Abstract
Chronic inflammation in adipose tissue, possibly related to adipose cell hypertrophy, hypoxia, and/or intestinal leakage of bacteria and their metabolic products, likely plays a critical role in the development of obesity-associated insulin resistance (IR). Cells of both the innate and adaptive immune system residing in adipose tissues, as well as in the intestine, participate in this process. Thus, M1 macrophages, IFN-γ–secreting Th1 cells, CD8+ T cells, and B cells promote IR, in part through secretion of proinflammatory cytokines. Conversely, eosinophils, Th2 T cells, type 2 innate lymphoid cells, and possibly Foxp3+ Tregs protect against IR through local control of inflammation.
Introduction
The prevalence of obesity has increased dramatically over the past three decades and has contributed to the increasing prevalence of insulin resistance (IR) and type 2 diabetes (T2D) as well as cardiovascular disease, fatty liver/cirrhosis, hypertension, and cancer (1, 2). Almost 400 million people worldwide have T2D. In the United States, one of every nine dollars spent on healthcare is put toward T2D management and care (3). Adverse health consequences related to obesity are largely mediated by IR, which affects approximately 40% of the US adult population (4) and greatly increases the risk of T2D. The biological basis linking obesity to metabolic dysfunction has not been fully elucidated. Accumulating evidence points to localized inflammation in adipose tissue (AT), which in turn promotes systemic inflammation and impaired insulin action. Ultimately, targeting inflammatory pathways or the stimuli responsible for inflammation may successfully prevent and/or treat T2D (5). This Review describes what is known about the roles of innate and adaptive immunity in the development of obesity-related metabolic disease.
Obesity and inflammation
The finding, over 20 years ago, that TNF-α is overexpressed in the AT of obese mice provided the first clear link between obesity, diabetes, and chronic inflammation (6). TNF-α impairs insulin action by promoting serine over tyrosine phosphorylation of insulin receptor substrate 1 (IRS1), thereby blocking insulin signaling through its receptor (6). Mice lacking functional TNF-α or its receptors are more insulin sensitive and glucose tolerant than WT controls (7), and administration of TNF-α to animals impairs insulin action (6). In humans, high levels of circulating inflammatory mediators such as IL-6 and markers of inflammation such as high-sensitivity C-reactive protein (hsCRP) correlate with obesity (8) and predict development of T2D (9). The levels of TNF-α gene and protein expression in human AT also correlate with BMI and decrease following dietary weight loss (10). IL-6, which like TNF-α is secreted by both adipocytes and macrophages, impairs lipoprotein lipase, thus contributing to disordered function of fat storage in AT (11).
Subsequent to the observations described above, macrophages were shown to populate subcutaneous AT (SAT) and visceral AT (VAT) in mice, correlating with total adiposity as well as with adipose cell size (12). In humans, expression of the macrophage marker CD68 in SAT also correlated with BMI and adipocyte size (12). Evaluation of the geographic relationships between AT macrophages (ATMs) and adipose cells in murine and human AT revealed that 90% of ATMs cluster around single enlarged and necrotic adipose cells in crown-like structures (CLSs), with evidence for scavenging of residual lipid in the interstitial space (13). A multitude of other studies in mice support these early findings. High-fat diet–induced (HFD-induced) expression of monocyte chemoattractant protein-1 (MCP-1) in AT (14) and adipose-specific overexpression of MCP-1 increased ATM frequency and systemic IR (15), whereas targeted deletion of MCP-1 or its receptor, CCR2, prevented inflammation and macrophage infiltration in AT and protected against diet-induced IR (16). Inflammation and macrophage-specific genes are progressively upregulated in white AT (WAT) in models of genetic- and HFD-induced obesity, and precede development of IR, implying causality (17). Finally, inflammatory cytokines and cellular stress induce kinases such as the NF-κB activator IKKB and JNK1, which are increased in obesity and directly impair insulin action (18, 19). Targeted disruption of both IKKB (20) and JNK (21) in mice prevents HFD-induced IR, highlighting a mechanistic link between inflammatory pathways and impaired insulin action.
Potential role of gut inflammation in disease development
In addition to AT, recent evidence suggests that the intestine is also a key site that becomes altered in obesity-related IR. These alterations include changes in the gastrointestinal flora, known as dysbiosis, which can impact body fat, systemic inflammation, and IR (22–24). Dysbiosis is believed to cause low-grade inflammation both systemically, through enhanced leakage of bacterial products such as LPS, and locally in the small bowel and colon (25, 26). Systemically, some of these bacterial products, including gut-derived antigens, are thought to accumulate and potentiate inflammation in AT, especially VAT (27, 28). Recent studies of diet-induced obese (DIO) mice have shown that obesity induces a chronic phenotypic proinflammatory shift in bowel lamina propria immune cell populations (29). Mice deficient in specific immune cell types such as B cells exhibit profound changes in their intestinal microbiomes, reflecting cross-talk between immune cells and gut (30). Suppression of the gut immune system, as in β7 integrin–deficient (β7-null) mice, has been shown to decrease IR, while treatment with the local gut antiinflammatory compound 5-aminosalicylic acid reverses bowel inflammation and improves metabolic parameters. These beneficial effects were dependent on adaptive and gut immunity, associated with reduced gut permeability and endotoxemia, and decreased VAT inflammation (29). Studies in humans have not yet addressed these relationships.
Innate immunity
Much of the research linking inflammation and metabolic disease has focused on the role of the innate immune response. Macrophages, as well as other innate cells such as mast cells, neutrophils, and DCs, have been shown to contribute to IR pathogenesis (31–33). Mast cells are present to a greater degree in obese mice compared with non-obese animals, and mast cell deficiency is associated with enhanced insulin sensitivity (31). Neutrophils have been shown to promote IR, mainly through elastase production (32). DCs are specialized antigen-presenting cells (APCs) that link the innate and adaptive immune responses. In particular, they play a role in inducing differentiation of naive CD4+ T cells into Th1, Th2, or Th17 cells or Tregs. Activated DCs were shown to be present in higher numbers in AT of obese non-diabetic humans and individuals with T2D as compared with lean control subjects (34). Eosinophils in murine VAT were shown to produce the antiinflammatory cytokines IL-4 and IL-13, which promote differentiation of macrophages into alternatively activated M2-polarized cells (35). Lastly, innate lymphoid type 2 cells (ILC2s) play a key role in maintenance of eosinophils and metabolic homeostasis in VAT through their production of IL-5 and IL-13. Recent studies in DIO mice showed that IL-5 deficiency led to worsened IR and adiposity and that depletion of ILC2s yielded diminished accumulation of eosinophils and alternatively activated macrophages in VAT (36).
Macrophages
Macrophages are key mediators of inflammation in AT and are the most abundant immune cells that infiltrate obese AT (37, 38). They comprise 40% to 60% of AT immune cells in obese mice compared to 10% to 15% of AT immune cells in lean mice, and secrete the majority of inflammatory cytokines in response to obesity (12, 17). Furthermore, macrophages are mainly found in interstitial spaces between adipocytes in the AT of lean individuals, but are primarily found in CLSs around large dying adipocytes during a state of obesity (39). Macrophages are a heterogeneous population characterized by varied surface markers and cytokine secretory patterns. Based partly on their surface phenotype, they are often classified as M1 or M2 cells, although many macrophages do not fall unequivocally into either classification. M1, or “classically activated,” macrophages are induced by proinflammatory mediators such as LPS and IFN-γ and secrete proinflammatory cytokines including IL-6, IL-1β, inducible NOS (iNOS), and TNF-α. M2, or “alternatively activated,” macrophages play a role in tissue repair, angiogenesis, and resolution of inflammation. These macrophages are induced by the antiinflammatory cytokines IL-4 and IL-13 and secrete high levels of antiinflammatory IL-10, IL-1 decoy receptor, and arginase, thereby blocking IL-1β and iNOS activity. Surface markers of M2 differ from those of M1; the classical differentiating marker is CD11c (negative in M2 and positive in M1), but other markers of inflammation also segregate with M1 (CD163, CD172, CD44) and M2 (arginase 1, CD206, CD301) phenotypes. Lean mice demonstrate a dominant M2 phenotype, whereas obese mice demonstrate a dominant M1 phenotype (40). In human obesity, classical M1 markers may not typify proinflammatory ATMs even in the face of functional and cytokine phenotypes typical of classical inflammation. Several studies have described mixed M1/M2 phenotypes in ATMs from obese mice and humans, suggesting that the ATMs adopt more complex states in vivo (41, 42). Indeed, one comparison of surface markers expressed in obese human ATMs versus bronchial macrophages from cystic fibrosis patients with chronic bacterial infection demonstrated absence of CD11c positivity and other classic M1 surface markers. A plasma membrane proteomics analysis in the latter study suggested that a unique metabolically activated macrophage phenotype was present, characterized by surface markers distinct from classic M1 markers (43). Macrophage phenotype also exhibits plasticity, changing rapidly in response to external stimuli. For example, while M2 macrophages dominate in AT of lean mice, a “phenotypic switch” occurs in DIO mice, characterized by a shift in polarization toward M1 over days to weeks (40, 44). An M2 phenotypic shift can also be induced by PPARγ activation, which protects against M1 activation and IR (45), possibly by promoting lipid storage in adipocytes and preventing lipotoxicity and adipocyte death (46).
The signals that regulate recruitment of macrophages to AT are not fully elucidated, but are almost certainly the product of cross-talk with adipocytes or with other immune cells. In general, these signals attract bone marrow–-derived monocytes to AT, where they become activated macrophages of varying phenotypes. The two leading mechanisms of macrophage recruitment to AT include secretion of chemokines and activation of pattern-recognition receptors (PRRs) on macrophages, including the NLR and TLR families, which recognize danger signals from dying and stressed cells, LPS from gut pathogens that reach the circulation, and lipid signals. MCP-1, a well-characterized macrophage chemoattractant, secreted by adipocytes as well as macrophages, is more abundant in obese versus lean mice and humans, and is elevated in VAT versus SAT (47). It is induced by IL-1β, TNF-α, IL-8, IL-4, and IL-6 and, when under- or overexpressed, alters monocyte recruitment to AT. Another chemokine that may contribute to macrophage recruitment is leukotriene B4 (LTB4), which is secreted by adipocytes and also stimulates chemotaxis of neutrophils; blockade of LTB4 prevents induction of diet-induced IR in mice (48). Fractaline (CX3CL1) and its receptor CX3CR1 participate in recruitment and adhesion of monocytes and T cells in atherosclerosis and have been implicated in macrophage recruitment to AT. CX3CL1 is expressed in adipocytes and upregulated in obese human AT, and contributes to adhesion of monocytes to adipocytes and glucose intolerance (49). In addition to the classical chemokines MCP-1 and LTB4, an axon-guiding molecule, semaphorin 3E (SEMA3E), also exhibits chemoattractant properties for macrophages. Expression of SEMA3E and its receptor, plexinD1, is increased in the AT of DIO mice. Blockade of the interaction between the SEMA3E and plexinD1 results in a decreased number of infiltrated macrophages in AT and improved IR (50). Finally, studies in mice implicate macrophage migration inhibitory factor, a proinflammatory chemokine secreted by numerous cell types, including adipocytes, in ATM accumulation, proinflammatory cytokine secretion, activation of JNK and IKKB pathways, and impaired insulin signaling in AT and liver (51).
Obesity not only induces the production of chemoattractants that direct the recruitment of macrophages into AT, but also results in the release of signals that promote macrophage retention. A recent study suggested that a neuronal guidance molecule, netrin-1, promotes macrophage retention in AT. Netrin-1 is increased in obese mice and humans and was reported to block macrophage emigration from AT via binding to its receptor, UNC5B, on macrophages (52). The overall importance of this process to macrophage accumulation and inflammation in AT remains to be determined.
PRRs, including TLRs, are present on macrophages and other innate immune cells, and recognize a variety of danger signals as well as metabolic alterations. PRRs play a critical role in the innate immune system by activating proinflammatory signaling pathways in response to microbial pathogens (53). For example, TLR4 binds to LPS on gram-negative bacterial cell walls and activates both IKKB and JNK, thereby triggering transcription of proinflammatory genes that encode cytokines, chemokines, and other effectors of the innate immune response (54). TLR4 responds not only to LPS, but also to free fatty acids (FFAs), which trigger inflammation and IR via TLR4 activation; this has been demonstrated in TLR4-knockout models, which exhibited decreases in NF-κB activation, proinflammatory gene expression, and IR in response to lipid infusion (55, 56). The NLR family is another group of PRRs that may participate in macrophage recruitment and activation. In macrophages these receptors recognize non-microbe signals, activating the NLRP3 inflammasome, which stimulates IL-1β and IL-18 production via caspase-1 activation. NLRP3-knockout animals are protected from IR and have more metabolically active adipose cells compared with WT animals (57). NLRP3 activation, with associated IL-1β and IL-18 secretion, has been observed in response to stimulation by lipotoxicity-related increases in intracellular ceramide (58), palmitate (59), islet amyloid polypeptide (60), serum amyloid A (61), cholesterol crystals (62), and oxidized LDL cholesterol (63). Other purported but not yet proven stimuli include AT hypoxia (64) and adipocyte death (65), both of which occur during obesity and have been shown to trigger inflammation through as yet unclear molecular pathways.
To date, few studies have evaluated macrophage activation and/or phenotype in obese humans. In one human study, M1 ATMs were localized to CLS surrounding adipocytes, expressed higher levels of proinflammatory genes including IL1B, IL6, IL8, TNFA, and CCL3, and were enriched for transcripts encoding mitochondrial, proteasomal, and lysosomal proteins, as well as fatty acid metabolism enzymes and T cell chemoattractants (66). These findings not only suggest that M1 macrophages in humans are proinflammatory and cluster around single adipocytes, as demonstrated in mice (13), but also indicate that they may play a role in lipid scavenging, as in mice (46), and antigen presentation to T cells, thus promoting an adaptive immune response. This study also demonstrated a direct association between the M1/M2 ratio and IR. Another study in non-obese humans with and without T2D found no association between M1/M2 ratio and T2D or insulin sensitivity, but did find a significant direct association with adipose cell size (67). A third study in non-obese humans showed that CD14+ macrophages in SAT, quantified by flow cytometry, correlated with central obesity, liver fat, and IR. Additionally, the expression of the macrophage activation marker CD11b correlated with expression of MCP-1 in SAT (68). Interestingly, weight gain of 2.7 kg over 28 days did not induce inflammation in AT despite an 11% worsening of IR. On the other hand, weight loss following bariatric surgery in morbidly obese subjects was associated with decreases in markers of activated macrophages and proinflammatory T cells in SAT (69) and peripheral blood (70–72). Independent of body weight, limited studies have shown a relationship between inflammation in human AT and IR, with greater frequency of mononuclear cells and expression of genes related to macrophage recruitment and activation including CD68, MCP1, IL6, and IL8 (73). Of note, the frequency of CLSs in human obesity is lower than in obese mice, which may reflect the fact that most human studies are cross-sectional (73), whereas obese mice are typically studied after weight perturbation.
Adaptive immunity
While most investigations of inflammation, obesity, and IR have focused on the role of macrophages, recent studies point to an important role for the adaptive immune system. T and B lymphocytes rely on antigen recognition and account for diverse populations of immune cells with either primarily proinflammatory or regulatory functions. Several studies have demonstrated that disease progression and severity are highly correlated with proinflammatory T and B cell phenotypes (74–77). Lymphocytes account for up to 10% of non-adipocyte cells in human AT and include T cells, B cells, NK cells, NKT cells, and ILC2s (67). T and B lymphocytes, along with macrophages, are present in CLSs surrounding necrotic adipocytes (78, 79). Rag-knockout mice, which are deficient in mature lymphocytes, exhibited greater weight gain associated with adipocyte hypertrophy and severely impaired glucose tolerance compared with WT mice fed a HFD (75). DIO mice lacking αβ T and B cells had substantially worsened inflammation in VAT and skeletal muscle as well as severe glucose intolerance compared with WT DIO mice (80). In accord with this observation, the predominant impact of T cells on glucose homeostasis, revealed by CD4+ T cell reconstitution studies in lymphocyte-free DIO mice, was improvement of glucose tolerance, enhanced insulin-sensitivity, and reduced weight gain (80). Nonetheless, treatment with either an anti-CD3 T cell–depleting or –non-depleting antibody or anti-CD20 B cell–depleting antibody led to reversal of IR in DIO mice (75, 81). Together, these data implicate both arms of the adaptive immune system in AT inflammation, systemic IR, and glucose dysregulation in the setting of caloric excess and obesity.
CD4+ T cells
CD4+ Th cells recognize antigen presented by MHC class II molecules on APCs such as macrophages, DCs, and B cells. Once activated, Th cells direct other immune cells to sites of infection or insult. CD4+ T cells are classified according to their signature cytokines as follows: Th1 cells by IFN-γ; Th17 cells by IL-17; Th2 cells by IL-4 and IL-13; and Tregs, which are identified on the basis of their expression of the Foxp3 transcription factor, secrete IL-10. Th1 and Th17 cells are considered to be proinflammatory, whereas Tregs and Th2 cells are generally antiinflammatory. In addition to their signature cytokines, Th cells secrete a myriad of other cytokines. Th1 cells are induced by IL-12 and IFN-γ and secrete the proinflammatory cytokines IFN-γ and TNF-α, which promote macrophage migration and differentiation into the proinflammatory M1 phenotype. Th2 cells are induced by IL-4 and secrete antiinflammatory cytokines IL-1, IL-4, IL-5, IL-10, and IL-13. Th17 cells secrete, in addition to IL-17, IL-21 and IL-22, and Tregs secrete IL-10 and TGF-β.
Recent studies in mice provide compelling support for a causal role of CD4+ Th1 cells in the development of obesity-associated IR. IFN-γ–expressing CD3+ and CD4+ cells increase in obesity (75, 82), and whole-body IFN-γ deletion improves obesity-induced IR and lowers macrophage infiltration in AT (82). Placing mice on a HFD produces a dramatic increase in VAT Th1 cells over static numbers of Th2 and Treg cells, in association with the development of glucose intolerance and localized inflammation in AT. Furthermore, T cell transfer into lymphocyte-free Rag1-null DIO mice reverses IR predominantly through the actions of Th2 cells. In addition, treatment of obese WT mice with CD3-specific antibody or its F(ab’)2 fragment reduces the predominance of Th1 cells over Tregs, reversing IR for months despite continuation of a HFD (81). Interestingly, lymphocyte transfer of CD4+ T cells from Stat6–/– mice, which have normal Th1 but impaired Th2 development, did not reverse IR in Rag1-null DIO mice, suggesting that Th2 cells protect against IR (75, 82). Finally, several studies have shown antigenic bias in the T cell receptor (TCR) of AT-associated but not lymphoid-associated CD4+ T cells, suggesting that AT T cells may undergo clonal expansion in response to specific, as yet unknown antigens (75, 83). Restriction of the TCR repertoire was shown to be more pronounced in CD4+ and CD8+ T cells in VAT as compared with SAT of DIO mice, and in obese versus lean mice, supporting the hypothesis that obesity leads to antigenic stimulation of T cells and may differ by fat depot (81). Human studies support the mouse data, with the frequency of Th2 cells in SAT, VAT, and blood correlating inversely with IR, and Th2 cells in VAT correlating inversely with plasma hsCRP. Conversely, the frequency of Th1 cells in SAT and VAT correlate directly with plasma hsCRP (84). Another human study documented an association between increasing obesity and CD4+ T cells in AT but not blood (85), although no correlation with markers of systemic inflammation was seen in this study.
CD4+ Th17 cells secrete IL-17, among other proinflammatory cytokines, which plays a role in mediating inflammation in autoimmune diseases such as psoriasis via activation of NF-κB (86). In vitro, IL-17 inhibits insulin-stimulated glucose uptake by skeletal muscle and hepatocytes and impairs adipocyte differentiation (87, 88). Surprisingly, studies in mice do not show a causal role for IL-17 in IR (89). In humans, however, several studies show that IL-17 is correlated with the severity of diabetes (90), is increased in association with IR, and inhibits glucose uptake in cultured human hepatocytes (91). Further, multiple Th17-related cytokines, including those not specific to Th17 cells (e.g., IL-21, IL-9), are more highly secreted by peripheral blood mononuclear cell (PBMC) in response to T cell–targeted stimuli αCD3/αCD28 in individuals with T2D than in controls (92).
Tregs
Tregs play a role in self-tolerance and decrease inflammation by suppressing autoreactive T cells and inflammatory macrophages. Upon induction of obesity in HFD-fed mice, Tregs in VAT decrease dramatically and are visible within CLSs in close contact with macrophages and other lymphoid cells (75, 93). In one study, induction of Tregs with IL-2 in DIO mice improved insulin sensitivity, reduced macrophage number, and lowered expression of TNF-α (75). However, another study in DIO mice failed to confirm a role for Tregs in promoting insulin sensitivity and suggested that Tregs may actually promote IR (94). Moreover, two cross-sectional studies in humans showed that expression of the Treg marker FoxP3 was increased in obese versus lean humans (85, 95). Two other studies have evaluated Tregs via flow cytometry in subcutaneous versus omental depots with differing results. One found significantly lower Tregs in omental fat, which correlated inversely with fasting glucose and MCP-1 but correlated positively with IR (96), whereas the other showed no difference in Treg numbers between depots and no association with IR as quantified by the modified insulin suppression test (84). Thus, the role of Tregs in obesity-associated inflammation and IR is unresolved.
CD8+ T cells
The primary function of CD8+ T cells is to kill infected or foreign cells by releasing perforin and granzyme B and secreting inflammatory cytokines. Obesity increases the frequency of CD8+ T cells in VAT and induces IFN-γ and granzyme B expression. CD8+ T cell increases in AT are seen within two weeks of HFD initiation in mice and continue until week 15, preceding both reductions in CD4+ T cells and Tregs and an increase in macrophages. Genetic and immunologic depletion of CD8+ T cells effectively lower proinflammatory M1 (but not M2) macrophage infiltration, AT inflammation, and systemic IR in mice. Furthermore, adoptive transfer of CD8+ T cells to CD8-deficient mice increased AT inflammation and IR (76). Conversely, another study demonstrated that blocking activation of T cells using anti-CD40L or CTLA-4–Ig reduced activated CD8+ T cells and inflammatory macrophages in AT, but no change was seen in whole body insulin sensitivity (97). These data indicate that CD8+ T cells may play a role in initiating inflammation in AT in obesity, and possibly also in modulating IR. Similar to mice, in obese humans, CD8+ T cells were noted to be more frequent in VAT as compared with SAT, but their frequency in these depots did not correlate with systemic IR (84). Furthermore, in a cross-sectional study in lean to moderately obese men, CD8+ T cells in SAT did not correlate with adiposity, although markers of activation (CD25 and CD69) were correlated with waist circumference (85).
ILCs
ILCs are a family of innate immune cells that mirror T cells. Among them, ILC2s are resident in VAT and play critical roles in regulating metabolic homeostasis.ILC2s are a major source of the Th2-associated cytokines, IL-5 and IL-13, in response to stimulation with IL-33 and IL-25. ILC2s promote the accumulation of eosinophils and alternatively activated macrophages to maintain glucose homeostasis through production of IL-5 and IL-13 (36). Additionally, ILC2s were recently found to contribute to AT beiging (98, 99). One study demonstrated that active ILC2s increased numbers of beige adipocytes in WAT by producing methionine-enkephalin peptides (98), while another study showed that activated ILC2s result in proliferation of adipocyte precursors and subsequent beige lineage commitment through ILC2- and eosinophil-derived IL-4 and IL-13 (99).
B cells
Like T cells, B cells have been shown to play an important role in the regulation of glucose metabolism, based mainly on studies in DIO mice. Thus, obesity leads to greater infiltration of B cells into VAT, particularly class-switched mature IgG+ B cells (81). Moreover, transfer of IgG from obese IR mice to young mice on a HFD/high-calorie diet resulted in accelerated development of AT inflammation and IR (68). In addition to their prominent role in antibody production, B cells can produce a wide range of cytokines and they can present antigens to T cells. Peripheral B cells in the blood of diabetic patients exhibit a proinflammatory phenotype, with enhanced IL-8, IL-6, and TNF-α production and dampened IL-10 secretion (90, 100). A similar trend has been found in splenic B cells in DIO mice (90, 100).
In the context of IR, B cell–knockout mice have improved insulin sensitivity and glucose tolerance compared with WT DIO mice. In one study DIO B cell–deficient (μMT) mice had a lower proinflammatory cytokine profile in the serum as well as an influx of Tregs in the VAT and spleen (100). Another study showed that B cell deficiency directly correlated with a reduction of TNF-α–producing M1 macrophages in VAT (81). When DIO mice were treated with a CD20-depleting antibody, a similar trend was observed with decreased TNF-α–producing macrophages (81). To assess whether the phenotypic change in B cells was correlative with obesity and disease, B cells from DIO mice were adoptively transferred into μMT mice. Transfer of B cells from obese but not lean mice resulted in diminished glucose tolerance and insulin sensitivity (81). These findings suggest that obesity fosters aberrant activation of B cells that may promote IR.
Two groups recently discovered an important role for populations of IL-10-producing regulatory B cells (Bregs) in the regulation of glucose metabolism (74, 101). Although these cell populations are not identical, both are more frequent in lean mice than obese mice, and their adoptive transfer into DIO mice ameliorated IR in an IL-10-dependent manner. Conversely, transfer of B-2 cells, which generate the majority of high affinity antibodies during an antigen challenge, led to worsened glucose metabolism in B cell-knockout mice, while removal of these cells from DIO mice led to improved glucose metabolism (74).
B cell antigen presentation can also promote IR. When B cells from obese mice that were wild type or deficient in MHC-1 or MHC-II were adoptively transferred to DIO mice, MHC-I or –II deficiency in B cells rescued disease, while transfer of WT B cells worsened IR (81). Overall, these studies support the notion that B cell subsets, like T cell subsets, can play both pathogenic and protective roles in IR and T2D via multiple mechanisms.
Molecular mechanisms of IR
Many mouse models have demonstrated that manipulating various components of the innate and/or adaptive immune system induces or ameliorates not only inflammation but also systemic IR and/or glucose intolerance. Several molecular pathways have been defined that explain this link. Multiple cytokines secreted by activated immune cells have been shown to impair insulin signaling via stimulation of stress kinases including IKKB and JNK1. These cytokines can have local effects on adipocytes or resident immune cells in AT or can circulate to peripheral tissues such as liver and muscle, where they exert their effects on insulin target cells. IL-6, TNF-α, and IL-1β as well as LPS, FFAs, and ER stress have all been shown to activate IKKB and/or JNK1, which are expressed in both myeloid cells and insulin target cells such as adipocytes, hepatocytes, and myocytes. Activation of these stress kinases impairs insulin action via phosphorylation of IRS1 on inhibitory serine instead of stimulatory tyrosine residues, thus blocking downstream insulin signaling (102). IKKB also promotes further inflammation by activating NF-κB, which is sequestered in the cytoplasm of myeloid and insulin-targeted cells by IκB. Upon activation, IκB is degraded, allowing NF-κB to translocate to the nucleus, where it promotes transcription of inflammatory genes such as pro–IL-1β, TNF-α, MCP-1, and IL-6. Support for a role of these kinases in obesity-induced IR resides in numerous reports of increased NF-κB (20) and JNK1 (19) activity in obese animals. Knockout studies of IKKB showed partial reversal of IR, including myeloid- (18) and liver-specific (103) strains. Mice in which JNK1 is knocked out or is in peripheral (non-hematopoietic) cells or AT only are protected from IR, whereas myeloid-specific deletion of JNK1 yields mixed results (104–106). Overall, there is strong evidence of a link between inflammatory cytokines and other obesity-related stimuli such as FFA/TLR4 signaling and activation of pathways that directly impair insulin action.
Clinical implications
A number of small clinical trials with antiinflammatory agents have yielded generally disappointing results, although in general the size of these trials has been small and the treatment duration short. Although a 19th century report suggested that high doses of salicylates, which inhibit IKKB/NF-κB, can reduce hyperglycemia in patients with diabetes (107), recent clinical trials with salsalate demonstrated only modest reductions in glycated hemoglobin A1C (0.37%) and fasting glucose (6%) in patients with T2D (108, 109), which may have been due to decreased insulin clearance rather than improved insulin sensitivity (110, 111). Similarly, small trials with TNF-α–neutralizing antibodies have demonstrated modest effects on glucose levels and mixed improvement in insulin sensitivity in obese patients with (112, 113) and without rheumatic disease (114, 115), and IL-1β blockade showed modest improvement in glycemic control that was attributed to enhanced β cell function rather than enhanced insulin sensitivity (116). Treatment with IL-6 antagonists has been associated with improvement in IR in patients with rheumatologic disease (117), but this effect is confounded by improvement in overall clinical state, and studies in subjects without rheumatologic disease are lacking. Conceivably, there are fundamental differences between humans and mice with respect to the role of inflammation in IR and T2D, but it is more likely that the limited amount of clinical efficacy data is explained by the dearth of appropriate clinical trials. Therefore, additional clinical studies of larger size and longer duration, evaluating immunomodulatory therapies in patients without underlying chronic inflammatory conditions, will be needed in order to ascertain whether disruption of inflammatory pathways can reverse IR and/or treat T2D.
Summary and conclusions
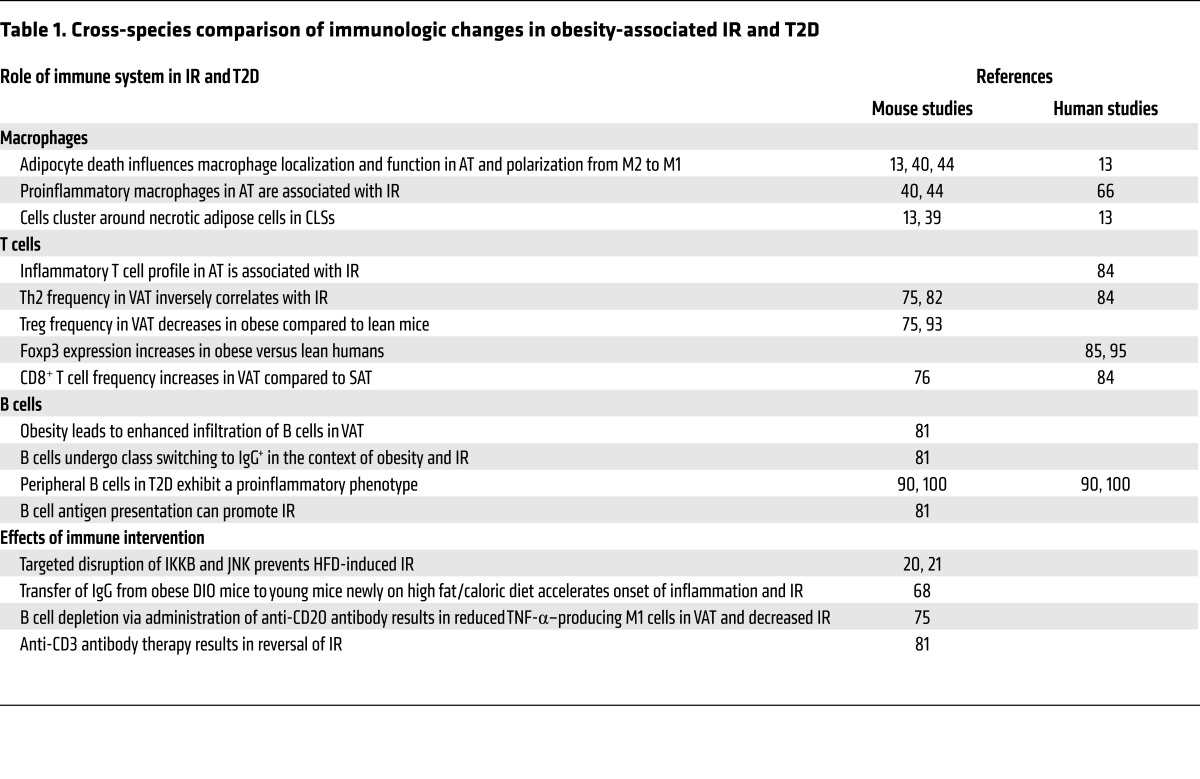
Extensive experimentation in animal models demonstrates that both the innate and adaptive arms of the immune system play critical roles in the regulation of glucose metabolism, with participation from multiple cell types. In particular, inflammation involving AT and the intestine appears to be critical to the development of IR. A schematic overview of the cells involved and their putative roles in both insulin sensitivity and IR is shown in Figure 1. Although studies of the immune system in humans with obesity-associated IR are limited, these studies have generally, but not always, yielded results consistent with those in experimental animals (Table 1). Despite the paucity of controlled clinical trials designed to evaluate antiinflammatory agents or more targeted immune therapies in obesity-associated IR or T2D, animal data indicate that IR is a reversible condition susceptible to immune manipulation. Therefore, evaluation of immunomodulatory approaches in humans to reverse IR and T2D is warranted.
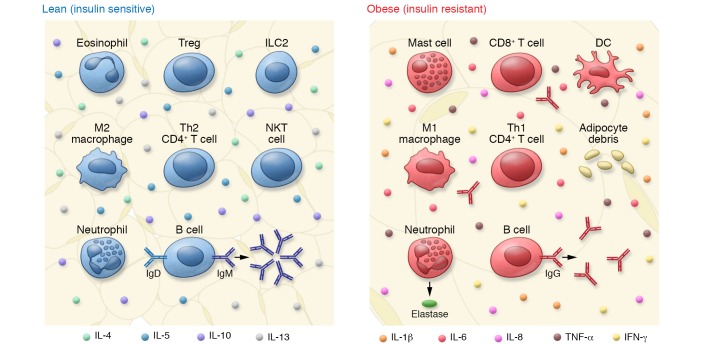
Figure 1. Changes in immune cell content and function in AT in lean versus obese settings influence IR.
In lean AT, immune cells play a predominant role in maintaining an antiinflammatory environment. For example, Th2 cells produce antiinflammatory cytokines including IL-4 and IL-10, which promote M2 macrophage polarization, and B cells produce IgM, which produces antiinflammatory IgM. During the course of obesity, significant changes in immune cell content and function occur that promote inflammation. Accumulation of M1 macrophages and Th1 cells results in excess production of proinflammatory cytokines including IFN-γ and TNF-α, and B cells undergo a class-switch, producing IgG.
Table 1. Cross-species comparison of immunologic changes in obesity-associated IR and T2D.
Acknowledgments
This work was supported by NIH grant DK096038. S.E. Ackerman receives financial support from the Stanford Bio-X Bowes Fellowship.
Footnotes
Conflict of interest: T. McLaughlin has an interest in Eiger Biopharmaceuticals in the form of intellectual property, company ownership, and consulting fees. E. Engleman has equity in Eiger Biopharmaceuticals, Bolt Biotherapeutics, and Medeor Therapeutics.
Reference information:J Clin Invest. 2017;127(1):5–13. doi:10.1172/JCI88876.
Contributor Information
Tracey McLaughlin, Email: tmclaugh@stanford.edu.
Lei Shen, Email: lshen@shsmu.edu.cn.
Edgar Engleman, Email: edengleman@stanford.edu.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Engelgau MM, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation. IDF Diabetes Atlas. IDF Web site. http://www.idf.org/diabetesatlas Accessed October 7, 2016.
- 4.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 7.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13(10):674–682. doi: 10.1016/S1047-2797(03)00053-X. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 10.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89(11):5577–5582. doi: 10.1210/jc.2004-0603. [DOI] [PubMed] [Google Scholar]
- 12.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Chen A, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13(8):1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 15.Kamei N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 16.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 19.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 20.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 21.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103(28):10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bäckhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Membrez M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22(7):2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 26.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caesar R, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. T-lymphocyte responses to intestinally absorbed antigens can contribute to adipose tissue inflammation and glucose intolerance during high fat feeding. PLoS One. 2010;5(11):e13951. doi: 10.1371/journal.pone.0013951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luck H, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21(4):527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Shulzhenko N, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17(12):1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanovic-Racic M, et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61(9):2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertola A, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrante AW. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 39.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeyda M, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31(9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 42.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kratz M, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 45.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prieur X, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 48.Spite M, et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187(4):1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah R, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60(5):1512–1518. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu I, et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 2013;18(4):491–504. doi: 10.1016/j.cmet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Finucane OM, et al. Macrophage migration inhibitory factor deficiency ameliorates high-fat diet induced insulin resistance in mice with reduced adipose inflammation and hepatic steatosis. PLoS One. 2014;9(11):e113369. doi: 10.1371/journal.pone.0113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramkhelawon B, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20(4):377–384. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Akira S. TLR signaling. Sˀemin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Kim JK. Fat uses a TOLL-road to connect inflammation and diabetes. Cell Metab. 2006;4(6):417–419. doi: 10.1016/j.cmet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niemi K, et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186(11):6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 62.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajamäki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 65.Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 66.Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acosta JR, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–570. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]
- 68.Tam CS, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59(9):2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cancello R, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 70.Kazankov K, et al. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J Gastroenterol Hepatol. 2015;30(8):1293–1300. doi: 10.1111/jgh.12943. [DOI] [PubMed] [Google Scholar]
- 71.Samaras K, Viardot A, Botelho NK, Jenkins A, Lord RV. Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia. 2013;56(12):2564–2572. doi: 10.1007/s00125-013-3033-7. [DOI] [PubMed] [Google Scholar]
- 72.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab. 2010;95(6):2845–2850. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 73.McLaughlin T, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51(12):2303–2308. doi: 10.1007/s00125-008-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen L, Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance. Diabetes. 2015;64(2):593–603. doi: 10.2337/db14-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 77.Wong N, et al. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology. 2011;152(10):3690–3699. doi: 10.1210/en.2011-0288. [DOI] [PubMed] [Google Scholar]
- 78.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32(3):451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 79.McDonnell ME, et al. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring) 2012;20(7):1372–1378. doi: 10.1038/oby.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan IM, et al. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis. 2014;233(2):419–428. doi: 10.1016/j.atherosclerosis.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103(5):467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaughlin T, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–2643. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Travers RL, Motta AC, Betts JA, Bouloumié A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes (Lond) 2015;39(5):762–769. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Zúñiga LA, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185(11):6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmed M, Gaffen SL. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Krüppel-like factors. Cytokine. 2013;61(3):898–905. doi: 10.1016/j.cyto.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng C, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med. 2012;90(2):175–186. doi: 10.1007/s00109-011-0816-5. [DOI] [PubMed] [Google Scholar]
- 90.Jagannathan-Bogdan M, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186(2):1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fabbrini E, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145(2):366–374.e1. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ip B, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity (Silver Spring) 2016;24(1):102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bapat SP, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pereira S, et al. Modulation of adipose tissue inflammation by FOXP3+ Treg cells, IL-10, and TGF-β in metabolically healthy class III obese individuals. Nutrition. 2014;30(7–8):784–790. doi: 10.1016/j.nut.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 96.Gyllenhammer LE, et al. Lower omental t-regulatory cell count is associated with higher fasting glucose and lower β-cell function in adults with obesity. Obesity (Silver Spring) 2016;24(6):1274–1282. doi: 10.1002/oby.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montes VN, et al. T cell activation inhibitors reduce CD8+ T cell and pro-inflammatory macrophage accumulation in adipose tissue of obese mice. PLoS One. 2013;8(7):e67709. doi: 10.1371/journal.pone.0067709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeFuria J, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishimura S, et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 2013;18(5):759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 102.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 103.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3(9):e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han MS, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baron SH. Salicylates as hypoglycemic agents. Diabetes Care. 1982;5(1):64–71. doi: 10.2337/diacare.5.1.64. [DOI] [PubMed] [Google Scholar]
- 108.Goldfine AB, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56(4):714–723. doi: 10.1007/s00125-012-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goldfine AB, et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152(6):346–357. doi: 10.7326/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hundal RS, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109(10):1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim SH, et al. Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study. Diabetes Care. 2014;37(7):1944–1950. doi: 10.2337/dc13-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Gay MA, et al. Anti-tumor necrosis factor-α blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(1):83–86. [PubMed] [Google Scholar]
- 113.Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ. Improved insulin sensitivity by anti-TNF-α antibody treatment in patients with rheumatic diseases. Ann Rheum Dis. 2007;66(4):558–559. doi: 10.1136/ard.2006.062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stanley TL, et al. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dominguez H, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42(6):517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 116.van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96(7):2119–2126. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- 117.Schultz O, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5(12):e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]