Abstract
Measured as elapsed time from first use to dependence syndrome onset, the estimated “induction interval” for cocaine is thought to be short relative to the cannabis interval, but little is known about risk of becoming dependent during first months after onset of use. Virtually all published estimates for this facet of drug dependence epidemiology are from life histories elicited years after first use. To improve estimation, we turn to new month‐wise data from nationally representative samples of newly incident drug users identified via probability sampling and confidential computer‐assisted self‐interviews for the United States National Surveys on Drug Use and Health, 2004–2013. Standardized modules assessed first and most recent use, and dependence syndromes, for each drug subtype. A four‐parameter Hill function depicts the drug dependence transition for subgroups defined by units of elapsed time from first to most recent use, with an expectation of greater cocaine dependence transitions for cocaine versus cannabis. This study's novel estimates for cocaine users one month after first use show 2–4% with cocaine dependence; 12–17% are dependent when use has persisted. Corresponding cannabis estimates are 0–1% after one month, but 10–23% when use persists. Duration or persistence of cannabis smoking beyond an initial interval of a few months of use seems to be a signal of noteworthy risk for, or co‐occurrence of, rapid‐onset cannabis dependence, not too distant from cocaine estimates, when we sort newly incident users into subgroups defined by elapsed time from first to most recent use. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: cocaine dependence, cannabis dependence, Hill function
Introduction
In recent epidemiological research with United States (US) samples, a novel functional analysis approach disclosed estimated transition probabilities for cocaine and for other drugs, with focus upon estimating risk of becoming a case of cocaine dependence within 12 months after onset of cocaine use, and with frequency of use as an underlying explanatory variable of central interest (Vsevolozhskaya and Anthony, 2016). In this research project, the aim is to extend the functional analysis approach for study of rapid transitions into cocaine dependence, which might depend upon or be interdependent with duration of use, with cannabis as a comparator agent in this inquiry. In addition, we hope to introduce the International Journal of Methods in Psychiatric Research (IJMPR) audience to the four parameter Hill equation functional analysis approach we are using for estimation of drug‐specific transition probabilities, and to encourage a more general application of functional analysis in drug dependence research as well as neuropsychiatry studies.
By way of background, before 1985, outside of the coca‐growing source countries, the United States, and Canada, few epidemiologists showed interest in cocaine. Thereafter, once its use had spread to Western Europe, cocaine provoked concern previously reserved for other internationally regulated drugs such as heroin, the amphetamines, and cannabis (United Nations, 2015). As such, this project's functional analysis approach might be interesting for international audiences, especially when one seeks to compare cocaine‐related harms with cannabis‐related harms in cross‐national studies. The functional analysis approach also should be of interest to psychiatric researchers in general because it can be applied elsewhere, as illustrated with three mood and anxiety disorder examples for which estimated functional analysis parameters would be useful in future research and clinical practice – namely, the estimated effect of time elapsed (1) from onset of adolescent anhedonia until the first episode of Major Depression (MD), (2) from MD onset until the first post‐MD suicide attempt, (3) from panic attack onset until newly incident agoraphobia.
With respect to current empirical estimates of transition probabilities for cocaine dependence once cocaine use starts, there is general consistency across published studies based on various data sources, different years of study, and the year‐wise retrospective measurement approach. Transition probability or risk estimates from these studies indicate that within 24 months after starting cocaine use, an estimated 5–6% of users have developed cocaine dependence. Corresponding estimates for cannabis transition probabilities are generally lower, at ~3% (Chen et al., 2005; Hasin et al., 2015; Lopez‐Quintero et al., 2011; O'Brien and Anthony, 2005; Wagner and Anthony, 2007) However, a recent Australian sample of twins provides evidence of the predictive and explanatory value of elapsed time from first to second occasion of cannabis use, with short elapsed time predicting much larger cumulative incidence of cannabis use disorder (Diagnostic and Statistical Manual of Mental Disorders [DSM] “abuse” and/or dependence) than the generally observed three percent value for cannabis dependence (Hines et al., 2015). In the Australia study, the twins had to think back for the assessment over relatively long spans of time since onset of cannabis use; in the present study, all temporal and diagnostic assessments have been completed within an interval of 12 months since first cannabis use.
We took these published values from year‐wise studies as a point of departure. Our hypothesis was that the drug‐specific transition probability estimates might vary considerably if one were to take into account the elapsed time from the very first use of the drug until the most recent use of the drug, evaluated month‐by‐month since the start of the drug use, and with a restriction to newly incident users so that the recall and reporting interval is constrained to be quite short (i.e. no more than 12 months duration).
We forecast potential observed interdependencies linking drug use duration with drug dependence processes, as well as some heterogeneity within duration‐stratified newly incident users. To illustrate, the first and most recent drug exposure can occur on the same day, as sometimes is true for drug users when initial cocaine or cannabis experiences are aversive or punishing from a behavioral analysis standpoint (e.g. when the drug experience serves to trigger an unexpected acute anxiety reaction). For these users, their first drug use might well be their last drug use. With such short drug use duration, a drug dependence process does not get started. Repetition of drug‐taking occasions is required before a dependence syndrome can emerge.
For some users, the first cocaine or cannabis experiences seem to be exceptionally reinforcing. Drug exposure might rapidly become a daily occurrence, sustained for many months. Here, elapsed time from first to most recent use might be short or can be quite lengthy, with observed dates of most recent use many months after first use.
In addition, there can be feedback loops. Once drug dependence processes start, these same processes can become explanations for subsequently extended durations of use, over and above any reinforcing function that drug use might have served when it was first initiated (Anthony, 2010).
As a manifestation of heterogeneity, among the newly incident users with very short elapsed time, the single‐occasion users are grouped with users whose month of first use (MFU) and month of survey assessment are one and the same, some of whom might have had a highly reinforcing first use but have not yet had time to accumulate more than one month of experience with the drug. Among newly incident users with multiple months of elapsed time from first use until most recent use, there are some who have become sustained daily users, as well as others who have used on no more than a few occasions, separated by long intervals of no use whatsoever.
The line of thinking for this study required a methodological extension of the initial Vsevolozhskaya–Anthony application of functional analysis in epidemiological research on estimated probability of a clinical outcome such as drug dependence, for which “frequency of drug use” was specified as an interdependent covariate. In this extension, we have replaced the “frequency of drug use” construct with the dimension of elapsed time from first to most recent drug exposure (i.e. observed “duration” of use).
In “functional analysis” we draw upon non‐linear regression models based on the Hill equation, which has somewhat more complexity than logistic regression models as generally applied in neuropsychiatric research to date. In binary response logistic regression, the slope parameters are the “estimands” of interest (e.g. Jacobi et al., 2015; Ringeisen et al., 2015), but there are four parameters of interest when Hill equation models are fit to observed data. These four parameters can be used to link an explanatory variable of interest with probability of a binary response. In this study's “month‐wise” context, we specify elapsed time from first to most recent drug as an interdependent covariate of interest, with the estimated probability of drug dependence as response, constraining the analysis to newly incident users who have had no more than 12 months since the month of first starting their drug use experiences. Here, the concept of “interdependence” is one that allows for feedback loops such that a dependence process can drive up duration of use. Multi‐wave longitudinal research with month‐by‐month assessments of newly incident users can build from this study's cross‐sectional estimates in order to characterize these acknowledged feedback loops (Anthony, 2010).
Two other salient methodological issues surface in the context of our model. First, cannabis dependence and cocaine dependence are measured as separable outcomes for which there is no question about whether the agent is the cause of the outcome (i.e. the cause‐effect association is not at issue; the agent qualifies as a necessary but not sufficient causal influence). A newly incident cannabis user cannot develop cocaine dependence until cocaine exposure occurs. A newly incident cocaine user cannot develop cannabis dependence until cannabis exposure occurs. Analogies to communicable disease research are pertinent. Vibrio cholerae exposure per se does not cause tuberculosis; it has been designated as the necessary cause for cholera as a clinical outcome. Tubercle bacillus exposure per se does not cause cholera; it has been designated as the necessary cause for tuberculosis as a clinical outcome. As such, we estimate comparative Hill function parameters for cocaine versus cannabis as one might estimate these parameters for Vibrio cholerae and tuberculosis (as in probability of transitioning from first effective agent exposure to onset of clinically apparent disease within 12 months after effective contact). Possibilities of subgroup variation in transition probabilities are pertinent here (e.g. males might be more likely to transition than females). Nonetheless, when the agent is designated as a necessary cause for the clinical outcome, there is no issue comparable to “confounding” as might surface when hypotheses about possibly confounded suspected causes are being evaluated in chronic disease epidemiology research.
Second, some readers might critique the cross‐sectional nature of the observed data or ask for high dimensional propensity scoring or other nuanced refinements as might be used to estimate the functional analysis parameters with adjustments for macro‐level differences (e.g. local area drug availability) or for individual‐level differences (e.g. prior use of other drugs, genetic susceptibility traits). We agree that these refinements might be useful, but this study's initial functional analysis estimates provide a foundation upon which future prospectively conducted research might be built, with design‐based or analysis‐based experimental control over important macro‐ and micro‐level covariates. In this respect, the work is analogous to derivation of starting estimates based on cross‐sectional data in order to lay plans for sample size requirements, optimal between‐assessment intervals, and other facets of subsequent prospective and longitudinal research projects.
Methods
Study population, sampling, and assessment procedures
The study population consists of non‐institutionalized civilian US community residents in each of the 50 states and the District of Columbia, age 12 years and older, as specified each year for the 2004–2013 National Surveys on Drug Use and Health (NSDUH). Each year's survey sample was drawn as an independent multistage area probability sample of this study population, with annual targeted sample size of ~67,500 individuals. NSDUH participants completed a multi‐module audio‐enhanced computer assisted self‐interview (ACASI). The ACASI assessment included standardized multi‐item DSM‐IV drug dependence syndrome diagnostic modules akin to those used in the Diagnostic Interview Schedule, the Composite International Diagnostic Interview, and the Alcohol Use Disorders and Associated Disabilities Interview Schedules (American Psychiatric Association, 2000). These case ascertainment modules were used to identify newly incident drug dependence cases among the newly incident drug users. (DSM‐5 was not yet published when the NSDUH 2004–2013 assessments were completed.) The study protocols were reviewed and approved by cognizant institutional review boards. NSDUH details and methods descriptions are widely available in prior articles and in numerous online reports (Parker and Anthony, 2014; Seedall and Anthony, 2013; United States, 2014a; Vsevolozhskaya and Anthony, 2014)
To protect respondents’ confidentiality, for each survey year, public use data files are created based on observations for ~55,000 respondents. We fit parametric non‐linear Hill models to weighted observations from the NSDUH “SDA” datasets (United States, 2014b).
Whereas each NSDUH is conducted as a cross‐sectional survey with an independently drawn replication sample, its month‐by‐month data on the first occasion of drug use make it possible to focus on the set of “newly incident drug users.” This set encompasses users for whom no more than 12 months passed between the MFU and the effective assessment date. In some instances month of assessment was not recorded, but the user reported the month of the most recent or “last” use (MLU). For a relatively small subset, only “calendar quarter of last use” is known, and the MLU value has been logically assigned to the middle month of the assessment quarter. For instance, when these newly incident users were assessed in the first quarter of 2012, the MLU was logically assigned to the quarter midpoint, or February 15, 2012. After this logical imputation, the resulting sample sizes were n = 3186 for newly incident cocaine users and n = 11,629 for newly incident cannabis users (all observed within 12 months since onset of use). Supplementary Material Table S1 provides additional sample characteristics.
Functional data analysis
The R statistical software “survey” analysis routines (Lumley, 2004) were used to account for the NSDUH complex survey design and to estimate weighted empirical probabilities of dependence, as well as their corresponding variances. When attention is paid to sampling units and clustering weights, tabulated values of the empirical probabilities of drug dependence by the “duration” of drug use in months can be estimated, where “duration” is estimated as elapsed time from first month of starting drug use until most recent month of using the drug. Standard errors of the empirical point estimates can be approximated via Taylor series linearization (Heeringa et al., 2010). Non‐linear interpolation to empirical probabilities of dependence is achieved via a parametric Hill equation as:
where y i is the empirical probability of drug dependence and x i is the exposure duration in months (i.e. x i = MLUi − MFUi) for subject i, i = 1, …, n. The ϵ i values are assumed to be independent and normally distributed with constant variance σ 2. The P min, P max, PD50 and k are Hill function parameters requiring estimation. An iterative estimation process requires users to supply plausible starting parameter values, supplied here as “eye‐balled” empirical point estimates. Alternative approaches are described elsewhere (Ritz and Streibig, 2008).
The four parameters that control the particular shape of the sigmoid Hill function have the following epidemiological interpretation in the context of current research: (1) P min is the estimated probability of drug dependence among the exposed for whom there are no more than a few repetitions of use within the first few months after onset, including those for whom initial experience might have been aversive or perhaps relatively non‐reinforcing, or for whom agent availability might be quite constrained; (2) P max is the probability of drug dependence among the exposed who seek out or otherwise have agent availability, as manifest in greater elapsed time from first to most recent drug use within the 12 months after initial exposure; (3) PD50 stands for the number of months of exposure after which the probability of drug dependence is halfway to P max (within the first 12 months after onset of drug use); and finally (4) k is the rate of transition from P min to P max, evaluated at month PD50. In some contexts, this study's estimate of PD50 × 2, based on the month‐wise data, will serve as a useful starting value for elapsed time until the peak risk of becoming a clinically apparent case has been reached, as an approximation of a maximum for each agent's induction interval after initial exposure.
Results
Based on functional analysis of data on newly incident users characterized in Table S1, Figure 1 shows estimated probability of observed cocaine dependence among newly incident cocaine users across a span of 12 months since cocaine use onset. Figure 2 is focused on the experiences of newly incident cannabis users, and depicts corresponding cannabis dependence risk estimates, each plotted in relation to the elapsed time from first to last occasion of use. Solid dots show empirical point estimates for the drug dependence proportions plotted for subgroups defined by elapsed time from MFU to MLU, with the origin anchored at 0.5 months when MFU = MLU such that maximum x = 12.5 ; vertical error bars depict 95% confidence intervals (CIs) via logit transformations (Vsevolozhskaya and Anthony, 2014). Solid lines display non‐linear Hill function estimates for transitions from first drug use to dependence as might be governed by, or interdependent with, elapsed time from first to most recent use, all observed within a fairly short interval after first use.
Figure 1.
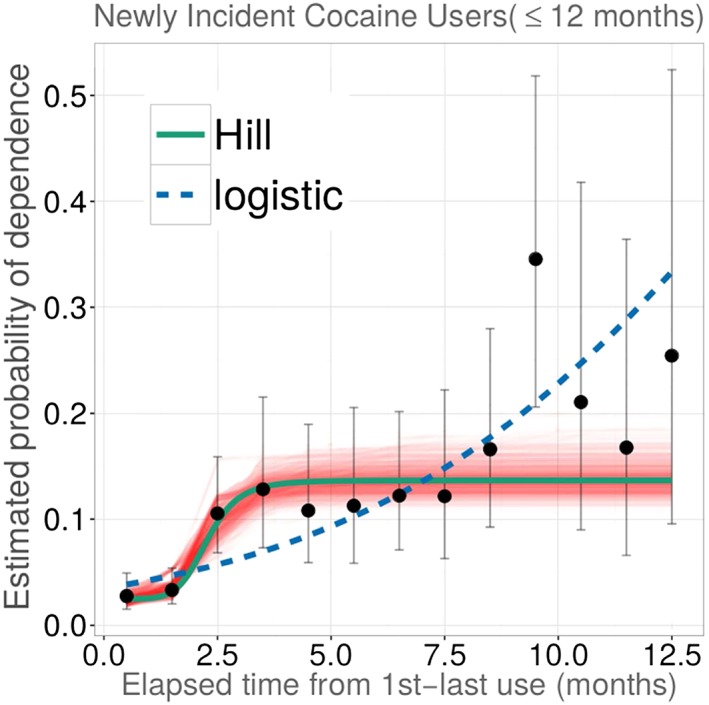
Estimated non‐linear parametric function for occurrence of cocaine dependence soon after onset of cocaine use, plotted in relation to elapsed time from first to most recent use (n = 3186 newly incident cocaine users). Data from the US National Surveys on Drug Use and Health, 2004–2013. (The function describes the estimated relationships linking “duration” of drug use among newly incident cocaine users and their estimated probability of becoming cocaine dependent within 12 months after first cocaine use.)
Figure 2.
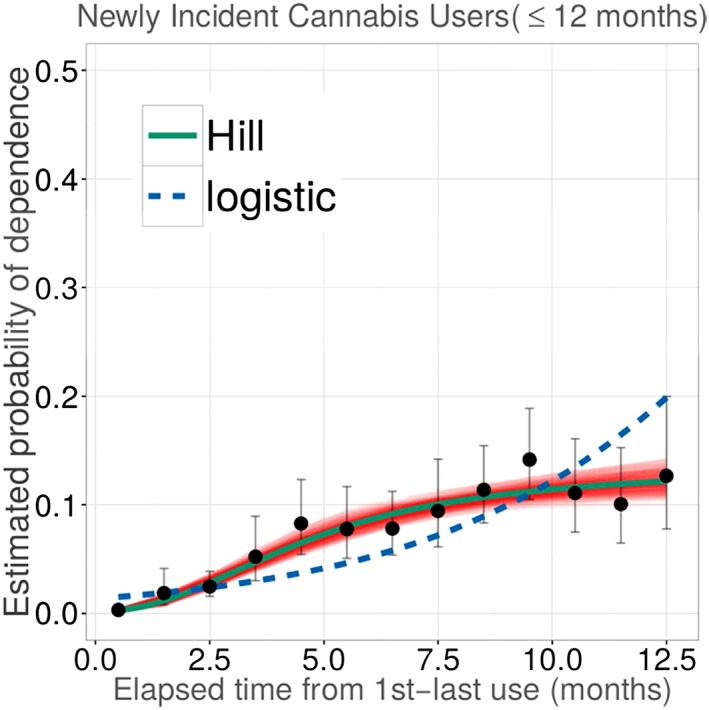
Estimated non‐linear parametric function for occurrence of cannabis dependence soon after onset of cannabis use, plotted in relation to elapsed time from first to most recent use (n = 11,629 newly incident cannabis users). Data from the US National Surveys on Drug Use and Health, 2004–2013. (The function describes the estimated relationships linking “duration“ of drug use among newly incident cannabis users and their estimated probability of becoming cannabis dependent within 12 months after first cannabis use.)
In Figures 1 and 2, bands around solid lines characterize variability in estimates for non‐linear Hill function parameters, derived from weighted residual bootstrap procedures (Vsevolozhskaya and Anthony, 2016). The weighted residual bootstrap procedure was used instead of Wald‐type CIs (e.g. plus/minus twice standard error) due to substantive departures from the model assumptions. Specifically, in both Figures 1 and 2, vertical error bars are getting wider along the x ‐axis, indicating violation of the constant variance assumption, which may result in biased and/or distorted standard errors. As such, we turned to the bootstrap approach, which does not rely on model assumptions.
Estimates from fitted Hill functions agree with a theoretically plausible specification that any rapid transition from first drug use to dependence among newly incident cannabis users should be smoother and slower than is observed for newly incident cocaine users, at least when elapsed time is relatively short. The tighter confidence bounds around the solid line for cannabis dependence are due to much larger numbers of newly incident cannabis users in the NSDUH samples.
Quantification of contrasting transitions to dependence for each of these two drugs is achieved in the comparison of the four governing parameters of estimated Hill functions. Table 1 depicts their 95% CIs from the weighted bootstrap procedure.
Table 1.
Comparative estimates of Hill function parameters characterizing probability of developing drug dependence soon after first occurrence of newly incident drug use, for two drugs: cocaine and cannabis. Data from the US National Surveys on Drug Use and Health, 2004–2013
| Parameters (95% bootstrap confidence intervals) | ||||
|---|---|---|---|---|
| P min | P max | PD50 | k | |
| Cocainea | 0.02 (0.02, 0.04) | 0.14 (0.12, 0.17) | 3 (2, 3) | 6.60 (2.75, 1.69) |
| Cannabisb | 0.00 (0.00, 0.01) | 0.13 (0.10,0.23) | 5 (3, 10) | 2.20 (1.38, 3.50) |
Unweighted number of newly incident cocaine users, n = 3186.
Unweighted number of newly incident cannabis users, n = 11,629.
The Hill function estimates in Table 1 for cocaine suggest that roughly 2% of cocaine users are becoming cocaine dependent within about two months of elapsed time from first until most recent cocaine use (P min 95% CI: 2%, 4%), after which risk shifts upward (slope, k = 6.6 at PD50 = three months) toward P max of 14% (95% CI: 12%,17%). The maximum is observed as early as an estimated “induction interval” of six months (PD50 × 2) of cocaine use.
For cannabis, Table 1 estimates show that relatively few are becoming cannabis dependent within first two months after first exposure. Relative to cocaine, there is a much more gradual upward shift in risk of dependence (estimated slope is k = 2.2 at PD50 = five months). Based on observations within 12 months after onset of cannabis use, estimated P max = 13% (95% CI: 10%, 23%). That is, roughly one in seven to eight newly incident users are becoming cannabis dependent when elapsed time from first to most recent cannabis use extends past 10 months (PD50 × 2).
To contrast our findings with estimates from a more familiar logistic regression (LR), we fit the LR model for a binary Y j = 0.1 drug dependence response, with the exposure duration as covariate, and with Hill function estimation routing retained to account for the NSDUH complex survey design. Resulting odds ratio estimates were 1.3 for cannabis (95% CI: 1.03, 1.6) and 1.2 for cocaine (95% CI: 1.2, 1.3). Fitted values from these models are shown in Figures 1 and 2 as dotted blue lines.
Discussion
Evaluated in relation to the few prior projects with large epidemiological community samples of newly incident or lifetime drug users, this study is the first one to substitute a month‐wise approach in place of a year‐wise approach for study of drug dependence processes emerging when no more than a few months have passed from the start of drug use until the most recent drug use. In contrast, all prior epidemiological studies of MLU to MFU differences among newly incident users have looked across the one or two years after drug use has started, facing larger constraints. Why? Because elapsed time almost always has been conceptualized with a timescale of years, not months. The timescale in years typically has been derived by taking differences between respondents’ age on an assessment date versus age of first drug use (Lopez‐Quintero et al., 2011; O'Brien and Anthony, 2005; Wagner and Anthony, 2002). Here, with a focus on newly incident users assessed no more than 12 months after first use, the month‐wise timescale is based on standardized responses to questions about the first month of use and the most recent month of use, as of the date of survey assessment.
This study's month‐wise approach to the elapsed time dimension, with estimation of Hill function P min and P max values, yields a noteworthy and somewhat unexpected discovery. Namely, cocaine's P min estimate is robustly larger than the P min estimate for cannabis, but the P max estimates do not differ statistically. An inference from P min is that the newly incident cocaine users are more likely to have become cocaine dependent during their first months after onset of use and with relatively short elapsed time from first use to most recent use, as compared to risk of cannabis dependence during the first months of cannabis use. The inference from P max is one of no corresponding cocaine–cannabis difference when elapsed time from first to most recent use has a value of about 10 months or larger.
Our introduction mentions the possibility of feedback loops such that an incipient drug dependence process might help determine duration or persistence of drug use (i.e. greater elapsed time from first to most recent use), with the feedback becoming more salient once a clinically recognizable drug dependence syndrome has formed (Anthony, 2010). In this respect, the P min comparison is noteworthy because it is during the P min interval of elapsed time (i.e. 0–2 months after first drug use) that feedback might be least salient when fewer users will have developed dependence. Given the observed cocaine–cannabis differences in P min, within 0–2 months after first use, future research projects might focus on the first three months of drug experience, after which the slope, k, for cocaine rises more sharply than is seen in the slope for cannabis.
While we appreciate that the Hill function point estimates for cocaine's PD50 and k parameters are numerically larger than the corresponding cannabis estimates, in this instance the bootstrapped CIs show at least modest overlap. This was not the case for P min.
Another discovery of interest is that the P max parameter estimates for cocaine and cannabis are not appreciably different from one another (i.e. when the elapsed time dimension has a value greater than about 10 months). Notably, the observed P max equivalencies serve as an indication of generally comparable cocaine and cannabis dependence transition probabilities for the subgroup of newly incident users observed with more than about 10 months from first to most recent use. We remind our readers about heterogeneity of the duration‐stratified subgroups, as mentioned in our introduction. The long duration subgroups include individuals who have become persistent daily users and for whom cessation of use has not yet materialized (within the first 12 months after first use). It also includes individuals who might be described as “chippers” who consistently use every weekend since first use, but do not use during the work week. Even with this heterogeneity, the Hill function P max parameter estimates and the fitted values from logistic regression models indicate a duration‐associated increased odds of seeing a drug dependence syndrome emerge within 12 months after initial drug‐taking for the subgroup of newly incident users with larger elapsed time values, irrespective of whether they have been using cannabis or cocaine. The degree to which the observed equivalence of cocaine and cannabis can be traced back to relative affordability or availability of these drug compounds cannot be evaluated with the data in hand. It is certain that other theoretically important influences also might be at play (e.g. greater clinically significant toxicity for sustained cocaine users relative to sustained cannabis users).
Before any additional discussion of these new findings, several study limitations deserve attention. First, self‐report gives rise to potential “methods effects” and a common method variance bias (Podsakoff et al., 2003). Nonetheless, self‐report assessment methods have been characterized as having both valid and reliable levels of validity in nationally representative samples (Del Boca and Darkes, 2003; Richman et al., 1999; Vignali et al., 2012).
We also note that longitudinal research commonly is regarded as a superior alternative to any cross‐sectional approach. We cannot disagree with this perspective. Nonetheless, we note that any meritorious proposal for longitudinal research includes a consideration of (a) sample size requirements, and (b) between‐assessment design intervals, and these considerations can be guided by starting estimates from cross‐sectional research of the type derived here. We also note that cross‐sectional derived estimates of this type do not suffer from differential attrition faced in longitudinal studies that compare experiences of users of various drug compounds (Anthony, 2010; Lopez‐Quintero et al., 2015; Seedall and Anthony, 2015).
Finally, our description of the functional analysis approach and specification of software for performing these analyses (available at http://www.epi.msu.edu/vsevoloz/scripts/Hill_function/) does not, at present, extend to the more complex problem of simultaneous adjustment for multiple possible confounding variables. We have planned additional functional analysis methods research that will make it possible to accommodate possibilities of confounding via covariate adjustments. For this reason, our introduction to the functional analysis approach does not include covariate‐controls seen when the generalized linear model is used with a logistic link (e.g. Hines et al., 2015).
Study limitations of this type motivate a cautious approach when these epidemiological estimates for cocaine and cannabis are interpreted. Some readers might wish to interpret the estimates as manifestations of “properties of the drug” such as an “addiction potential” or as a “dependence liability” manifestation. In our view, this interpretation of epidemiological estimates is premature. The relatively uncontrolled community context of epidemiological research does not take into account macro‐level environmental conditions such as local drug availability or micro‐level conditions such as individual susceptibility traits that might be pertinent to some drug exposures but not to others (Caulkins et al., 2015; Cerda et al., 2012; Hines et al., 2015).
Notwithstanding limitations of this type, we hope readers will appreciate this initial application of a functional analysis approach, with study of elapsed time from first use to most recent use as an important dimension generally not considered in prior studies. To illustrate, an American child psychiatrist seeing an adolescent patient with untreated newly incident anhedonia of two weeks duration now can turn to published epidemiological estimates to learn that MD in adulthood is a likely outcome, with approaching 50% probability (Wilcox and Anthony, 2004).If the Hill function approach were applied to new data on duration of adolescent‐onset anhedonia relative to adult‐onset MD, analogously useful probability estimates could be derived for anhedonia of one, two, or three weeks, or longer duration. Similar applications to MD and suicide attempt, as well as panic attack and agoraphobia, can be worked out as future functional analysis applications. Post‐traumatic stress disorder as a response to specific types of qualifying traumatic events also can be studied using the Hill equation functional analysis approach and the software we have shared.
Finally, one of our findings suggested greater equivalence between drug dependence odds for cocaine and cannabis users with 10–12 months separating first and most recent use. Commentary about the implications of these epidemiological estimates for drug policy decision‐making now would be speculative and premature. Nonetheless, it does not escape our attention that cannabis dependence estimates at this level might have some importance in relation to judgments made by jurisdictions that are changing their cannabis policies toward increased availability and reduced “cost”, which might be expected to increase duration of use among newly incident cannabis users. Nonetheless, little more than speculation is possible when trying to link this study's estimates to the recent cannabis policy debates. Under the best policy analysis circumstances, empirical risks of clinically important outcomes such as drug dependence must be evaluated relative to the clear harmfulness of criminal records and incarceration (United States, 1972).
Funding
This work was supported by a National Institutes of Health National Institute on Drug Abuse T32 research training program grant award (T32DA021129) for OAV's postdoctoral fellowship, by JCA's NIDA Senior Scientist and Mentorship Award (K05DA015799), and by the Michigan State University. The sponsoring agencies and university had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper and the content are the sole responsibility of the authors and do not necessarily represent official views of Michigan State University, the National Institute on Drug Abuse, or the National Institutes of Health.
Declaration of interest statement
The authors have no competing interests.
Supporting information
Supporting info item
Acknowledgements
The authors would like to thank the US Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality, which sponsors the National Survey on Drug Use and Health and makes available public use datasets. Both authors participated in the design of the study; analysis and interpretation of the data; and preparation, review, and approval of the manuscript.
The sponsoring agencies and university had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper are solely those of the authors.
Vsevolozhskaya, O. A. , and Anthony, J. C. (2017) Estimated probability of becoming a case of drug dependence in relation to duration of drug‐taking experience: a functional analysis approach. Int J Methods Psychiatr Res, 26: e1513. doi: 10.1002/mpr.1513.
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual – Text Revision (DSM‐IV‐TR, 2000), Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anthony J.C. (2010) Novel phenotype issues raised in cross‐national epidemiological research on drug dependence. Annals of the New York Academy of Sciences, 1187, 353–369. DOI: 10.1111/j.1749-6632.2009.05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulkins J.P., Kilmer B., Reuter P.H., Midgette G. (2015) Cocaine's fall and marijuana's rise: questions and insights based on new estimates of consumption and expenditures in US drug markets. Addiction, 110(5), 728–736. DOI: 10.1111/add.12628. [DOI] [PubMed] [Google Scholar]
- Cerda M., Wall M., Keyes K.M., Galea S., Hasin D. (2012) Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug and Alcohol Dependence, 120(1–3), 22–27. DOI: 10.1016/j.drugalcdep.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., O'Brien M.S., Anthony J.C. (2005) Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug and Alcohol Dependence, 79(1), 11–22. DOI: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Del Boca F.K., Darkes J. (2003) The validity of self‐reports of alcohol consumption: state of the science and challenges for research. Addiction, 98(s2), 1–12. [DOI] [PubMed] [Google Scholar]
- Hasin D.S., Saha T.D., Kerridge B.T., Goldstein R.B., Chou S.P., Zhang H., Jung J., Pickering R.P., Ruan W.J., Smith S.M., Huang B., Grant B.F. (2015) Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry, 72(12), 1235–1242. DOI: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa S.G., West B.T., Berglund P.A. (2010) Applied Survey Data Analysis, Boca Raton, FL: CRC Press. [Google Scholar]
- Hines L.A., Morley K.I., Strang J., Agrawal A., Nelson E.C., Statham D., Martin N.G., Lynskey M.T. (2015) The association between speed of transition from initiation to subsequent use of cannabis and later problematic cannabis use, abuse and dependence. Addiction, 110(8), 1311–1320. DOI: 10.1111/add.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi F., Hofler M., Strehle J., Mack S., Gerschler A., Scholl L., Busch M.A., Hapke U., Maske U., Seiffert I., Gaebel W., Maier W., Wagner M., Zielasek J., Wittchen H.U. (2015) Twelve‐months prevalence of mental disorders in the German Health Interview and Examination Survey for Adults – Mental Health Module (DEGS1‐MH): a methodological addendum and correction. International Journal of Methods in Psychiatric Research, 24(4), 305–313. DOI: 10.1002/mpr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Quintero C., Perez de los Cobos J., Hasin D.S., Okuda M., Wang S., Grant B.F., Blanco C. (2011) Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug and Alcohol Dependence, 115(1–2), 120–130. DOI: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Quintero C., Roth K.B., Eaton W.W., Wu L.T., Cottler L.B., Bruce M., Anthony J.C. (2015) Mortality among heroin users and users of other internationally regulated drugs: a 27‐year follow‐up of users in the Epidemiologic Catchment Area Program household samples. Drug and Alcohol Dependence, 156, 104–111. DOI: 10.1016/j.drugalcdep.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. (2004) Analysis of complex survey samples. Journal of Statistical Software, 9(1), 1–19. [Google Scholar]
- O'Brien M.S., Anthony J.C. (2005) Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 30(5), 1006–1018. DOI: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Parker M.A., Anthony J.C. (2014) Should anyone be riding to glory on the now‐descending limb of the crack‐cocaine epidemic curve in the United States? Drug and Alcohol Dependence, 138, 225–228. DOI: 10.1016/j.drugalcdep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsakoff P.M., MacKenzie S.B., Lee J.‐Y., Podsakoff N.P. (2003) Common method biases in behavioral research: a critical review of the literature and recommended remedies. Journal of Applied Psychology, 88(5), 879. [DOI] [PubMed] [Google Scholar]
- Richman W.L., Kiesler S., Weisband S., Drasgow F. (1999) A meta‐analytic study of social desirability distortion in computer‐administered questionnaires, traditional questionnaires, and interviews. Journal of Applied Psychology, 84(5), 754. [Google Scholar]
- Ringeisen H., Aldworth J., Colpe L.J., Pringle B., Simile C. (2015) Estimating the prevalence of any impairing childhood mental disorder in the national health interview survey. International Journal of Methods in Psychiatric Research, 24(4), 266–274. DOI: 10.1002/mpr.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz C., Streibig J.C. (2008) Nonlinear Regression with R, Berlin: Springer Science & Business Media. [Google Scholar]
- Seedall R.B., Anthony J.C. (2013) Risk estimates for starting tobacco, alcohol, and other drug use in the United States: male–female differences and the possibility that ‘limiting time with friends’ is protective. Drug and Alcohol Dependence, 133(2), 751–753. DOI: 10.1016/j.drugalcdep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedall R.B., Anthony J.C. (2015) Monitoring by parents and hypothesized male–female differences in evidence from a nationally representative cohort re‐sampled from age 12 to 17 years: an exploratory study using a "mutoscope" approach. Prevention Science: The Official Journal of the Society for Prevention Research, 16(5), 696–706. DOI: 10.1007/s11121-014-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . (2015) World Drug Report https://www.unodc.org/wdr2015/
- United States . (1972) National Commission on Marihuana and Drug Abuse. The official report of the National Commission on Marihuana and Drug Abuse. http://www.druglibrary.org/schaffer/library/studies/nc/ncmenu.htm
- United States . (2014a) Office of Applied Studies SAMHSA. Methodology Reports and Questionnaires. http://www.samhsa.gov/data/sites/default/files/NSDUH-MethodSummDefs2014/NSDUH-MethodSummDefs2014.htm
- United States . (2014b) Substance Abuse and Mental Health Data Archive. National Survey on Drug Use and Health (NSDUH) Series. http://www.icpsr.umich.edu/icpsrweb/ICPSR/series/64/studies?q=nsduh&searchSource=find-analyze-home&sortBy=&paging.startRow=1
- Vignali C., Stramesi C., Vecchio M., Groppi A. (2012) Hair testing and self‐report of cocaine use. Forensic Science International, 215(1), 77–80. [DOI] [PubMed] [Google Scholar]
- Vsevolozhskaya O.A., Anthony J.C. (2014) Confidence interval estimation in R‐DAS. Drug and Alcohol Dependence, 143, 95–104. DOI: 10.1016/j.drugalcdep.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vsevolozhskaya O.A., Anthony J.C. (2016) Transitioning from first drug use to dependence onset: illustration of a multiparametric approach for comparative epidemiology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(3), 869–876. DOI: 10.1038/npp.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F.A., Anthony J.C. (2002) From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 26(4), 479–488. DOI: 10.1016/s0893-133x(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wagner F.A., Anthony J.C. (2007) Male–female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug and Alcohol Dependence, 86(2–3), 191–198. DOI: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wilcox H.C., Anthony J.C. (2004) Child and adolescent clinical features as forerunners of adult‐onset major depressive disorder: retrospective evidence from an epidemiological sample. Journal of Affective Disorders, 82(1), 9–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


