Abstract
1) the objective
Porphyromonas gingivalis is considered a major pathogen of chronic periodontitis, which also may be implicated with systemic diseases such as atherosclerosis. Therefore, the aim of this study was to evaluate natural products from medical plants to develop a new therapeutic agent against periodontal disease.
2) the background data discussing the present status of the field
Secreted cysteine proteases, gingipains Rgp and Kgp, are essential for P. gingivalis virulence. Some polyphenols and flavonoids are known to inhibit gingipains activity and interfere with biofilm formation by P. gingivalis. Lots of bioactive compounds have been isolated from Epimedium species, but availability of these compounds on gingipains and P. gingivalis is still unclear.
3) methods
Prenylated flavonoids were isolated from Epimedium species plant using column chromatographies. The inhibitory effect of the prenylated flavonoids against protease activity of gingipains were examined using purified gingipains and fluorogenic substrates. Anti-P. gingivalis activity was evaluated to analyze planktonic growth and biofilm formation in BHI medium in the presence of the prenylated flavonoids.
4) results
We isolated 17 prenylated flavonoids (Limonianin and Epimedokoreanin B etc.) from Epimedium species. We found that some prenylated flavonoids inhibited gingipains activity in a non-competitive manner with Ki values at µM order. The prenylated flavonoids also hindered growth and biofilm formation of P. gingivalis, in a manner independent of gingipain inhibition by the compounds.
5) conclusion
The results clearly indicated inhibitory effect of the prenylated flavonoids against P. gingivalis and would provide useful information for future development of periodontitis treatment that suppress gingipains, P. gingivalis growth and biofilm formation.
Keywords: Prenylated flavonoids, gingipains, Porphyromonas gingivalis growth, biofilm
INTRODUCTION
Porphyromonas gingivalis, a Gram-negative oral anaerobe, is a major etiologic factor in the onset of adult periodontitis, a disease characterized by breakdown of tooth supporting tissues and resultant tooth loss (1, 2). Gingipains, cysteine proteases secreted by P. gingivalis occur in cell membrane-bound and soluble forms and are main virulence factors of this bacterium (3, 4). Based on substrate specificity, gingipains are classified into arginine-specific (Rgp) and lysine-specific (Kgp) proteases (5). Gingipains contribute to acquisition of nutrients, processing of outer membrane proteins, biofilm formation, and evasion of the host defense system through proteolytic inactivation of key components of this system (3, 4). Moreover, gingipains are implicated to play a role in development of systemic diseases, such as atherosclerosis (6, 7). Therefore, inhibition of gingipains may impede progression of periodontitis and related systemic diseases. P. gingivalis forms biofilm, matrix-enclosed structure in subgingival plaque. Bacteria enclosed in the biofilm structure are highly resistant to attack by the host immune system and antibacterial agents (8, 9), which enables persistent infection. Thus, inhibition of biofilm formation facilitates P. gingivalis elimination.
Flavonoids are natural, low molecular weight polyphenolic compounds composed of two aromatic rings (A and B) connected with three carbons that form an oxygenated heterocircle (C-ring). Some polyphenols and flavonoids are present in plant extracts and are able to inhibit gingipains activity and interfere with biofilm formation by P. gingivalis. For examples, a high-molecular-weight fraction from the cranberry extract that contains polyphenols inhibited biofilm formation (10–12) by P. gingivalis, and a flavonoid catechin and its derivatives from green tea suppressed the inflammatory reaction (13, 14). Both efficiently inhibited activity of gingipains. Prenylated flavonoids are widely distributed in the plant world and prenylation elevates hydrophobicity on the basic structure of the molecule, enhancing flavonoid biological functions (15, 16). Prenylation may facilitate flavonoid interaction with biofilm and uptake into bacteria through the membrane, enhancing inhibitory effects of the flavonoids on P. gingivalis growth and biofilm formation.
Lots of bioactive compounds have been isolated from Epimedium species, a genus of the family Berberidaceae (17). From a flavonoid fraction of the extract of Epimedium species, we purified 17 prenylated flavonoids and the derivatives. In order to explore an inhibitory effect of prenylated flavonoids on periodontitis, we examined these compounds for inhibition of gingipains, P. gingivalis growth and biofilm formation.
MATERIALS AND METHODS
Reagents and Materials
Fluorogenic substrates, t-butyloxycarbonyl-L-valyl-L-prolyl-L-arginine-4-methylcoumaryl-7-amide (Boc-Val-Pro-Arg-MCA) for 50-kDa Rgp (RgpB) and t-butyloxycarbonyl-l-valyl-l-leucyl-l-lysine (Boc-Val-Leu-Lys-MCA) for Kgp were purchased from the Peptide Institute (Minoh, Japan). Epimedium species was purchased from Uchida Wakan-yaku Co. Ltd. (Tokyo, Japan, lot number: C1S1504). Quercetin was purchased from Wako Pure Chemical Industries (Osaka, Japan). Luteolin was obtained from Cayman chemical (Michigan, USA). Epicatechin (EC), epicatechin gallate (ECg), epigallocatechin (EGC), and epigallocatechin gallate (EGCg) were purchased from Nagara Science Corp. (Gifu, Japan). The flavonoids were defined as 98% purity by supplier (luteorin, EC, ECg, EGC, and EGCg) or high performance liquid chromatography using XXX (quercetin).
Extraction and Isolation of flavonoids
The aerial parts of Epimedium species (3.0 kg) were extracted with methanol by sonication for 6 h (30 min×12) at room temperature. The extract was concentrated with an evaporator to obtain a residue (485.0 g). The residue was partitioned between n-hexane and 80% methanol, and the 80% methanol layer was concentrated with an evaporator to give a residue (408.1 g), which was loaded onto a MCI gel CHP20P column (ϕ50×300 mm, Mitsubishi Chemical Co., Tokyo, Japan) and eluted stepwise with H2O-methanol (1.5 L of 0%, 50% and 100% methanol solutions) to give 3 fractions. The 2nd fraction (46.5 g, eluted by 50% methanol) was further applied to a MCI gel CHP20P column (ϕ50×300 mm) and eluted stepwise with H2O-methanol (1.5 L of 50%, 60%, 70%, 80% and 100% methanol solutions) to give 5 fractions (frs. 2–1~ 2–5). Fr. 2–3 (5.3 g, eluted with 70% methanol) and Fr. 2–4 (1.1 g, eluted with 80% methanol) were applied to a Sephadex LH-20 column (ϕ20×1000 mm, GE Healthcare Bioscience Co., Uppsala, Sweden), then separated with a μ-Bonda Pak C18 (ϕ25×200 mm, Waters Co., Massachusetts, USA), eluted stepwise with H2O-methanol (135 mL of 60%, 70%, 80% methanol solutions). In these procedures, eluted solutions were divided by silica gel thin layer chromatography using pre-coated silica gel 60 F254 (Merck Ltd., Frankfurter, Germany) and compounds were detected by spraying with 10% H2SO4 followed by heating. The solutions containing prenyl flavonoids from Fr. 2–3 and Fr. 2–4 were applied to a COSMOSIL AR-II column (5 µm, ϕ10.0×250 mm, Nacalai Tesque Inc., Kyoto, Japan) at a flow rate 2.0 mL/min. at 40°C and eluted with 70% methanol to give compounds 5 (4.5 mg) and 4 (1.5 mg), respectively. The 3rd fraction (65.0 g, eluted by 100% methanol) was further applied to a MCI gel CHP20P column [ϕ50×300 mm, eluted with H2O-methanol (1.5 L of 0%, 50%, 60%, 70%, 80%, 90%, 100% methanol solutions)] to give 7 fractions (frs. 3–1~ 3–7). Fr. 3–4 (9.0 g, eluted by 70% methanol from MCI gel) was loaded on a Sephadex LH-20 column (ϕ20×1000 mm, eluted with methanol), then separated with a μ-Bonda Pak C18 [ϕ25×200 mm, eluted stepwise with H2O-methanol (135 mL of 60%, 70%, 80% each methanol solutions)]. Eluted solutions were loaded on a silica gel column [(ϕ10×100 mm, 230–400 mesh, Merck Ltd., Frankfurter, Germany), eluted with CHCl3:methanol:H2O = 9:1:0.1 (v/v)], and then separated with a Sunfire Prep C18 column (5 µm, ϕ10.0×250 mm, Waters Co., Massachusetts, USA) by elution with 70% methanol for compounds 1 (28.9 mg) and 14 (47.3 mg), with a X-Bridge Prep C18 column (5 µm, ϕ10.0×250 mm, Waters Co., Massachusetts, USA) by elution with 65% methanol for compound 17 (6.3 mg). Fr. 3–5 (10.4 g, eluted by 80% methanol from MCI gel) was subjected to a Sephadex LH-20 column (ϕ20×1000 mm, eluted with methanol), and then separated with a COSMOSIL 5C18 AR-II column by elution with 70% methanol for compounds 2 (3.3 mg) and 3 (28.1 mg), with an X-Bridge Prep C18 column by elution with 70% methanol for compound 10 (4.0 mg), with a COSMOSIL π-Nap column (5 µm, ϕ10.0×250 mm, Nacalai Tesque Inc., Kyoto, Japan) by elution with 80% methanol for compounds 9 (1.2 mg) and 15 (17.1 mg). Fr. 3–6 (12.2 g, eluted by 90% methanol from MCI gel) was subjected to a Sephadex LH-20 column (ϕ20×1000 mm, eluted with methanol) and then separated with a μ-Bonda Pak C18 column [ϕ25×200 mm, eluted stepwise with H2O-methanol (135 mL of 70%, 80%, 90% methanol solutions)]. Eluted solutions were further separated with a COSMOSIL 5C18 AR-II column by elution with 85% methanol for compounds 6 (7.7 mg), 7 (4.6 mg), 8 (33.7 mg) and 11 (4.0 mg), with a Triart PFP column (5 µm, ϕ4.6×150 mm, YMC Co. Ltd., Kyoto, Japan) by elution with 75% methanol for compound 13 (2.5 mg). Compounds 12 and 16 were obtained by an enzymatic hydrolysis of glucosides 2 and 5, respectively. Compound 2 (2.0 mg) in acetate buffer (1.0 mL, pH 5.0, 100 mM) was incubated for 12 h at 37°C in the presence of β-glucosidase from Almond (5.0 mg, EC 3.2.1.21, Sigma). The reaction was quenched by adding methanol and the solvent was evaporated in vacuo to get a residue. The residue was separated with a silica gel column [ϕ8×40 mm, eluted with CHCl3:methanol = 20: 1 (v/v)] to afford compound 12 (1.0 mg). In the same manner described above, compound 16 (1.0 mg) was prepared from compound 5 (2.0 mg).
1H- and 13C-NMR spectra of compounds were measured with a JEOL ECA 500 NMR spectrometer. HR-ESI-MS was recorded with a JEOL JMS-T100LP spectrometer. According to the spectrum data, the chemical structure of compounds 1~17 were identified to Icariin, Ikariside I, Ikariside II, Ikariside A, Epimedoside C, Limonianin, 8,5’-diprenyl apigenin, Epimedokoreanin B, Neophellamuretin, 8-prenyl luteolin, Broussonol D, Anhydroicaritin, Euchrestaflavanone A, Sagittatoside A, Korepimedoside A, Desmethylicaritin, Epimedokoreanin C, respectively, using the authentic data of respective compounds. These compounds were dissolved in dimethyl sulfoxide (DMSO) to make a 1 mM stock solution.
Purification and activation of gingipains
RgpB and Kgp were purified from culture media of P. gingivalis (HG66 strain) and activated with cysteine according to the method described previously (18). The activated proteases were diluted with 0.1 M Tris-HCl (pH 7.6) buffer containing 50 mM NaCl and 5 mM CaCl2 directly before assays.
Protease inhibition assay
Fifty µL of flavonoid sample in 0.1 M Tris-HCl (pH 7.6) buffer containing 50 mM NaCl and 5 mM CaCl2 and 50 µL of 5 nM RgpB or Kgp were mixed in a 96 well plate. After a preincubation at 37°C for 5 min, 50 µL of substrate solution (500 µM) was added to the mixture. Release of aminomethyl-coumarin (AMC) was measured using a fluorescence spectrophotometer (Wallac 1420 ARVO Multilabel Counter, Perkin Elmer) with an excitation at 380 nm and an emission at 440 nm. The linear increase of AMC release was recorded for 10 min. Residual activity in the presence of a sample was expressed as relative protease activity for the activity in the absence of the sample.
The inhibition pattern of compound 8 against gingipains was determined by Dixon plot ([S]/v vs. [I]), where [I] is compound 8 concentration, v is substrate cleaving velocity and [S] is substrate concentration. Ki values were calculated by nonlinear analysis using GraphPad Prism 5.0 software (Prism Software).
P. gingivalis growth assay
P. gingivalis ATCC 33277 was grown in brain heart infusion (BHI) broth supplemented with hemin and menadione (HM) or on BHI blood agar plate with HM in an anaerobic chamber (miniMACS anaerobic workstation; Don Whitley Scientific Ltd., Shipley, United Kingdom) in 80% N2, 10% H2, and 10% CO2. Influence of prenylated flavonoids on P. gingivalis growth was investigated by measuring the turbidity of bacterial suspension in a 96- well microplate format (3595, Corning, New York, NY). Two µL of a prenylated flavonoid at various concentrations was added to P. gingivalis suspension standardized at 2 × 107 CFU in 200 µL of BHI-HM broth (1 × 108 CFU/mL) in the wells. Absorbance at 620 nm was measured at different time periods using a microplate reader (Multiskan Ascent; Thermo Electron Oy, Vantaa, Finland).
Biofilm formation assay
To examine the effect of prenylated flavonoids on biofilm formation, P. gingivalis was assayed using a method described previously (19) with modifications. Briefly, to produce biofilms, 2 × 107 CFU of P. gingivalis in 200 µL of BHI-HM broth (1 × 108 CFU/mL) was added to microtiter plate wells (3595, Corning). After the plates were anaerobically incubated at 37°C for 48 h, planktonic cells in liquid medium were discarded and the plates were washed twice with distilled water. The plates were then air-dried, and attached biofilms were stained with 200 µL of 0.25% safranin for 30 min. Then, the plates were rinsed twice with distilled water to remove excess dye and air-dried. All dye associated with the attached biofilms was dissolved with 200 µL of 100% ethanol, and then absorbance at 492 nm was measured with a microplate reader (Multiskan Ascent; Thermo Electron Oy, Vantaa, Finland) to determine the amount of biofilm formation.
RESULTS
Inhibition of gingipains by prenylated flavonoids
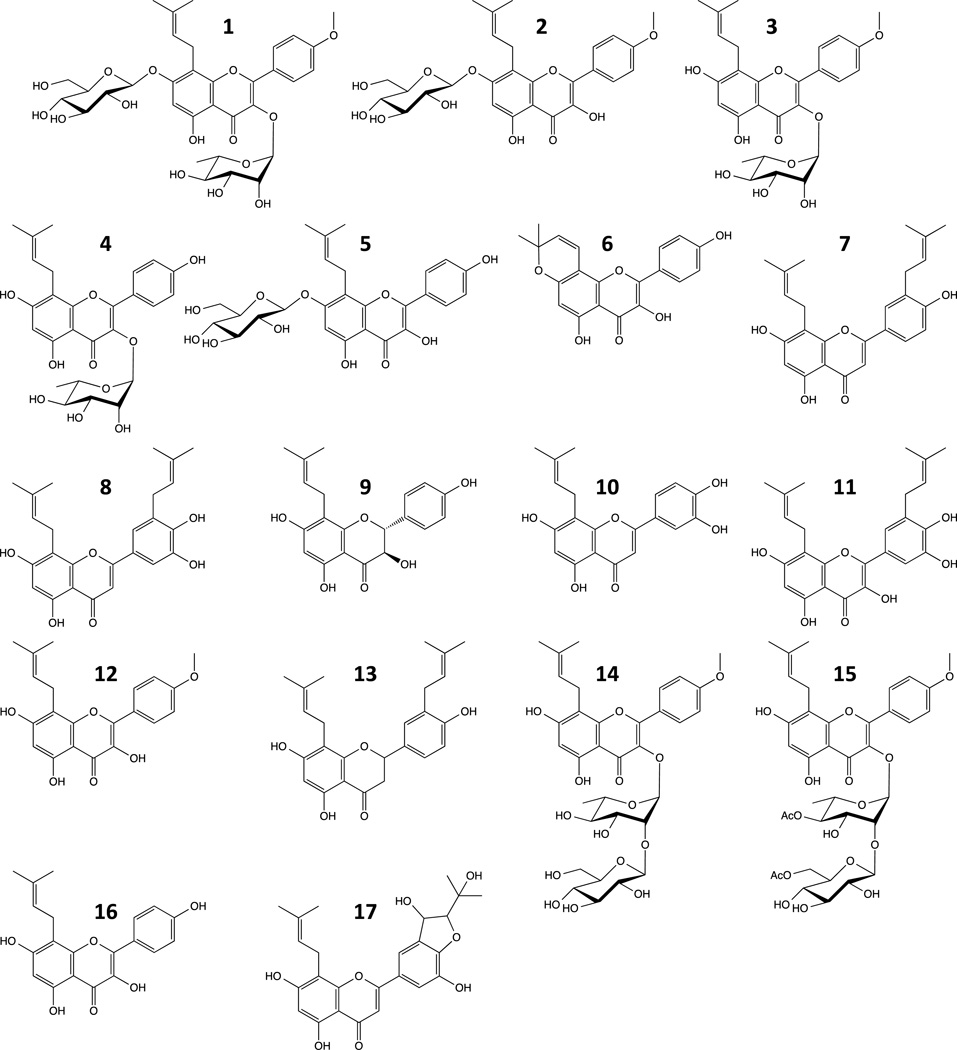
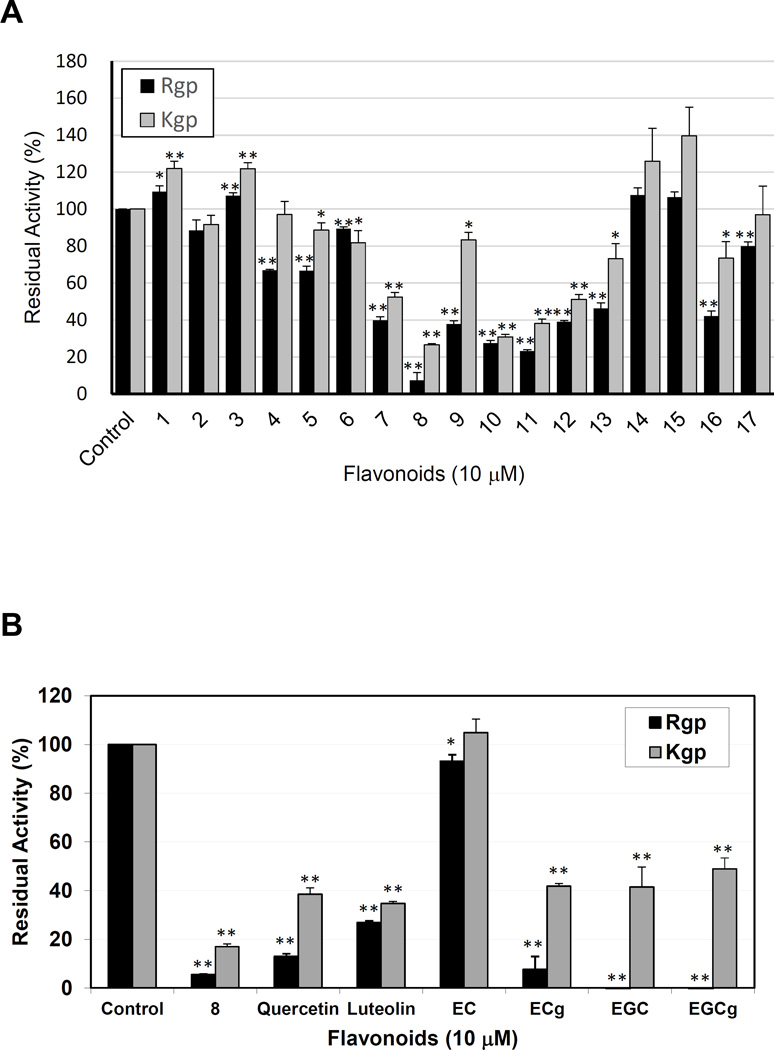
From the extract of Epimedium species, we isolated more than 30 flavonoids, including glycosylated flavonoids (Icariin, compound 1; Icariside I, compound 2; etc.), non-glycosylated flavonoids (Limonianin, compound 6; Epimedokoreanin B, compound 8; etc.) (Fig. 1), and novel flavonoids (data not shown). All of isolated flavonoids were prenylated. In our preliminary experiments, some of prenylated flavonoids showed inhibitory effect against gingipains at few to 30 µM order in a dose-dependent manner (data not shown). Screening of the known flavonoids for gingipain inhibition activity revealed that at 10 µM concentration compounds 8, 10, and 11 inhibited gingipains in this order of efficiency (Fig. 2A). Inhibitory activity of compound 8 against Rgp was more potent than that of quercetin, luteolin, and EC (P < 0.01) and compound 8 was a more potent Kgp inhibitor (P < 0.05) than any of the unprenylated flavonoids (Fig. 2B). Compared with these unprenylated flavonoids, the compound 8 was a potent inhibitor against both Rgp and Kgp.
Figure 1. Structure of flavonoids from Epimedium species.
Compound 1, Icariin; 2, Icariside I; 3, Icariside II; 4, Icarisoside A; 5, Epimedoside C; 6, Limonianin; 7, 8–5’ diprenyl apigenin; 8, Epimedokoreanin B; 9, Neophellamuretin; 10, 8-prenyl luteolin; 11, Broussonol D; 12, Anhydroicaritin; 13, Euchresta flavanone A; 14, Sagittatoside A; 15, Korepimeside A; 16, Desmethylicaritin; 17, Epimedokoreanin C.
Figure 2. Inhibition of Rgp and Kgp by prenylated flavonoids isolated from Epimedium species (A) and selected flavonoids (B).
Rgp and Kgp (each at 1.67 nM final concentration) were preincubated with 10 µM of prenylated flavonoid and residual enzyme activity was measured. The enzyme activity in the absence of flavonoid was taken as 100%. EC, Epicatechin; ECg. Epicatechin gallate; EGC, Epigallocatechin; EGCg, Epigallocatechin gallate. All values are expressed as average ± standard error in triplicate assay. Asterisk (P < 0.05) and double asterisk (P < 0.01) indicates significant difference of the values versus control values.
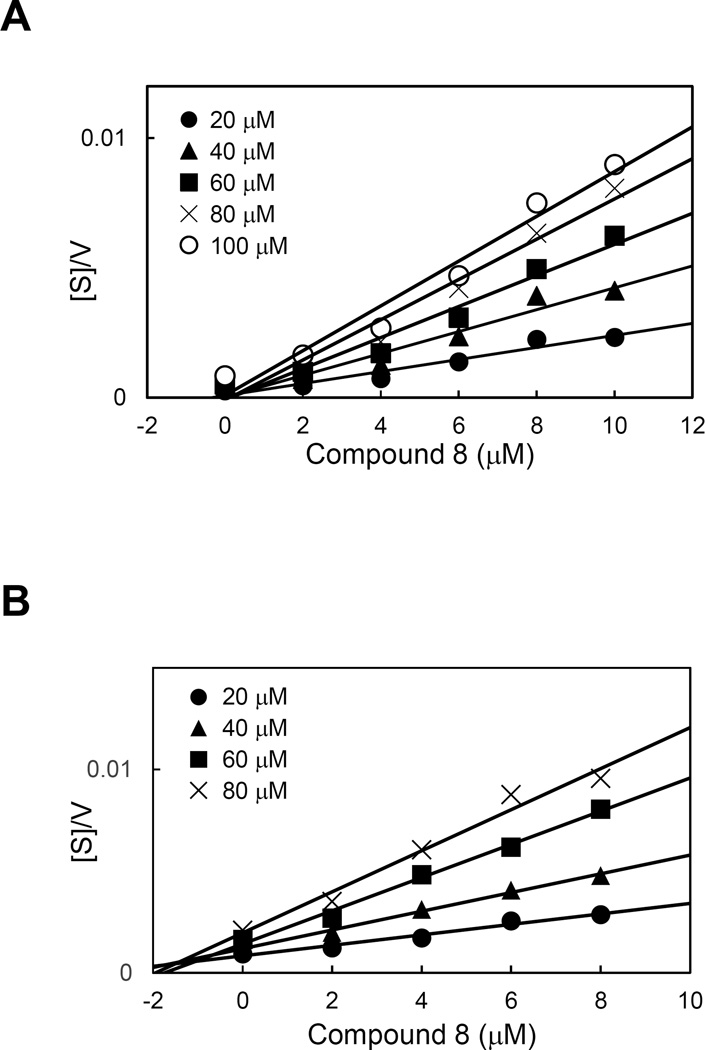
Gingipain inhibition pattern of compound 8
To determine the mode of the gingipain inhibition by compound 8, the kinetic of inhibitory reaction was analyzed. The plot of [S]/v versus [I] intersected the x-axis, which indicates that compound 8 is a non-competitive inhibitor of the gingipains (Fig. 3A and B). From the x-axis intersection points the Ki values were determined as 1.67±0.07 µM for Rgp and 2.71±0.22 µsM for Kgp (Fig. 3).
Figure 3. Determination of the mode of inhibition of RgpB (A) and Kgp (B) by Epimedokoreanin B (compound 8).
The velocity (v) of the reaction was determined at the substrate concentration [S] in the range from 20 µM to 100 µM in the presence of various concentrations (0–10 µM) of Epimedokoreanin B and the ratio of [S]/v for each inhibitor concentration [I] was plotted (the
Inhibition of P. gingivalis growth and biofilm formation by prenylated flavonoids
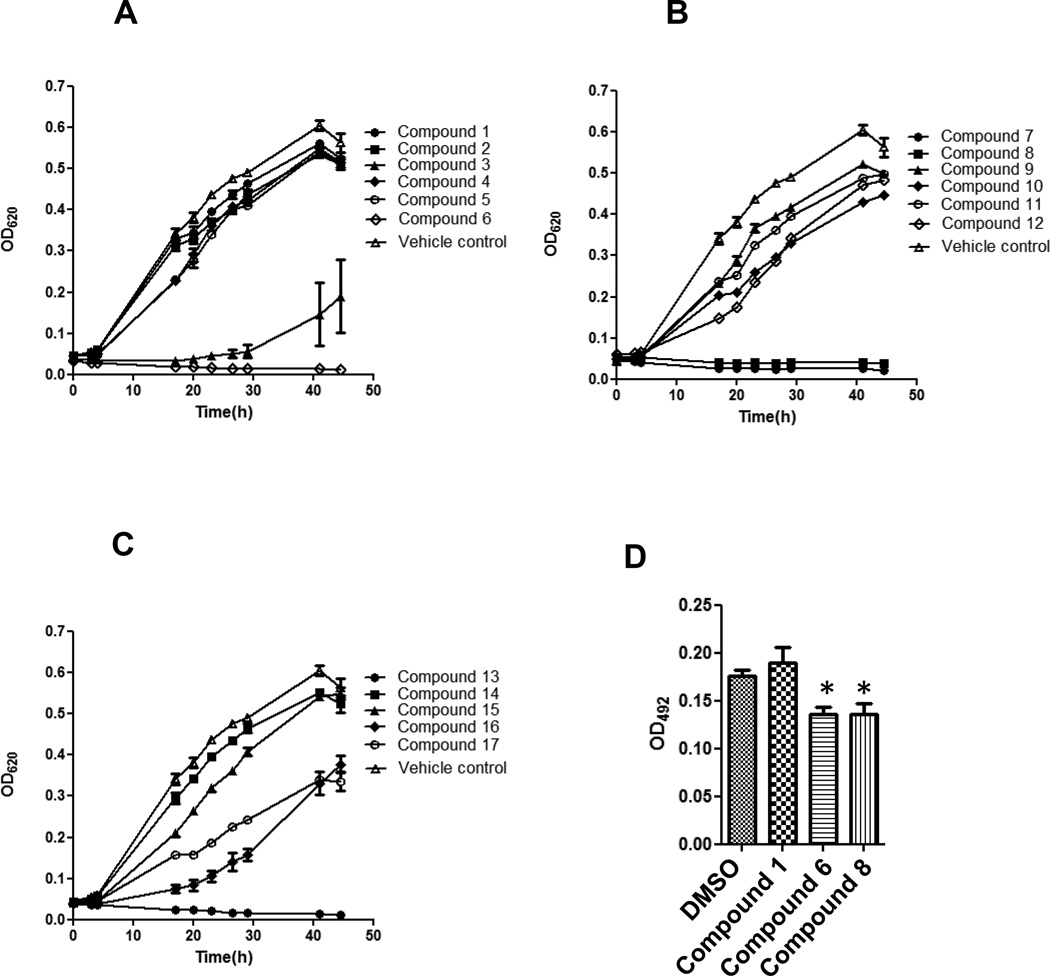
Gingipains are essential for acquisition of nutritious peptides by P. gingivalis, which are used as source of carbon and energy in the asaccharolytic fermentative metabolism. Thus, we have next investigated an effect of 17 prenylated flavonoids on the growth of the bacterium in BHI-HM broth medium. Compounds 6, 7, 8 and 13 inhibited completely P. gingivalis growth at 12.5 µM while compound 3 exerted a partial effect (Fig. 4A–C). Other compounds showed a negligible inhibitory effect on the P. gingivalis growth.
Figure 4. Inhibitory effect of prenylated flavonoids on growth and biofilm formation of P. gingivalis.
(A–C) P. gingivalis growth in the presence of prenylated flavonoids at 12.5 µM is shown by measuring absorbance at 620 nm different time points. Results were similar in each of three independent experiments and representative data are shown as means ± SD in a triplicate assay. (D) Biofilm formation (absorbance at 492 nm after staining with safranin) was assessed after a 24 h-culture in the presence of a compound at 1.25 µM. All the compounds at 1.25 M had no effect on growth of P. gingivalis. Results were similar in three independent experiments and representative data are shown. Results are expressed as means ± SD in a triplicate assay. Data were statistically analyzed by one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison test. Asterisks indicate significant difference (P < 0.05) from values obtained in the absence of flavonoids.
P. gingivalis exists in vivo in biofilms called subgingival plaques that accrete to the surface of the tooth root (20, 21). Host defense systems are not effective in elimination of P. gingivalis in biofilms, thus inflammatory responses to the pathogen continue resulting in tissue destruction and ultimately in tooth loss (22). Because gingipains directly and indirectly (prefimbrillin processing and fimbriae assembly) participate in colonization of a subgingival plaque (23), we investigated the effect of prenylated flavonoids on biofilm formation by P. gingivalis. The biofilm formation was assessed in 96-well microplates in the presence or absence of compound 1, 6, or 8 at 1.25 µM. These compounds are a representative glycosylated prenylated flavonoids, aglycone with moderate inhibitory activity, and aglycone with strong inhibitory activity against gingipain (?), respectively. The concentration at which these compounds do not affect the P. gingivalis growth (Supplemental Fig. 1A–C). This assay revealed that compounds 6 and 8, but not compound 1, significantly inhibited biofilm formation by P. gingivalis (Fig. 4D).
Discussion
Gingipains play critical roles in growth and biofilm formation of P. gingivalis thus are essential virulence factors in the periodontal disease pathology, such as tissue destruction, host-defense system dysregulation, vascular permeability induction, etc (3, 4). Therefore, inhibitors targeting these cysteine proteinases can be promising therapeutic agent for periodontitis. Prenyl flavonoids are widely distributed in plant kingdom and some of them are well-known bioactive components of medicinal plants (24, 25). Prenylation enhances hydrophobicity of flavonoids and augments flavonoid bioactivity and tissue accumulation (15, 26). In contrast, glycosylation increases the molecular size and polarity of flavonoids upsetting their planar structure thus negatively affecting their inhibition activity against some enzymes (27). Here, we analyzed gingipain inhibition by prenylated flavonoids (Fig. 2). The gingipain inhibition profile revealed the following structures as favorable for inhibition: (1) catechol group in the benzenoide (B-ring of flavonoid) as shown from comparison between compounds 7 and 8; (2) flavone (compound 7 and 8) rather than flavonol (compound 11) and flavanone (compound 13); (3) prenylation (addition of isoprenyl group) at C5’ carbon of the B-ring as shown from comparison of luteolin with compounds 8 and 10; (4) non-glycosylated flavonoids rather than O-glycosylated flavonoids as shown from comparison between compound 4 and 16, and of compound 12 with compounds 3, 14 and 15. These findings can be used to design synthesis of modified prenyl flavonoids to increase their inhibitory potency against gingipains.
Gingipain-deficient P. gingivalis that lacks the protease genes rgpA, rgpB, and kgp is unable to grow in a defined medium containing bovine serum albumin as a sole carbon/energy source (28). In addition, small peptide analogs (KYT inhibitors) and compounds in a rice protein extract, which are known to inhibit gingipains, suppress growth of wild type P. gingivalis in the defined medium (29, 30). These reports indicate that proteolysis of extracellular protein by gingipains is essential for bacterial growth. Compound 6, 7, 8, 13 inhibited the planktonic growth of P. gingivalis in this order, followed by compound 3 (Fig. 4A–C). Thus, these prenyl flavonoids and related compounds are potent suppressors of the P. gingivalis growth. However, BHI-HM broth used in this study is a nutrition-rich media in which gingipain null-P. gingivalis mutants grow as well as the wild-type strain. This result strongly suggests that prenylated flavonoids may target other than nutrition acquisition function of gingipains or, more likely they also hinder vital metabolic pathways of P. gingivalis. Also it needs to be kept in mind that besides gingipains P. gingivalis produces a number of extracellular and cell-associated proteinases (31). This corroborates with the finding by Blankenvoorde et al. that inhibition of cysteine proteases did not cause any suppression of P. gingivalis growth (32), suggesting an involvement of proteases other than cysteine proteases in the growth of this bacterium. It is likely that prenylated flavonoids also inhibit these proteases. The structure-activity relationship of prenyl flavonoid in inhibition of P. gingivalis planktonic growth is unclear. The exception is the strongly reduced growth-inhibitory activity of compound 4 in comparison to 16 which is clearly caused by O-glycosylation. Of note, some prenylated flavonoids (compound 6 and 13) with weak gingipains inhibitory activity (Fig. 2 and 4A–C) strongly suppressed planktonic growth. This supports a contention that the prenylated flavonoids inhibit P. gingivalis growth by undefined mechanism(s) independent of gingipain inhibition. Taken together, prenylated flavonoids can be considered as potential novel therapeutic agents capable of inhibiting both gingipains-dependent virulence and bacterial growth in periodontitis sites where proteins are the sole source of nutrition.
Location of P. gingivalis in subgingival biofilm in a periodontal pocket facilitates evasion of host immune responses and resistance to antibacterial agents. The finding that biofilm formation by P. gingivalis was significantly reduced in the presence of compound 6 or 8 (Fig. 4D) also indicates therapeutic applicability of these compounds for treatment of periodontitis. Interestingly, compound 6 suppressed P. gingivalis biofilm formation at 1.25 µM (Fig. 4D) but did not affect the bacterium growth even at 2.5 µM concentration (Supplementary Fig. 1B). This result indicates that some flavonoids exert efficient suppression of the biofilm formation via mechanisms other than gingipains- and growth-inhibition. The similar finding was described for tea catechin, lactoferrin, and cranberry proanthocyanidin, all of which hampered the P. gingivalis biofilm formation independent of gingipain inhibition (11, 13, 33). Using gingipain-null mutants, Kuboniwa et al. showed that gingipains affects the biofilm structure and maturation (34), although they are dispensable for the biofilm formation per se. Cumulatively, apart from inhibition of gingipain and P. gingivalis growth by flavonoids and prenylated flavonoids they may act through an unknown mechanism, which impedes the biofilm formation. Further studies are necessary to elucidate the mechanism. The present study provides useful information for future development of periodontitis treatment using the prenylated flavonoids that suppress gingipains, P. gingivalis growth and biofilm formation.
Supplementary Material
References
- 1.Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease: introduction. Periodontol 2000. 1999;20:7–13. doi: 10.1111/j.1600-0757.1999.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 2.Oliver RC, Brown LJ. Periodontal diseases and tooth loss. Periodontol 2000. 1993;2:117–127. doi: 10.1111/j.1600-0757.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 3.Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74:111–118. doi: 10.1902/jop.2003.74.1.111. [DOI] [PubMed] [Google Scholar]
- 4.NM OB-S, Veith PD, Dashper SG, Reynolds EC. Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire. Curr Protein Pept Sci. 2003;4:409–426. doi: 10.2174/1389203033487009. [DOI] [PubMed] [Google Scholar]
- 5.Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Current Protein and Peptide Science. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- 6.Lourbakos A, Yuan YP, Jenkins AL, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. 2001;97:3790–3797. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annual review of microbiology. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 9.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 10.Bodet C, Piche M, Chandad F, Grenier D. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J Antimicrob Chemother. 2006;57:685–690. doi: 10.1093/jac/dkl031. [DOI] [PubMed] [Google Scholar]
- 11.La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 54:1778–1784. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42:589–592. doi: 10.1111/j.1600-0765.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 13.Asahi Y, Noiri Y, Miura J, et al. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J Appl Microbiol. 116:1164–1171. doi: 10.1111/jam.12458. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto M, Sugimoto A, Leung KP, Nakayama K, Kamaguchi A, Maeda N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19:118–120. doi: 10.1046/j.0902-0055.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 15.Mukai R, Fujikura Y, Murota K, et al. Prenylation enhances quercetin uptake and reduces efflux in Caco-2 cells and enhances tissue accumulation in mice fed long-term. J Nutr. 143:1558–1564. doi: 10.3945/jn.113.176818. [DOI] [PubMed] [Google Scholar]
- 16.Botta B, Vitali A, Menendez P, Misiti D, Monache GD. Prenylated flavonoids: pharmacology and biotechnology. Current medicinal chemistry. 2005;12:713–739. doi: 10.2174/0929867053202241. [DOI] [PubMed] [Google Scholar]
- 17.Zhao HY, Sun JH, Fan MX, et al. Analysis of phenolic compounds in Epimedium plants using liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr A. 2008;1190:157–181. doi: 10.1016/j.chroma.2008.02.109. [DOI] [PubMed] [Google Scholar]
- 18.Imamura T, Matsushita K, Travis J, Potempa J. Inhibition of trypsin-like cysteine proteinases (gingipains) from Porphyromonas gingivalis by tetracycline and its analogues. Antimicrob Agents Chemother. 2001;45:2871–2876. doi: 10.1128/AAC.45.10.2871-2876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao R, Senpuku H, Watanabe H. Porphyromonas gingivalis galE is involved in lipopolysaccharide O-antigen synthesis and biofilm formation. Infect Immun. 2006;74:6145–6153. doi: 10.1128/IAI.00261-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey ME, Costerton JW. Molecular genetics analyses of biofilm formation in oral isolates. Periodontol 2000. 2006;42:13–26. doi: 10.1111/j.1600-0757.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 21.Marsh PD. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–1438. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 22.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J Biol Chem. 1998;273:29072–29076. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- 24.Tahara S, Ibrahim RK. Prenylated isoflavonoids: an update. Phytochemistry. 1995;38:1073–1094. [Google Scholar]
- 25.Barron D, Ibrahim RK. Isoprenylated flavonoids- survey. Phytochemistry. 1996;43:921–982. [Google Scholar]
- 26.Ming LG, Lv X, Ma XN, et al. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology. 154:1202–1214. doi: 10.1210/en.2012-2086. [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Chen T, Cao H. Flavonoid glycosylation and biological benefits. Biotechnol Adv. doi: 10.1016/j.biotechadv.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka S, Baba A, Suda Y, et al. A novel, potent dual inhibitor of Arg-gingipains and Lys-gingipain as a promising agent for periodontal disease therapy. Faseb j. 2014;28:3564–3578. doi: 10.1096/fj.14-252130. [DOI] [PubMed] [Google Scholar]
- 30.Taiyoji M, Yamanaka T, Tsuno T, Ohtsubo S. Potential value of a rice protein extract, containing proteinaceous inhibitors against cysteine proteinases from Porphyromonas gingivalis, for managing periodontal diseases. Biosci Biotechnol Biochem. 2013;77:80–86. doi: 10.1271/bbb.120585. [DOI] [PubMed] [Google Scholar]
- 31.Gharbia SE, Shah HN. Hydrolytic enzymes liberated by black-pigmented gram-negative anaerobes. FEMS Immunol Med Microbiol. 1993;6:139–145. doi: 10.1111/j.1574-695X.1993.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 32.Blankenvoorde MF, van't Hof W, Walgreen-Weterings E, et al. Cystatin and cystatin-derived peptides have antibacterial activity against the pathogen Porphyromonas gingivalis. Biol Chem. 1998;379:1371–1375. [PubMed] [Google Scholar]
- 33.Dashper SG, Pan Y, Veith PD, et al. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob Agents Chemother. 56:1548–1556. doi: 10.1128/AAC.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuboniwa M, Amano A, Hashino E, et al. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009;9:105. doi: 10.1186/1471-2180-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.