 24 hours post-injection, dissecting the kidneys and fixing them in 4% formaldehyde/10% sucrose on ice for 2 hours; the fixed specimens were then rinsed and incubated in PBS containing 30% sucrose at 4°C overnight, and then embedded in an equal volume mixture of 30% sucrose and OCT medium. Frozen sections of 5~10 μm were then cut and mounted in medium containing DAPI and viewed on the microscope and digitally imaged with the 60× objective; for some images, a 3× or 4× digital zoom was applied, and some images were obtained in digital interference contrast mode.
Immunostaining of kidney sections for protein kinase A (PKA) substrate proteins was performed by treating the sections on a glass slide successively with PBS solutions containing: 0.1% SDS (1 × 5 minutes); 5% BSA (3 × 5 minutes); rabbit antibody to phospho-Ser/Thr-PKA-substrate (Cell Signaling Technology, Danvers, MA, USA; cat. no. 9621) diluted 1:100 (1 hour); high-salt (2.7% NaCl; 2 × 5 minutes); Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific, Waltham, MA, USA); diluted 1:500; and then high-salt (2 × 5 minutes). Control sections were treated with the Alexa Fluor 488 F(ab′) 2 fragment but not with the anti-phospho-PKA antibody.
Quantitative real-time RT-PCR (qRT-PCR)
RNA was extracted from the kidney and tibia obtained from mice 24 hours after injection with a modified PTH analog using TRIzol Reagent and was reverse-transcribed into cDNA using a Superscript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). A SYBR Green PCR Kit (Invitrogen) was used for qPCR assessment of mRNA levels. Measured mRNA levels were normalized to levels of mRNA encoding Gapdh. The targeted gene products and PCR primer sequences used were as follows: 1α-OHase (Cyp27B), forward 5-aagtcactgtccagagcgctg-3 and reverse 5-gttgtccagagttccagcata-3; Npt2a (sodium-dependent phosphate co-transporter-IIa, Scl34A1), forward 5-ccttcacaagactcatcatcc-3 and reverse 5-atggtggtgtttgcaaggctg-3; Npt2c (sodium-dependent phosphate co-transporter-IIc, Scl34A3), forward 5-cttggaagagggaggtacaga-3 and reverse 5-aagcagagctgaggatgtcca-3; TRPV5 (transient receptor potential vanilloid 5), forward 5-ctccatacttggtcacagagt-3 and reverse 5-gtagatgaggttgtgtgaact-3; RANKL (receptor activator of NF-κB ligand), forward 5-cacagcgcttctcaggagctc-3 and reverse 5-gagatcttggcccagcctcga-3; OPG (osteoprotegerin), forward 5-agtccgtgaagcaggagtgca-3 and reverse 5-aagtctcacctgagaagaacc; TRAP (tartrate-resistant acid phosphatase), forward 5-agtgcacgatg ccagcgacaa-3 and reverse 5-ccagcgcttggagatcttaga-3; MMP-9 (matrix metallopeptidase 9), forward 5-gcacgccttggtgtagca caa-3 and reverse 5-ctggtcatagttggctgtggt-3; sclerostin (SOST), forward 5-ggtggcctcgtgcaagtgcaa-3 and reverse 5-taggcgttctccagctccg-3; FGF23 (Fibroblast growth factor 23), forward 5-acttgtcgcagaagcatc-3 and reverse 5-gtgggcgaacagtgtagaa-3, and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), forward 5-tggagtggtgtcttcactact-3 and reverse 5-aagcagttggtggtgcaggat-3.
Data analysis
Data were processed using Microsoft Excel and GraphPad Prism 7.0 software packages and analyzed statistically using Student’s t test (two-tailed and unequal variances).
Results
Bioactivity of pegylated PTH in PTHR1-expressing GP-2.3 cells
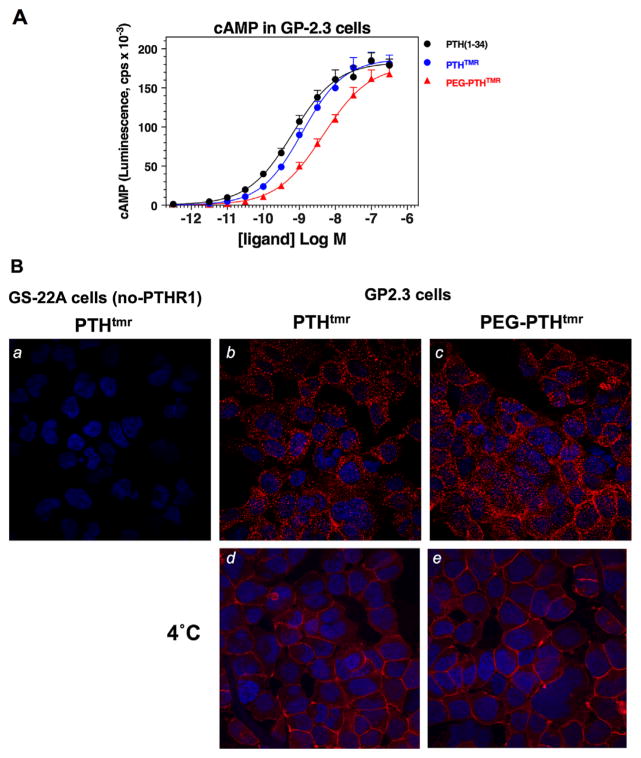
The capacity of the PEG-PTHTMR analog to stimulate cAMP signaling via the PTHR1 was assessed in an HEK293-derived cell line, GP-2.3, in which is stably expressed the human PTHR1 along with the luciferase-based glosensor cAMP reporter. The PEG-PTHTMR analog stimulated an increase in cAMP-dependent luminescence in these cells that reached the same maximum level as that observed with PTH(1-34) or with the non-pegylated parent peptide, PTHTMR, and was characterized by a potency value (EC50) that was about three- to fivefold weaker than that exhibited by PTH(1-34) and PTHTMR (Fig. 1A; Table 1). Fluorescent confocal microscopy analysis of the same GP-2.3 cells treated for 15 minutes at 21°C with either PEG-PTHTMR or PTHTMR, each at a concentration of 100 nM, revealed each ligand to be located predominantly in internalized vesicles, or at the cell surface if the treatments were at 4°C (Fig. 1B). No such TMR fluorescence was observed for either ligand in the parental HEK293/glosensor cells (GS-22A cells), which are not transfected with the PTHR1. The results of these cell-based assays establish that the pegylated PTH analog binds specifically to the PTHR1 and can induce PTHR1-dependent signaling and internalization responses that parallel closely those induced by a non-pegylated agonist control PTH peptide.
Fig. 1.
cAMP signaling and internalization properties of PEG-PTHTMR in HEK-293-derived GP-2.3 cells. (A) Dose-response analysis of the capacity of PEG-PTHTMR, PTHTMR, and PTH(1-34) to stimulate cAMP formation in GP-2.3 (HEK-293/PTHR1/glosensor) cells. (B) Confocal fluorescence microscopy analysis of the internalization properties of PEG-PTHTMR and PTHTMR in GP-2.3 cells. Also shown are the non-PTHR1-transfected parental GS-22A (HEK-293/ glosensor) cells treated with PTHTMR. The cells were treated with the ligands (100 nM) at 21°C for 15 minutes (a–c) or, to block internalization, at 4°C for 30 minutes (d, e), then rinsed, fixed, and mounted for imaging (magnification = 400×, TMR = red; DAPI = blue). Data in A are means (±SEM) of 10 experiments; curve-fitting parameters are reported in Table 1. Images in B are representative of three other experiments.
Table 1.
cAMP Dose-Response Analyses in GP-2.3 Cellsa
| pEC50
|
Emax(cps × 10−3)
|
|||||
|---|---|---|---|---|---|---|
| p versus PTH(1-34) | p versus PTHTMR | p versus PTH(1-34) | p versus PTHTMR | |||
| PTH(1-34) | 9.15 ± 0.08 0.60 nM |
1.00 | 188 ± 10 | 1.00 | ||
| PTHTMR | 8.90 ± 0.09 0.92 nM |
0.05 | 184 ± 12 | 0.77 | ||
| PEG-PTHTMR | 8.35 ± 0.14 2.87 nM |
0.0014 | 0.004 | 178 ± 14 | 0.55 | 0.70 |
Assays were performed in GP-2.3 cells; values of half-maximal stimulatory concentration (as pEC50 and corresponding nanomolar value shown below) and Emax (as cAMP-dependent luminescence counts per second ×10−3) were derived from curve fitting dose-response data; the maximum luminescence observed in vehicle-treated cells was 1.3 ± 0.1 cpsx10−3. Data are means ±SEM of 10 experiments; PTHTMR, K13(TMR),C35-PTH(1-35); PEG-PTHTMR, K13(TMR), C35(PEG-20kDa)-PTH(1-35).
Pharmacodynamic and pharmacokinetic properties of PEG-PTHTMR in mice
Effects on blood Ca2+ levels
Ligand injection studies in mice were performed to assess the biological properties of the PEG-PTHTMR analog, in comparison to the non-pegylated control peptide, PTHTMR, in vivo. The peptides were injected iv, via the tail vein at a dose of 50 nmole/ kg-body weight, and blood was withdrawn for analysis at times after injection or immediately before injection (t = 0). A major effect of PTH is to elevate blood Ca2+ levels by inducing the release of calcium from bone, increasing the reabsorption of filtered calcium by the kidney, and, secondarily, by increasing synthesis of 1,25(OH)2D, which enhances intestinal calcium absorption. Upon injection of the non-pegylated control peptide PTHTMR, blood Ca2+ levels increased to a peak value at one hour post-injection that was ~8% greater than the pre-injection (t = 0) level, and the levels then decreased to baseline by 24 hours (Fig. 2A). Injected at the same dose, PEG-PTHTMR resulted in a much larger and more prolonged overall increase in blood Ca2+, such that the peak level was attained at the 24-hour time point and was ~21% above the pre-injection level (Fig. 2A). The Ca2+ levels were still elevated at the 48-hour time point and did not return to baseline until the final measurement at 96 hours post-injection. The overall blood calcium response profile observed with PEG-PTHTMR was characterized by two distinct phases: an initial phase that paralleled closely the overall profile observed with PTHTMR and which included a brief deflection in Ca2+ between 2 and 4 hours; and a subsequent phase marked by a gradual increase in Ca2+ that continued over the next 20 hours.
Fig. 2.
Pharmacodynamic comparison of PEG-PTHTMR and PTHTMR in mice. Mice were injected intravenously with PEG-PTHTMR (filled squares) or PTHTMR (open circles) each at a dose of 50 nmol/kg, and tail vein blood and spot urine samples were collected for analyses at various times thereafter and immediately before injection (t = 0). (A) Blood levels of ionized calcium (Ca2+). (B) Plasma levels of inorganic phosphorus (Pi). (C) Spot urine levels of total calcium expressed as a fractional excretion index of calcium (FECa; 100 flurine Ca / (urine creatinine x blood Ca2+)). (D) Spot urine levels of total Pi expressed as a fractional excretion index of phosphorus (FEPi; 100 flurine Pi / (urine creatinine × plasma Pi)). (E) Urine levels of cAMP corrected for creatinine. (F) Plasma levels of collagen-1 C-terminal peptide (CTX). Data represent means ± SEM (n = 6 mice per group). Insets in A, B, D, and E show the same data for the first 24 hours using an expanded x axis. Asterisks indicate comparisons of PEG-PTHTMR to PTHTMR at the same time point: *<0.05; **<0.01.
Effects on plasma Pi
In addition to stimulating increases in blood levels of Ca2+, PTH acts to reduce blood levels of inorganic phosphorus (Pi) by suppressing functional expression of the sodium-dependent phosphate transporter proteins, Npt2a and Npt2c, in the kidney. Consistent with this known PTH-regulated action, blood levels of Pi were reduced in both the mice injected with PTHTMR and those injected with PEG-PTHTMR, but the reductions were more pronounced and extended with the pegylated analog (Fig. 2B). Thus, in PTHTMR-treated mice, blood phosphate levels declined by 27% at the nadir, which was attained at 1 hour after injection and then returned to baseline by 4 hours, whereas in PEG-PTHTMR-treated mice, the Pi levels declined by 42% at the nadir, which was attained at 2 hours after injection, and they did not return to baseline until between the 24- and 48-hour time points (Fig. 2B).
Effects on urinary Ca/Pi excretion and CTX bone resorption marker
Consistent with the observed increases in blood calcium and decreases in blood Pi levels, injection of each analog caused a reduction in the fractional excretion of calcium and an increase in the fractional excretion of Pi into the urine, and these effects on urinary excretion were significantly greater and longer-lasting (sustained for up to 24 hours) with PEG-PTHTMR than with PTHTMR (Fig. 2C, D). In addition, whereas both injected analogs resulted in increases in urinary levels of cAMP and blood levels of collagen-1 C-terminal peptide (CTX), markers of PTH action in the kidney and in bone, respectively, the increases were more pronounced and longer-lasting with the pegylated peptide than with the lower-molecular weight control ligand analog (Fig. 2E, F).
Effects on blood 1,25(OH)2 vitamin D and kidney and bone mRNA levels
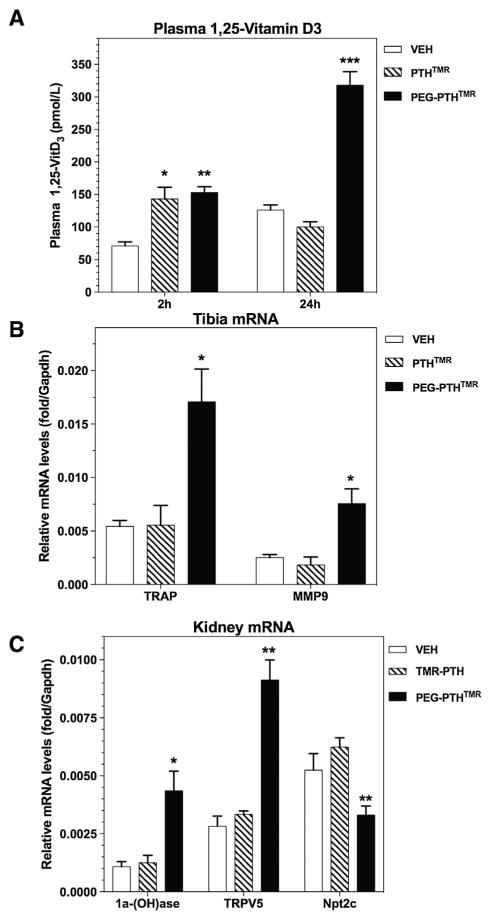
A separate experiment was conducted to evaluate the effects of the pegylated PTH analog on blood levels of 1,25(OH)2D, as well as on gene expression patterns in bone and kidney tissue. At 2 hours after injection, blood levels of 1,25(OH)2D were elevated in both PTHTMR- and PEG-PTHTMR-injected mice by about threefold, relative to levels in vehicle-injected mice, whereas at 24 hours after injection, the 1,25(OH)2D levels were elevated only in mice injected with the pegylated analog (Fig. 3A). In bone and kidney samples obtained from the mice at 24 hours post-injection, PEG-PTHTMR induced more robust changes in mRNAs encoding known PTH-regulated genes—TRAP and MMP9 in tibia and 1alpha-hydroxylase, TRPV5 and Npt2c in kidney—than did PTHTMR (Fig. 3B, C). Levels of mRNA for SOST, RANKL, OPG, and FGF23 in tibia and for Npt2a and 24-hydroxylase in kidney were not significantly altered by either PTH ligand at this time point. Note that the marked increases in the levels of 1,25(OH)2D in blood, as well as 1alpha-hydroxylase mRNA in kidney found at 24 hours in the mice injected with PEG-PTHTMR, but not in mice injected with PTHTMR, could underlie, in part, via effects on intestinal calcium absorption, the prolonged increases in blood Ca2+ found in the PEG-PTHTMR treated mice in the experiment of Fig. 2.
Fig. 3.
Ligand effects on 1,25(OH)2D in blood and PTH-responsive mRNA levels in bone and kidney. Mice were injected via the tail vein with vehicle, PTHTMR, or PEG-PTHTMR, each ligand at a peptide dose of 50 nmol/kg, and subsequently blood was collected at 2 and 24 hours post-injection and analyzed for changes in blood levels of 1,25(OH)2D (A), and tibia (B) and kidneys (C) were isolated at 24 hours post-injection and analyzed by quantitative real-time RT-PCR for levels of mRNA encoding the indicated gene products. Data in B and C are normalized to the level of mRNA encoding GAPDH detected in each tissue. Asterisks indicate comparisons of PEG-PTHTMR or PTHTMR to vehicle: *<0.05; **<0.01; ***<0.001. Not shown for mice injected with vehicle, PTHTMR or PEG-PTHTMR are the relative levels of kidney mRNA encoding Npt2a (0.49 ± 0.03; 0.58 ± 0.02, and 0.52 ± 0.01, respectively) and 24-(OH) ase (0.05 ± 0.01; 0.09 ± 0.01, and 0.17 ± 0.01, respectively), and tibia mRNA encoding RANKL (0.014 ± 0.001; 0.014 ± 0.001, and 0.019 ± 0.003, respectively); OPG (0.009 ± 0.003; 0.005 ± 0.001, and 0.005 ± 0.001, respectively); FGF23 (0.019 ± 0.003; 0.018 ± 0.001, and 0.020 ± 0.004) and sclerostin (0.0018 ± 0.0005; 0.0012 ± 0.0003, and 0.0012 ± 0.0002, respectively), for which ligand-injected levels were not significantly different from vehicle-injected levels.
Assessment of PEG-PTHTMR in the blood and urine
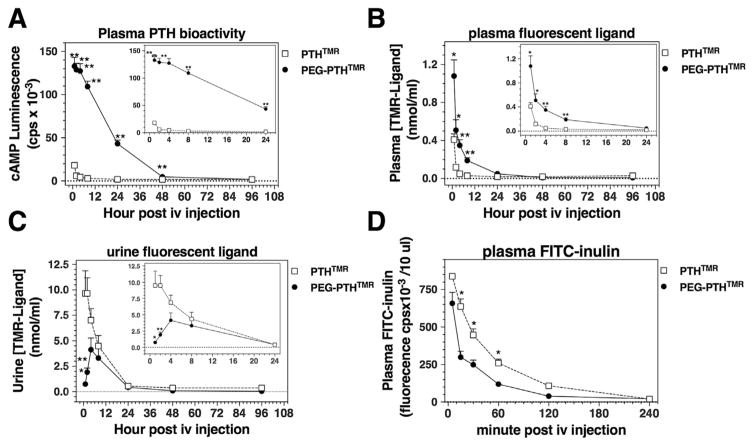
The extended pharmacodynamic responses observed with the pegylated PTH analog were consistent with the hypothesis that this ligand persists in the circulation for longer times than does the non-pegylated control ligand. To test this, we assessed blood samples collected at times after ligand injection for the presence of the bioactive PTH ligands by applying a portion of the plasma onto PTHR1-expressing GP-2.3 cells and measuring effects on intracellular cAMP formation (ie, changes in glosensor-dependent luminescence). Plasma collected from mice injected with PEG-PTHTMR over the initial 24-hour post-injection period induced significantly greater increases in cAMP than did plasma from mice injected with PTHTMR, such that whereas PEG-PTHTMR bioactivity was readily detectable to at least the 24-hour time point, no PTHTMR-dependent bioactivity was detectable after the 4-hour time point (Fig. 4A).
Fig. 4.
Pharmacokinetic assessment of injected PTH analogs in blood and urine. Mice were injected via the tail vein with PEG-PTHTMR or PTHTMR each at a dose of 50 nmol/kg, and blood and spot urine was collected at times thereafter and immediately before injection (t = 0), and analyzed for PTH ligand content. (A) Blood plasma was assessed for PTH bioactivity by applying 1 μL of plasma to GP-2.3 cells and measuring the resulting changes in cAMP-dependent luminescence. (B) Blood plasma was assessed for fluorescent PTH ligand by measuring ligand-dependent TMR fluorescence (λexc. = 510 nm; λexc. = 580 nm) in 1 μL of sample and converting the resulting values to peptide concentration using for interpolation a standard curve of TMR-fluorescence versus ligand concentration generated with the same corresponding peptide ligand. (C) Urine was assessed as in B for fluorescent PTH ligand content. No change in cAMP-dependent luminescence or TMR fluorescence, relative to blank controls, was detected in plasma or urine samples collected before (time 0) injection. Data in A–C were obtained from samples generated in the same experiment reported in Fig. 2 and represent means ± SEM (n = 6 mice per group). The absence of samples for early time points (0 to 60 minutes) precluded derivation of formal pharmacokinetic parameters. (D) Mice (n = 3) were co-injected via the tail vein with FITC-inulin and either PTHTMR or PEG-PTHTMR (50 nmol/kg), and tail vein blood was collected at times thereafter and the plasma was assessed for FITC fluorescence. Asterisks indicate comparisons of PEG-PTHTMR to PTHTMR at the same time point: *<0.05; **<0.01.
The same plasma samples were also assessed for the presence of the injected ligands by measuring TMR-based fluorescence (λexc. 531 nm; λemm. 580 nm) derived from the TMR-modification of the ligands. The fluorescent signal obtained for each sample was converted to a specific ligand concentration by interpolation from a dose-fluorescence standard curve generated with the respective stock ligand. For the non-pegylated PTHTMR control peptide, TMR fluorescence was detected only at the 1-and 2-hour two time points, whereas for PEG-PTHTMR, fluorescence was detected out to at least the 8-hour time point, and the calculated peptide concentration at the peak 1-hour time point was about threefold greater than that observed for PTHTMR (Fig. 4B). Thus, both fluorescent- and bioactivity-based methods of analyses indicated that the PEG-PTHTMR analog persisted in the circulation for longer times than did the PTHTMR ligand. Consistent with a persistence of PEG-PTHTMR in the blood, there was less TMR fluorescence detected in the urine of mice injected with PEG-PTHTMR than in the urine of mice injected with PTHTMR (Fig. 4C).
A separate experiment was performed to determine if the persistence of PEG-PTHTMR in the circulation might have been attributable to some unfavorable effect of the ligand on renal function that resulted in reduced renal filtration and clearance. We thus co-injected mice with FITC-inulin together with either PEG-PTHTMR or PTHTMR and measured the amount of FITC-inulin-derived fluorescence (λexc. 485 nm; λemm. 535 nm) present in the plasma at times after injection.(14) The FITC fluorescence disappeared from the blood plasma of mice co-injected with the PEG-PTHTMR analog if anything more rapidly than it did from the blood of mice co-injected with PTHTMR (Fig. 4D). These results indicate that renal filtration was not negatively impacted by the PEG-PTHTMR analog. As expected, TMR fluorescence for the PEG-PTHTMR analog persisted in the blood for longer times and at higher levels than did that of the PTHTMR analog (50,405 ± 5624 cps versus 603 ± 197 cps; at 240 minutes post-injection, p = 0.02; n = 3).
Localization of TMR fluorescence and PKA activation in kidneys of ligand-injected mice
TMR localization
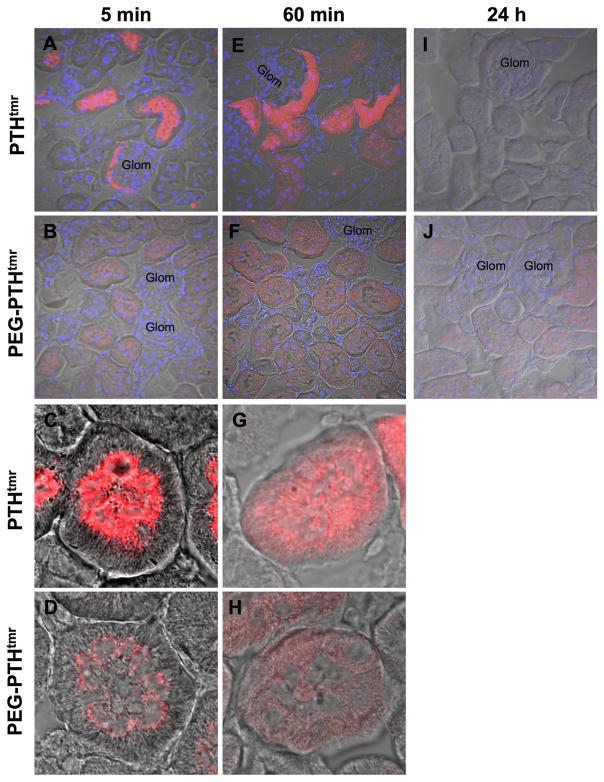
The above pharmacokinetic data were consistent with a reduced rate of renal filtration for the pegylated PTH analog compared with the non-pegylated peptide. This possibility was examined more directly by analyzing kidney sections obtained from mice at different times after ligand injection for TMR fluorescence using fluorescence confocal microscopy. At 5 minutes after injection, kidneys from mice injected with PTHTMR exhibited strong TMR fluorescence that was localized mainly within the brush-border luminal regions of the proximal tubules and adjacent glomeruli, whereas kidneys from mice injected with PEG-PTHTMR also exhibited TMR fluorescence in the brush border luminal region of the tubules but the overall intensity was much less (Fig. 5A–D).
Fig. 5.
Confocal microscopy analysis of TMR-peptide localization in kidney. Confocal fluorescence with superimposed differential interference contrast (DIC) microscopy images of renal cortex sections derived from mice at 5 minutes (A–D), 60 minutes (E–H), or 24 hours (I, J) after intravenous injection with PEG-PTHTMR or PTHTMR (50 nmol/kg). Data shown are representative of two independent experiments. Samples were imaged in confocal mode for TMR (red) with or without DAPI (blue) fluorescence, as well as in DIC mode, and the channels were merged. Glom = glomerulus. Total magnification = 600× (A, B, E, F, I, J) or 2400× (C, D, G, H).
At 60 minutes after injection, the overall level of fluorescence with the non-pegylated peptide was still more intense with PTHTMR than with PEG-PTHTMR, and the fluorescence appeared dispersed in the cytoplasm of the proximal tubule cells, extending broadly from the luminal to the basal membrane borders (Fig. 5E–H). By 24 hours post-injection, little or no TMR fluorescence was apparent in the kidneys obtained from mice injected with PTHTMR, whereas a low-level of fluorescence continued to be detected in the proximal tubule cells of mice injected with PEG-PTHTMR (Fig. 5I, J). The relatively reduced appearance of overall TMR fluorescence in the luminal regions of the tubules and glomeruli observed in these kidney sections for the PEG-PTHTMR analog is consistent with an impeded rate of filtration of the pegylated PTH analog through the glomerular capillary meshwork and thus a persistence of the analog in the blood of the injected animals.
Protein kinase A activation
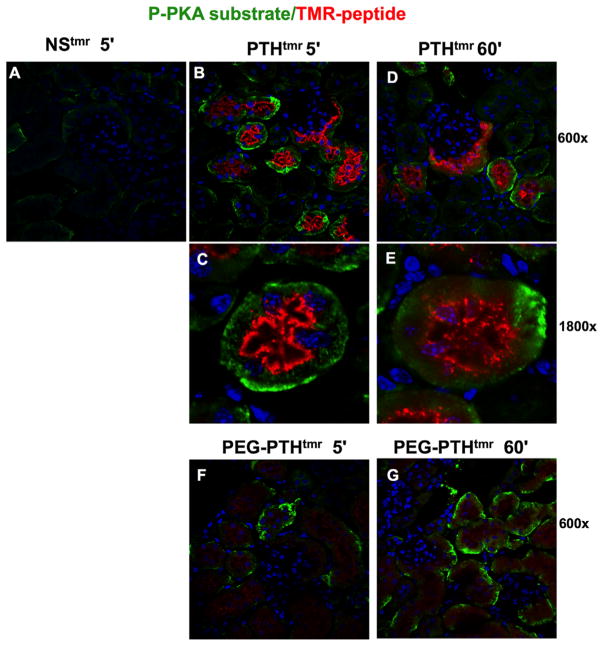
The isolated kidney sections were further evaluated for effects of injected PEG-PTHTMR and PTHTMR on PTHR1-mediated signaling responses by immunostaining for phosphorylated forms of protein kinase A (PKA) substrate target proteins. The antibody used for these studies does not permit identification of any specific PKA target protein phosphorylated, as it reacts broadly with PKA-targeted substrates and potentially other AGC-family kinases, such as PKC or AKT, but it nevertheless can provide information on the extent of activation of the cAMP/PKA pathway, as well as on the location of at least some downstream PKA targets. As a control for possible nonspecific effects, a parallel group of mice were injected with a non-PTH-based 12-amino acid (NNGEDHSQITKV) peptide labeled with TMR (NSTMR). Kidney sections isolated from these control mice at 5 minutes post-injection exhibited little or no green fluorescence corresponding to immunostained pPKA substrate, as well as little or no red fluorescence corresponding to the TMR-peptide (Fig. 6A). In contrast, kidneys obtained from mice at 5 or 60 minutes after injection with PTHTMR exhibited clear staining for pPKA substrate, and this positive pPKA substrate signal tended to be located in proximal tubules that were also positive for PTH-dependent TMR fluorescence (Fig. 6B–E). Of interest, the PTHTMR fluorescence in these tubules was localized mainly on the luminal/brush-border surfaces, as was observed in the images of Fig. 5, whereas the pPKA substrate staining was localized mainly at or near the basolateral surfaces and thus in contralateral position to the bulk of the PTHTMR fluorescence. Kidneys from mice injected with PEG-PTHTMR exhibited a level of immuno-stained pPKA substrate that was at least comparable to that observed with the PTHTMR ligand, especially at the 60-minute time point, even though the overall level of TMR fluorescence was considerably lower than that found in mice injected with PTHTMR, and again, the pPKA substrate was localized mainly on the basolateral surfaces of the proximal tubule cells (Fig. 6F, G).
Fig. 6.
Confocal microscopy analysis of TMR-peptide and phosphorylated PKA substrate in kidney. Confocal microscopy images of sections of renal cortex derived from mice at 5 minutes, 60 minutes, or 24 hours after intravenous injection with either a nonspecific control peptide, NSTMR (A), PTHTMR (BE), or PEG-PTHTMR (F, G). Sections were immunostained (green) using a primary IgG antibody directed against phosphorylated PKA substrates and a secondary anti-rabbit IgG antibody labeled with Fluor-488. Sections were imaged for pKA-substrate (green), TMR-peptide (red), and DAPI (blue) at a total magnification of 600× or 1800× (C, E). No immunostaining was detected in sections not treated with primary antibody.
Discussion
The approach of attaching a polyethylene glycol chain to a bioactive molecule for the purpose of improving pharmacologic utility has been applied to a number of peptide or protein ligands, including the interferons,(27,28) TNF receptor 1,(29) interleukin-6,(30) and granulocyte colony-stimulating factor.(31) The main benefit derived from pegylation is a prolongation of the serum half-life of the target drug, which can result from alterations in a number of biophysical parameters that influence ligand pharmacokinetics, such as the capacity to bind to serum or tissue proteins, the susceptibility to protease degradation, immunogenicity, and the rate of renal filtration.(19,20,32) The only PTH-based therapies evaluated so far in hypoparathyroidism are unmodified PTH(1-34) and PTH(1-84), administered by repeated (at least daily) subcutaneous injection, or, to mitigate fluctuations in calcium associated with repetitive injections, by continuous infusion via an insulin pump.(4,6,11,33)
Our current studies show that a 20-kiloDalton polyethylene glycol group can be attached to the C-terminus of PTH(1-34), via a Cys35 thiol linkage, without severely compromising the signaling actions of the ligand on the PTHR1 and to thus result in a ligand that mediates markedly prolonged calcemic and phosphaturic responses upon injection into mice. In mice, the sustained increases in blood calcium were accompanied by sustained reductions in the fractional excretion of calcium into the urine, consistent with the known capacity of PTH to increase the rate of calcium reabsorption in the renal tubules. Further, consistent with a more sustained increase in the rate of calcium reabsorption, PEG-PTHTMR induced a greater increase in the renal expression of the TRPV5 channel, which is a key mediator of the effects of PTH on calcium reabsorption in the distal tubule of the nephron,(34) than did PTHTMR (Fig. 3C). It also worth noting that patients with hypoparathyroidism tend to show an increased prevalence of nephrocalcinosis and renal function impairment, which is thought to be related to the excessive rates of urinary calcium excretion that can occur with the conventional therapy of oral calcium and vitamin D supplementation, as such a non-PTH-based mode of treatment lacks a direct beneficial effect on calcium excretion.(35)
The prolonged pharmacodynamic responses observed with our PEG-PTHTMR analog were clearly associated with a prolonged persistence in the serum, as we could detect the pegylated analog in the blood using a cAMP-based bioassay of active PTH ligand, as well as by assessment of the attached TMR moiety by direct fluorescence, for much longer times—at least 24 hours—after injection, than we could the non-pegylated counterpart peptide, PTHTMR, which was undetectable after 4 hours. The extended pharmacokinetic profile of PEG-PTHTMR, in turn, could be attributed, in large part, to a reduced rate of glomerular filtration, as the amount of TMR fluorescence detected in the urine after injection was substantially lower with PEG-PTHTMR as compared to with PTHTMR. Thus, C-terminal pegylation effectively extended the pharmacokinetic and pharmacodynamic profile of this PTH(1-34)-based agonist peptide.
The finding that PEG-PTHTMR was cleared into the urine at a reduced rate is consistent with the capacity of pegylation, via increasing molecular mass, to impede passage through the glomerular complex of the kidney.(19) The reduced rate of urinary clearance suggests that such pegylated PTH analogs might be useful in dissecting mechanisms of physiological action in the kidney. In particular, the extent to which PTH regulates the renal phosphate transporters, NPT2a and NPT2c, in proximal tubule cells by acting at PTH receptors on the luminal/ brush-border membrane surfaces of the cells (ie, the urine side of the tubule) or on the basolateral surface (ie, the blood side) has yet to be fully elucidated. Two prior studies in which isolated mouse proximal tubules were used provide evidence that PTH can act on both surfaces, as the application of PTH(1-34) to the basolateral bathing solutions or to the luminal perfusates was found to result in a reduction of NPT2a protein abundance on the brush border surface,(36) as well as a reduction in the rate of Pi absorption.(24) On the other hand, a study using cultured chicken primary proximal tubule cells found that PTH(1-34) reduced transcellular Pi transport when applied to the basolateral surface of the cells but not when applied to the luminal surface.(37)
Given that PEG-PTHTMR, when injected into a mouse, exhibited a markedly reduced rate of filtration into the urine, yet induced a phosphaturic effect that was, if anything, greater than that induced by PTHTMR, our studies provide data in vivo to support a major role for PTH receptors located on the basolateral surface of the proximal tubule cells in mediating the acute effects of PTH on renal Pi handling. In addition, injection of PEG-PTHTMR induced increases in blood levels of 1,25(OH)2D that were similar to those induced by PTHTMR as early as 2 hours after injection and were sustained at 24 hours, and it also induced markedly greater increases in the expression of the 1-alpha-hydroxylase gene in kidney tissue, as measured at 24 hours post-injection. These findings are consistent with a role for PTH receptors located on the basolateral surfaces of the proximal tubule cells in mediating the effects of PTH ligands on renal vitamin D metabolism. Further, consistent with the interpretation that PTH ligand action on basolateral surfaces of the renal proximal tubule cells contributes importantly to effects on Pi transport and vitamin D metabolism, our confocal microscopy analyses of kidney sections obtained from mice as early as 5 minutes after injection revealed increases in the levels of immunoreactive substrates phosphorylated by pPKA, and potentially other AGC-family kinases, such as PKC or AKT, in proximal tubule cells that were at least similar for PEG-PTHTMR and PTHTMR, despite markedly reduced amounts of the pegylated ligand in the luminal compartment of the tubules. Moreover, the increases in pPKA substrate were, for each ligand, located primarily on the basolateral surfaces of the proximal tubule cells.
Our findings nevertheless do not exclude the possibility that ligand action at receptors located on the brush-border membranes of the proximal tubule cells plays some role in mediating the renal effects of the ligand on Pi transport and vitamin D metabolism. In fact, initially (at 5 minutes post-injection) the ligand-derived TMR-fluorescence was localized most prominently on the luminal surfaces of the proximal tubule cells, albeit at low levels for PEG-PTHTMR, and although this fluorescence became more dispersed in the cytoplasm by 60 minutes, there was little, if any, of it detected specifically on the basolateral surfaces of the cells at either the early or later time point.
The failure to detect TMR-labeled ligand on the basolateral surfaces of the proximal tubule cells may seem paradoxical in relation to the above-mentioned functional data supporting a role for PTH action at such basolateral receptors. However, it is possible that the relative abundance of the basolateral surface receptors is simply too low to detect with our current TMR-ligand analogs and fluorescent tracking methods. In this regard, we note that we have not been able to detect TMR-labeled PTH analogs in bone sections obtained from injected mice, nor binding of the analogs, at room temperature or 4°C, to cultured osteoblastic cells (ie, SaOS-2 and UMR-106 cells) that express the PTH receptor endogenously and at levels lower than in our transfected HEK-293-derived cells, but which nevertheless give robust cAMP responses to the applied PTH ligands.(5) In addition, rapid internalization of agonist-bound complexes might also account for the apparent absence of such complexes on the basolateral surfaces and may underlie at least some of the cytoplasmic TMR fluorescence that had accumulated at the later times (60 minutes). Using immunocytochemical methods, Ba and colleagues detected some PTH receptor staining on the basolateral surfaces of the proximal tubules, but the signal was slight compared with the luminal signal.(24) We also note that the extent to which the TMR fluorescence staining we observed with our TMR-labeled PTH ligands on the luminal surfaces of the tubules might reflect binding to sites other than the PTHR1, for example, to megalin/cubulin scavenging proteins,(38) is uncertain. Our non-PTH-based, 12-amino-acid, TMR-labeled control peptide (NSTMR) resulted in little or no such staining on the lumenal surfaces, suggesting that nonspecific peptide scavenging may not be a major contributing factor to the luminal staining patterns observed with the PTH-TMR ligands, although the smaller size and distinct primary structure of that control peptide leave open the possibility that it utilizes filtration mechanisms that differ from those used by PTH ligands. In any case, although our data by no means exclude the possibility of PTH actions at luminal surfaces, they do provide support for the notion that PTH action at PTH receptors located on the basolateral surfaces of proximal tubule cells can play a major role in mediating the effects of the ligand on renal Pi transport and metabolism of 1,25(OH)2D.
Pegylated PTH analogs could be potentially useful in deciphering the roles that different signaling pathways play in mediating the actions of the hormone in different tissues. For example, both the cAMP/PKA and PLC/IP3 pathways have been implicated as important mediators of the phosphaturic response to PTH in the kidney,(39–41) but the roles that the two pathways play in the response are not well understood. In a prior study performed in wild-type mice, we found that the PLC-defective analog, Trp1-M-PTH(1-28) reduced blood Pi levels, as well as the abundance of NPT2a and NPT2c proteins in renal brush-border membranes obtained from the mice nearly as effectively as did the bifunctional control ligand M-PTH(1-28).(42) On the other hand, in mice expressing a PLC-signaling defective PTHR1 mutant, DSEL, in place of the wild-type PTHR1, infusion of PTH(1-34) via a surgically implanted mini-pump was less effective at maintaining a chronic hypophosphatemic state than it was in wild-type mice, suggesting that the PLC-pathway is required for the continued suppression of renal Pi transport by PTH.(43) Pegylated PTH analogs modified with Trp1 or some other structural change that alters signaling bias could prove useful in dissecting further the mechanisms by which PTH functions in different target tissue compartments (eg, filtered versus serosal surfaces of the tubule) and under different durations of exposure (chronic versus acute).
Certain PTH and PTHrP peptide analogs have recently been shown to form highly stable complexes with the PTHR1 and thus mediate functional responses that persist for some hours after initial application. One such analog, a PTH(1-14)/PTHrP(15-36) hybrid ligand, forms particularly stable complexes and thus induces calcemic and hypophosphatemic responses in rodents that persist for many hours after initial application and thus for considerably longer times than those induced by PTH(1-34).(14,15) Studies performed with fluorescently labeled versions of such PTH analogs in PTHR1-expressing HEK-293 cells suggest that the prolonged signaling responses induced by the ligands may be mediated from within the intracellular compartments, via a mechanism termed “non-canonical” endosomal signaling.(44) The overall biological responses found with any such long-acting PTH analog might differ from that attained by an analog that achieves prolonged action via extended pharmacokinetics, such as the PEG-PTHTMR ligand described here, or the recently developed PTH(1-34) analogs that contain beta-amino acid modifications that reduce susceptibility to serum/tissue proteases.(17) Comparing and contrasting the properties of these different types of long-acting PTH analogs in vitro and in vivo could help reveal new aspects of basic PTH mechanisms of action.
Acknowledgments
Authors’ roles: J.G. performed experiments, A.K. prepared peptides, all authors contributed to experimental planning, data analysis and writing of the manuscript. The authors thank Braden Corbin, Monica Reyes and Tom Dean of the M.G.H. Endocrine Unit for technical assistance. This work was supported by NIH grants DK11794 and AR066261.
Footnotes
Disclosures
A.M. is an employee of Chugai Pharmaceutical Co., Ltd. and contributed to the work as an MGH appointee. All other authors declare no conflict of interest.
References
- 1.Martin TJ. Bone biology and anabolic therapies for bone: current status and future prospects. J Bone Metab. 2014;21(1):8–20. doi: 10.11005/jbm.2014.21.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau JE, Collins MT. The PTH-vitamin D-FGF23 axis. Rev Endocr Metab Disord. 2015;16(2):165–74. doi: 10.1007/s11154-015-9318-z. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317–37. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1(4):275–83. doi: 10.1016/S2213-8587(13)70106-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Mannstadt M, Guo J, et al. A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30(10):1803–13. doi: 10.1002/jbmr.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer KK, Fulton KA, Albert PS, Cutler GB., Jr Effects of pump versus twice-daily injection delivery of synthetic parathyroid hormone 1-34 in children with severe congenital hypoparathyroidism. J Pediatr. 2014;165(3):556–63. e551. doi: 10.1016/j.jpeds.2014.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polyzos SA, Makras P, Efstathiadou Z, Anastasilakis AD. Investigational parathyroid hormone receptor analogs for the treatment of osteoporosis. Expert Opin Investig Drugs. 2015;24(2):145–57. doi: 10.1517/13543784.2015.973021. [DOI] [PubMed] [Google Scholar]
- 8.Fraher LJ, Klein K, Marier R, et al. Comparison of the pharmacokinetics of parenteral parathyroid hormone-(1-34) [PTH-(1-34)] and PTH-related peptide-(1-34) in healthy young humans. J Clin Endocrinol Metab. 1995;80(1):60–4. doi: 10.1210/jcem.80.1.7829640. [DOI] [PubMed] [Google Scholar]
- 9.Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1-34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int. 2010;87(6):485–92. doi: 10.1007/s00223-010-9424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammerle SP, Mindeholm L, Launonen A, et al. The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone. 2012;50(4):965–73. doi: 10.1016/j.bone.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L. PTH(1-84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res. 2013;28(10):2232–43. doi: 10.1002/jbmr.1964. [DOI] [PubMed] [Google Scholar]
- 12.Clarke BL, Kay Berg J, Fox J, Cyran JA, Lagast H. Pharmacokinetics and pharmacodynamics of subcutaneous recombinant parathyroid hormone (1-84) in patients with hypoparathyroidism: an open-label, single-dose, phase I study. Clin Ther. 2014;36(5):722–36. doi: 10.1016/j.clinthera.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT, Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci USA. 2008;105(43):16525–30. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda A, Okazaki M, Baron DM, et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA. 2013;110(15):5864–9. doi: 10.1073/pnas.1301674110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi R, Fan Y, Lauter K, et al. Diphtheria toxin- and GFP-based mouse models of acquired hypoparathyroidism and treatment with a long-acting parathyroid hormone analog. J Bone Miner Res. 2016;31(5):975–84. doi: 10.1002/jbmr.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu M, Joyashiki E, Noda H, et al. Pharmacodynamic actions of a long-acting PTH analog (LA-PTH) in thyroparathyroidectomized (TPTX) rats and normal monkeys. J Bone Miner Res. 2016;31(7):1405–12. doi: 10.1002/jbmr.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheloha RW, Maeda A, Dean T, Gardella TJ, Gellman SH. Backbone modification of a polypeptide drug alters duration of action in vivo. Nat Biotechnol. 2014;32(7):653–5. doi: 10.1038/nbt.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serada M, Sakurai-Tanikawa A, Igarashi M, et al. The role of the liver and kidneys in the pharmacokinetics of subcutaneously administered teriparatide acetate in rats. Xenobiotica. 2012;42(4):398–407. doi: 10.3109/00498254.2011.622811. [DOI] [PubMed] [Google Scholar]
- 19.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97(10):4167–83. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 20.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83(4):601–6. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 22.Youn YS, Na DH, Lee KC. High-yield production of biologically active mono-PEGylated salmon calcitonin by site-specific PEGylation. J Control Release. 2007;117(3):371–9. doi: 10.1016/j.jconrel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Lee S, Youn YS, et al. Synthesis, characterization, and pharmacokinetic studies of PEGylated glucagon-like peptide-1. Bioconjug Chem. 2005;16(2):377–82. doi: 10.1021/bc049735+. [DOI] [PubMed] [Google Scholar]
- 24.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol. 2003;285(6):F1233–43. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 25.Binkowski BF, Fan F, Wood KV. Luminescent biosensors for real-time monitoring of intracellular cAMP. Methods Mol Biol. 2011;756:263–71. doi: 10.1007/978-1-61779-160-4_14. [DOI] [PubMed] [Google Scholar]
- 26.Hattersley G, Dean T, Gardella TJ. Differential binding selectivity of abaloparatide (BA058) compared to PTH and PTHrP for PTH type 1 receptor conformations. Paper presented at: Endocrine Society’s 96th Annual Meeting and Expo: Novel Signaling Mechanisms and Bone Cell Biology; June 21–24, 2014; Chicago, IL, USA. [Google Scholar]
- 27.Bailon P, Palleroni A, Schaffer CA, et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001;12(2):195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- 28.Khan UT, Tanasescu R, Constantinescu CS. PEGylated IFNbeta-1a in the treatment of multiple sclerosis. Expert Opin Biol Ther. 2015;15(7):1077–84. doi: 10.1517/14712598.2015.1053206. [DOI] [PubMed] [Google Scholar]
- 29.Wang JL, Qian X, Chinookoswong N, et al. Polyethylene glycolated recombinant TNF receptor I improves insulitis and reduces incidence of spontaneous and cyclophosphamide-accelerated diabetes in nonobese diabetic mice. Endocrinology. 2002;143(9):3490–7. doi: 10.1210/en.2002-220412. [DOI] [PubMed] [Google Scholar]
- 30.He XL, Yin HL, Wu J, et al. A multiple-dose pharmacokinetics of polyethylene glycol recombinant human interleukin-6 (PEG-rhIL-6) in rats. J Zhejiang Univ Sci B. 2011;12(1):32–9. doi: 10.1631/jzus.B1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H, Satake-Ishikawa R, Ishikawa M, Matsuki S, Asano K. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor conjugated to polyethylene glycol in rats. Cancer Res. 1991;51(14):3710–4. [PubMed] [Google Scholar]
- 32.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–6. [PubMed] [Google Scholar]
- 33.Cusano NE, Rubin MR, Bilezikian JP. Parathyroid hormone therapy for hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2015;29(1):47–55. doi: 10.1016/j.beem.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Groot T, Lee K, Langeslag M, et al. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20(8):1693–704. doi: 10.1681/ASN.2008080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97(12):4507–14. doi: 10.1210/jc.2012-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1-34 and 3-34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol. 2000;278(5):F792–8. doi: 10.1152/ajprenal.2000.278.5.F792. [DOI] [PubMed] [Google Scholar]
- 37.Dudas PL, Villalobos AR, Gocek-Sutterlin G, Laverty G, Renfro JL. Regulation of transepithelial phosphate transport by PTH in chicken proximal tubule epithelium. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R139–46. doi: 10.1152/ajpregu.00427.2001. [DOI] [PubMed] [Google Scholar]
- 38.Guo J, Song L, Liu M, Mahon MJ. Fluorescent ligand-directed co-localization of the parathyroid hormone 1 receptor with the brush-border scaffold complex of the proximal tubule reveals hormone-dependent changes in ezrin immunoreactivity consistent with inactivation. Biochim Biophys Acta. 2012;1823(12):2243–53. doi: 10.1016/j.bbamcr.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fenton RA, Murray F, Dominguez Rieg JA, Tang T, Levi M, Rieg T. Renal phosphate wasting in the absence of adenylyl cyclase 6. J Am Soc Nephrol. 2014;25(12):2822–34. doi: 10.1681/ASN.2013101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Archiv. 2003;446(1):52–60. doi: 10.1007/s00424-002-0969-8. [DOI] [PubMed] [Google Scholar]
- 41.Capuano P, Bacic D, Roos M, et al. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol. 2007;292(2):C927–34. doi: 10.1152/ajpcell.00126.2006. [DOI] [PubMed] [Google Scholar]
- 42.Nagai S, Okazaki M, Segawa H, et al. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286(2):1618–26. doi: 10.1074/jbc.M110.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo J, Song L, Liu M, et al. Activation of a non-cAMP/PKA signaling pathway downstream of the PTH/PTHrP receptor is essential for a sustained hypophosphatemic response to PTH infusion in male mice. Endocrinology. 2013;154(5):1680–9. doi: 10.1210/en.2012-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheloha RW, Sullivan JA, Wang T, et al. Consequences of periodic alpha-to-beta(3) residue replacement for immunological recognition of peptide epitopes. ACS Chem Biol. 2015;10(3):844–54. doi: 10.1021/cb500888q. [DOI] [PMC free article] [PubMed] [Google Scholar]