Abstract
Because of elevations in IOP and other forces, cells in the trabecular meshwork (TM) are constantly subjected to mechanical strain. In order to preserve cellular function and regain homeostasis, cells must sense and adapt to these morphological changes. We and others have already shown that mechanical stress can trigger a broad range of responses in TM cells; however, very little is known about the strategies that TM cells use to respond to this stress, so they can adapt and survive.
Autophagy, a lysosomal degradation pathway, has emerged as an important cellular homeostatic mechanism promoting cell survival and adaptation to a number of cytotoxic stresses. Our laboratory has reported the activation of autophagy in TM cells in response to static biaxial strain and high pressure. Moreover, our newest data also suggest the activation of chaperon-assisted selective autophagy, a recently identified tension-induced autophagy essential for mechanotransduction, in TM cells under cyclic mechanical stress.
In this review manuscript we will discuss autophagy as part of an integrated response triggered in TM cells in response to strain, exerting a dual role in repair and mechanotransduction, and the potential effects of dysregulated in outflow pathway pathophysiology.
1. Introduction
The trabecular meshwork (TM)/Schlemm’s Canal (SC) conventional outflow pathway is a complex tissue primarily responsible for regulating the outflow of aqueous humor (AH) from the anterior chamber of the eye. Aqueous humor is continuously produced by the ciliary body and enters the anterior chamber through the pupil, draining passively out of the eye in a pressure gradient manner via first the TM and then through the SC. The rate of AH drainage must be equal to AH production. Resistance to AH outflow through the TM/SC causes elevated intraocular pressure (IOP), and with that the risk of developing glaucoma, the second leading cause of irreversible permanent blindness worldwide (Stamer and Acott, 2012).
Cells in the TM are constantly subjected to mechanical forces and deformations resulting from changes in IOP and eye movement. It is expected TM cells to possess adaptive mechanisms, which allow them to cope to this stress and prevent further injury. Here, we will review the existing data supporting autophagy, a catabolic mechanism that involves the degradation of unnecessary or dysfunctional cellular components through the action of lysosomes, as part of a homeostatic integrated regulatory response triggered in TM cells in response to strain. In addition, we will include additional new data obtained in our laboratory showing activation of a new described tension-induced autophagy in cyclically stretched TM cells.
2. Mechanical Forces in the Outflow Pathway: Critical Regulators of IOP Homeostasis
Direct recordings of IOP in a human volunteer revealed that IOP is not stable, but it experiences a high level of fluctuations with normal ocular activities (Coleman and Trokel, 1969; Johnstone, 2004). These variations in IOP include transient pressure changes of up to 10 mmHg resultant from blinking and eye movement, as well as continuous small cyclic oscillations (2–3 mmHg) associated with the ocular pulse (Johnstone, 2004). Long lasting high-magnitude changes occur under pathological conditions in ocular hypertensive eyes.
Such elevations in IOP generate an increase in pressure gradient across the TM, which is translated into distension and compaction of the elastic tridimensional meshwork. Studies performed in human enucleated eyes from postmortem donors fixated at different pressures, as well as in eyes from rhesus monkeys subjected to graded levels of physiologic IOP levels in vivo, have documented dramatic changes in the morphology of the outflow pathway under the influence of changing IOP (Grierson and Lee, 1975a; 1975b; Johnstone and Grant, 1973; Stamer and Acott, 2012). Increased IOP results in distention and stretching of the TM and its contained cells, while decreased IOP leads to relaxation of the tissue. According to measurements conducted by Grierson and Lee, changes in pressure from 8 to 30 mm Hg could result in a level of stretching of the outflow pathway cells that could reach as much as 50% (Grierson and Lee, 1975b; 1975a; Stamer and Acott, 2012). Additional sources of strain are those originated by ciliary muscle contraction, with mechanical forces stretching it from Schwalbe’s line to the scleral spur, and inwards towards the SC lumen (Coleman and Trokel, 1969; Johnstone and Grant, 1973).
Schlemm’s canal cells are also exposed to a variety of mechanical forces, specifically shear stress exerted by the flowing of AH within Schlemm’s canal and basal-to-apical pressure gradient, which result in the alignment of the SC parallel to the flow and in large cellular deformations, respectively (Ethier et al., 2004; Johnstone, 2004).
2.1 Mechanosensors in Outflow Pathway Cells
Numerous in vitro studies aimed at investigating the effects of mechanical stress in both perfused organ culture and cell culture have proven that cells in the outflow pathway are capable of sensing and responding to fluctuations in IOP and mechanical strain (cyclic and static). Despite its importance, identification of the mechanosensor/s has not been however a subject of extensive number of reports in the literature. Trabecular meshwork cells have been shown to express several known mechanotransduction channels (Piezo1, Piezo2, TASK1, TREK1, TRPA1, TRPC1, TRPC2, TRPC3, TRPC6, TRPM2, TRPP2) in tissue as well as in cultured conditions (Grierson and Lee, 1975b; 1975a; Johnstone and Grant, 1973; Li et al., 2007; Tran et al., 2014). Which, if any, does indeed function as mechanosensor in TM cells is currently unknown. Cochlin, an ECM protein linked to the pathogenesis of glaucoma, has also been reported to be involved in mechanosensasion of fluid flowing and mechanotransduction, in conjunction with the TREK-1 channel. In this specific case, it was proposed that the mechanotransduction initiated by cochlin/TREK-1 would modulate the expression of several cytosolic proteins, i.e., profilin, which in turn would cause cytoskeletal remodeling concomitant with increased outflow facility and fluid flow, thus resulting in reduction of IOP (Goel et al., 2011). Most recently, in an elegant study using multiple complementary approaches, Luo et al. identified primary cilia as the pressure-sensing organelle in the TM (Luo et al., 2014). Furthermore, proper cilia function was found to be essential for pressure sensation in these cells. Primary cilia in TM cells shorten in response to fluid flow and elevated hydrostatic pressure, and promote increased transcription of TNF-α, TGF-β, and GLI1 genes. These effects were found to require both, OCRL, an inositol polyphosphate 5-phosphatase, and transient receptor potential vanilloid 4 (TRPV4), a ciliary mechanosensory channel, in a manner where OCRL appears to be required for proper localization and function of TRPV4 in the cilia. This in turn is necessary for calcium flux that regulates the transcriptional programs that coordinate cilia function with pressure sensing.
2.2 Mechanical Stress in Cellular and Tissue Homeostasis
Mechanical forces are known to elicit a broad range of responses in TM cells, including alterations to the extracellular matrix, changes in cytoskeleton, induction of gene expression, secretion of cytokines, and activation of regulatory and, as we will discuss later, degradative pathways (Bradley et al., 2001; 2003; Chudgar et al., 2006; Keller et al., 2007; Liton et al., 2005a; 2005c; Luna et al., 2009b; 2009c; Matsuo et al., 1996; Mitton et al., 1997; Okada et al., 1998; Sato et al., 1999; Stamer and Acott, 2012; Tumminia et al., 1998; Vittal et al., 2005; WuDunn, 2001). Cells in the inner wall of the SC have been shown to release nitric oxide with application of shear stress and rearrangement of their F-actin architecture (Ashpole et al., 2014; Ethier et al., 2004). Although the specific or detailed mechanisms are not really yet elucidated, it is logical to think that the ultimate goal of these stretch-induced responses is to maintain cellular and tissue homeostasis.
Experiments conducted in vitro in perfused eyes clearly indicated the existence of some sort of homeostatic mechanism responsible for IOP regulation in the TM/SC outflow pathway tissue, defining IOP homeostasis as a long-term response to mechanical stretch (Acott et al., 2014). Elevation of the perfusion rate was followed by a consequent expected elevation in pressure. However, over the days, pressure was found to return to its baseline level, even though inflow was kept high (Borrás et al., 2002; Bradley et al., 2001). These observations, which have been corroborated by different groups, manifest the presence of a compensatory mechanism that contributes to maintaining IOP homeostasis (Baetz et al., 2009; Booth et al., 1999; Borrás, 2003; Bradley et al., 2001; 2003; Chudgar et al., 2006; Gasull et al., 2003; Keller et al., 2007; 2009; Liton et al., 2005b; 2005d; Luna et al., 2009c; 2011; Matsuo et al., 1996; Mitton et al., 1997; Okada et al., 1998; Ramos et al., 2009; Sato et al., 1999; Tamm et al., 1999; Tumminia et al., 1998; Vittal et al., 2005; WuDunn, 2009; 2001). Sensing the resistance or pressure misbalance in the form of mechanical stretch by the cells in the outflow pathway is thought to be the triggering response.
At the cellular level, strain causes profound changes to cell morphology, affecting a variety of cellular properties such as motility, stiffness, contraction, orientation and cell alignment. Continuous exposure to strain predisposes cells to stretch-induced injury. Therefore, cellular responses elicited by stretching and fluid flow must be also directed to adapt TM cells to such mechanical forces and to repair them from potential stretch-induced damage. A very recent study performed in our laboratory suggests that autophagy could be one relevant cellular adaptive mechanism in response to strain (Porter et al., 2014).
3. Autophagy: A Stress Response Adaptive Pathway
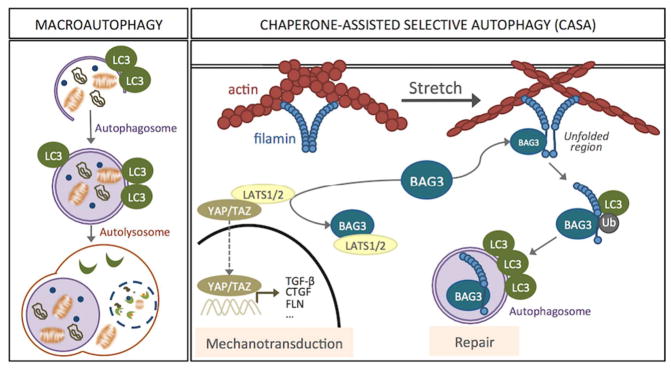
Autophagy is arising as one on the central cellular adaptive response to stress. Autophagy, which means “self-eating”, is a general term that refers to the catabolic process in which cellular components, including organelles, are degraded by the machinery of the lysosomes. Three different types of autophagy have been described in mammalian cells based on the delivery route of the cytoplasmic material to the lysosomal lumen: macroautophagy, microautophagy, and chaperon-mediated autophagy. Among them, macroautophagy, commonly known simply as autophagy is the most extensively studied. This particular type of autophagy is characterized by the formation of a double membrane-bound organelle, the autophagosome, which engulfs the material targeted for degradation. Autophagosomes then fuse with lysosomes to form autolysosomes, in which the cytoplasmic cargos are degraded by resident hydrolases into their minor constituents. Degradation products are then transported back into the cytosol through the activity of membrane permeases, so they can be recycled and used for different purposes, such as new protein synthesis, energy production, and gluconeogenesis (Fig 1). Macroautophagy then consists of four sequential differentiated steps: (1) sequestration and autophagosome formation, (2) fusion of autophagosomes with lysosomes, (3) lysosomal degradation of cargo material, and (4) recycling. All these steps are controlled by a number of evolutionary conserved autophagy related genes (ATG genes) (Mizushima, 2007).
Figure 1.
Descriptive illustration of macroautophagy and CASA.
Although initially believed to be a primary response to starvation, macroautophagy is known to occur at basal levels in most tissues as it contributes to routine housekeeping functions. Autophagy exerts a wide variety of physiological roles, like amino acid pool maintenance, intracellular quality control, development, cell death, tumor suppression and anti-aging (Mariño and López-Otín, 2004; Mizushima, 2009). The primary role of basal autophagy is to contribute to the intracellular quality control and housekeeping by removing misfolded or aggregated proteins, clearing damaged organelles (i.e. mitochondria, endoplasmic reticulum, peroxisomes), as well as eliminating intracellular pathogens. In addition to performing basal functions, autophagy acts as a cellular survival pathway that is quickly activated in response to different types of stress, including ER stress, unfolded protein response, oxidative stress, metabolic stress, and mechanical stress. Activation of autophagy represents an essential mechanism by which organisms can repair stress-induced damage and adapt to the new environmental conditions. Dysfunction in the autophagy pathway has been associated with several human diseases, including glaucoma (Levine and Kroemer, 2008; Porter et al., 2015).
A growing list of intracellular and extracellular stimuli are known to activate autophagy (Kroemer et al., 2010); e.g. nutrient depletion, hypoxia, drug and radiation treatment, reactive oxygen species (ROS) or, as we will discuss later, mechanical strain. Many signaling pathways and second messengers have been shown to regulate the activity of the autophagy machinery. The majority of them converge on mTOR (mammalian target of rapamycin), which is a central regulator of macroautophagy (Sarkar, 2013). Regardless the selectivity of the process, recent evidence support the notion that macroautophagic cargo is not incorporated in the nascent autophagosome in a bulky randomly manner, but that it can occur selectively for substrates. Selective macroautophagy utilizes the same core machinery used for nonselective macroautophagy. Selectivity is achieved by the recognition of the cargo ligand (scaffold) by a specific receptor that recruits it to the phagophore assembling site, where an autophagosome forms. In many cases, the receptor proteins subsequently bind one of the LC3 family proteins. This interaction may connect the cargo directly with the macroautophagy machinery (Johansen and Lamark, 2011). Examples of selective autophagy are degradation of aggregated proteins (aggrephagy), lipid droplets (lipophagy), ribosomes (ribophagy), mitochondria (mitophagy), and chaperon-selective assisted autophagy (CASA), a specific type of autophagy recently described in response to tension.
4. Autophagy and Mechanical Stretch: Dual Role in Repair and Mechanotransduction
4.1 Stretch-Induced Autophagy
The majority of the cells in all organisms are eventually exposed to mechanical stressed caused by physical changes in their microenvironment. Mechanical forces may be experienced in the form of compression, strain, or shear stress. When exposed to these physical forces, cells need to undergo a number of changes, i.e. changes in cytoskeletal stiffness or increase in cortical rigidity, to successfully cope with the stress. The ability to respond and adapt to alterations in the physical environment is therefore a universal and essential property required to maintain cell functionality. This is true even for specialized mechanosensitive cells, like TM cells, whose metabolism is anticipated to be at least partly regulated by mechanical forces. The responses to mechanical stress are complex and still not completely understood.
The induction of autophagy by mechanical stress was first documented in 2011 by King et al. in Dictyostellium (King et al., 2011). In this study, investigators found that mechanical compressive stress rapidly induces autophagosome formation greater than 20-fold. Similar response was corroborated in mammalian cells, thus indicating that such is an evolutionary conserved, general response to mechanical stress. The response to changes in mechanical pressure was graduated, with half-maximal responses at ~0.2 kPa, similar to other mechano-sensitive responses. They further showed that the mechanical induction of autophagy is mTOR-independent and transient, lasting until the cells adapt to their new environment and recover their shape. Activation of autophagy with mechanical compression has been also described in injury models of cartilage explants and in the spinal cord with increase intracranial pressure and in conditions of intervertebral disk degeneration. In all these cases, pharmacological stimulation of autophagy with rapamycin exerted a protective role decreasing both damage and cell death (Caramés et al., 2012; Tanabe et al., 2011; Q. J. Wang et al., 2006).
Induction of autophagy has also been recently reported with mechanical strain (Porter et al., 2014). The signaling pathways and the physiological roles varies depending on the cell type and experimental model. Autophagy was found to protect end plate chondrocytes from intermittent cyclic mechanical tension-induced calcification, playing a key role in bone homeostasis (Xu et al., 2014). In contrast, application of cyclic mechanical stress caused autophagic cell death in tenofibroblast through activation of PGE2 production (Chen et al., 2015). Similarly, excessive activation of autophagy through AT1 receptor-mediated activation of p38MAPK in culture cardiomyocytes and mouse hearts has been linked to cardiac hypertrophy (Lin et al., 2014).
4.1.1 Autophagy, a Physiological Response to Mechanical Stress and High Pressure in Trabecular Meshwork Cells
Recently, our laboratory reported the activation of autophagy in TM cells in response to biaxial static mechanical stretch (20% elongation), an experimental model that mimics acute sustained elevation of IOP, similar to that occurring in the outflow pathway in vivo with ocular hypertension. We observed a quick activation of autophagy as early as 30 minutes after application of mechanical forces. Activation of autophagy was characterized by a dramatic elevation in the levels of LC3-II, which could be prevented by pharmacological blockage of autophagosome formation with 3-MA. Electron microscopy qualitatively confirmed the increased presence of autophagic structures in mechanically-strained cells. Moreover, the number and the size of autolysosomes was also found to be higher indicating that the maturation process was not impaired by potential disruption of the microtubular system with the mechanical forces.
Corroborating earlier observations by Grierson et al. describing increased size and number of lysosomes and lysosomal complexes in the outflow pathway cells of Rhesus monkeys subjected to high IOP (Grierson and Lee, 1975b), activation of autophagy in TM cells was also confirmed in situ in response to high pressure (Porter et al., 2014). Similarly, autophagy was characterized by elevated LC3-II levels and the presence of autophagic figures. While these autophagic figures were preferentially found in the cells of the corneoscleral meshwork in the porcine outflow pathway, they were described in both the JCT and the cells lining the trabeculae in the monkey eye. It is likely that the distinct anatomy of the porcine outflow pathway compared to primates, which might influence the stretch-sensing tissue properties, or differences in the experimental procedure (in vivo versus in vitro) could account for this dissimilarity rather than a cell-specific response.
The signaling pathway triggering stretch-induced autophagy in TM cells has still not been identified. As mentioned earlier, MTOR is the best well-known pathway regulator of autophagy, having an inhibitory function. Activation of MTOR leads to inhibition of autophagy through (i) the phosphorylation of multiple autophagy-related proteins, which promote autophagy initiation and autophagosome nucleation, and (ii) by preventing the nuclear translocation of the transcription factor TFEB, a master regulator of lysosomal and autophagy gene expression (Sarkar, 2013). Mechanical stretch activates the MTOR pathway in TM cells (Bradley et al., 2003); therefore indicating that stretch-induced autophagy must occur in TM cells in an MTOR-independent manner. A similar finding has been reported in breast cancer cells subjected to mechanical compression (King et al., 2011). Together these studies indicate the existence of a still unknown alternative mechanosensitive activator of autophagy that override the inhibitory signals triggered from stretch-induced MTOR activation. Various signaling pathways regulate autophagy independently of MTOR, including cAMP/PLCε, Ca2+/calpain and inositol signalling pathways (Sarkar, 2013). Whether any of these pathways is implicated in stretch-induced autophagy in TM cells is currently under investigation.
4.2 Chaperon-Assisted Selective Autophagy (CASA), a Tension-Induced Autophagy
Chaperon-assisted autophagy (CASA) is a type of selective autophagy recently described in muscle cells in response to tension, essential for muscle maintenance (Ulbricht et al., 2013). This type of autophagy integrates tension sensing, autophagosome formation and transcription regulation. The CASA complex, comprised of the molecular chaperones Hsc7, HspB8 and the cochaperone BAG3 (BCL2-Associated Athanogene 3), senses the mechanical unfolding of the actin-crosslinking protein filamin and initiates the ubiquitin-dependent autophagic sorting of damaged filamin to lysosomes for degradation. Intriguingly, at the same time that BAG3 facilitates the autophagic degradation of mechanically damaged cytoskeleton components, it triggers a transcriptional response to compensate that disposal. This is performed by activation and nuclear translocation of the transcription factors YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif). Very interestingly YAP/TAZ were previously identified as key downstream elements and mediators of mechanical cues (Dupont et al., 2011). BAG3 utilizes its WW domain to bind the YAP/TAZ inhibitors LATS1/2 or AmotL1/2 and promote nuclear translocation of YAP/TAZ and concomitant transcriptional activation of proteins involved in cell adhesion and ECM remodeling, including filamin and BAG3 (Dupont et al., 2011) (Fig 1).
4.2.1 Induction of CASA and Mechanotransduction in TM cells
Our laboratory investigated the possibility that the observed induction of autophagy in TM cells with static biaxial stretch could be mediated by CASA (Porter et al., 2014). First, we checked whether filamin was being degraded through the lysosomal pathway in response to mechanical injury. For this, human TM cells were stretched in the presence of bafilomycin A1, a lysosomal inhibitor, or cycloheximide, a protein biosynthesis inhibitor. None of the treatments caused any differences in the filamin protein levels in stretched cells compared to non-stretched ones, suggesting that CASA was not being activating in our experimental model. To further confirm these results, stretch-induced autophagy was investigated in TM cells with knocked down BAG3 expression levels. Downregulation of BAG3 did not cause any differential expression in LC3-I, nor did it affect the increase in LC3-II with mechanical stress. Altogether, these results indicated that CASA was not being activated in TM cells in response to biaxial stretch.
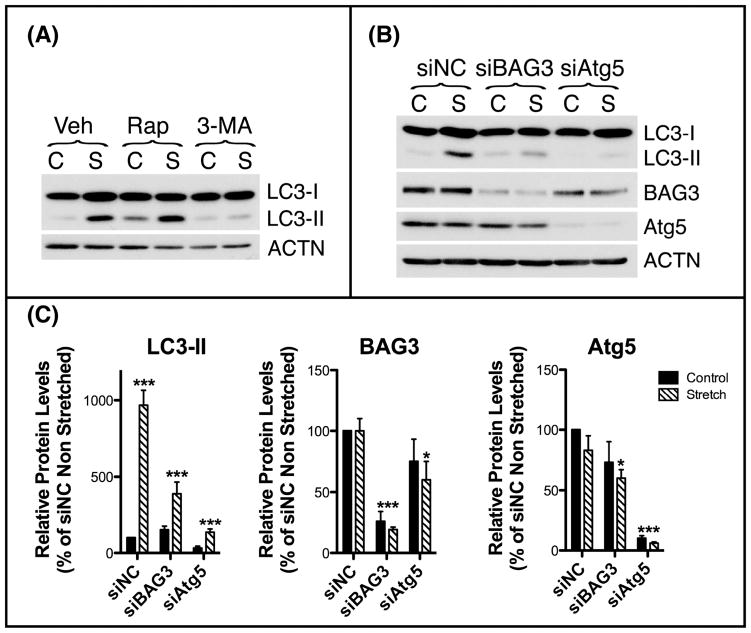
Gene expression profile analysis showed, however, upregulated mRNA levels of BAG3, filamin and several of the genes that are known to be modulated by YAP/TAZ (i.e. TGF-β, and CTGF) in TM cells subjected to cyclic mechanical stretch (20% elongation, 1 cycle/second, (Luna et al., 2009b)). In view of this, we decided to evaluate CASA under cyclic mechanical stretch conditions in primary cultures of human TM cells. As seen in Fig. 2, cyclic mechanical stress (20% elongation, 1 cycle/sec, 16 hours) also resulted in increased levels of LC3-II. The stretch-induced increase in LC3-II was blocked with 3-MA, but did not suffer major changes with rapamycin, an mTOR-dependent activator of autophagy (Fig. 2A). Down-regulation of Atg5, an ubiquitin ligase required for lipidation of LC3-I to LC3-II, via Amaxa nucleoporation using a specific siRNA targeting human Atg5 (siAtg5), completely abolished the stretched-induced activation of autophagy. Intriguingly, in this case, down-regulation of BAG3, also significantly diminished the increase in LC3-II in the stretched cultures (Fig. 2B–C), indicating a potential role of CASA in the induction of autophagy with cyclic mechanical stress.
Figure 2.
Stretch-induced activation of CASA in cyclically stretched human TM cells. (A) Primary cultures of human TM cells at passage 3 were grown on collagen-I coated Flexcell plates to confluency and subjected to cyclic mechanical stretch (20% elongation, 1 cycle/second) for 24 hours in the presence of vehicle, the autophagy activator rapamycin (1uM), or the autophagy inhibitor 3-MA (10mM) added in fresh culture media prior application of mechanical forces. Lipidation of LC3-I to LC3-II were evaluated by WB. (B) Primary cultures of human TM cells were transfected with siRNA against human BAG3 (siBAG3), Atg5 (siAtg5), or control (siNC) and subjected to cyclic mechanical stretch (20% elongation, 1 cycle/second, 24 hours) at 48 hours post-transfection. Protein levels of BAG3, Atg5, and LC3-II were evaluated by WB. Three different cell lines were used for these experiments. (C) Normalized relative protein levels calculated from densitometric analysis of the blots. Data are the means ± SD, n = 3, *p<0.05, ***p < 0.0001. C: Control; S: Stretched.
Still we do not know why this differential response between static and cyclic stress, although it is not surprising. Cyclic regimes of mechanical stress are known to exert different effects from static stretching (J. H.–C. Wang and Thampatty, 2006). Therefore, it should be expected that cyclic mechanical stimulation of TM cells might elicit different responses from those observed after static stretching. One potential explanation is that continuous cycles of contraction/relaxation might more extensively damage cytoskeleton components, but it definitively has to be studied.
Other two related questions that remain to be investigated are how BAG3 controls initiation of autophagy and autophagosome formation to induce autophagic degradation, and whether this regulation is MTOR dependent or independent. A recent report in the literature has shown that BAG3 actually regulates the basal amount of total cellular LC3B by controlling its mRNA translation. This was specific for LC3, since no other ATG proteins were found to be affected in that study (Rodríguez et al., 2016). We have observed, however, decreased Atg5 protein levels in siBAG3-transfected TM cells. There is little information about the regulation of key autophagy classic checkpoints by BAG3. There is only one description showing that autophagy is controlled by BAG3 through a Beclin-independent mechanism suggesting that BAG3 could control the autophagy signaling through a nontraditional mechanism (Liu et al., 2013). Recently, BAG3 has been implicated in the non-canonical activation of autophagy by estrogen receptor (Felzen et al., 2015). This process was independent of MTOR signaling network. If non-canonical activation of autophagy were the case for CASA, we would have expected, however, Atg5 knockdown to have not effect on stretched-induced autophagy. This, together with the fact that siBAG3 does not completely abolish the increase in LC3-II levels with mechanical stress, highlights the complexity of the regulating signaling pathways controlling stretch-induced autophagy. Similarly, based on our data, we cannot discard that non-canonical and canonical autophagy occur simultaneously, resulting from other types of stress or simply basal autophagy.
4.3 Autophagy and Shear Stress
As seen earlier, cells in the inner wall of the SC are subjected to an additional type of stress, shear stress. Although the effect of shear stress on autophagic function in SC cells has still not been investigated, a couple of very recent studies in the literature have reported that shear stress induces activation of autophagy in both ex vivo perfused vascular endothelial cells and vascular vessel segment (Bharath et al., 2014; Guo et al., 2014). Moreover, autophagy was found to regulate vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress, at least in the ex vivo perfused system (Guo et al., 2014). Compared with steady laminar shear stress alone, pretreatment with the autophagy inducer rapamycin further strengthened the effects, while treatment with the autophagy inhibitor 3-MA abolished it. In addition to playing a critical role in maintaining nitric oxide (NO) bioavailability, autophagy has been proposed to also be a key regulator of oxidant-antioxidant balance and inflammatory-anti-inflammatory balance that ultimately regulate endothelial cell responses to shear stress (Bharath et al., 2014). It would be extremely interesting to see whether the reported NO production by shear stress in SC cells is also mediated through activation of autophagy (Ashpole et al., 2014).
4.3 Role of Autophagy in TM Functionality and IOP Homeostasis
The physiological or pathophysiological implications of mechanically-induced autophagy and/or CASA are not known at present. Induction of autophagy with mechanical stress-either strain, compression or shear stress – is an emerging area of research with just a few reports in the literature (King, 2012; King et al., 2011; Lien et al., 2013; Tanabe et al., 2011). Likely, the major roles of stretch-induced autophagy would be adaptation and repair or protection against long-term stretch-induced injury. When subjected to forces or elevated pressure, TM cells must modify and reinforce their cytoskeleton to increase cortical rigidity. Induction of autophagy can help cells to undergo these changes by increasing protein and organelle turnover, which might in turn affect other cellular and metabolic processes and help rebalance cellular and tissue function. Whether autophagy is a primary response to elevated pressure or, in contrast, it is a secondary response to morphological tissue deformations (Battista et al., 2008) is still to be determined, but the fact that activation of autophagy was observed as early as 30 min post-stretch reasonably supports its role as one of the initial responses elicited in TM cells to cope with the stress and regain homeostasis.
Of utmost relevance for outflow pathway physiology might be the activation of CASA. Long-term perfusion experiments have suggested the existence of a compensatory pressure-lowering mechanism in the trabecular outflow pathway in response to more physiologic pressure elevations (Borrás et al., 2002; Bradley et al., 2001). It is very plausible that CASA forms part of this IOP compensatory homeostatic mechanism by facilitating secondary adaptation, such as ECM remodeling or mechanical signaling to stretch (Acott et al., 2014), through the activation and nuclear translocation of the transcriptional regulators YAP/TAZ. As briefly mentioned earlier, YAP/TAZ were recently identified as sensors and mediators of mechanical cues instructed by the cellular microenvironment, nuclear relays of mechanical signals exerted by ECM rigidity and cell shape (Dupont et al., 2011). Interestingly, this regulation was independent of Hippo cascade, but required Rho GTPase activity and tension of the actomyosin cytoskeleton, which are well-known major players in outflow pathway tissue homeostasis. YAP/TAZ regulate the activity of multiple transcription factors. Specifically, YAP has been shown to modulate the expression of greater than 60 genes including genes previously described to be upregulated with mechanical stress in TM cells and/or be significantly elevated in glaucoma like, for example, connective tissue growth factor, transforming growth factor β, transglutaminase-2, and plasminogen-activator inhibitor-1, among others.
Increased NO production by shear stress during SC collapse at elevated intraocular pressures have been proposed to mediate, in part, IOP homeostasis by both, increasing the permeability of the inner wall of the SC and decreasing contractility of the juxtacanalicular tissue (Ashpole et al., 2014). Supporting this hypothesis, overexpression of eNOS in transgenic mice resulted in decreased IOP and increased outflow facility (Stamer et al., 2011). It has been therefore postulated that elements that regulate eNOS activity and expression, such as shear stress, may impact intraocular pressure. In this regard, based on the reports in other vascular endothelial cells, activation of autophagy by shear stress might represent a very important mechanism regulating NO production and IOP homeostasis, in particular at elevated IOPs.
5. Dysregulation of autophagy with oxidative stress and in the glaucomatous TM: Potential Effect in Mechanotransduction and IOP Homeostasis
Autophagy is generally regarded as a prosurvival mechanism, however, if continuously activated it can lead to autophagic cell death (Kroemer and Levine, 2008; Lenardo et al., 2009). Thus, mechanical activation of autophagy under normal physiological forces may help remove damaged components and suppress cell death. In contrast, if mechanical forces exceed the physiological ranges or under pathological conditions, dysregulation of autophagy might trigger cell death and contribute to disease (Tanabe et al., 2011).
Increasing number of evidence in the literature suggests that the autophagic lysosomal function is altered in glaucomatous outflow pathway cells. First indirect evidence come from older studies describing increased hydrolase activities in the TM of glaucomatous eyes (Coupland et al., 1993), as well as ultrastructural changes, such as the presence of membrane-limited vesicles filled with granular material and accumulation of autophagic vacuoles and pigment granules in the cytoplasm of glaucomatous TM cells (Cracknell et al., 2006; Rohen, 1982; Tektas and Lütjen-Drecoll, 2009). More direct experimental evidence linking autophagy to outflow pathway pathophysiology comes from our latest study, in which we evaluated autophagic lysosomal function in TM cells isolated from glaucomatous donors and compared it to that in age-matched donor eyes (Porter et al., 2015). Glaucomatous TM cells displayed a significant decrease in the steady-state levels of LC3-II, reduced lysosomal proteolysis, and chronic activation of the mTOR autophagy inhibitory pathway, indicating that autophagy was constitutively suppressed in these cultures. Not only that, we also found that their ability to induce autophagy in response to, at least, oxidative stress, as we previously reported for non-glaucomatous TM cells (Porter et al., 2013), was similarly compromised. Basification of the lysosomal compartment with subsequent dysfunction of autophagy and reduced autophagic flux was also observed with chronic oxidative stress in TM cells (Porter et al., 2013).
The impact of impaired lysosomal function on mechanotransduction and IOP homeostasis has not been studied so far, nor has been evaluated whether chronic mechanical stress affects lysosomal function itself. The most obvious consequence of failure of the autophagic system would be the accumulation of stretch-induced damage in outflow pathway cells leading to lack of adaptation, and progressive failure of cellular and, ultimately, TM tissue function. Dysfunction of autophagy might also affect, as seen in other vascular cells, NO production by SC cells. Intriguingly, SC cells isolated from glaucomatous eyes were found to be shear stress unresponsive and displayed lower NO levels (Ashpole et al., 2014). Whether this is a consequence of impaired autophagic function is an exciting possibility that needs still to be investigated.
Alterations in CASA have the potential to additionally affect mechanotransduction. In this regard, different studies by Paul Russell’s group showed the activation of YAP/TAZ in TM cells in response to stiffness (Raghunathan et al., 2013), as well as increased stiffness in glaucomatous TM cells (Russell and Johnson, 2012). Activation of CASA in response to tension caused by substrate stiffness has been reported in muscle cells (Arndt et al., 2010). Although there is still no experimental evidence, it is plausible that continuous CASA activation in the stiffened glaucomatous TM results in disrupted mechanotransduction and the upregulation of downstream genes like TGF-β or CTGF, further contributing to the stiffness. Finally, overactivation of autophagy, as discussed earlier, might also cause autophagic cell death and contribute to the loss in cellularity described in the glaucomatous outflow pathway (Alvarado et al., 1981).
Concluding Remarks
In conclusion, although there are still relatively few data in the literature, stretch-induced autophagy is emerging as a key cellular system part of an integrated response triggered in TM cells in response to strain, exerting a dual role in repair and mechanotransduction and contributing to IOP homeostasis. Dysregulation of this response with aging and in disease would very elegantly explain the molecular basis underlying critical questions in outflow pathway pathogenesis such as why the decreased NO production and increased TGF-β, ECM deposition and stiffness in glaucoma. Future studies are needed to investigate the significance of the differential response between static and cyclic mechanical forces and the precise role of macroautophagy and CASA in mechanotransduction and outflow pathway tissue homeostasis. Understanding the exact roles of autophagy and the signaling pathways involved in the autophagic response in TM cells can help to identify novel therapeutic targets for the modulation of the autophagic lysosomal function in ocular hypertension and glaucoma.
Acknowledgments
Special thanks to my mentor David L. Epstein, MD for teaching me “almost” everything I know about the outflow pathway. Also, the authors would like to thank the funding sources: National Institute of Health Grants R01EY020491 (Liton) and P30EY005722, and the Glaucoma Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular Pressure Homeostasis: Maintaining Balance in a High-Pressure Environment. J Ocul Pharmacol Ther. 2014 doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727. [PubMed] [Google Scholar]
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Höhfeld J. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Ashpole NE, Overby DR, Ethier CR, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm’s canal cells. Invest Ophthalmol Vis Sci. 2014;55:8067–8076. doi: 10.1167/iovs.14-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Hoffman EA, Yool AJ, Stamer WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89:95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol. 2014;92:605–612. doi: 10.1139/cjpp-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A, Nguyen T, Polansky J. TIGR and stretch in the trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:1888–1889. [PubMed] [Google Scholar]
- Borrás T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003;22:435–463. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Borrás T, Rowlette LLS, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Bradley JMB, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Caramés B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chen L, Cheng B, Jiang C. Cyclic mechanical stretching induces autophagic cell death in tenofibroblasts through activation of prostaglandin E2 production. Cell Physiol Biochem. 2015;36:24–33. doi: 10.1159/000374050. [DOI] [PubMed] [Google Scholar]
- Chudgar SM, Deng P, Maddala R, Epstein DL, Rao PV. Regulation of connective tissue growth factor expression in the aqueous humor outflow pathway. Mol Vis. 2006;12:1117–1126. [PubMed] [Google Scholar]
- Cochlin induced TREK-1 co-expression and annexin A2 secretion: role in trabecular meshwork cell elongation and motility. Cochlin induced TREK-1 co-expression and annexin A2 secretion: role in trabecular meshwork cell elongation and motility. 2011;6:e23070. doi: 10.1371/journal.pone.0023070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Heimann H, Hoffmann F, Penfold PL, Billson FA. Increased hydrolase activities in the human trabecular meshwork of glaucomatous eyes. Ger J Ophthalmol. 1993;2:107–112. [PubMed] [Google Scholar]
- Cracknell KPB, Grierson I, Hogg P, Majekodunmi AA, Watson P, Marmion V. Melanin in the trabecular meshwork is associated with age, POAG but not Latanoprost treatment. A masked morphometric study. Exp Eye Res. 2006;82:986–993. doi: 10.1016/j.exer.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- eNOS, a pressure-dependent regulator of intraocular pressure. eNOS, a pressure-dependent regulator of intraocular pressure. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier CR, Read AT, Chan D. Biomechanics of Schlemm’s canal endothelial cells: influence on F-actin architecture. Biophys J. 2004;87:2828–2837. doi: 10.1529/biophysj.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzen V, Hiebel C, Koziollek-Drechsler I, Reißig S, Wolfrum U, Kögel D, Brandts C, Behl C, Morawe T. Estrogen receptor α regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015;6:e1812. doi: 10.1038/cddis.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull X, Ferrer E, Llobet A, Castellano A, Nicolás JM, Palés J, Gual A. Cell membrane stretch modulates the high-conductance Ca2+-activated K+ channel in bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:706–714. doi: 10.1167/iovs.02-0384. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp Eye Res. 1975a;20:505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (2) Pressures outside the physiological range (0 and 50 mmHg) Exp Eye Res. 1975b;20:523–530. doi: 10.1016/0014-4835(75)90219-5. [DOI] [PubMed] [Google Scholar]
- Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng. 2014;42:1978–1988. doi: 10.1007/s10439-014-1033-5. [DOI] [PubMed] [Google Scholar]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Acott TS. Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:5769–5777. doi: 10.1167/iovs.09-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2007;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- King JS. Mechanical stress meets autophagy: potential implications for physiology and pathology. Trends in molecular medicine. 2012;18:583–588. doi: 10.1016/j.molmed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- King JS, Veltman DM, Insall RH. The induction of autophagy by mechanical stress. Autophagy. 2011;7:1490–1499. doi: 10.4161/auto.7.12.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo MJ, McPhee CK, Yu L. Methods in Enzymology, Methods in Enzymology. Elsevier; 2009. Chapter 2 Autophagic Cell Death; pp. 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wolfgang L, Pratap C, David E, Pedro G, Paloma L. Expression of Transient Receptor Potential (TRP) Channels in the Outflow Pathway and Ciliary Body of Porcine Eyes. Presented at the ARVO Meeting Abstracts; 2007. p. 2066. [Google Scholar]
- Lien SC, Chang SF, Lee PL, Wei SY, Chang MDT, Chang JY, Chiu JJ. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta. 2013;1833:3124–3133. doi: 10.1016/j.bbamcr.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lin L, Tang C, Xu J, Ye Y, Weng L, Wei W, Ge J, Liu X, Zou Y. Mechanical stress triggers cardiomyocyte autophagy through angiotensin II type 1 receptor-mediated p38MAP kinase independently of angiotensin II. PLoS ONE. 2014;9:e89629. doi: 10.1371/journal.pone.0089629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Liu X, Challa P, Epstein DL, Gonzalez P. Induction of TGF-beta1 in the trabecular meshwork under cyclic mechanical stress. J Cell Physiol. 2005a;205:364–371. doi: 10.1002/jcp.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Liu X, Challa P, Epstein DL, Gonzalez P. Induction of TGF-beta1 in the trabecular meshwork under cyclic mechanical stress. J Cell Physiol. 2005b;205:364–371. doi: 10.1002/jcp.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005c;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005d;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BQ, Du ZX, Zong ZH, Li C, Li N, Zhang Q, Kong DH, Wang HQ. BAG3-dependent noncanonical autophagy induced by proteasome inhibition in HepG2 cells. Autophagy. 2013;9:905–916. doi: 10.4161/auto.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Liton PB, Epstein DL, Gonzalez P. Alterations in gene expression induced by cyclic mechanical stress in trabecular meshwork cells. Mol Vis. 2009a;15:534–544. [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Liton PB, Epstein DL, Gonzalez P. Alterations in gene expression induced by cyclic mechanical stress in trabecular meshwork cells. Mol Vis. 2009b;15:534–544. [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009c;50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, Sun Y. Primary cilia signaling mediates intraocular pressure sensation. Proceedings of the National Academy of Sciences. 2014;111:12871–12876. doi: 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G, López-Otín C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci. 2004;61:1439–1454. doi: 10.1007/s00018-004-4012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Uchida H, Matsuo N. Bovine and porcine trabecular cells produce prostaglandin F2 alpha in response to cyclic mechanical stretching. Jpn J Ophthalmol. 1996;40:289–296. [PubMed] [Google Scholar]
- Mitton KP, Tumminia SJ, Arora J, Zelenka P, Epstein DL, Russell P. Transient loss of alphaB-crystallin: an early cellular response to mechanical stretch. Biochem Biophys Res Commun. 1997;235:69–73. doi: 10.1006/bbrc.1997.6737. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Okada Y, Matsuo T, Ohtsuki H. Bovine trabecular cells produce TIMP-1 and MMP-2 in response to mechanical stretching. Jpn J Ophthalmol. 1998;42:90–94. doi: 10.1016/s0021-5155(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Porter K, Hirt J, Stamer WD, Liton PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim Biophys Acta. 2015;1852:379–385. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KM, Jeyabalan N, Liton PB. MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochim Biophys Acta. 2014;1843:1054–1062. doi: 10.1016/j.bbamcr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Dreier B, Reilly CM, Thomasy SM, Wood JA, Ly I, Tuyen BC, Hughbanks M, Murphy CJ, Russell P. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest Ophthalmol Vis Sci. 2013;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RF, Sumida GM, Stamer WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest Ophthalmol Vis Sci. 2009;50:3826–3832. doi: 10.1167/iovs.08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AE, López-Crisosto C, Peña-Oyarzún D, Salas D, Parra V, Quiroga C, Morawe T, Chiong M, Behl C, Lavandero S. BAG3 regulates total MAP1LC3B protein levels through a translational but not transcriptional mechanism. Autophagy. 2016;12:287–296. doi: 10.1080/15548627.2015.1124225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohen JW. Presence of matrix vesicles in the trabecular meshwork of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 1982;218:171–176. doi: 10.1007/BF02150090. [DOI] [PubMed] [Google Scholar]
- Russell P, Johnson M. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012;53:117. doi: 10.1167/iovs.11-9314. [DOI] [PubMed] [Google Scholar]
- Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- Sato Y, Matsuo T, Ohtsuki H. A novel gene (oculomedin) induced by mechanical stretching in human trabecular cells of the eye. Biochem Biophys Res Commun. 1999;259:349–351. doi: 10.1006/bbrc.1999.0797. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tanabe F, Yone K, Kawabata N, Sakakima H, Matsuda F, Ishidou Y, Maeda S, Abematsu M, Komiya S, Setoguchi T. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy. 2011;7:1462–1471. doi: 10.4161/auto.7.12.17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Tran VT, Ho PT, Cabrera L, Torres JE, Bhattacharya SK. Mechanotransduction channels of the trabecular meshwork. Curr Eye Res. 2014;39:291–303. doi: 10.3109/02713683.2013.842593. [DOI] [PubMed] [Google Scholar]
- Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- Ulbricht A, Eppler FJ, Tapia VE, van der Ven PFM, Hampe N, Hersch N, Vakeel P, Stadel D, Haas A, Saftig P, Behrends C, Fürst DO, Volkmer R, Hoffmann B, Kolanus W, Höhfeld J. Cellular Mechanotransduction Relies on Tension-Induced and Chaperone-Assisted Autophagy. Curr Biol. 2013 doi: 10.1016/j.cub.2013.01.064. [DOI] [PubMed] [Google Scholar]
- Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Kohtz S, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D. Mechanobiology of trabecular meshwork cells. Exp Eye Res. 2009;88:718–723. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- WuDunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr Eye Res. 2001;22:394–397. doi: 10.1076/ceyr.22.5.394.5500. [DOI] [PubMed] [Google Scholar]
- Xu HG, Yu YF, Zheng Q, Zhang W, Wang CD, Zhao XY, Tong WX, Wang H, Liu P, Zhang XL. Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone. 2014;66:232–239. doi: 10.1016/j.bone.2014.06.018. [DOI] [PubMed] [Google Scholar]