Abstract
Background
Calcific aortic valve disease (CAVD) is the most common valvular heart disease and likely evolves from inflammatory pre-conditions in the valve. Type II diabetes mellitus (DMII) has been associated with pathogenesis of CAVD, however, the mechanism initiating CAVD in DMII is not well understood and the human valve pathology in DMII has not been described. We therefore performed quantitative histological analyses of aortic valves of CAVD patients with and without DMII.
Methods
CAVD human aortic valves (n=45) obtained after surgical valve replacement were examined macroscopically with gross measurements of calcified areas. Inflammation and calcification were assessed by immunohistochemistry and immunofluorescence staining.
Results
Calcification was increased in diabetic patients according to gross measurements (p<0.01) and alizarin red staining (p=0.05). Early calcification markers, including Runx2 (p=0.02) and alkaline phosphatase (ALP, p=0.03) were significantly elevated in diabetic patients. Furthermore, in diabetic patients we found significantly increased expression of annexin II (p=0.04) and annexin V (p=0.04), both of which are thought to play a role in microcalcification formation via apoptosis or extracellular vesicle release. Macrophage numbers were comparable in both groups (p=0.41), while the expression of the pro-inflammatory protein S100A9 (p<0.01) was significantly decreased in diabetic individuals. Evaluation of lymphocytes revealed similar CD8 (p=0.45) and CD4 (p=0.92) T cell counts in diabetic and non-diabetic aortic valves.
Conclusion
Aortic valves from diabetic patients show more calcification, while inflammation is similar in both patient populations. Considering the generally accepted theory of an inflammation-dependent mechanism of calcification, these data suggest that in patients with CAVD requiring valve replacement, diabetic patients could be molecularly in a more advanced disease stage with a higher grade of mineralization than non-diabetic patients.
Keywords: calcific aortic valve disease (CAVD), type II diabetes mellitus, calcification, inflammation
1. Introduction
Calcific aortic valve disease (CAVD) is the most common valvular heart disease and is a significant cause of morbidity and mortality (Otto, 2006; Bertazzo et al., 2013). It is viewed as a progressive disease that is initiated by alterations in valvular cell biology that progresses to leaflet thickening, neovascularization and calcium deposition to calcific aortic stenosis (Aikawa, Nahrendorf, Sosnovik, et al., 2007; Otto, 2008; Rajamannan, 2009). Surgical or interventional valve replacement is the only effective treatment of symptomatic aortic valve stenosis, as pharmacological therapies have not proven to be sufficient (Cowell et al., 2005; Rossebo et al., 2008; Chan et al., 2010; Green et al., 2014; Martínez-Martínez, 2014).
Calcification evolving from inflammatory conditions predominantly results from active osteogenesis within aortic valve leaflets (Mohler et al., 2001). Previous observations suggest that this is mediated through osteoblast-like differentiation of valvular interstitial cells (Yetkin and Waltenberger, 2009). Three stages of CAVD have been proposed (Aikawa and Otto, 2012): During the inflammatory initiation phase, pro-osteogenic factors, which are released by activated macrophages, induce the transformation of valvular interstitial myofibroblasts into osteoblast-like cells. Apart from macrophages, immune cells, including CD4 and CD8 T cells, and the pro-inflammatory protein S100A9, which is predominantly expressed by activated macrophages, indicate these inflammatory processes. In the propagation phase, an early stage of calcification, the pro-inflammatory conditions promote the formation of microcalcification consisting of hydroxyapatite accumulation on a bone-like matrix of collagen and bone-matrix proteins (Mohler et al., 1997; Mohler et al., 2001), leading to calcium deposition and leaflet stiffening. Regulation of valvular osteogenesis occurs via activation of specific transcription factors including Runx2 (Rajamannan et al., 2011), which is necessary for osteoblast and osteocyte differentiation and maturation (Wirrig and Yutzey, 2013). Runx2 and its downstream target alkaline phosphatase (ALP), which also serves as an early marker of osteoblast differentiation, have been increased in high fat diet rabbit and mouse models of CAVD (Rajamannan et al., 2002; Aikawa, Nahrendorf, Figueiredo, et al., 2007; Miller et al., 2009). Since annexins are implicated in various processes, including microcalcification, apoptosis and extracellular vesicle release (Shanahan et al., 2011; New et al., 2013), their expression in aortic valve leaflets was investigated. Annexins are calcium-regulated membrane-binding proteins (Gerke et al., 2005), described to play a role in matrix mineralization in hypertrophic bone and cartilage - possibly via modulating Ca2+ entry into extracellular matrix vesicles during hydroxyapatite formation. In contrast to irreversible late-stage calcification, earlier stages might be targeted by medical therapies aiming at the attenuation of osteogenic and pro-inflammatory changes whereas the advanced late stage is characterized by reduced inflammation and excessive calcification progressing to the point of no return (Aikawa and Otto, 2012).
Inflammation plays a central role in the pathogenesis of adult glucose disorders, notably type II diabetes mellitus (DMII) (Barzilay et al., 2001; Sjoholm and Nystrom, 2006). In addition to the observation that chronic inflammation and activation of the immune system are involved in the pathogenesis of DMII (Esser et al., 2014), numerous studies have identified diabetes as an important risk factor for CAVD (Gleissner et al., 2007; Wilhelmsen et al., 2008; Emerging Risk Factors et al., 2010; Yeh et al., 2010; Gleissner, 2015). However, relatively little is known about the effect of diabetes on progression of CAVD. In fact, the disease progression in DMII patients may be accelerated and involve different pro-calcific pathways. Therefore, the current study was designed to evaluate the inflammation and calcification processes in the aortic valves in CAVD patients with or without diabetes.
It has been previously concluded that DMII contributes to CAVD pathogenesis by promoting early mineralization of the aortic valve (Le Quang et al., 2014). However, this conclusion was derived from observations in a mouse model and inflammation was not assessed. Recently published data point towards significant differences regarding inflammatory and calcification mechanisms in mouse and human (Thubrikar et al., 1986; Ingersoll et al., 2010; Miller et al., 2011; Cheek et al., 2012; Seok et al., 2013).
2. Methods
2.1. Tissue Collection
Human aortic valves were obtained with patient consent (Institutional Review Board protocol 2010P002567/BWH) from CAVD patients aged 51–88 years, undergoing aortic valve replacement surgery (17 diabetic patients, 28 non-diabetic patients) at Brigham and Women’s Hospital, Boston, USA (Table 1). Patients with renal failure (GFR<30 ml/min/1.73m2) were excluded. Patient medical history including past surgical history, laboratory results and pharmacological therapies were collected.
Table 1.
Clinical Baseline Characteristics. Continuous variables are recorded as mean +/− SD. Categorical variables are recorded as number and percentage
| All patients (n = 45) |
Non-diabetic pa- tients (n = 28) |
Diabetic patients (n = 17) |
p value | |
|---|---|---|---|---|
| Age (years) | 68 ± 11 | 67 ± 11 | 71 ± 10 | NS |
| Gender (male) | 27(60.0%) | 15 (55.6%) | 12 (70.6%) | NS |
| BMI (kg/m2) | 28.6 ± 5.8c | 26.9 ± 4.6 c | 31.4 ± 6.7 c | 0.020* |
| Smoker a, n (%) | 18 (46.5%) | 13 (46.4%) | 6 (46.7%) | NS |
| Bicuspid valves, n (%) | 14 (31.11%) | 11 (39.29%) | 3 (17.65%) | 0.002* |
| AVAa (cm2) | 0.72 ± 0.18 c | 0.68 ± 0.03 c | 0.80 ± 0.04 c | 0.032* |
| LVEFa (%) | 61 ± 9 | 61 ± 10 | 60 ± 9 | NS |
| MPGa (mmHg) | 48 ± 14c | 51 ± 14 c | 43 ± 11 c | 0.037* |
| Diastolic BPa (mmHg) | 76 ± 12 | 76 ± 13 | 75 ± 9 | NS |
| Systolic BPa (mmHg) | 135 ±17 | 136 ± 16 | 133 ± 19 | NS |
| Total cholesterol (mg/dl)a |
174 ± 62 | 159 ± 41 | 194 ± 84 | NS |
| HbA1c (%)a | 6.2 ± 1.3 | 5.5 ± 0.4 | 7.1 ± 1.5 c | < 0.0001* |
| Glucose (mg/dl)a | 110 ± 40 | 96 ± 13 | 132 ± 56 c | 0.007* |
| Hemoglobin (g/dl)a | 13.9 ± 1.6 | 14.2 ± 1.3 | 13.4 ± 2.0 | NS |
| GFR (ml/min/1.73m2) | 71.0 ± 14.2c | 73.7 ± 15.0 c | 66.6 ± 12.2 c | NS |
| Antihypertensive me- dicationa |
40 (93.0%) | 26 (92.9%) | 14 (93.3%) | NS |
| Statin n (in %) |
35 (77.8%) | 23 (82.2%) | 12 (70.6%) | NS |
| Insulin n (in %) |
- | - | 5 (29.4%) | - |
Data are shown as n (in %) or mean ±SD.
statistically significant (p < 0.05).
data not for all patients available.
calculated with CKD-EPI formula.
deviant values.
2.2. Measurements of gross calcification
Standardized overview pictures of the aortic valves were obtained by adhering to the same recording conditions: valve fragment on a white background, exposure time smaller than hundredth of a second (standard adjustment of front camera of Samsung Galaxy S4 mini), same angle (90°) and same distance (approx. 30 cm/12 inch) to leaflet. The percentage of positive area of calcified nodules was calculated with the software ImagePro Plus (6.0 Media Cybernetics, Inc., Rockville, MD, USA). All aortic valves had portions of the attachment of the aortic root and the tip of the valve, which enabled us to calculate uncalcified distance measurements for conclusions about disease progression.
2.3. Histology and immunohistochemistry
Tissue samples were frozen in OCT compound and 6-µm sections were prepared using a Cryostat CM3050 S (Leica Microsystems, Buffalo Grove, IL, USA). All samples were stained with hematoxylin and eosin (Sigma-Aldrich, St. Louis, MO, USA) for general morphology. Tissue sections were fixed in 37% buffered formaldehyde (American MasterTech Scientific, Lodi, CA, USA). For the staining process, Bluing Reagent (VWR International, Radnor, PA, USA), buffered with Tris-buffered saline, was used to ensure a constant pH during staining. Tissue sections were dehydrated in 70%, 95% and 100% ethanol (Fisher Scientific, Waltham, MA, USA) and cleared with xylene (Fisher Scientific, Waltham, MA, USA). Slides were mounted with a permanent mounting medium (Vector Laboratories, Burlingame, CA, USA) and coverslipped.
Alizarin red staining (American MasterTech Scientific, Lodi, CA, USA) was performed for all samples for detection of calcium deposits. Tissue sections were fixed and dehydrated with −20°C cold acetone (Fisher Scientific, Waltham, MA, USA) and cleared with an acetone/xylene solution (50% acetone and 50% SafeClear II Xylene substitute, both purchased from Fisher Scientific, Waltham, MA, USA). Slides were mounted with a permanent mounting medium (Vector Laboratories, Burlingame, CA, USA) and coverslipped.
For immunohistochemistry, tissue sections were fixed in −20°C acetone (Fisher Scientific, Waltham, MA, USA) and blocked with 0.3% hydrogenperoxidase (Fisher Scientific, Waltham, MA, USA) and Protein Block Serum-Free (DAKO, Carpinteria, CA, USA). For washing and dilution steps phosphate-buffered saline (PBS, Boston BioProducts, Ashland, MA) was used.
All primary antibodies were diluted in 5% normal horse serum (Vector Laboratories, Burlingame, CA, USA), which was prepared with PBS (see Table 2). All primary antibodies were incubated for 90 minutes at room temperature. Secondary antibodies were a ready-to-use biotinylated goat anti-mouse and anti-rabbit link (Dako, LSAB Kit) and biotinylated rabbit anti-goat IgG (Vector Laboratories Burlingame, CA, USA) used at 1:100 dilution (diluted in 5% normal horse serum; see Table 3). All secondary antibodies were incubated for 45 minutes at room temperature. The conventional streptavidin peroxidase method (Dako, LSAB Kit) was performed for each antibody and the reaction was visualized with a 3-amino-9-ethylcarbazol substrate (AEC Substrate Chromogen, Dako). Tissues were counterstained with Gill’s No. 3 Hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), mounted with glycerol gelatin (Sigma-Aldrich, St. Louis, MO, USA) and coverslipped. For negative control samples, primary antibodies were replaced by PBS.
Table 2.
Primary Antibodies for IHC Staining
| Antibody | Source | Clonality | Dilution | Developing time |
Company | Catolog number |
|---|---|---|---|---|---|---|
| CD 4 | mouse | monoclonal | 1:25 | 15 min. | Dako, Clone 4B12 |
M7310 |
| CD8 | mouse | monoclonal | 1:50 | 10 min. | Dako, Clone C8/144B |
M7103 |
| CD68 | mouse | monoclonal | 1:700 | 15 – 20 min. | Dako, Clone KP1 |
M0714 |
| annexin II | mouse | monoclonal | 1:200 | 6 – 12 min. | Invitrogen, Clone ZO14 |
03-4400 |
| annexin V | goat | polyclonal | 1:75 | 10 – 12 min. | Santa Cruz, R- 20 |
Sc-1929 |
| S100A9 | rabbit | polyclonal | 1:35 | 20 min. | Proteintech | 14226-1- AP |
| Runx2 | mouse | monoclonal | 1:25 | 9 min. | Abcam | Ab115899 |
| ALP | rabbit | monoclonal | 1:100 | 12 min. | Abcam | Ab108337 |
Table 3.
Secondary Antibodies for IHC Staining
| Antibody | Source | Directed against | Dilution | Company | Catalog number |
|---|---|---|---|---|---|
| biotinylated link | goat | anti-mouse, anti- rabbit |
- | Dako, LSAB Kit | K0675 |
| biotinylated IgG | rabbit | anti-goat | 1:100 | Vector Labora- tories |
BA-5000 |
To confirm coexpression annexin V with CD68, double immunofluorescence staining was performed. Tissue sections were fixed in acetone (-20°C) and blocked with 5% normal serum of the species of the secondary antibody diluted in PBS with 1% albumin from bovine serum.
Goat anti-annexin V was first primary antibody (1:100; R-20, Santa Cruz, Dallas, TX, USA) and coupled with rabbit Alexa Red 594 (1:500; Life Technologies, Grand Island, NY, USA) as second primary antibody. Mouse second primary antibody against CD68 (Dako, Clone KP1, Carpinteria, CA, USA) was coupled with goat Alexa Green 488 (1:300; Life Technologies, Grand Island, NY, USA). Primary antibodies were incubated overnight (14 hours), secondary antibodies were incubated for 40 minutes. Nuclei were counterstained with DAPI (4′,6-Diamidin-2-phenylindol; Nuc Blue Fixed Cell Stain from Life Technologies, Grand Island, NY, USA). Slides were mounted with fluorescent mounting medium (Dako, Carpinteria, CA, USA) and coverslipped.
2.4. Quantitative analysis
For measurements of gross valve pictures, the area of calcification nodules as well as the distance from nodule to tip of the valve were measured using the software ImagePro Plus (6.0 Media Cybernetics, Rockville, MD, USA). To measure the calcified areas after alizarin red staining, whole tissue images were taken with an Omnyx™ VL4 slide scanner (GE Healthcare, Pittsburgh, PA, USA). The percentage of positive staining was calculated using the software ImagePro Plus (6.0 Media Cybernetics, Rockville, MD, USA).
For quantification of immunohistochemistry staining, five high power field images per tissue were captured at 400× magnification (Eclipse 50i, Nikon Instruments, Melville, NY, USA) with a cooled CCD camera (DS-Fi1c, Nikon Instruments, Melville, NY, USA). Positive stained cells in the fibrosa layer of the valve and in the area around calcified nodules were quantified. Adjacent positive staining in the spongiosa layer was also included while the ventricularis layer was always excluded.
The RGB images were transformed into binary images using color deconvolution software as described previously (Ruifrok and Johnston, 2001; Taylor and Levenson, 2006). These images were then analyzed using quantification features of the imaging software NIS-Elements AR (Advanced Research) 3.1 (Nikon Instruments, Melville, NY, USA). For each antibody, a threshold was determined in order to quantify positive stained area.
2.5. Statistic Analysis
Differences between non-diabetic and diabetic patients were evaluated by a two-tailed unpaired Mann Whitney test. P<0.05 was considered significant. Unless otherwise noted, all values are expressed as means ± standard error of the mean (mean ± SEM). Statistical analyzes were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Patient cohort
Our study included 17 diabetic and 28 non-diabetic CAVD patients. As CAVD is an age-dependent disease, similarly aged groups are necessary to minimize the influence of age on the assessed parameters. Diabetic patients showed characteristic metabolic features, such as higher BMI, glucose and HbA1c values. Clinically, non-diabetic and diabetic patients had similar AS severity, as expressed by measurements of aortic mean pressure gradient, ejection fraction and blood pressure. Aortic valve area was slightly larger in diabetic compared to non-diabetic patients at the time of valve replacement (Table 1).
3.2 Aortic valve leaflet macrocalcification is increased in diabetic patients
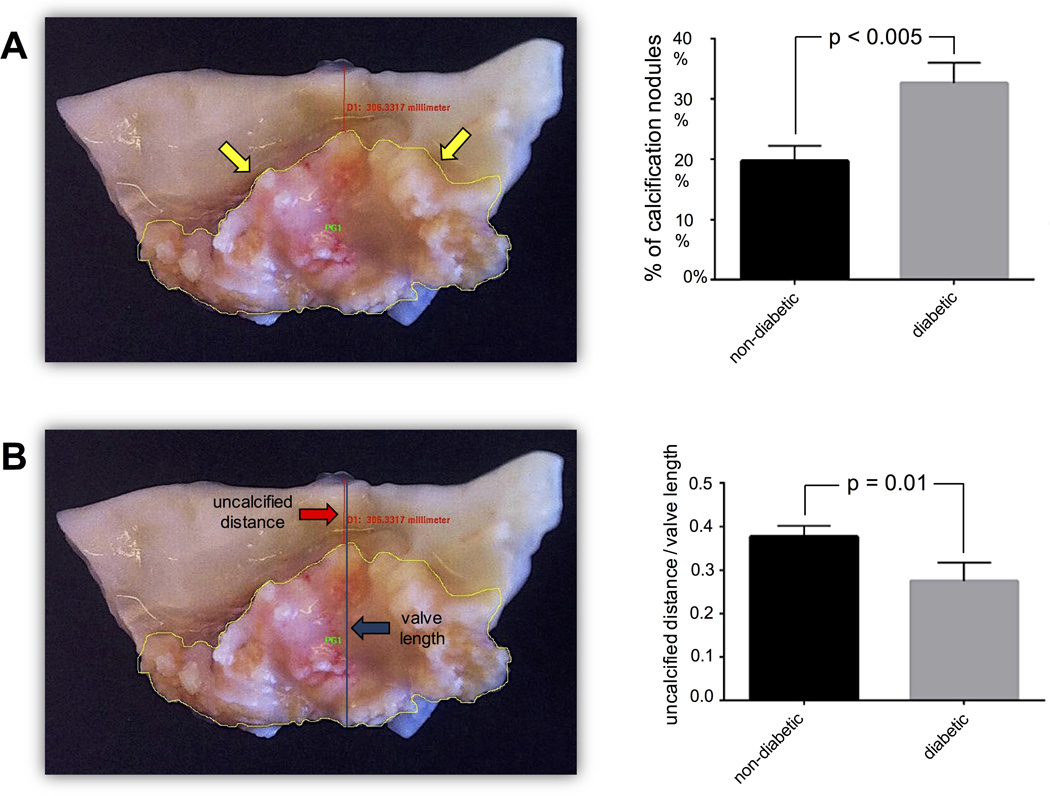
Gross overview images of the aortic valve leaflets removed from CAVD patients were used to identify macroscopic valve calcification. The percentage of positive area of calcified nodules within valve tissue was significantly greater in diabetic compared to non-diabetic individuals (Fig. 1A). Consistently, the distance between the calcification nodules and the tip of the leaflet was significantly smaller in diabetic patients suggesting a more advanced stage of calcification in diabetic patients (Fig. 1B). However, potential contraction of calcified leaflets may have influenced the measurements of the distance to the tip of the leaflet.
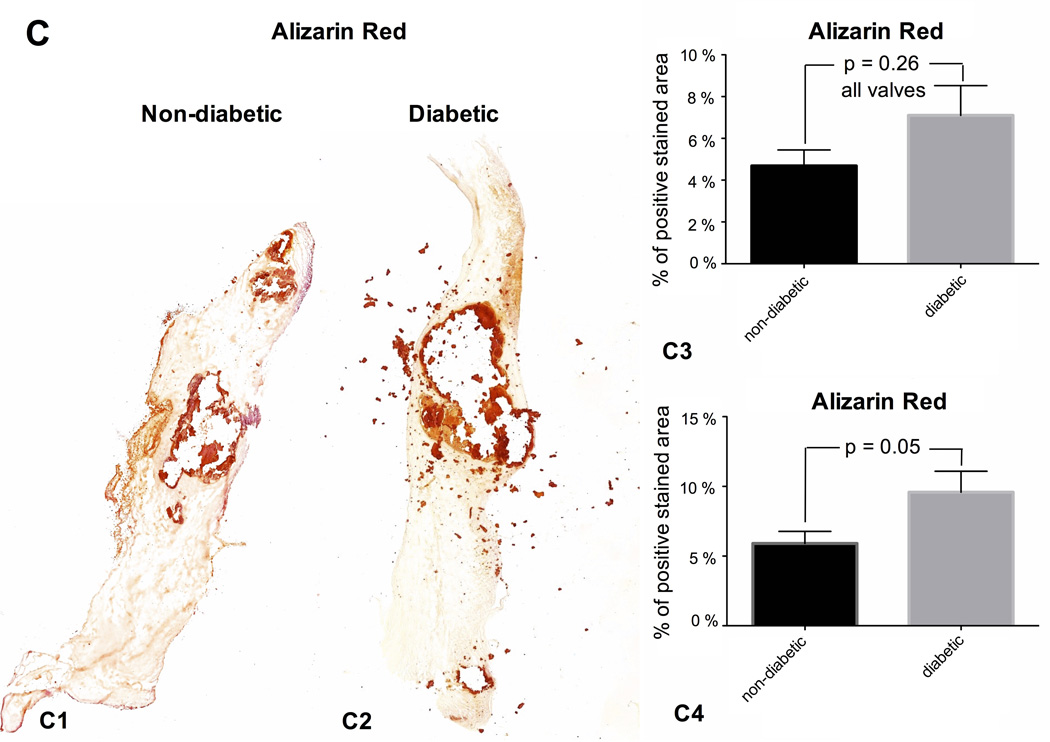
Fig. 1. MACROCALCIFICATION.
Yellow arrows – border of calcification area; red arrow – distance from calcification nodule to tip of the valve; blue arrow – total valve length.
The area of macroscopic calcification nodules is larger in diabetic than in non-diabetic valve tissue (n = 64) (1A). The ratio of the distance from calcification nodule to the tip of the valve relative to the total length of the valve is shorter in diabetic compared to non-diabetic valve tissue (n = 69) (1B). Alizarin red staining reveals larger calcification nodules in diabetic than non-diabetic valve tissue. Whereas the comparison of all valve tissues (1C3; n = 45 (17 – 28)) did not reveal a major distinction between the two patient groups, there was a significant difference when comparing valve tissue with macroscopically detectable calcification (1C4; n = 34 (12 – 22)). Representative valve tissue sections of a non-diabetic (1C1) and a diabetic patient (1C2) (×40 magnification).
Histological staining for calcium deposition using alizarin red in resected aortic valve leaflets of diabetic patients was stronger than in non-diabetic patients (Fig. 1C3). Those samples with macroscopic visible calcification showed an even greater difference between diabetic and non-diabetic patients (p=0.05; Fig. 1C4).
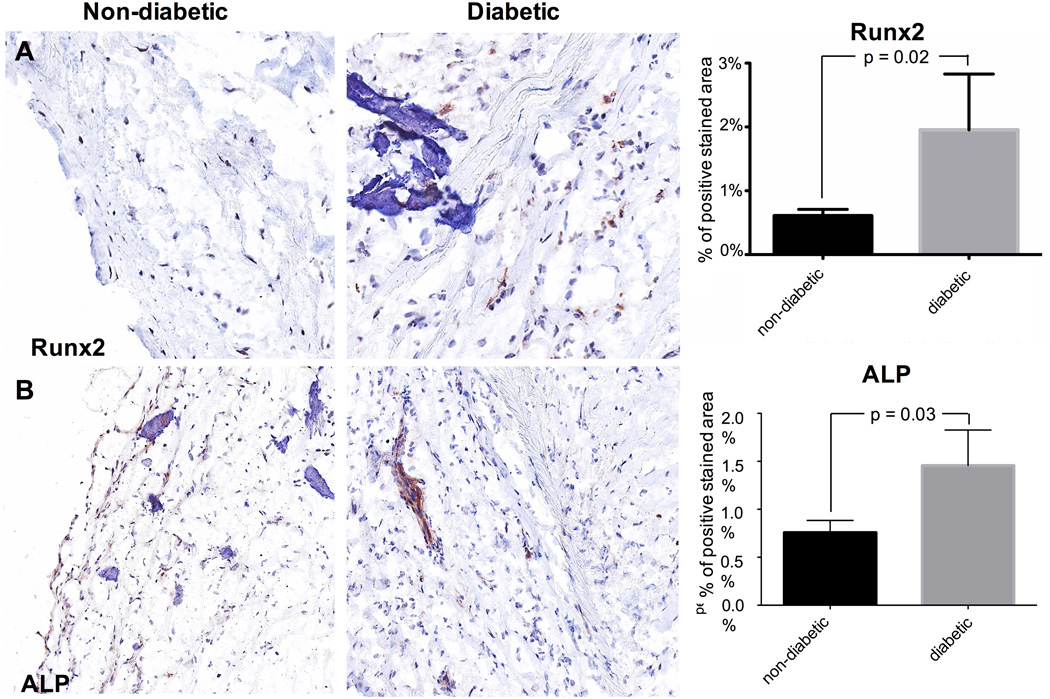
3.3. Early calcification markers are increased in diabetic patients
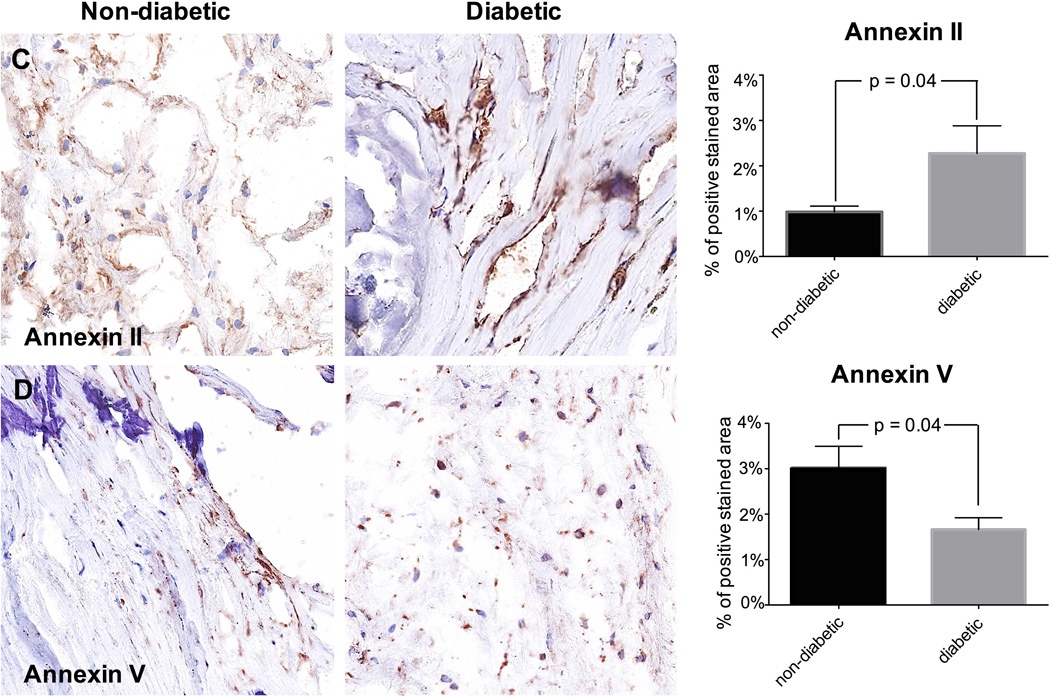
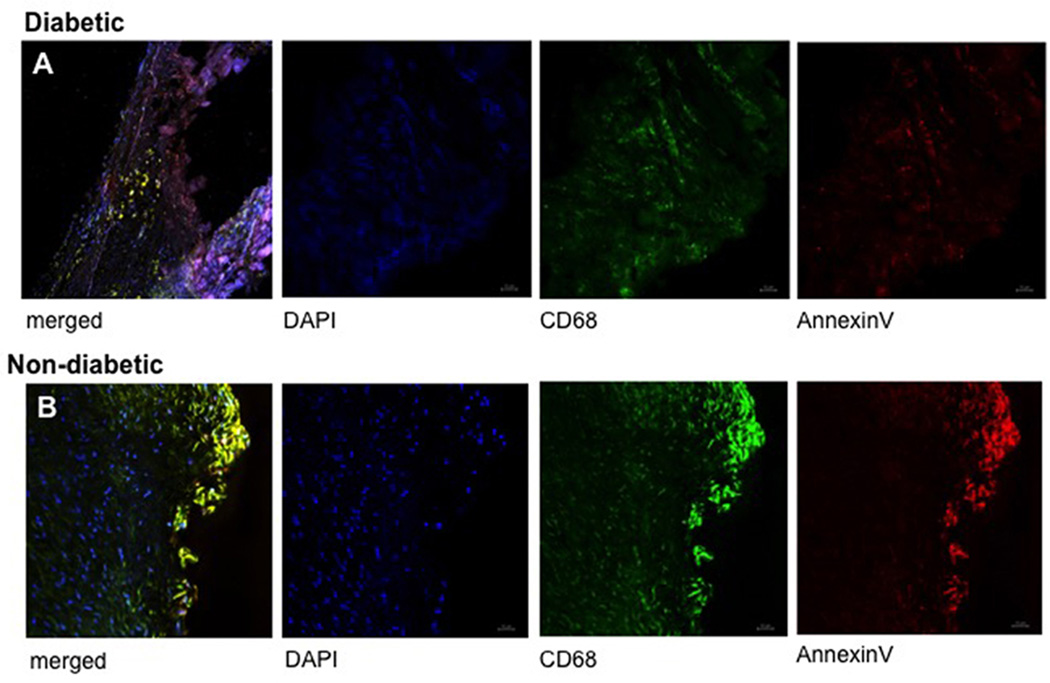
Based on differences in macrocalcification, molecular markers for early calcification were investigated. Expression of Runx2 (p=0.02; Fig. 2A) and ALP (p=0.03; Fig. 2B) were significantly higher in diabetic patients suggesting that many cells may undergo osteogenic differentiation and that calcification is still active in these individuals. In diabetic patients we found higher expression of annexin II (p=0.04; Fig. 2C). However, annexin V expression was lower in the diabetic group (p=0.04; Fig. 2D). Since Annexin V may be released from macrophages (New et al., 2013), we performed co-labeling of Annexin V and CD68, a macrophage marker. Immunofluorescence staining confirmed colocalization of annexin V with the CD68-positive macrophages (Fig. 3).
Fig. 2. MICROCALCIFICATION.
Runx2 is more expressed in diabetic than in non-diabetic patients. Representative valve tissue sections of a non-diabetic and a diabetic patient (×400 magnification) (2A; n = 45 (17 – 28)). ALP is more expressed in diabetic than in non-diabetic patients. Representative valve tissue sections of a non-diabetic and a diabetic patient (×200 magnification) (2B; n = 45 (17 – 28)). Annexin II is more expressed in diabetic than in non-diabetic tissue sections that are strongly calcified (n = 34 (12 – 22)). Representative valve tissue sections of a non-diabetic and a diabetic patient (×40 magnification) (2C). Annexin V is more expressed in non-diabetic than in diabetic tissue sections that are strongly calcified (n = 34 (12 – 22)). Representative valve tissue sections of a non-diabetic and a diabetic patient (×400 magnification) (2D).
Fig. 3. COLOCALIZATION ANNEXIN V AND CD68.
Immunoflourescence double labeling confirms colocalization of annexin V with CD68-positive macrophages, suggesting that annexin V may be released from macrophages via extracellular vesicles or apoptotic bodies. Representative valve tissue sections of a non-diabetic (3A) and a diabetic patient (3B) (×400 magnification).
3.4. Inflammation and immune response are not increased in diabetic patients
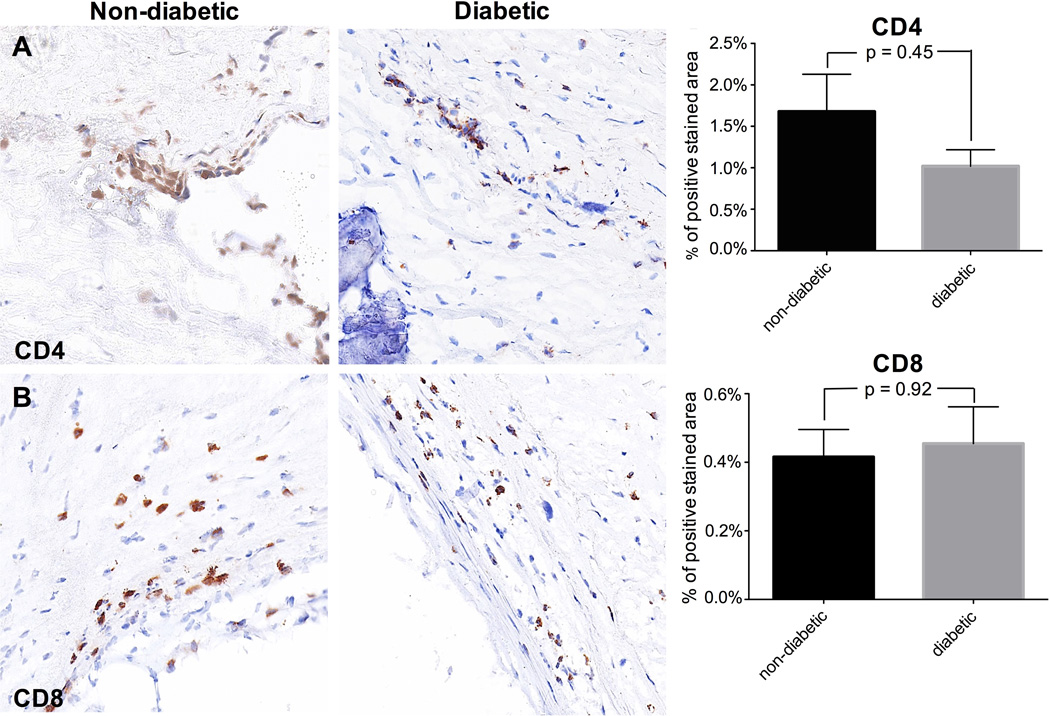
Insulin resistance, as a central feature of the metabolic syndrome and DMII, is thought to be associated with the pathogenesis of CAVD (Utsunomiya et al., 2014). Therefore, it was expected that diabetic patients more frequently show signs of inflammation. In early aortic valve lesions, T lymphocytes, such as CD4 and CD8 T cells, (Olsson et al., 1994; Wallby et al., 2002) and macrophages (Otto et al., 1994) are the predominant inflammatory cell types.
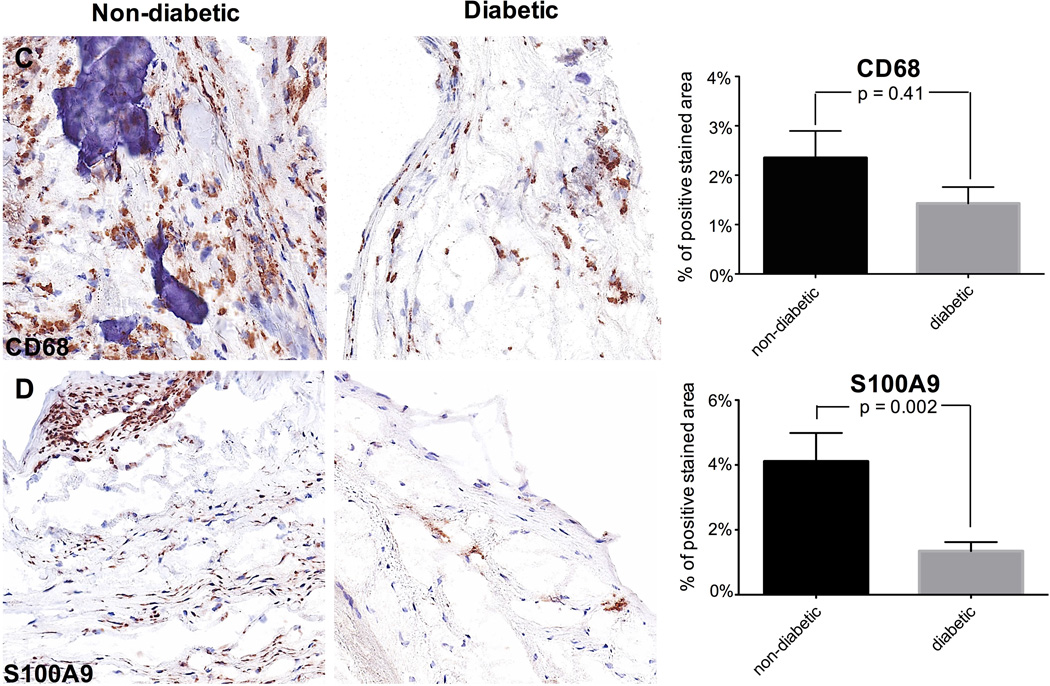
There was no difference in the number of CD4 and CD8 T cells between the two patient groups (Fig. 4A, 4B). Macrophages (CD68 cells) were similarly infrequent in both groups (Fig. 4C). Previous studies have shown that the pro-inflammatory calcium-binding S100A proteins associate with inflammation and calcification, as found in serum analysis and histological assessments (Yan and Bowman, 2014). S100A9 is preponderantly expressed by macrophages (Croce, 2010) and described to mediate macrophage recruitment into inflamed tissue. (Croce et al., 2009) We found a significantly lower expression of S100A9 in diabetic patients compared to non-diabetic patients (p=0.002; Fig. 4D).
Fig. 4. INFLAMMATION.
Representative valve tissue sections of a non-diabetic and a diabetic patient (×400 magnification) (4A-D). No difference could be detected in the presence of CD4+ and CD8+ T cells (4A, 4B) nor CD68-positive macrophages (4C) between the two patient groups (n = 45 (17 – 28)). By contrast, S100A9 is significantly lower expressed in diabetic patients compared to non-diabetic patients (4D; n = 45 (17 – 28)).
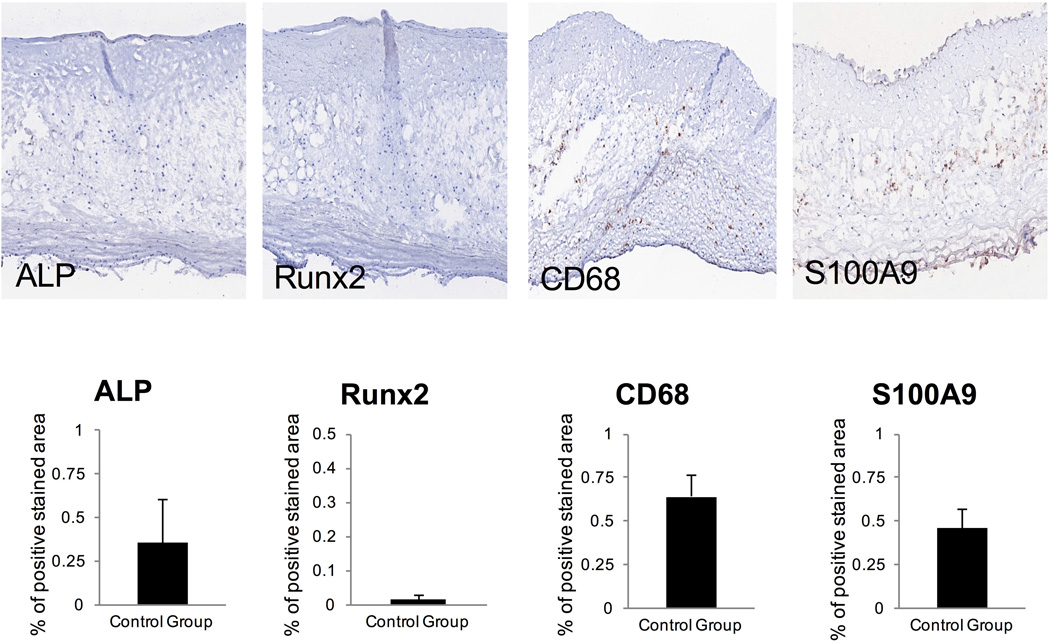
Control stainings were performed for Runx2, ALP, CD68 and S100A9 on healthy aortic valves (n = 3) obtained from autopsies and expectedly showed no relevant stainings (Fig. 5).
Fig. 5. CONTROL CAVD STAINING.
4. Discussion
It has been previously concluded that DMII contributes to CAVD pathogenesis by promoting early mineralization of the aortic valve (Le Quang et al., 2014). However, this conclusion was derived from observations in a mouse model and inflammation was not assessed. Recently published data point towards significant differences in inflammatory and calcification mechanisms between mouse and human (Thubrikar et al., 1986; Ingersoll et al., 2010; Miller et al., 2011; Cheek et al., 2012; Seok et al., 2013). Therefore, the present study specifically examined the role of DMII to provide insights into the pathology of human CAVD. Here we show that diabetic CAVD is accompanied by a greater degree of calcification, whereas inflammation is similar in diabetic and non-diabetic individuals. This data suggests that, at the time of valve replacement, diabetic patients may be in a more advanced disease stage than non-diabetic patients. Whereas clinical severity of AS appeared to be comparable in both groups or even less severe in the diabetic individuals (Table 3), diabetic patients show less or equal inflammatory signs and more early calcification. Likewise, glucose levels seem to play a role in the severity of calcification as diabetic patients with higher HbA1c levels (> 6.5%) show a trend towards more macrocalcification as detected by alizarin red staining (data not shown). In addition, the calcification process may also differ in diabetic individuals with progression via different mechanisms unassociated with inflammation.
The presence and upregulation of Runx2 in diabetic patients found in the present study strongly supports the concept that CAVD is an actively regulated process (Wirrig and Yutzey, 2013). It indicates that at the time of aortic valve surgery more valve interstitial cells (VIC) are undergoing osteogenic differentiation, a process promoted by Runx2 (Yang et al., 2009); (Ladich et al., 2011). This interpretation related to early calcification is supported by our data that the direct downstream target of Runx2, ALP, was likewise elevated in diabetic patients. Notably, ALP promotes mineral deposition into the extracellular matrix by providing inorganic phosphate through hydrolysis of pyrophosphate (Orimo, 2010).
Annexins II, V and VI are abundant in bone matrix vesicles (Kirsch et al., 1997; Wu et al., 1997; Kirsch et al., 2000; Thouverey et al., 2009) and play a role in microcalcification via facilitating Ca2+ entry into the vesicles and the formation of hydroxyapatite (Genge et al., 1990). Most reports on the role of annexins in humans were focused on their role in bone tissue. We found that annexin II expression, primarily detected in close proximity to calcific lesions, was increased in diabetic CAVD patients, suggesting participation of annexins in microcalcification, with similarities to skeletal bone development.
By contrast, we found decreased annexin V expression in diabetic patients. Immunofluorescence double labeling revealed co-localization of annexin V and CD68 suggesting that annexin V may be released from macrophages via extracellular vesicles or apoptotic bodies. However, since the number of macrophages was low, the levels of annexin V were also decreased in diabetic patients. Several annexins have been reported to interact with S100 proteins (Rety et al., 1999; Lewit-Bentley et al., 2000; Rety et al., 2000) and the formation of phosphatidylserine - annexin V - S100A9 - hydroxyapaptite complexes may be involved in the secretion of calcifying matrix vesicles from macrophages as described by our group (New et al., 2013). The significant decrease of S100A9 in diabetic patients may be a consequence of reduced macrophage or rather granulocyte numbers and annexin V expression. It could also be envisaged that diabetic factors such as hyperlipidemia and high oxidative stress, which are present in the diseased valve, may conjoin with inflammatory processes within the tissue and together enhance calcification. Likewise, it is plausible that calcification in diabetic patients may undergo different pathways independent from inflammation (e.g. apoptosis). Due to multifaceted roles of annexins, further investigation is necessary to pinpoint their exact functions in aortic stenosis and DMII.
Calcification of the aortic valve is also triggered by inflammation induced by radiation, infectious disease and metabolic syndrome (Aikawa, Nahrendorf, Sosnovik, et al., 2007). Metabolic syndrome has been shown to be independently associated with presence (Katz et al., 2006) and progress of CAVD (Katz et al., 2009) and aortic stenosis (Briand et al., 2006). Contrary to expectations, inflammation was diminished in diabetic patients. T lymphocytes, which have been suggested to have a mediating role in the pathogenesis of aortic stenosis, (Wu et al., 2007) and particularly S100A9, which was found in different inflammatory disorders, (Bouma et al., 2004; Burke et al., 2004; Johansson et al., 2008; Lood et al., 2011) were less expressed in valves explanted from diabetic individuals. These results are in contrast with the findings of Natorska et al. who reported elevated signs of inflammation in diabetic patients by analyzing the expression of C-reactive protein (CRP) in aortic stenosis, concluding that enhanced inflammation might contribute to faster disease progression (Natorska et al., 2012). However, in our observational study we were restricted to surgically removed valve leaflets from patients with end-stage disease. This might have contributed to lower detection of inflammation, which limits the force of conclusions for earlier disease stages.
At present, plasma levels of S100A8/A9 (MRP-8/14) serve as indicators of the risk for cardiovascular disease and can be used as biomarkers for acute cardiovascular events, such as atherosclerotic plaque rupture and thrombosis (Healy et al., 2006; Morrow et al., 2008; Wang, 2014). Serum analysis in addition to histological measurements of inflammatory biomarkers in CAVD individuals could potentially differentiate between general inflammation in the body and locally confined inflammation within the valve tissue. Potentially, S100A9 may also be used in the staging of aortic stenosis severity, high levels arguing for early disease stages, low levels for advancement. As S100A9 is involved in the regulation of vascular inflammation, early targeting of S100A9 may furthermore provide a novel therapeutic approach to diverse forms of appearances of vascular injuries.
As the calcification process during CAVD is currently seen as an inflammation-dependent process divided into three phases – initiation, propagation, and late stage – (Aikawa and Otto, 2012), our findings may suggest that surgically explanted aortic valves with severe aortic stenosis of diabetic patients are more mineralized with higher expression of osteogenic markers than the tissue of non-diabetic patients in a similar end-stage phase. In our study, the criteria for grading aortic stenosis severity, aortic valve area and mean pressure gradient, differ significantly between both patient groups. Aortic valve area is higher and mean pressure gradient lower in diabetic patients, which classifies them clinically as less advanced in disease, whereas on the contrary molecular markers such as Runx2 and ALP have shown that CAVD progresses faster in diabetic patients. Consequently, the inevitable question is if at the time of aortic valve replacement diabetic patients are indeed clinically in less severe stages but molecularly considerably further in disease progression, notably calcification. Future studies involving comparison proteomics and transcriptomics analyses with larger sample sizes are warranted.
Acknowledgments
This study was supported by the National Institutes of Health grants (R01HL114805; R01HL109506 to E.A.).
Abbreviations
- AVA
aortic valve area
- BMI
body mass index
- BP
blood pressure
- CAVD
calcific aortic valve disease
- DMII
type II diabetes mellitus
- LVEF
left ventricular ejection fraction
- MPG
mean pressure gradient
- VIC
valve interstitial cell
References
- Aikawa E, Otto CM. Look more closely at the valve: Imaging calcific aortic valve disease. Circulation. 2012;125:9–11. doi: 10.1161/CIRCULATIONAHA.111.073452. [DOI] [PubMed] [Google Scholar]
- Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: The cardiovascular health study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nature materials. 2013;12:576–583. doi: 10.1038/nmat3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of mrp-8/14 in type 1 diabetes induce an increased expression of cd11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–1986. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP, Arsenault M, Couet J, Pibarot P. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J. Am. Coll. Cardiol. 2006;47:2229–2236. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: A postmortem study. Arterioscler. Thromb. Vasc. Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (astronomer) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J. Mol. Cell. Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- Croce K. S100a8/a9 complex: More than just a biomarker of cardiovascular risk? Circ. J. 2010;74:626–627. doi: 10.1253/circj.cj-10-0192. [DOI] [PubMed] [Google Scholar]
- Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors C. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Genge BR, Wu LN, Wuthier RE. Differential fractionation of matrix vesicle proteins. Further characterization of the acidic phospholipid-dependent ca2(+)-binding proteins. J. Biol. Chem. 1990;265:4703–4710. [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: Linking ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gleissner CA. The vulnerable vessel. Vascular disease in diabetes mellitus. Hamostaseologie. 2015;35:267–271. doi: 10.5482/HAMO-14-11-0059. [DOI] [PubMed] [Google Scholar]
- Gleissner CA, Galkina E, Nadler JL, Ley K. Mechanisms by which diabetes increases cardiovascular disease. Drug Discov. Today Dis. Mech. 2007;4:131–140. doi: 10.1016/j.ddmec.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Ramey DR, Emneus M, Iachina M, Stavem K, Bolin K, McNally R, Busch-Sorensen M, Willenheimer R, Egstrup K, Kesaniemi YA, Ray S, Basta N, Kent C, Pedersen TR. Incidence of cancer and mortality in patients from the simvastatin and ezetimibe in aortic stenosis (seas) trial. Am. J. Cardiol. 2014;114:1518–1522. doi: 10.1016/j.amjcard.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in ldl receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O'Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: The multi-ethnic study of atherosclerosis (mesa) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Harrison G, Golub EE, Nah HD. The roles of annexins and types ii and x collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- Ladich E, Nakano M, Carter-Monroe N, Virmani R. Pathology of calcific aortic stenosis. Future Cardiol. 2011;7:629–642. doi: 10.2217/fca.11.53. [DOI] [PubMed] [Google Scholar]
- Le Quang K, Bouchareb R, Lachance D, Laplante MA, El Husseini D, Boulanger MC, Fournier D, Fang XP, Avramoglu RK, Pibarot P, Deshaies Y, Sweeney G, Mathieu P, Marette A. Early development of calcific aortic valve disease and left ventricular hypertrophy in a mouse model of combined dyslipidemia and type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2014;34:2283–2291. doi: 10.1161/ATVBAHA.114.304205. [DOI] [PubMed] [Google Scholar]
- Lewit-Bentley A, Rety S, Sopkova-de Oliveira Santos J, Gerke V. S100-annexin complexes: Some insights from structural studies. Cell Biol. Int. 2000;24:799–802. doi: 10.1006/cbir.2000.0629. [DOI] [PubMed] [Google Scholar]
- Lood C, Stenstrom M, Tyden H, Gullstrand B, Kallberg E, Leanderson T, Truedsson L, Sturfelt G, Ivars F, Bengtsson AA. Protein synthesis of the pro-inflammatory s100a8/a9 complex in plasmacytoid dendritic cells and cell surface s100a8/a9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:R60. doi: 10.1186/ar3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martínez E, Aikawa E. Identification of early pathological events in calcific aortic valve disease by molecular imaging. In: Rajamannan NM, editor. Molecular biology of valvular heart disease. Springer. London. Heidelberg. New York. Dordrecht: 2014. [Google Scholar]
- Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: Methods, models, and mechanisms. Circ. Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler. Thromb. Vasc. Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein-8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the prove it-timi 22 trial. Am. Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natorska J, Wypasek E, Grudzien G, Sobczyk D, Marek G, Filip G, Sadowski J, Undas A. Does diabetes accelerate the progression of aortic stenosis through enhanced inflammatory response within aortic valves? Inflammation. 2012;35:834–840. doi: 10.1007/s10753-011-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of t lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J. Am. Coll. Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010;77:4–12. doi: 10.1272/jnms.77.4. [DOI] [PubMed] [Google Scholar]
- Otto CM. Valvular aortic stenosis: Disease severity and timing of intervention. J. Am. Coll. Cardiol. 2006;47:2141–2151. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Otto CM. Calcific aortic stenosis--time to look more closely at the valve. N. Engl. J. Med. 2008;359:1395–1398. doi: 10.1056/NEJMe0807001. [DOI] [PubMed] [Google Scholar]
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM. Calcific aortic stenosis: Lessons learned from experimental and clinical studies. Arterioscler. Thromb. Vasc. Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, Russo-Marie F, Lewit-Bentley A. The crystal structure of a complex of p11 with the annexin ii n-terminal peptide. Nat. Struct. Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- Rety S, Osterloh D, Arie JP, Tabaries S, Seeman J, Russo-Marie F, Gerke V, Lewit-Bentley A. Structural basis of the ca(2+)-dependent association between s100c (s100a11) and its target, the n-terminal part of annexin i. Structure. 2000;8:175–184. doi: 10.1016/s0969-2126(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm A, Nystrom T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab. Res. Rev. 2006;22:4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Levenson RM. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment ii. Histopathology. 2006;49:411–424. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula S. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like saos-2 cells. J. Cell. Biochem. 2009;106:127–138. doi: 10.1002/jcb.21992. [DOI] [PubMed] [Google Scholar]
- Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am. J. Cardiol. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Yamamoto H, Kunita E, Hidaka T, Kihara Y. Insulin resistance and subclinical abnormalities of global and regional left ventricular function in patients with aortic valve sclerosis. Cardiovasc. Diabetol. 2014;13:86. doi: 10.1186/1475-2840-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallby L, Janerot-Sjoberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non-rheumatic aortic stenosis: A comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–351. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Platelet-derived s100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Invest. 2014;124:2160–2171. doi: 10.1172/JCI70966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen L, Welin L, Svardsudd K, Wedel H, Eriksson H, Hansson PO, Rosengren A. Secular changes in cardiovascular risk factors and attack rate of myocardial infarction among men aged 50 in gothenburg, sweden. Accurate prediction using risk models. J. Intern. Med. 2008;263:636–643. doi: 10.1111/j.1365-2796.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- Wirrig E, Yutzey K. Developmental pathways in cavd. 2013 [Google Scholar]
- Wu HD, Maurer MS, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stewart AS, Winchester R. The lymphocytic infiltration in calcific aortic stenosis predominantly consists of clonally expanded t cells. J. Immunol. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- Wu LN, Genge BR, Dunkelberger DG, LeGeros RZ, Concannon B, Wuthier RE. Physicochemical characterization of the nucleational core of matrix vesicles. J. Biol. Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- Yan L, Bowman MA. Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: A new link between s100/rage and fgf23. Inflamm. Cell. Signal. 2014;1 doi: 10.14800/ics.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Jr, Fullerton DA. Bone morphogenic protein 2 induces runx2 and osteopontin expression in human aortic valve interstitial cells: Role of smad1 and extracellular signal-regulated kinase 1/2. J. Thorac. Cardiovasc. Surg. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- Yetkin E, Waltenberger J. Molecular and cellular mechanisms of aortic stenosis. Int. J. Cardiol. 2009;135:4–13. doi: 10.1016/j.ijcard.2009.03.108. [DOI] [PubMed] [Google Scholar]