Abstract
The oxidation hypothesis of atherosclerosis proposes that oxidized LDL is a major causative factor in the development of atherosclerosis. Although this hypothesis has received strong mechanistic support and many animal studies demonstrated profound atheroprotective effects of antioxidants, which reduce LDL oxidation, the results of human clinical trials with antioxidants were mainly negative, except in selected groups of patients with clearly increased systemic oxidative stress. We propose that even if reducing lipoprotein oxidation in humans might be difficult to achieve, deeper understanding of mechanisms by which oxidized LDL promotes atherosclerosis and targeting these specific mechanisms will offer novel approaches to treatment of cardiovascular disease. In this review article, we focus on oxidized cholesteryl esters (OxCE), which are a major component of minimally and extensively oxidized LDL and of human atherosclerotic lesions. OxCE and OxCE-protein covalent adducts induce profound biological effects. Among these effects, OxCE activate macrophages via toll-like receptor-4 (TLR4) and spleen tyrosine kinase and induce macropinocytosis resulting in lipid accumulation, generation of reactive oxygen species and secretion of inflammatory cytokines. Specific inhibition of OxCE-induced TLR4 activation, as well as blocking other inflammatory effects of OxCE, may offer novel treatments of atherosclerosis and cardiovascular disease.
Introduction
Low-density lipoprotein (LDL), which gradually accumulates in the vascular wall in humans and in hypercholesterolemic animal models, is highly susceptible to oxidative damage due to its complex lipid-protein composition and a large number of polyunsaturated fatty acyl (PUFA) chains. Strong evidence for the presence of oxidized LDL (OxLDL) in human atherosclerotic lesions provided a scientific rationale for the “oxidation hypothesis of atherosclerosis,” stating that OxLDL is a major causative factor in the development of atherosclerosis [1, 2]. This hypothesis generated widespread enthusiasm for antioxidants as a therapeutic approach to delay the development of atherosclerosis and reduce the incidence of cardiovascular events. Indeed, many animal studies demonstrated profound atheroprotective effects of various antioxidants [2]. However, the results of human clinical trials with antioxidants were mainly negative, except in selected groups of patients with clearly increased systemic oxidative stress, such as patients on hemodialysis or diabetics with haptoglobin 2-2 genotypes associated with higher hemoglobin-mediated oxidative stress [3-5].
If reducing lipoprotein oxidation in the vessel wall is difficult to achieve in the majority of human populations, can deleterious effects of OxLDL be contained? In our opinion, deeper understanding of the biological effects of OxLDL and its components may identify specific targets for future therapy, offering better focused and, potentially, personalized treatment of cardiovascular disease (CVD).
We and others focus on inflammatory responses to OxLDL because inflammation in atherosclerotic lesions is believed to be a major contributor to plaque vulnerability to rupture, which causes acute cardiovascular and cerebrovascular events. The concept of host-derived, damage-associated molecular patterns (DAMPs) helps understand why OxLDL becomes inflammatory [6]. It appears that DAMPs share structural motifs with microbial pathogen-associated molecular patterns and activate pattern-recognition, innate immune receptors. Ensuing inflammatory responses can be harmful if unconstrained and prolonged, such as in atherosclerosis, a life-long, chronic inflammation of large arteries leading to CVD. OxLDL epitopes detected in human vulnerable plaques by specific antibodies become increasingly more prominent as lesions progress and rupture [7]. These epitopes are particularly prominent in advanced coronary and carotid lesions in macrophage-rich areas, lipid pools, the necrotic core and in ruptured plaques. The presence of OxLDL in clinically relevant human lesions provides a strong rationale to use OxLDL epitopes as biomarkers in plasma and as a molecular imaging target in atherosclerotic plaques for clinical applications.
Among OxLDL components, oxidized phospholipids (OxPL) and oxysterols were the major focus of much research in the field, resulting in identification of molecular structures, receptors and signaling pathways leading to inflammatory responses in vascular cells [8-13]. Less attention was given to oxidized cholesteryl esters (OxCE). In this article, we review studies of OxCE, with the goal to describe remarkable features of CE oxidation and OxCE-activated pathways and responses and to demonstrate the importance of containment of OxCE-induced inflammation in atherosclerosis.
Cholesteryl ester oxidation and its prevalence in atherosclerotic plaque
Cholesterol esterified with a fatty acyl chain is the form in which cholesterol is transported within LDL from liver to the periphery and stored in lipid droplets inside the cell. Even though CE reside in the hydrophobic core of LDL, CE with PUFA chains are readily oxidized, via enzymatic and free radical mechanisms. Inducers and mechanisms of LDL oxidation have been extensively investigated and reviewed elsewhere [14]. These include reactions catalyzed by 12/15-lipoxygenase (12/15-LO), myeloperoxidase, nitric oxide synthases and NADPH oxidases, as well as those mediated by transition metals, heme and hemoglobin [15]. Several studies suggest that CE is a preferential substrate for 12/15-LO [16, 17]. In an in vitro reaction of LDL oxidation by rabbit 15-LO, a close homolog of human 15-LO and mouse 12/15-LO, even when the LDL particle was loaded with free linoleic acid, cholesteryl linoleate constituted the major 15-LO substrate [16]. Remarkably, intracellular OxCE hydrolysis and subsequent incorporation of an oxidized fatty acyl chain into PL often is the source of OxPL in the cell [17]. The authors used wild type and 12/15-LO-deficient murine peritoneal macrophages and radioisotope labeled cholesteryl linoleate and cholesteryl arachidonate to demonstrate that both intracellular CE and the CE in LDL are effectively oxidized by macrophage 12/15LO and that the oxidized fatty acyls originated in OxCE can then be found as part of OxPL molecules.
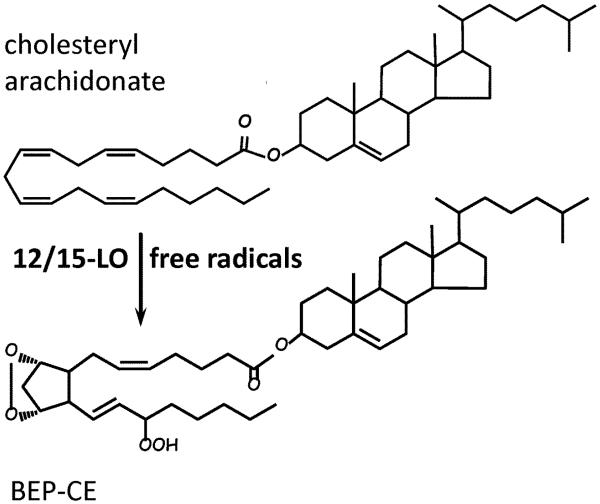
Specific 12/15-LO products of CE oxidation can be further oxidized in free radical-mediated reactions, forming numerous and complex isoprostane OxCE products, with up to 6 oxygen atoms inserted in the molecule of cholesteryl arachidonate [18, 19]. Only the PUFA chain of the CE undergoes oxidation, whereas the sterol remains unmodified. Prominent among these polyoxygenated CE products were molecules with a bicyclic endoperoxide group [18, 19], such as cholesteryl (9,11)-epidioxy-15-hydroperoxy-(5Z,13E)-prostadienoate shown in Fig. 1 (abbreviated as BEP-CE for the presence of bicyclic endoperoxide and hydroperoxide groups).
Figure 1. Oxidized cholesteryl esters.
Cholesteryl arachidonate has 4 unsaturated bonds in the fatty acyl chain, which makes it susceptible to oxidation in general and a preferable substrate for 12/15-lipoxygenase (12/15-LO). Initial 12/15-LO enzymatic oxygenation of cholesteryl arachidonate is often followed by free radical oxidation, resulting in a variety of polyoxygenated CE molecules. The example shown is a product with bicyclic endoperoxide and hydroperoxide groups (BEP-CE, or cholesteryl (9,11)-epidioxy-15-hydroperoxy-(5Z,13E)-prostadienoate), which has specific biological activity as illustrated in Fig. 2.
Importantly, specific OxCE molecules first identified in test-tube oxidation reactions were also found in cellular systems, in vascular lesions of experimental animals and in plasma and atherosclerotic plaques isolated from human CVD patients. Cholesteryl hydroperoxyoctadecadienoate (HPODE) was a major oxidized lipid in LDL incubated with activated human monocytes [20]. Cholesteryl HPODE can further decompose to form cholesteryl 9-oxononanoate (9-ON), a core aldehyde, which was found in human atherosclerotic lesions and can react with ε-aminogroup of lysines and form covalent adducts with proteins [21], particularly in small, dense LDL under oxidative conditions [22].
Earlier studies estimated that 2% of total CE and 30% of cholesteryl linoleate are oxidized in human plaques [23, 24]. A recent study employing advanced mass spectrometry techniques identified many OxCE species in human atherosclerotic lesions and quantified that, on average, 23% of cholesteryl linoleate, 16% of cholesteryl arachidonate and 12% of cholesteryl docosahexaenoate were oxidized [25]. In another mass spectrometry study, OxCE comprised from 11% to as much as 92% of the CE-PUFA pool in human plaques [26]. Among cholesteryl arachidonate oxidation products, BEP-CE was detected in human plasma from CVD patients and in human atherosclerotic lesions [27], as well as in experimental studies with hypercholesterolemic mice and zebrafish [6, 28]. Admittedly, it is difficult to establish if OxCE in human atherosclerotic plaque are mainly associated with lipoproteins retained in the extracellular matrix or reside intracellularly. Human atherosclerotic lesions contain OxCE not only in a free lipid form, but also as covalent adducts to proteins, including apoB-100, as detected with a monoclonal antibody raised against proteins modified with cholesteryl 9-ON [29].
Biological effects of OxCE
It was noticed that OxLDL inactivates PDGF, and cholesteryl HPODE, likely forming covalent adducts with PDGF, was identified as a lipid in OxLDL responsible for this effect [30]. Hydrogen peroxide and short fatty acid hydroperoxides did not inactivate PDGF. Cholesteryl HPODE also inactivated bFGF and TFG-β but not EGF [30]. Both cholesteryl HPODE and cholesteryl 9-ON activated PKC and ERK1/2 in endothelial cells and resulted in expression of connecting segment-1 and enhanced adhesion of monocytes to endothelial cells [31]. Cholesteryl 9-ON induced expression of both TFG-β and TGF-β receptor type I in human U937 promonocytic cells, the effect mediated by ERK1/2 and potentially involved in sustaining vascular remodeling in atherosclerosis [32]. Cholesteryl HPODE was the active component of moderately oxidized LDL that activated PPARα-dependent expression of CD36 in human monocyte-derived macrophages, whereas PL-esterified HPODE and 7-ketocholesterol did not produce this effect [33].
There is a mechanism to detoxify OxCE in circulation, which involves CETP-mediated exchange of OxCE between LDL and HDL [34] and selective uptake of HDL-associated OxCE by liver via an SR-BI-mediated mechanism [35]. The selective uptake of OxCE was 2-3 fold higher compared with selective uptake of non-oxidized CE, and high-cholesterol feeding of rats significantly decreased OxCE uptake by liver parenchymal cells but dramatically increased OxCE uptake by Kupffer cells [35].
OxCE activation of TLR4/MD-2
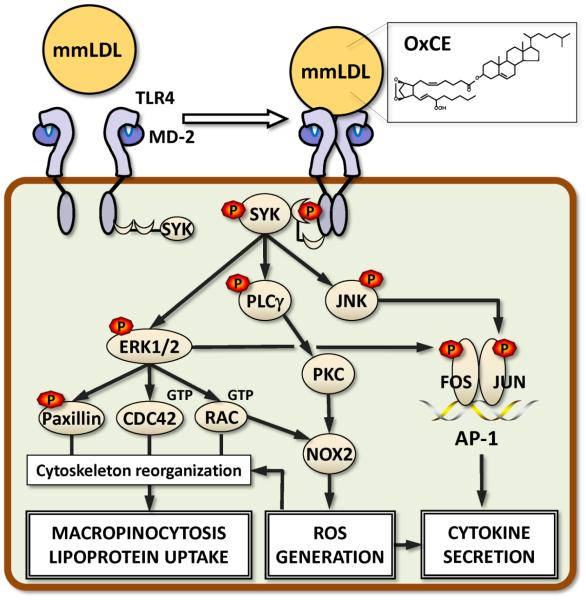
Work from our laboratory demonstrated that minimally oxidized LDL (mmLDL), produced by LDL oxidation with 15-LO-expressing cells, induced profound cytoskeleton changes in macrophages, including actin polymerization, cell spreading, membrane ruffling and macropinocytosis [36, 37] (Fig. 2). Macropinocytosis resulted in robust uptake of OxLDL, mmLDL and native LDL by macrophages and foam cell formation. Moreover, mmLDL induced PLCγ, PKC and NOX2 dependent ROS production, which regulated expression of RANTES (CCL5), IL-1β and IL-6 [38]. Expression of MCP-1 (CCL2), TNFα, MIP-2 (CXCL2) and MIP-1α (CCL3) was induced by mmLDL in a NOX2-independent manner [38]. The mmLDL-induced expression of cytokines was also mediated by ERK1/2 and JNK phosphorylation of FOS and JUN, and synergized with NF-κB activation induced by low-dose LPS [39, 40].
Figure 2. Biological activity of OxCE.
Minimally oxidized LDL (mmLDL) contains many OxCE moieties, one of them BEP-CE shown in Fig. 1. Both mmLDL and isolated BEP-CE induce dimerization of TLR4, presumably, via cholesterol binding to MD-2. While MyD88 plays a minimal role, SYK mediates the majority of mmLDL and BEP-CE effects downstream from TLR4. SYK binds to TLR4, becomes phosphorylated and activates ERK1/2, JNK and PLCγ. ERK1/2, via Paxillin, CDC42 and RAC, induces cytoskeleton reorganization and membrane ruffling in macrophages, leading to macropinocytosis. Macropinocytosis is a robust mechanism of macrophage lipid accumulation and foam cell formation in which all lipoproteins, native and oxidized, are internalized. PLCγ-activated PKC and ERK1/2-activated RAC contribute to activation of NOX2, a major NADPH oxidase in macrophages. Resulting reactive oxygen species regulate cytoskeletal changes and contribute to cytokine expression. MIP-2 (CXCL2) is a major cytokine induced by BEP-CE in macrophages via TLR4 and SYK. Its expression depends on ERK1/2-mediated phosphorylation of FOS and JNK-mediated phosphorylation of JUN. Together these two transcription factors form an AP-1 complex, which regulates expression of CXCL2.
OxCE was identified as an active component in mmLDL responsible for the majority of mmLDL effects [27, 28, 37], and 15-LO-oxidized cholesteryl arachidonate reproduced many of mmLDL effects [28]. mmLDL also bound to CD14 [36], a receptor that binds bacterial lipopolysaccharide (LPS) and presents it to TLR4/MD-2. The mmLDL binding to CD14 implied the involvement of TLR4/MD-2 in mmLDL effects. Indeed, Tlr4 mutant and Tlr4−/− primary macrophages failed to respond to mmLDL or OxCE [27, 36, 37]. OxCE induced MD-2 recruitment to TLR4 and TLR4 dimerization [27]. Interestingly, MyD88, a TLR4 adaptor which mediates the majority of LPS effects, minimally contributed to macrophage responses to mmLDL. Instead, spleen tyrosine kinase (SYK) was identified as a kinase, which was recruited to TLR4 and mediated the majority of mmLDL- and OxCE-induced effects in macrophages [37, 38, 40] (Fig. 2). This dichotomy between LPS- and OxCE-mediated TLR4 responses attests, in addition to the pattern-recognition character of TLR4, to the TLR4 biased agonism, or functional selectivity. This emerging concept in the field of G-protein-coupled receptors (GPCR), explaining the instances when different ligands of the same receptor preferentially activate one signaling pathway over another, integrates new discoveries showing multidimensionality of GPCR signaling rather than the linear spectrum of GPCR responses [41, 42]. More work is needed to understand mechanisms responsible for biased agonism in TLR4 signaling and its implications for the development of chronic inflammatory diseases.
In the TLR4/MD-2 receptor complex, MD-2 is an LPS-binding receptor, which provides a hydrophobic pocket for five of the six saturated fatty acyl chains of LPS. The sixth fatty acyl forms a hydrophobic interaction with the phenylalanines of TLR4 of a different TLR4/MD-2 pair, and phosphate groups of LPS contribute to receptor multimerization by forming ionic interactions with a cluster of positively charged residues in TLR4 and MD-2 [43]. In this LPS-induced receptor complex, the intracellular TIR domains of two molecules of TLR4 dimerize and recruit adaptor molecules, which initiate signaling cascades, resulting in robust inflammatory responses [43].
MD-2 has a β-cup fold structure composed of two antiparallel β sheets forming a hydrophobic pocket, with positively charged residues located near the opening rim of the pocket [43, 44]. The hydrophobic pocket accommodates fatty acyl chains of LPS, and positively charged residues at its opening bind negatively charged phosphate groups of LPS. In the molecule of cholesterol, a hydrocarbon chain together with the steroid form an elongated hydrophobic structure, which may dock in the hydrophobic pocket of MD-2, and a hydroxyl group linked to the other side of the steroid may stabilize cholesterol at the positively charged entrance to the pocket. Indeed, we found that MD-2 binds cholesterol [45]. MD-2 exists in cell-surface-expressed and soluble forms, and soluble MD-2 is found in human blood plasma. Upon LPS binding, soluble MD-2 associates with cell surface-expressed TLR4 and activates it [46]. We demonstrated the presence of cholesterol associated with soluble MD-2 in human plasma and in mouse atherosclerotic lesions [45]. It is unlikely that unesterified cholesterol binding to MD-2 activates TLR4. However, both LPS and, importantly, OxCE-modified BSA compete with cholesterol for MD-2 binding [45]. Future structural studies will test the hypothesis that the polyoxygenated fatty acyl chain in BEP-CE and/or components of OxCE-protein conjugates provide additional interaction surfaces, which, in combination with cholesterol anchoring in the MD-2 hydrophobic pocket, provide sufficient interfaces for OxCE-induced TLR4 dimerization, which was observed experimentally [27].
Conclusions
Compared with OxPL, little is known about inflammatory and atherogenic effects of OxCE. Yet, OxCE are a major component of OxLDL and atherosclerotic lesions. Oxidation of CE, particularly by 12/15-LO, may contribute to the formation of OxPL. Atherogenic and inflammatory responses to OxCE include monocyte adhesion to endothelial cells, CD36 expression, production of ROS, expression of inflammatory cytokines, and macrophage lipid accumulation. Cholesterol binds to MD-2 and this likely explains that OxCE activates macrophages via TLR4/MD-2. Better understanding of mechanisms by which specific OxCE moieties induce inflammatory responses in vascular cells may pave the way to new therapies to reduce atherosclerosis burden and treat CVD. Additional studies are required to demonstrate whether, similar to the wide use of OxPL in biomarker and molecular imaging applications [47], detection of OxCE in blood and atherosclerotic plaques will be effective as diagnostic and prognostic tool.
Highlights.
Oxidized cholesteryl esters (OxCE) are a major component of atherosclerotic lesions
OxCE and OxCE-protein adducts induce profound biological effects
OxCE induce atherogenic, TLR4- and SYK-dependent macrophages responses
Blocking inflammatory effects of OxCE may suggest novel atherosclerosis therapy
Acknowledgments
Work in authors’ laboratory is supported by grants HL124174, HL055798 and HL088093 (Y.I.M.) from the National Institutes of Health, and SDG14710028 (S.-H.C.) from the American Heart Association.
Abbreviations
- 12/15-LO
12/15-lipoxygenase
- 9-ON
9-oxononanoate
- CE
cholesteryl esters
- CVD
cardiovascular disease
- DAMPs
damage-associated molecular patterns
- HPODE
hydroperoxyoctadecadienoate
- LDL
low-density lipoprotein
- MD-2
myeloid differentiation-2
- mmLDL
minimally oxidized LDL
- OxCE
oxidized CE
- OxLDL
oxidized LDL
- OxPL
oxidized phospholipid
- PUFA
polyunsaturated fatty acid or acyl
- TLR4
toll-like receptor-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- [2].Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J. Lipid Res. 2009;50:S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler. Thromb. Vasc. Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- [4].Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. The Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- [5].Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- [6].Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Dijk RA, Kolodgie F, Ravandi A, Leibundgut G, Hu PP, Prasad A, Mahmud E, Dennis E, Curtiss LK, Witztum JL, Wasserman BA, Otsuka F, Virmani R, Tsimikas S. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of Phospholipid Oxidation Products in Atherosclerosis. Circ. Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr. Opin. Pharmacol. 2013;13:186–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014;115:770–780. doi: 10.1161/CIRCRESAHA.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oh MJ, Zhang C, LeMaster E, Adamos C, Berdyshev E, Bogachkov Y, Kohler EE, Baruah J, Fang Y, Schraufnagel DE, Wary KK, Levitan I. Oxidized LDL signals through Rho-GTPase to induce endothelial cell stiffening and promote capillary formation. J. Lipid Res. 2016;57:791–808. doi: 10.1194/jlr.M062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colles SM, Maxson JM, Carlson SG, Chisolm GM. Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential roles for oxysterols. Trends Cardiovasc.Med. 2001;11:131–138. doi: 10.1016/s1050-1738(01)00106-2. [DOI] [PubMed] [Google Scholar]
- [14].Tsimikas S, Miller YI. Oxidative modification of lipoproteins: Mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr. Pharm. Des. 2011;17:27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- [15].Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem. 2010;51:229–251. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- [16].Belkner J, Stender H, Kuhn H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. The Journal Of Biological Chemistry. 1998;273:23225–23232. doi: 10.1074/jbc.273.36.23225. [DOI] [PubMed] [Google Scholar]
- [17].Hutchins PM, Murphy RC. Cholesteryl ester acyl oxidation and remodeling in murine macrophages: formation of oxidized phosphatidylcholine. J. Lipid Res. 2012;53:1588–1597. doi: 10.1194/jlr.M026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yin H, Havrilla CM, Morrow JD, Porter NA. Formation of isoprostane bicyclic endoperoxides from the autoxidation of cholesteryl arachidonate. J. Am. Chem. Soc. 2002;124:7745–7754. doi: 10.1021/ja0201092. [DOI] [PubMed] [Google Scholar]
- [19].Yin H, Morrow JD, Porter NA. Identification of a Novel Class of Endoperoxides from Arachidonate Autoxidation. J. Biol. Chem. 2004;279:3766–3776. doi: 10.1074/jbc.M307137200. [DOI] [PubMed] [Google Scholar]
- [20].Folcik VA, Cathcart MK. Predominance of esterified hydroperoxy-linoleic acid in human monocyte-oxidized LDL. J. Lipid Res. 1994;35:1570–1582. [PubMed] [Google Scholar]
- [21].Hoppe G, Ravandi A, Herrera D, Kuksis A, Hoff HF. Oxidation products of cholesteryl linoleate are resistant to hydrolysis in macrophages, form complexes with proteins, and are present in human atherosclerotic lesions. J. Lipid Res. 1997;38:1347–1360. [PubMed] [Google Scholar]
- [22].Chancharme L, Therond P, Nigon F, Lepage S, Couturier M, Chapman MJ. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler. Thromb. Vasc. Biol. 1999;19:810–820. doi: 10.1161/01.atv.19.3.810. [DOI] [PubMed] [Google Scholar]
- [23].Suarna C, Dean RT, May J, Stocker R. Human Atherosclerotic Plaque Contains Both Oxidized Lipids and Relatively Large Amounts of α-Tocopherol and Ascorbate. Arterioscler. Thromb. Vasc. Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- [24].Upston JM, Niu X, Brown AJ, Mashima R, Wang H, Senthilmohan R, Kettle AJ, Dean RT, Stocker R. Disease Stage-Dependent Accumulation of Lipid and Protein Oxidation Products in Human Atherosclerosis. Am. J. Pathol. 2002;160:701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hutchins PM, Moore EE, Murphy RC. Electrospray tandem mass spectrometry reveals extensive and non-specific oxidation of cholesterol esters in human peripheral vascular lesions. J. Lipid Res. 2011;52:2070–2083. doi: 10.1194/jlr.M019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA, Witztum JL, Tsimikas S. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J. Am. Coll. Cardiol. 2014;63:1961–1971. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Choi S-H, Yin H, Ravandi A, Armando A, Dumlao D, Kim J, Almazan F, Taylor AM, McNamara CA, Tsimikas S, Dennis EA, Witztum JL, Miller YI. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J. Biol. Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawai Y, Saito A, Shibata N, Kobayashi M, Yamada S, Osawa T, Uchida K. Covalent binding of oxidized cholesteryl esters to protein: implications for oxidative modification of low density lipoprotein and atherosclerosis. J. Biol. Chem. 2003;278:21040–21049. doi: 10.1074/jbc.M212426200. [DOI] [PubMed] [Google Scholar]
- [30].Van Heek M, Schmitt D, Toren P, Cathcart MK, DiCorleto PE. Cholesteryl hydroperoxyoctadecadienoate from oxidized low density lipoprotein inactivates platelet-derived growth factor. J. Biol. Chem. 1998;273:19405–19410. doi: 10.1074/jbc.273.31.19405. [DOI] [PubMed] [Google Scholar]
- [31].Huber J, Boechzelt H, Karten B, Surboeck M, Bochkov VN, Binder BR, Sattler W, Leitinger N. Oxidized cholesteryl linoleates stimulate endothelial cells to bind monocytes via the extracellular signal-regulated kinase 1/2 pathway. Arterioscler. Thromb. Vasc. Biol. 2002;22:581–586. doi: 10.1161/01.atv.0000012782.59850.41. [DOI] [PubMed] [Google Scholar]
- [32].Gargiulo S, Gamba P, Sottero B, Biasi F, Chiarpotto E, Serviddio G, Vendemiale G, Poli G, Leonarduzzi G. The core-aldehyde 9-oxononanoyl cholesterol increases the level of transforming growth factor beta1-specific receptors on promonocytic U937 cell membranes. Aging cell. 2009;8:77–87. doi: 10.1111/j.1474-9726.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- [33].Jedidi I, Couturier M, Therond P, Gardes-Albert M, Legrand A, Barouki R, Bonnefont-Rousselot D, Aggerbeck M. Cholesteryl ester hydroperoxides increase macrophage CD36 gene expression via PPAR[alpha] Biochem. Biophys. Res. Commun. 2006;351:733–738. doi: 10.1016/j.bbrc.2006.10.122. [DOI] [PubMed] [Google Scholar]
- [34].Christison JK, Rye KA, Stocker R. Exchange of oxidized cholesteryl linoleate between LDL and HDL mediated by cholesteryl ester transfer protein. J. Lipid Res. 1995;36:2017–2026. [PubMed] [Google Scholar]
- [35].Fluiter K, Sattler W, De Beer MC, Connell PM, van der Westhuyzen DR, van Berkel TJ. Scavenger receptor BI mediates the selective uptake of oxidized cholesterol esters by rat liver. J. Biol. Chem. 1999;274:8893–8899. doi: 10.1074/jbc.274.13.8893. [DOI] [PubMed] [Google Scholar]
- [36].Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- [37].Choi S-H, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages Generate Reactive Oxygen Species in Response to Minimally Oxidized Low-Density Lipoprotein: Toll-Like Receptor 4- and Spleen Tyrosine Kinase-Dependent Activation of NADPH Oxidase 2. Circ. Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor {kappa}B and activator protein-1. Possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Choi SH, Wiesner P, Almazan F, Kim J, Miller YI. Spleen Tyrosine Kinase Regulates AP-1 Dependent Transcriptional Response to Minimally Oxidized LDL. PLoS One. 2012;7:e32378. doi: 10.1371/journal.pone.0032378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rankovic Z, Brust TF, Bohn LM. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 2016;26:241–250. doi: 10.1016/j.bmcl.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Anderson CA, Solari R, Pease JE. Biased agonism at chemokine receptors: obstacles or opportunities for drug discovery? J. Leukoc. Biol. 2016;99:901–909. doi: 10.1189/jlb.2MR0815-392R. [DOI] [PubMed] [Google Scholar]
- [43].Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- [44].Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [45].Choi SH, Kim J, Gonen A, Viriyakosol S, Miller YI. MD-2 binds cholesterol. Biochem. Biophys. Res. Commun. 2016;470:877–880. doi: 10.1016/j.bbrc.2016.01.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tissieres P, Dunn-Siegrist I, Schappi M, Elson G, Comte R, Nobre V, Pugin J. Soluble MD-2 is an acute phase protein and an opsonin for Gram-negative bacteria. Blood. 2008;111:2122–2131. doi: 10.1182/blood-2007-06-097782. [DOI] [PubMed] [Google Scholar]
- [47].Miller YI, Tsimikas S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: biomarkers, molecular imaging and therapeutics. Curr. Opin. Lipidol. 2013;24:426–437. doi: 10.1097/MOL.0b013e328364e85a. [DOI] [PMC free article] [PubMed] [Google Scholar]