Abstract
Cataract is the major cause of blindness worldwide. The WHO has estimated around 20 million people have bilateral blindness from cataract, and that number is expected to reach 50 million in 2050. The cataract surgery is currently the main treatment approach, though often associated with complications, such as Posterior Capsule Opacification (PCO)-also known as secondary cataract. The lens is an avascular ocular structure equipped with an unusually high level of glutathione (GSH), which plays a vital role in maintaining lens transparency by regulating lenticular redox state. The lens epithelium and outer cortex are thought to be responsible for providing the majority of lens GSH via GSH de novo synthesis, assisted by a continuous supply of constituent amino acids from the aqueous humor, as well as extracellular GSH recycling from the gamma-glutamyl cycle. However, when de novo synthesis is impaired, in the presence of low GSH levels, as in the aging human lens, compensatory mechanisms exist, suggesting that the lens is able to uptake GSH from the surrounding ocular tissues. However, these uptake mechanisms, and the GSH source and its origin, are largely unknown. The lens nucleus does not have the ability to synthesize its own GSH and fully relies on transport from the outer cortex by yet unknown mechanisms. Understanding how aging reduces GSH levels, particularly in the lens nucleus, how it is associated with age-related nuclear cataract (ARNC), and how the lens compensates for GSH loss via external uptake should be a major research priority. The intent of this review, which is dedicated to the memory of David C. Beebe, is to summarize our current understanding of lens GSH homeostasis and highlight discrepancies and gaps in knowledge that stand in the way of pharmacologically minimizing the impact of declining GSH content in the prevention of age-related cataract.
Introduction
The lens has evolved as an anaerobic biological system with millimolar concentrations of glutathione (GSH). The critical role of GSH in maintaining lens redox status and transparency is well recognized and has been, over the years, the subject of several excellent reviews(Giblin, 2000; Lou, 2000; Reddy, 1990; Truscott, 2005). While one could argue that nothing new was to be expected concerning the protective role of GSH in the lens, our interest in GSH homeostasis in the lens was rekindled with the unexpected finding that lenticular GSH levels were not completely suppressed in the LEGSKO mouse in spite of complete absence of γ-glutamylcysteine ligase(Fan et al., 2012). This issue, which is the subject of intense investigation in our laboratory, is closely linked to lenticular ascorbate metabolism and cataractogenesis and the work of David Beebe who has pioneered the importance of the vitreous as a source of oxidative stress to the lens(Beebe et al., 2014; Holekamp et al., 2005; Li et al., 2013b; Shui et al., 2009). These paradigm shifting studies inspired us to study lens biology in connection not only to the aqueous humor but also to the vitreous humor. Additionally, Dr. Beebe’s pioneer studies provide the mechanistic framework for a potential therapeutic treatment of high risk (>90%) and rapid (within two years) nuclear cataract formation after vitrectomy surgery(Petrash, 2013).
In order to provide a complete coverage of lens GSH homeostasis, we have to discuss the lenticular GSH dynamics from the perspective of both protein conjugated GSH and free GSH/oxidized GSH (GSSG). Since several excellent reviews have covered the protein glutathionylation(Lou, 2000, 2003; Lou and Dickerson, 1992; Lou et al., 1990; Lou et al., 1995), we will mainly focus on the roles of latter. Below we review the established mechanisms and pathways that are involved in lens GSH homeostasis. We also provide a brief summary of recent progress regarding lens nucleus GSH homeostasis, as well as the impact of aging on lens GSH homeostasis, since age-related nuclear cataract (ARNC) is often believed to be, in part, associated with declining nuclear GSH levels in the aging human lens(Giblin, 2000).
GSH de novo synthesis and its constituent amino acids transport
Intracellular GSH is synthesized by two ATP-dependent enzymes: γ-glutamylcysteine ligase (GCL) and glutathione synthase (GS) to produce γ-glutamylcysteine and GSH, respectively. The mammalian GCL is a heterodimer enzyme consisting of a 73-kDa catalytic subunit, Gclc, and a 28-kDa modulatory subunit, Gclm. The catalytic subunit, Gclc has the enzymatic activity and is regulated via a GSH feedback inhibition mechanism(Richman and Meister, 1975). Gclm has no enzymatic activity, but heterodimer formation of Gclm and Gclc significantly decreases the Km value for glutamate and increases the Ki value for the feedback inhibition by GSH(Chen et al., 2005).
Like other tissue systems, the lens has a functional GSH de novo synthesis machinery, which mostly lies in the epithelial and cortical layers, while mature fibers cells sit in inner layers of the lens that have lost cell organelles such as nucleus and mitochondria(Bassnett and Beebe, 1992). However, due to the avascularity of the lens, for GHS biosynthesis to take place, the constituent amino acids, glutamic acid, glycine and cysteine have to be transported to the epithelial and outer cortical fibers cells. Pioneering work from Reddy et al. has demonstrated that these amino acids are delivered to the lens from the plasma via the aqueous humor and the lens epithelium, from which they are delivered to the rest of the lenticular system(Reddy, 1973, 1979) (Fig.1). For GSH synthesis, the Km value of GCL for cysteine is ~0.15 mM, while that for glutamate is ~1.7 mM, and that of GS for glycine is ~0.8 mM(McBean, 2012). In order for GSH biosynthesis to take place, the required intracellular concentration of these amino acids is thought to be close to their Km value. However, various studies suggest species-specific results regarding these amino acids levels in the lens epithelium and outer cortex. For example, Lim et al.(Lim et al., 2007) find that the concentration of the three amino acids in the rat lens cortex is lower than that required for the GCL and GS Km value of its proper constituent amino acids. In contrast, other studies in human, rabbit and bovine lens demonstrate much higher values than these required for the Km(Barber, 1968; Kern and Ho, 1973; Reddy, 1973). It has to be pointed out that these studies measured the total GSH from the homogenate of the lens tissue, and that this does not exclude the possibility that intracellular amino acid level might be much higher to fulfill the needs of GSH de novo synthesis. Nevertheless, similar to other body systems, such as the central nervous system (CNS)(Aoyama et al., 2012), cysteine level is relatively lower than glutamic acid and glycine. It is, therefore, the rate-limiting substance in lenticular de novo GSH synthesis and an adequate cysteine supply is essential wherever GSH de novo synthesis is taking place.
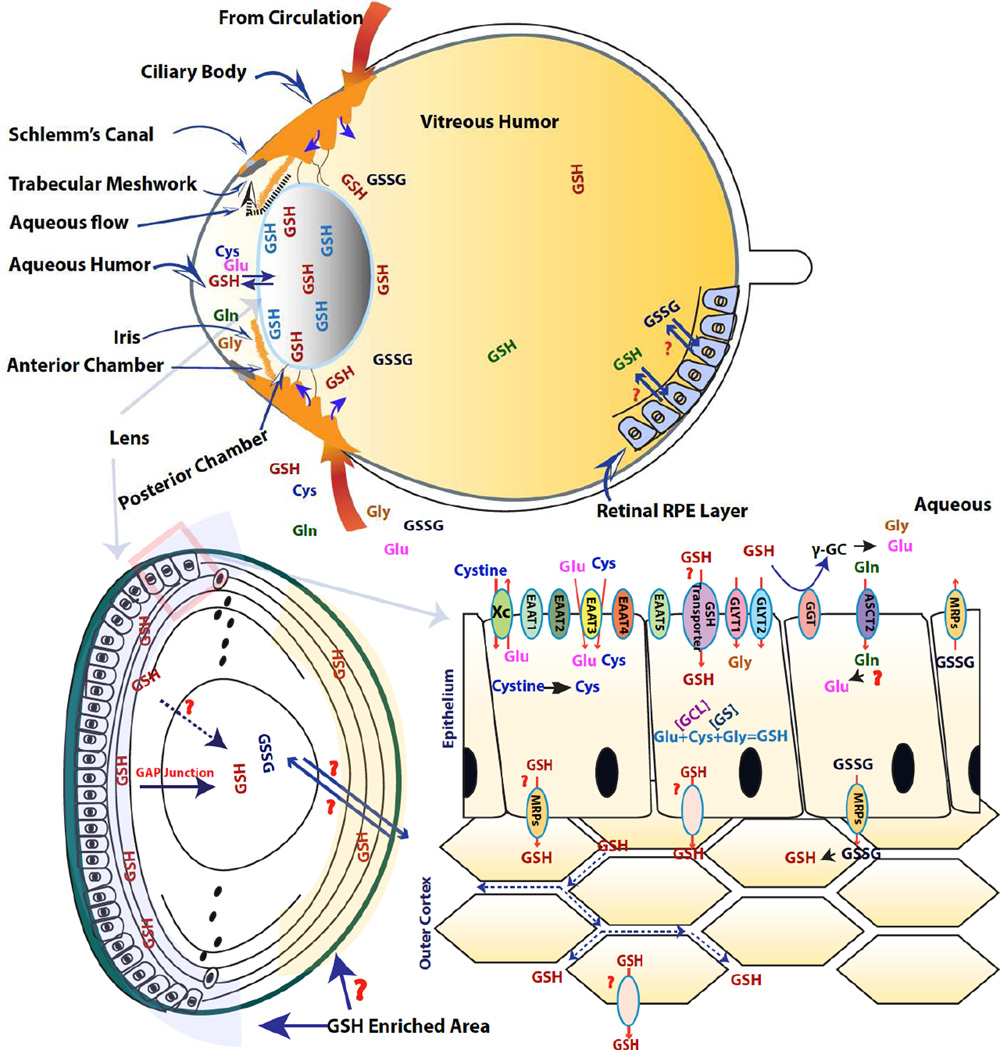
Figure 1. Lens glutathione homeostasis.
(Top panel): Illustration of the lens and its conjunction ocular tissues. The ciliary body with its vascular circulation system continuously produces the aqueous humor that contains both the constituent amino acids for GSH synthesis and GSH/GSSG. It first enters the posterior chamber, then reaches to the anterior aqueous humor and then drains out via trabecular meshwork and Shclemm’s canal. Both ciliary body and retina also produce molecular solute into the vitreous humor to maintain vitreous humor in a more dynamic, and this may also include GSH and GSSG. The lens will then utilize the amino acids, GSH or GSSG supply to maintain its GSH homeostasis via de novo synthesis and uptake, respectively. The pathway with a question marker indicates that it is currently unknown.
(Lower left panel): The illustration of lens structure. The anterior of the lens is assembled with monolayer of epithelial cells, and their continuously proliferation and differentiation produce lens fiber cells to form outer and inner cortical fiber layer. The lens nucleus is assembled with the embryonic nucleus and the fetal nucleus. The lens epithelial cells and outer cortical fiber cells have the ability to synthesis GSH, while lens nuclear GSH is completely reliant on transport from outer cortex. The outer cortex has been found possess high concentration of GSH than lens nucleus, though whether this is also true in posterior outer cortex is still unclear.
(Lower right panel): Illustration of lens epithelial and outer fiber layer. The epithelial cells are responsible for transporting the constituent amino acids from aqueous humor for GSH synthesis, which is taking place in both epithelial and outer fiber cell layer. The lens epithelial cells may also take up GSH or GSSG from aqueous humor. The high GSH concentration gradient between lens and aqueous humor implicates it might be a carried based transporting mechanisms, though the identity of the transporter is still unknown. The lens fiber cells are enriched with gap junctions, and it has been suggested that gap junction might playing a role in lens nuclear GSH homeostasis.
1. Cysteine/cystine transport
From the above considerations, it is clear that cellular cysteine and GSH synthesis are tightly linked. Multiple mechanisms have been postulated in terms of intracellular cysteine homeostasis in studies of the central nervous system (CNS) or hepatocytes(Lu, 1999; McBean and Flynn, 2001). The sodium independent cystine/glutamate exchanger (Xc−) has been shown to take up cystine into cells, which is subsequently reduced into cysteine for GSH synthesis(Lewerenz et al., 2013; Lim and Donaldson, 2011). In one set of studies, the Xc− exchanger was found present in the entire rat lens, predominantly in the cytoplasm in the outer cortex cells, while it was more membranous in the inner cortex region(Lim et al., 2005). In the human lens, Xc− is present in the entire lens region at a young age, but no immunoreactivity is found in the central lens region of aged human lenses(Lim et al., 2013). In contrast, in other studies, Xc− was reported to be predominately present in the membranes of outer cortex cells, and no detection was observed in the nuclear region of the dog lens(Lall et al., 2008). These studies provide evidence for the presence of Xc−, but whether this exchanger is important for lens cysteine homeostasis is still not very clear. Several findings point to quite different research directions. In a vascular eye perfusion study in guinea pigs, radiolabeled cysteine, cystine and methionine were injected through the common carotid artery(Mackic et al., 1997). In this study, cysteine, but not cystine, was readily taken up by the lens epithelial and cortical fiber layers, while infused cystine failed to incorporate into GSH synthesis. Other evidence in support of cysteine rather than cystine uptake is that high levels of free cysteine, but not cystine, are found in human(Barber, 1968), monkey(Gaasterland et al., 1979) and calf(Kern and Ho, 1973) aqueous humor, though different results was reported in the aqueous humor of guinea pigs(Mackic et al., 1997). Furthermore, recent reports(Martis, 2015) indicate has no impact on lens GSH level based on tests with Xc− knockout mice.
On the other hand, earlier studies indicate that over 90% of cystine transport is actually processed by sodium-dependent high affinity glutamate transporters (XAG-), based on rat brain tissue uptake experiments(Flynn and McBean, 2000) and a cultured astrocytes study(Bender and Norenberg, 2000). Also, cysteine was found to be able to inhibit XAG- facilitated transport(McBean and Flynn, 2001). Altogether, five subtypes of high-affinity glutamate transporters (excitatory amino acid transporters 1–5 (EAAT1–5)) have been identified in mammalian tissues(Aoyama and Nakaki, 2013; Bridges and Esslinger, 2005). More recent studies demonstrate that EAAT3, also named “excitatory amino acid carrier 1” (EAAC1), is more functional in cysteine transport than the control of extracellular glutamate levels(Aoyama and Nakaki, 2013; Holmseth et al., 2012). The brain cysteine and GSH levels are significantly reduced in EAAC1-deficent mice, and this can be attenuated by the treatment with N-acetylcysteine (NAC), whose uptake proceeds via different uptake mechanisms than cysteine/cystine(Aoyama et al., 2006). All five types of EAATs are found present in rat lens based on RT-PCR and western-blot analysis(Lim et al., 2005). We therefore speculate that lenticular cysteine transport from aqueous humor occurs, most likely via a sodium-dependent transporter system, such as EAAC1, and that high cysteine transport activity occurs at the lens equator region, as reported by Truscott’s group(Sweeney et al., 2003).
The metabolic pathway transsulfuration can also supply cysteine from methionine via the transmethylation pathway(McBean, 2012). The transsulfuration pathway is also present in the lenticular system(Persa et al., 2004). Cystathionine-beta-synthase (CBS), one of the enzymes utilizing methionine to produce cysteine via transmethylation is elevated in human lens nucleus but decreased in the epithelial layer with aging. CBS expression can also be stimulated with oxidative stress, such as H2O2 in lens epithelial cell culture(Persa et al., 2004). In addition, the betaine-homocysteine S-methyltransferase 1(BHMT1), a remethylation enzyme that converts homocysteine to methionine, is found to be down-regulated in aged human lens nuclei(Zhou et al., 2015). This may explain the findings that the free cysteine levels are elevated in the lens nucleus under oxidative stress(Giblin et al., 1995; Lou, 2000). However, in a vascular eye perfusion study in guinea pigs(Mackic et al., 1997), methionine failed to produce cysteine and incorporate into GSH, suggesting minimal contribution of methionine in circulation to lenticular cysteine homeostasis(Mackic et al., 1997). Apparently, more study is needed to clarify whether the transsulfuration pathway plays a significant role in lens cysteine and GSH homeostasis.
2. Glutamate and glycine transporter
As mentioned above, high affinity sodium-dependent glutamate transporters have been located in rat lenses(Lim et al., 2005), though no report exists so far about their expression level in the human lens. We anticipate that these transporters will also be present in human lenses. Extensive studies and reviews have addressed their roles in the CNS(Divito and Underhill, 2014; Vandenberg and Ryan, 2013). On the other hand, it is well established that cellular glutamate originates from glutamine, which is readily taken up by the lens via a transport mechanism that is hundreds of times more efficient than that of glutamate. Glutamine was shown to convert into glutamate and be incorporated into GSH synthesis in ex vivo calf and rat lens culture systems(Kern and Ho, 1973). However, no in vivo confirmation of this in vitro finding was reported. Since the anterior, but not posterior, part of the lens is equipped with a monolayer of epithelial cells(Beebe, 2008), it is anticipated that the anterior will be more selective and specific for amino acid transport than the posterior of the lens. In that regard, it is our point of view that ex vivo lens culture systems using total immersion are convenient, but not suitable for lens transporter studies because lens anterior and posterior surfaces have completely different structures. More work is needed to test whether glutamine uptake and conversion to glutamate plays a significant role in lens GSH homeostasis. At least one neutral amino acid transporter, ASCT2, has been found present in rat lenses(Lim et al., 2006), and free glutamine measured in calf aqueous humor is more than double that of glutamate(Kern and Ho, 1973).
Intracellular glycine homeostasis, like other neurotransmitters, is mainly regulated by the high affinity sodium-dependent transporters GLYT1 and GLYT2, which belong to the solute transporter family (SLC6)(Chen et al., 2004). Both glycine transporters have been identified in rat lenses(Lim et al., 2007; Lim et al., 2006) (Fig.1).
3. Glutathione metabolism/γ-glutamyl cycle
Gamma-glutamyl transpeptidase (GGT), also known as gamma-glutamyl transferase, a glycoprotein, is localized at the cell surface and anchored to the cell membrane via a single N-terminus transmembrane domain. It cleaves only the extracellular GSH, oxidized GSH (GSSG), as well as glutathione S-conjugates(Ikeda et al., 1995; Wickham et al., 2012), therefore providing the cells with the amino acids necessary for intracellular GSH synthesis(Hanigan, 2014). GGT was reported present in the lens, ciliary body and cornea over 40 years ago in two independent studies(Reddy and Unakar, 1973; Ross et al., 1973) (Fig.1). GGT activity enables the cells to maintain their intracellular GSH levels, thus coping with reactive oxygen species (ROS) attack. Both GGT knockout and mutant mice develop cataract in a very short time period after birth(Chevez-Barrios et al., 2000; Yamada et al., 2013). GGT deficient mice were found to have severe cysteine deficiency (~20% of WT plasma cysteine level)(Lieberman et al., 1996). Interestingly, despite high plasma GSH level in GGT deficient mice, eye and lens GSH levels are markedly reduced (~5% of WT)(Chevez-Barrios et al., 2000). The drastic reduction of lens GSH content in GGT KO mice cannot simply be explained by impaired GSH de novo synthesis due to cysteine deficiency. The lens Gclc conditional knockout mouse (LEGSKO mouse) recently created by our group(Fan et al., 2012) is able to maintain ~50% GSH level (1–2mM) relative to wild type (WT) mice on the C57 black background (unpublished), while levels were lower in the FVB/B6 hybrid strain (Fan et al., 2012). We believe that both low cysteine and GSH supply to the lens are necessary for drastic GSH reduction. In other words, lenses lacking efficient de novo GSH synthesis may take up either GSH or GSSG from surrounding ocular structures, i.e. the aqueous humor or the vitreous humor. We will discuss this aspect in the following section. Needless to say, the gamma-glutamyl cycle is an important amino acid recycling mechanism for maintenance of lens GSH homeostasis, particularly in the metabolically active regions of the lens.
Lens GSH/GSSG uptake
An in situ vascular eye perfusion study in guinea pig has come to the very surprising conclusion that the de novo GSH synthesis from circulating and aqueous sulfur amino acids, such as cysteine, cystine or methionine can be only a minor source of the millimolar concentration of GSH in the epithelium(Mackic et al., 1997). A t1/2 of 5480 hours was estimated for lens epithelium GSH to be replaced entirely if solely based on circulating sulfur amino acids, the limiting amino acids in intracellular GSH biosynthesis. This obviously is not the case based on human lens GSH turn over studies, whose t1/2 is around 85.4 hours(Rathbun and Murray, 1991). Studies indicate that GSH can be taken up into ex vivo-cultured lenses, such as rabbit and bovine lenses(Hockwin et al., 1985; Reddy, 1973). Again, studies carried out utilizing in situ vascular eye perfusion with [S35-cysteine] GSH and/or [H3-glycine] GSH in guinea pigs have demonstrated that the lens is capable of taking up circulating GSH(Mackic et al., 1996; Zlokovic et al., 1994). The rate for lens epithelium GSH uptake from aqueous humor is more than three times than the rate of aqueous humor uptake from plasma within only 10 minutes of vascular eye perfusion, and only simple diffusion is observed when GSH enter aqueous humor from plasma(Mackic et al., 1996; Zlokovic et al., 1994). Based on this study, a t1/2 of 85.4 hours is estimated if endogenous epithelial GSH has to be replaced entirely by plasma-derived GSH(Mackic et al., 1996), which is similar to the t1/2 estimated in human lens (90 hr)(Rathbun and Murray, 1991). By perfusing with an equal ratio of [S35-cysteine] GSH /[H3-glycine] GSH, then determining the ratio in plasma, aqueous humor and lens epithelium, this study demonstrates that lens GSH uptake from aqueous humor is not due to the gamma-glutamyl cycle(Zlokovic et al., 1994). However, this study only tested the amino acids and GSH levels in aqueous and lens without measurements in vitreous humor. The secretion from both ciliary body and retina could also boost vitreous humor GSH or amino acids level in a significant manner. The GSH or amino acids uptake from posterior of the lens can then contribute to lens GSH or amino acid level, but these amino acids stay in lens posterior are not able to incorporated into GSH biosynthesis due to mature fiber cells that lack translational machinery. Overall, it will interfere the estimation of t1/2 if only based on the calculation from aqueous humor and entire lens. Furthermore, the LEGSKO mouse(Fan et al., 2012), which lacks GSH biosynthesis, maintains around 50% of its lenticular GSH content relative to WT in the C57BL6 strain (unpublished). This indicates that the uptake from surrounding ocular tissue can only make up approximately half of lens GSH content. Stewat-DeHaan et al(Stewart-DeHaan et al., 1999) performed an in vivo transport study in rats by intraperitoneal injection of either H3 or S35 labeled GSH, then measured the plasma and lens GSH accumulation. Over a 4 hour period, the lens can accumulate 12.3% of its total GSH from injected GSH, though other radiolabeled GSH species appear as fractionated GSH based on HPLC analysis. GSH fractionation would be expected, since the majority of GSH will be metabolized in the liver following i.p. injection. Nevertheless, ~12% of intact GSH accumulated in the lens indicating a remarkable uptake force by the lens.
Taken together, these studies support existence of compensatory mechanisms, i.e. somehow taking it up from circulating GSH, also plays an important role in lenticular GSH homeostasis. Thus, the LEGSKO mouse model of GSH depletion provides a useful tool for studying the mechanisms of weakened lenticular GSH homeostasis in old age. By measuring the differential in GSH level between aqueous humor to plasma, and lens epithelium to aqueous, respectively, Zlokovic et al(Zlokovic et al., 1994) predicted that a high affinity sodium-dependent GSH transporter might be present in lens epithelium, since very low levels (<50µM) of GSH are present in aqueous humor, particular in rodents. In order for the lens to take up GSH from the aqueous, it must pump across a GSH concentration gradient, and only an energy-dependent carrier-like transport system can fulfill such a task. However, whether a molecular GSH influx transporter exists in mammals is a still a debated topic(Bachhawat et al., 2013; Gukasyan et al., 2007; Kaplowitz et al., 1996), despite several studies supporting the existence of a GSH transporter in the lenticular system(Kannan et al., 1996a; Kannan et al., 1996b; Li et al., 2010). Additionally, a high affinity GSH transporter (Hgt1) that has been identified in baker’s yeast (Saccharomyces cerevisiae)(Bourbouloux et al., 2000). However, the Kannan studies(Kannan et al., 1996b; Kannan et al., 1995) were dismissed as a cloning artifact(Li et al., 1997), leaving Li’s studies(Li et al., 2010) as the sole evidence in support of a specific GSH transporter in the lens. There have also been several studies, particularly in corneal GSH homeostasis, which suggest that, GSSG, but not GSH, is a better substrate for uptake in ocular tissues and then reduction back to GSH(Nakamura et al., 1994; Veltman et al., 2004). Whether this mechanism significantly contributes to the lens GSH pool requires further clarification.
1. Aqueous humor as the potential source of GSH
Aqueous humor is a clear fluid that fills and helps form the anterior and posterior chamber of the eye (Fig.1). The aqueous humor serves as “ocular blood” to provide nutrition, remove excretory products from metabolism and contribute to homeostatic regulation of these avascular structures, i.e. the lens and cornea(Goel et al., 2010). Aqueous humor is secreted by the ciliary epithelium lining the ciliary processes and first enters the posterior chamber, then reaches to the anterior aqueous humor chamber after passing through the capillary wall, stroma and epithelial bilayer(Goel et al., 2010). The aqueous humor is a dynamic system, and therefore helps to supply nutrition and remove metabolic waste in a continuous fashion.
Much effort has been focused on GSH supply from the aqueous humor, as mentioned above. Plasma GSH can indeed reach the aqueous humor by either vascular eye perfusion(Mackic et al., 1996) or in vivo GSH intraperitoneal injection(Stewart-DeHaan et al., 1999). A large aqueous humor GSH concentration (~1.5mM) can also be achieved by giving large, pharmaceutical doses of GSH (20mM) via perfusion, suggesting the importance of this channel in maintaining lens GSH homeostasis (Mackic et al., 1996). GSH/GSSG efflux transporter, such as the multidrug resistance-associated protein family, have been found expressed in ciliary body in human as well as in rat(Li et al., 2013a; Pelis et al., 2009). However, more study is needed to decipher the exact role that aqueous humor plays in maintaining lens GSH homeostasis besides supplying constituent amino acids for GSH biosynthesis, arguably due to low level of GSH in the aqueous humor.
2. The vitreous as a potential source of GSH
The vitreous humor accounts for up to 80% of the volume of the eyeball. It is an optically transparent gel formed by fine fibers and small molecular solutes that attach to lens and retina. However, the vitreous humor has functions beyond just being a space filling gel. Soluble extracellular proteins and small molecule solutes present in the vitreous are anticipated to play a vital role in maintaining homeostasis of adjacent ocular tissues, i.e. the lens and retina(Berman, 1991; Lund-Andersen, 2003; Monteiro et al., 2015). Outstanding work from Dr. Beebe’s group suggestes another important functional mechanisms of vitreous humor, namely the regulation of molecular oxygen levels(Barton et al., 2007; Beebe et al., 1986; Filas et al., 2013; Harocopos et al., 2004; Holekamp et al., 2008; Shui et al., 2009). The gel-structure and high ascorbic acid concentration in vitreous humor is believed to continuously prevent oxygen from reaching the lens, thus maintaining the lens under anaerobic conditions.
GSH is one of many small molecule solutes in vitreous humor, and it works to restore dehydroascorbic acid (DHA) into ascorbic acid in the so-called ascorbic-GSH cycle(May et al., 2001). Additional studies conclude that the vitreous humor is indeed in a more dynamic state than previously thought(Lumi et al., 2015; Monteiro et al., 2015). The soluble proteins and small molecular solutes are constantly transported in and out of the vitreous humor. Therefore, we speculate that vitreous GSH may also help regulate adjacent ocular tissue glutathione homeostasis. The vitreous humor GSH can come either from ciliary body secretion(Li et al., 2013a; Pelis et al., 2009) or from retinal metabolism, i.e. via retinal-pigmented epithelial (RPE) cells(Garcia et al., 2011; Lu et al., 1995). In addition, the vitreous humor is a nonhomogeneous tissue that displays a graded density at different anatomical locations, which have been subdivided into three main anatomical regions: the vitreous core, vitreous base, and vitreous cortex. Though, no regional GSH concentrations have been reported to our knowledge, one would expect these to vary; being e.g. much higher at the vitreous base if ciliary body is the actual source of GSH secretory tissue, or in vitreous cortex if the retina is the main provider of vitreous GSH. The total vitreous GSH varies according to species as recently observed in our laboratory (unpublished data) and reported by others(Nozal et al., 1997; Sulochana et al., 1999). Rabbit, rat, guinea pig and mouse are reported to have high GSH concentration (>250 µM) in their vitreous humor, while bovine and human have much lower GSH content (<50 µM). One reasonable explanation is that rodent lens is relative larger and rounder than human lens, and, as such, occupies much of the eye space, which leaves a very small vitreous humor volume. In rodent, the GSH obtained from its appropriate ocular tissue can be quickly mixed in the vitreous humor and the total GSH level will be high. In contrast, bovine and human have much larger vitreous humor volumes, and even if high GSH concentration is maintained at the vitreous base or cortex, the vitreous core may have very low GSH levels, and the total GSH concentration is still extremely low. In terms of the possibility of lens GSH uptake approaching from the vitreous humor, the GSH content at the vitreous base is apparently more important than other anatomical regions. The gel-like vitreous structure and large volume in human rules out the possibility for the vitreous cortex to serve as a source for lens GSH homeostasis. The measurement of GSH content in different regions of the vitreous humor in different species will shed much needed light on deciphering the GSH vitreous humor dynamics, and thus provide insight into the relationship between lens and vitreous GSH exchange, and the question of the extent to which the lens might be the GSH supplier for adjacent ocular tissues, as suggested by Umapathy et al.(Umapathy, 2014).
Lens nucleus GSH homeostasis
From the inner core to the periphery, the lens nucleus is constituted by an embryonic nucleus, a fetal nucleus, and an adult nucleus formed by differentiated fiber cells. Each of these can be delicately peeled off the human lens using simple tweezers(Beebe, 2008). These mature fibers cells have no capacity for protein or small molecule synthesis, including that of GSH. However, the lens nucleus still contains substantial level of GSH for maintenance of lens nucleus redox status(Giblin, 2000; Truscott, 2005). Obviously, lens nucleus GSH content is reliant on transport mechanisms from the outer cortex. Similar to the lens epithelium and outer cortex, whether a GSH transporter is responsible for such tasks has not yet been determined. Currently, the well-recognized concept is that of concentration gradient diffusion into the nucleus via gap junctions, which connect the mature fiber cells(Beebe et al., 2011). More recently, Slavi N. et al found a significant GSH reduction in lens nucleus in Cx46 (connexin 46) knockout mouse(Slavi et al., 2014). Cx46 and Cx50 (connexin 50) are two gap junction channel proteins expressed in the lens. The remarkable reduction of lens nucleus GSH in Cx46 KO mouse suggests the importance of gap junctions in lens nucleus GSH homeostasis. However, only a low degree of GSH permeation is found in single channels formed by either Cx46 or Cx50. Whether gap junctions are playing a major role in maintaining lens nucleus GSH homeostasis is thus still unclear. In addition, rapid nuclear cataract formation in Cx46 KO mice (Xia et al., 2006) further complicate the content of lens nucleus GSH. In a nutshell, much more work is needed in order to better understand the mechanisms regulating lens nucleus GSH homeostasis and its role in age-related nuclear cataract formation. It has been proposed that approximately 1 mM is the threshold GSH concentration for human lenses for avoiding rapid cataract formation(Giblin, 2000). The LEGSKO mouse in the FVB/B6 hybrid background has significant decreases in lens nucleus GSH, contributing to the development of nuclear cataract at four months of age(Fan et al., 2012). An alternative hypothesis is that the lens “microcirculation” might be responsible for supplying and removing metabolites in the lens. However, such a hypothesis, as suggested by Beebe et al.(Beebe et al., 2011), is not a suitable mechanism, at least for GSH circulation in the lens. One argument is that the water, flowing from center to lens periphery, will block the GSH diffusion.
Aging impact on lens GSH homeostasis
The GSH level declines with age in the lens, particularly in the lens nucleus, and this is widely believed to be a major mechanism for age-related nuclear cataract formation(Giblin, 2000; Lou, 2003; Truscott, 2005). Multiple causes have been proposed for this decrease:
The age-related loss of synthetic enzymes for de novo GSH synthesis is considered one of the major mechanisms. GCL, the GSH de novo synthesis rate limiting enzyme, has a 16-fold decrease in activity over a 83-year time frame(Rathbun, 1984). Interestingly, such drastic activity loss is not unequivocally associated with lens GSH content, which is only reduced around 2–3 fold over several decades in normal lenses without cataract(Lou, 2003). This finding further suggests a compensatory mechanism is in place for maintenance of lens GSH homeostasis, which is most likely linked to uptake from surrounding ocular structures, as already argued above. The mechanisms of this loss of function in GSH biosynthesis enzyme is unknown and probably due to a combination of stochastic damage by oxidation and glycation(Beebe et al., 2010; Nagaraj et al., 2012) and other factors, as described in several reviews(Knight, 2000; Liu et al., 2004).
Concerning the source of extra-lenticular GSH or its constituent amino acids, the aging ciliary body might have a down-regulation of its amino acid, GSH and GSSG secretory function to the aqueous humor or vitreous humor. Studies have found that the stroma of the aging ciliary body processes becomes collagenized, and that the processes become less vascularized(Folberg, 1996; Grossniklaus et al., 2013), therefore slowing down secretory functions. Similarly, aging also has a major impact on the retina. The retinal pigment epithelial (RPE) cell numbers are found decreasing at continuous rate from the 20s to 90s in human(Gao and Hollyfield, 1992), and this will have a remarkable impact on GSH efflux if, indeed, RPE cells are responsible for vitreous humor GSH homeostasis.
Several studies have found elevated lens stiffness and barrier formation with age(Beebe et al., 2011; Heys et al., 2004; Heys and Truscott, 2008; Sweeney and Truscott, 1998; Truscott, 2000), and a decline in GSH diffusion into the inner layer of the lens. Nuclear cataract is just an extreme example of lens hardening and impaired GSH diffusion, where barely detectable GSH is found in the cataract region, while millimolar levels of GSH are still available in the outer cortex(Pau et al., 1990). More work is needed to understand the molecular mechanisms responsible for the lens metabolic barrier formation during aging.
GSH usually works as a key partner with glutathione peroxidase (GPx), glutathione reductase (GR) and glutaredoxin to form a “GSH antioxidant network”, and GSH can be regenerated from this network system(Lou, 2003). However, during aging of the lens, many of the enzymes involved in this network are impaired(Zhu et al., 2010), including the thiol repair system(Wei et al., 2015). This is also reflected by the elevated protein-S-S-glutathione (PSSG) mixed disulfides in aged human lens which have been very well documented in the past(Lou, 2000, 2003; Lou and Dickerson, 1992; Lou et al., 1990; Lou et al., 1995). All of these changes are strongly associated with increased crystallin oxidation and crosslinking(Fan et al., 2006; Fan et al., 2009; Wang et al., 2014) and may ultimately hamper GSH regeneration.
Concluding remarks and perspectives
The lens is an avascular ocular tissue equipped with an unusually high level of GSH. The high concentration of GSH works with its antioxidant partners to keep the lens redox system in check and maintain lens transparency for several decades in humans. In addition to GSH de novo synthesis that only occurs in the epithelium and outer cortex, the lens is believed to also take up large portions of GSH from surrounding ocular tissues, i.e. the aqueous humor and the vitreous humor. However, more studies are needed to address the following questions: First, urgent study is needed to understand the mechanisms by which the lens manages to take up GSH from the circulation. If it is bycarrier-based mechanisms, most likely at the anterior pole of the lens, then what is the identity of the transporter? If it is by passive diffusion, most likely at posterior pole of the lens, then where are the sources and the origin of the GSH? Second, what is the contribution of lens GSH uptake vs. de novo synthesis in young and aged human lenses? We speculate that GSH uptake from surrounding ocular tissue will take a more important role in aged human lenses due to impaired de novo synthesis,. In that case, we need to understand what kinds of genes are adapted with aging. Third, we need to understand the role of the vitreous humor in lens GSH homeostasis including its dynamics and which ocular tissue is the main source of vitreous GSH. Fourth, more work is needed to understand the transport mechanisms for maintenance of lens nucleus GSH homeostasis, and verify whether gap junctions are indeed responsible for lens nucleus GSH homeostasis. Are other mechanisms rather than gap junctions or those that work together with gap junction playing a role in lens nucleus GSH homeostasis? Lastly, since GSSG is unable to permeate through gap junction channels(Slavi et al., 2014), the obvious question is how does the lens eliminate GSSG if it is beyond the lens regeneration capacity? We believe that addressing these critical questions is urgently needed to understand the age-related decline in lens GSH homeostasis, and open the door to novel therapeutic approaches for the prevention or delay of age-related nuclear cataract. Dr. Beebe has been the pioneer in many research areas of the lens field, such as his recent work on the oxygen regulatory role by the vitreous humor. This has provided us with the new concept that we should place the lens in the context of the eye rather than as an independent island when we study its many biological phenomena.
Highlights.
Glutathione is playing vital role in lens biology.
The lens GSH homeostasis is maintained via biosynthesis and transport.
The aqueous humor and gamma-glutamyl cycle continuously supplies constituent amino acids for GSH synthesis.
Aqueous and vitreous humor are providing glutathione for transporting into lens.
The mechanisms of lens GSH transport and nucleus GSH homeostasis are unknown.
Acknowledgments
We thank the National Eye Institute (Grant EY024553 to XF, and EY 07099 to VMM), and the Case Western Reserve University Visual Science Research Center (NEI P30EY-11373) for supporting our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama K, Nakaki T. Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1) Amino acids. 2013;45:133–142. doi: 10.1007/s00726-013-1481-5. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Modulation of neuronal glutathione synthesis by EAAC1 and its interacting protein GTRAP3–18. Amino acids. 2012;42:163–169. doi: 10.1007/s00726-011-0861-y. [DOI] [PubMed] [Google Scholar]
- Bachhawat AK, Thakur A, Kaur J, Zulkifli M. Glutathione transporters. Biochimica et biophysica acta. 2013;1830:3154–3164. doi: 10.1016/j.bbagen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Barber GW. Free amino acids in senile cataractous lenses: possible osmotic etiology. Investigative ophthalmology. 1968;7:564–583. [PubMed] [Google Scholar]
- Barton KA, Shui YB, Petrash JM, Beebe DC. Comment on: the Stokes-Einstein equation and the physiological effects of vitreous surgery. Acta ophthalmologica Scandinavica. 2007;85:339–340. doi: 10.1111/j.1600-0420.2007.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Developmental dynamics : an official publication of the American Association of Anatomists. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Seminars in cell & developmental biology. 2008;19:125–133. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic research. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1293–1300. doi: 10.1098/rstb.2010.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Latker CH, Jebens HA, Johnson MC, Feagans DE, Feinberg RN. Transport and steady-state concentration of plasma proteins in the vitreous humor of the chicken embryo: implications for the mechanism of eye growth during early development. Developmental biology. 1986;114:361–368. doi: 10.1016/0012-1606(86)90200-9. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Shui YB, Siegfried CJ, Holekamp NM, Bai F. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Japanese journal of ophthalmology. 2014;58:225–231. doi: 10.1007/s10384-014-0318-4. [DOI] [PubMed] [Google Scholar]
- Bender AS, Norenberg MD. Effect of ammonia on GABA uptake and release in cultured astrocytes. Neurochem Int. 2000;36:389–395. doi: 10.1016/s0197-0186(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Berman ER. Biochemistry of the eye. New York: Plenum Press; 1991. [Google Scholar]
- Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. The Journal of biological chemistry. 2000;275:13259–13265. doi: 10.1074/jbc.275.18.13259. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. The Journal of biological chemistry. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- Chevez-Barrios P, Wiseman AL, Rojas E, Ou CN, Lieberman MW. Cataract development in gamma-glutamyl transpeptidase-deficient mice. Experimental eye research. 2000;71:575–582. doi: 10.1006/exer.2000.0913. [DOI] [PubMed] [Google Scholar]
- Divito CB, Underhill SM. Excitatory amino acid transporters: roles in glutamatergic neurotransmission. Neurochemistry international. 2014;73:172–180. doi: 10.1016/j.neuint.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu X, Hao S, Wang B, Robinson ML, Monnier VM. The LEGSKO mouse: a mouse model of age-related nuclear cataract based on genetic suppression of lens glutathione synthesis. PLoS One. 2012;7:e50832. doi: 10.1371/journal.pone.0050832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Zhang J, Theves M, Strauch C, Nemet I, Liu X, Qian J, Giblin FJ, Monnier VM. Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. J Biol Chem. 2009;284:34618–34627. doi: 10.1074/jbc.M109.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filas BA, Shui YB, Beebe DC. Computational model for oxygen transport and consumption in human vitreous. Investigative ophthalmology & visual science. 2013;54:6549–6559. doi: 10.1167/iovs.13-12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J, McBean GJ. Kinetic and pharmacological analysis of L-[35S]cystine transport into rat brain synaptosomes. Neurochem Int. 2000;36:513–521. doi: 10.1016/s0197-0186(99)00151-5. [DOI] [PubMed] [Google Scholar]
- Folberg R. The eye. Philadelphia, PA: W.B. Saunders, Co.; 1996. [Google Scholar]
- Gaasterland DE, Pederson JE, MacLellan HM, Reddy VN. Rhesus monkey aqueous humor composition and a primate ocular perfusate. Investigative ophthalmology & visual science. 1979;18:1139–1150. [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investigative ophthalmology & visual science. 1992;33:1–17. [PubMed] [Google Scholar]
- Garcia TB, Oliveira KR, do Nascimento JL, Crespo-Lopez ME, Picanco-Diniz DL, Mota TC, Herculano AM. Glutamate induces glutathione efflux mediated by glutamate/aspartate transporter in retinal cell cultures. Neurochemical research. 2011;36:412–418. doi: 10.1007/s11064-010-0356-3. [DOI] [PubMed] [Google Scholar]
- Giblin FJ. Glutathione: a vital lens antioxidant. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Experimental eye research. 1995;60:219–235. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. The open ophthalmology journal. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Nickerson JM, Edelhauser HF, Bergman LA, Berglin L. Anatomic alterations in aging and age-related diseases of the eye. Investigative ophthalmology & visual science. 2013;54:ORSF23–ORSF27. doi: 10.1167/iovs.13-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukasyan HJ, Kim KJ, Lee VH, Kannan R. Glutathione and its transporters in ocular surface defense. The ocular surface. 2007;5:269–279. doi: 10.1016/s1542-0124(12)70093-9. [DOI] [PubMed] [Google Scholar]
- Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Advances in cancer research. 2014;122:103–141. doi: 10.1016/B978-0-12-420117-0.00003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Investigative ophthalmology & visual science. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Molecular vision. 2004;10:956–963. [PubMed] [Google Scholar]
- Heys KR, Truscott RJ. The stiffness of human cataract lenses is a function of both age and the type of cataract. Experimental eye research. 2008;86:701–703. doi: 10.1016/j.exer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Hockwin O, Korte I, Noll E, Heiden M, Konopka R, Hagenah J, Hurtado R. Is it possible to maintain a normal glutathione level in lenses in vitro? Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1985;222:142–146. doi: 10.1007/BF02173539. [DOI] [PubMed] [Google Scholar]
- Holekamp NM, Harocopos GJ, Shui YB, Beebe DC. Myopia and axial length contribute to vitreous liquefaction and nuclear cataract. Archives of ophthalmology. 2008;126:744. doi: 10.1001/archopht.126.5.744-a. author reply 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. American journal of ophthalmology. 2005;139:302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32:6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Fujii J, Taniguchi N, Meister A. Expression of an active glycosylated human gamma-glutamyl transpeptidase mutant that lacks a membrane anchor domain. Proc Natl Acad Sci U S A. 1995;92:126–130. doi: 10.1073/pnas.92.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Yi JR, Tang D, Li Y, Zlokovic BV, Kaplowitz N. Evidence for the existence of a sodium-dependent glutathione (GSH) transporter. Expression of bovine brain capillary mRNA and size fractions in Xenopus laevis oocytes and dissociation from gamma-glutamyltranspeptidase and facilitative GSH transporters. The Journal of biological chemistry. 1996a;271:9754–9758. doi: 10.1074/jbc.271.16.9754. [DOI] [PubMed] [Google Scholar]
- Kannan R, Yi JR, Tang D, Zlokovic BV, Kaplowitz N. Identification of a novel, sodium-dependent, reduced glutathione transporter in the rat lens epithelium. Investigative ophthalmology & visual science. 1996b;37:2269–2275. [PubMed] [Google Scholar]
- Kannan R, Yi JR, Zlokovic BV, Kaplowitz N. Molecular characterization of a reduced glutathione transporter in the lens. Investigative ophthalmology & visual science. 1995;36:1785–1792. [PubMed] [Google Scholar]
- Kaplowitz N, Fernandez-Checa JC, Kannan R, Garcia-Ruiz C, Ookhtens M, Yi JR. GSH transporters: molecular characterization and role in GSH homeostasis. Biological chemistry Hoppe-Seyler. 1996;377:267–273. doi: 10.1515/bchm3.1996.377.5.267. [DOI] [PubMed] [Google Scholar]
- Kern HL, Ho CK. Transport of L-glutamic acid and L-glutamine and their incorporation into lenticular glutathione. Experimental eye research. 1973;17:455–462. doi: 10.1016/0014-4835(73)90226-1. [DOI] [PubMed] [Google Scholar]
- Knight JA. The biochemistry of aging. Advances in clinical chemistry. 2000;35:1–62. doi: 10.1016/s0065-2423(01)35014-x. [DOI] [PubMed] [Google Scholar]
- Lall MM, Ferrell J, Nagar S, Fleisher LN, McGahan MC. Iron regulates L-cystine uptake and glutathione levels in lens epithelial and retinal pigment epithelial cells by its effect on cytosolic aconitase. Investigative ophthalmology & visual science. 2008;49:310–319. doi: 10.1167/iovs.07-1041. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxidants & redox signaling. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Li L, Donaldson PJ, Lim JC. Dynamic regulation of GSH synthesis and uptake pathways in the rat lens epithelium. Experimental eye research. 2010;90:300–307. doi: 10.1016/j.exer.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Li B, Umapathy A, Tran LU, Donaldson PJ, Lim JC. Molecular identification and scellular localisation of GSH synthesis, uptake, efflux and degradation pathways in the rat ciliary body. Histochemistry and cell biology. 2013a;139:559–571. doi: 10.1007/s00418-012-1049-6. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Ballatori N. Functional re-evaluation of the putative glutathione transporters, RcGshT and RsGshT. The Yale journal of biology and medicine. 1997;70:301–310. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yan H, Ding TB, Han J, Shui YB, Beebe DC. Oxidative responses induced by pharmacologic vitreolysis and/or long-term hyperoxia treatment in rat lenses. Current eye research. 2013b;38:639–648. doi: 10.3109/02713683.2012.760741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lam YC, Kistler J, Donaldson PJ. Molecular characterization of the cystine/glutamate exchanger and the excitatory amino acid transporters in the rat lens. Investigative ophthalmology & visual science. 2005;46:2869–2877. doi: 10.1167/iovs.05-0156. [DOI] [PubMed] [Google Scholar]
- Lim J, Li L, Jacobs MD, Kistler J, Donaldson PJ. Mapping of glutathione and its precursor amino acids reveals a role for GLYT2 in glycine uptake in the lens core. Investigative ophthalmology & visual science. 2007;48:5142–5151. doi: 10.1167/iovs.07-0649. [DOI] [PubMed] [Google Scholar]
- Lim J, Lorentzen KA, Kistler J, Donaldson PJ. Molecular identification and characterisation of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Experimental eye research. 2006;83:447–455. doi: 10.1016/j.exer.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Lim JC, Donaldson PJ. Focus on molecules: the cystine/glutamate exchanger (System x(c)(−)) Experimental eye research. 2011;92:162–163. doi: 10.1016/j.exer.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Lim JC, Lam L, Li B, Donaldson PJ. Molecular identification and cellular localization of a potential transport system involved in cystine/cysteine uptake in human lenses. Experimental eye research. 2013;116:219–226. doi: 10.1016/j.exer.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. Annals of the New York Academy of Sciences. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- Lou MF. Thiol regulation in the lens. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- Lou MF. Redox regulation in the lens. Progress in retinal and eye research. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Experimental eye research. 1992;55:889–896. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE, Jr, Garadi R. The role of protein-thiol mixed disulfides in cataractogenesis. Experimental eye research. 1990;50:819–826. doi: 10.1016/0014-4835(90)90133-f. [DOI] [PubMed] [Google Scholar]
- Lou MF, Xu GT, Cui XL. Further studies on the dynamic changes of glutathione and protein-thiol mixed disulfides in H2O2 induced cataract in rat lenses: distributions and effect of aging. Current eye research. 1995;14:951–958. doi: 10.3109/02713689508995135. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1169–1183. [PubMed] [Google Scholar]
- Lu SC, Sun WM, Nagineni CN, Hooks JJ, Kannan R. Bidirectional glutathione transport by cultured human retinal pigment epithelial cells. Investigative ophthalmology & visual science. 1995;36:2523–2530. [PubMed] [Google Scholar]
- Lumi X, Hawlina M, Glavac D, Facsko A, Moe MC, Kaarniranta K, Petrovski G. Ageing of the vitreous: From acute onset floaters and flashes to retinal detachment. Ageing research reviews. 2015;21:71–77. doi: 10.1016/j.arr.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H, Sander B. The vitreous. Mosby: St. Lous; 2003. [Google Scholar]
- Mackic JB, Jinagouda S, McComb JG, Weiss MH, Kannan R, Kaplowitz N, Zlokovic BV. Transport of circulating reduced glutathione at the basolateral side of the anterior lens epithelium: physiologic importance and manipulations. Experimental eye research. 1996;62:29–37. doi: 10.1006/exer.1996.0004. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Kannan R, Kaplowitz N, Zlokovic BV. Low de novo glutathione synthesis from circulating sulfur amino acids in the lens epithelium. Experimental eye research. 1997;64:615–626. doi: 10.1006/exer.1996.0260. [DOI] [PubMed] [Google Scholar]
- Martis RM, Donaldson P, Lim J. Role of the cystine/glutamate antiporter in maintaining glutathione levles in ocular tissues. The 3rd International Conference on the Lens Kona; Hawaii. 2015. p. 36. [Google Scholar]
- May JM, Qu Z, Morrow JD. Mechanisms of ascorbic acid recycling in human erythrocytes. Biochimica et biophysica acta. 2001;1528:159–166. doi: 10.1016/s0304-4165(01)00188-x. [DOI] [PubMed] [Google Scholar]
- McBean GJ. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- McBean GJ, Flynn J. Molecular mechanisms of cystine transport. Biochemical Society transactions. 2001;29:717–722. doi: 10.1042/0300-5127:0290717. [DOI] [PubMed] [Google Scholar]
- Monteiro JP, Santos FM, Rocha AS, Castro-de-Sousa JP, Queiroz JA, Passarinha LA, Tomaz CT. Vitreous humor in the pathologic scope: insights from proteomic approaches. Proteomics. Clinical applications. 2015;9:187–202. doi: 10.1002/prca.201400133. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino acids. 2012;42:1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nakano T, Hikida M. Effects of oxidized glutathione and reduced glutathione on the barrier function of the corneal endothelium. Cornea. 1994;13:493–495. [PubMed] [Google Scholar]
- Nozal MJ, Bernal JL, Toribio L, Marinero P, Moral O, Manzanas L, Rodriguez E. Determination of glutathione, cysteine and N-acetylcysteine in rabbit eye tissues using high-performance liquid chromatography and post-column derivatization with 5,5'-dithiobis(2-nitrobenzoic acid) Journal of chromatography. 1997;A 778:347–353. doi: 10.1016/s0021-9673(97)00473-1. [DOI] [PubMed] [Google Scholar]
- Pau H, Graf P, Sies H. Glutathione levels in human lens: regional distribution in different forms of cataract. Experimental eye research. 1990;50:17–20. doi: 10.1016/0014-4835(90)90005-f. [DOI] [PubMed] [Google Scholar]
- Pelis RM, Shahidullah M, Ghosh S, Coca-Prados M, Wright SH, Delamere NA. Localization of multidrug resistance-associated protein 2 in the nonpigmented ciliary epithelium of the eye. The Journal of pharmacology and experimental therapeutics. 2009;329:479–485. doi: 10.1124/jpet.108.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persa C, Pierce A, Ma Z, Kabil O, Lou MF. The presence of a transsulfuration pathway in the lens: a new oxidative stress defense system. Experimental eye research. 2004;79:875–886. doi: 10.1016/j.exer.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Investigative ophthalmology & visual science. 2013;54:ORSF54–ORSF59. doi: 10.1167/iovs.13-12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun WB. Lenticular glutathione synthesis: rate-limiting factors in its regulation and decline. Current eye research. 1984;3:101–108. doi: 10.3109/02713688408997190. [DOI] [PubMed] [Google Scholar]
- Rathbun WB, Murray DL. Age-related cysteine uptake as rate-limiting in glutathione synthesis and glutathione half-life in the cultured human lens. Experimental eye research. 1991;53:205–212. doi: 10.1016/0014-4835(91)90075-p. [DOI] [PubMed] [Google Scholar]
- Reddy VN. Transport of organic molecules in the lens. Experimental eye research. 1973;15:731–750. doi: 10.1016/0014-4835(73)90007-9. [DOI] [PubMed] [Google Scholar]
- Reddy VN. Dynamics of transport systems in the eye. Friedenwald Lecture. Investigative ophthalmology & visual science. 1979;18:1000–1018. [PubMed] [Google Scholar]
- Reddy VN. Glutathione and its function in the lens--an overview. Experimental eye research. 1990;50:771–778. doi: 10.1016/0014-4835(90)90127-g. [DOI] [PubMed] [Google Scholar]
- Reddy VN, Unakar NJ. Localization of gamma-glutamyl transpeptidase in rabbit lens, ciliary process and cornea. Experimental eye research. 1973;17:405–408. doi: 10.1016/0014-4835(73)90219-4. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. The Journal of biological chemistry. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Ross LL, Barber L, Tate SS, Meister A. Enzymes of the gamma-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci U S A. 1973;70:2211–2214. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Archives of ophthalmology. 2009;127:475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavi N, Rubinos C, Li L, Sellitto C, White TW, Mathias R, Srinivas M. Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. The Journal of biological chemistry. 2014;289:32694–32702. doi: 10.1074/jbc.M114.597898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-DeHaan PJ, Dzialoszynski T, Trevithick JR. Modelling cortical cataractogenesis XXIV: uptake by the lens of glutathione injected into the rat. Molecular vision. 1999;5:37. [PubMed] [Google Scholar]
- Sulochana KN, Biswas J, Ramakrishnan S. Eales' disease: increased oxidation and peroxidation products of membrane constituents chiefly lipids and decreased antioxidant enzymes and reduced glutathione in vitreous. Current eye research. 1999;19:254–259. doi: 10.1076/ceyr.19.3.254.5312. [DOI] [PubMed] [Google Scholar]
- Sweeney MH, Garland DL, Truscott RJ. Movement of cysteine in intact monkey lenses: the major site of entry is the germinative region. Experimental eye research. 2003;77:245–251. doi: 10.1016/s0014-4835(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Experimental eye research. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Age-related nuclear cataract: a lens transport problem. Ophthalmic research. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Experimental eye research. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Umapathy A, Li B, Donaldson PJ, Lim JC. Characterising glutathione efflux pathways in the rat lens-is the lens a reservior of glutathione for other ocular tissues?. International Conference on the Lens Kailua-Kona; Hawaii. 2014. p. 67. [Google Scholar]
- Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiological reviews. 2013;93:1621–1657. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- Veltman JC, Podval J, Mattern J, Hall KL, Lambert RJ, Edelhauser HF. The disposition and bioavailability of 35S-GSH from 35S-GSSG in BSS PLUS in rabbit ocular tissues. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2004;20:256–268. doi: 10.1089/1080768041223639. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lyons B, Truscott RJ, Schey KL. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging cell. 2014;13:226–234. doi: 10.1111/acel.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Xing KY, Fan YC, Libondi T, Lou MF. Loss of thiol repair systems in human cataractous lenses. Investigative ophthalmology & visual science. 2015;56:598–605. doi: 10.1167/iovs.14-15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham S, Regan N, West MB, Kumar VP, Thai J, Li PK, Cook PF, Hanigan MH. Divergent effects of compounds on the hydrolysis and transpeptidation reactions of gamma-glutamyl transpeptidase. J Enzyme Inhib Med Chem. 2012;27:476–489. doi: 10.3109/14756366.2011.597748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Experimental eye research. 2006;83:688–696. doi: 10.1016/j.exer.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Yamada K, Tsuji T, Kunieda T. Phenotypic characterization of Ggt1(dwg/dwg) mice,a mouse model for hereditary gamma-glutamyltransferase deficiency. Exp Anim. 2013;62:151–157. doi: 10.1538/expanim.62.151. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Yan H, Wang LL, Yan WJ, Shui YB, Beebe DC. Quantitative proteomics analysis by iTRAQ in human nuclear cataracts of different ages and normal lens nuclei. Proteomics. Clinical applications. 2015;9:776–786. doi: 10.1002/prca.201400061. [DOI] [PubMed] [Google Scholar]
- Zhu X, Korlimbinis A, Truscott RJ. Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation research. 2010;13:553–560. doi: 10.1089/rej.2009.1009. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Mackic JB, McComb JG, Kaplowitz N, Weiss MH, Kannan R. Blood-to-lens transport of reduced glutathione in an in situ perfused guinea-pig eye. Experimental eye research. 1994;59:487–496. doi: 10.1006/exer.1994.1134. [DOI] [PubMed] [Google Scholar]