Abstract
Obesity is associated with chronic inflammation, which contributes to insulin resistance and type 2 diabetes mellitus. Under normal conditions, skeletal muscle is responsible for the majority of insulin-stimulated whole-body glucose disposal; thus, dysregulation of skeletal muscle metabolism can strongly influence whole-body glucose homeostasis and insulin sensitivity. Increasing evidence suggests that inflammation occurs in skeletal muscle in obesity and is mainly manifested by increased immune cell infiltration and proinflammatory activation in intermyocellular and perimuscular adipose tissue. By secreting proinflammatory molecules, immune cells may induce myocyte inflammation, adversely regulate myocyte metabolism, and contribute to insulin resistance via paracrine effects. Increased influx of fatty acids and inflammatory molecules from other tissues, particularly visceral adipose tissue, can also induce muscle inflammation and negatively regulate myocyte metabolism, leading to insulin resistance.
Introduction
Obesity is becoming a global epidemic, increasing the health burden of associated complications of insulin resistance and diseases such as cardiovascular disease (1). Because insulin resistance leads to type 2 diabetes (T2D) (2), T2D incidence and prevalence are also increasing rapidly. The number of adults with diagnosed diabetes in the United States nearly quadrupled over 32 years, from 5.5 million in 1980 to 21.3 million in 2012; 90% to 95% of these individuals have T2D (3).
Molecular links between obesity and insulin resistance and T2D remain incompletely understood but may include chronic inflammation, particularly in adipose tissue (AT) (4–8). AT inflammation may contribute to whole-body insulin resistance and T2D via the endocrine effects of inflammatory molecules secreted by AT (known as adipokines) on insulin sensitivity in various tissues, particularly skeletal muscle (SM) and liver. Additionally, dysregulation of preadipocyte/adipocyte functions accelerates fat spillover from AT to SM and liver, resulting in ectopic fat deposition and insulin resistance in these tissues, which contribute to systemic insulin resistance and T2D (5, 7, 9–13). SM is the most important organ for whole-body glucose homeostasis (14, 15) and is responsible for approximately 80% of insulin-stimulated whole-body glucose uptake and disposal under normal conditions (15–18). Insulin resistance in SM is the major defect in T2D (16–18) and is therefore central to systemic insulin resistance and T2D.
While studies have focused on the roles of intramyocellular lipids, mitochondrial defects, and endocrine effects of adipokines on SM insulin resistance (10, 12, 15), emerging evidence indicates that inflammation also occurs in SM in the setting of obesity and may exert autocrine or paracrine effects on myocyte metabolic functions. In this Review we focus on obesity-linked SM inflammation and its roles in muscle insulin resistance.
Inflammation in SM
Although obesity-linked inflammation is less well studied and documented in SM than in AT, available evidence suggests that SM myocytes can secrete large numbers of cytokines and other molecules and may become inflamed in obesity. In addition, immune cells can infiltrate into SM and increase SM inflammation in obesity.
SM as a secretory organ and myocyte secretion of inflammatory molecules.
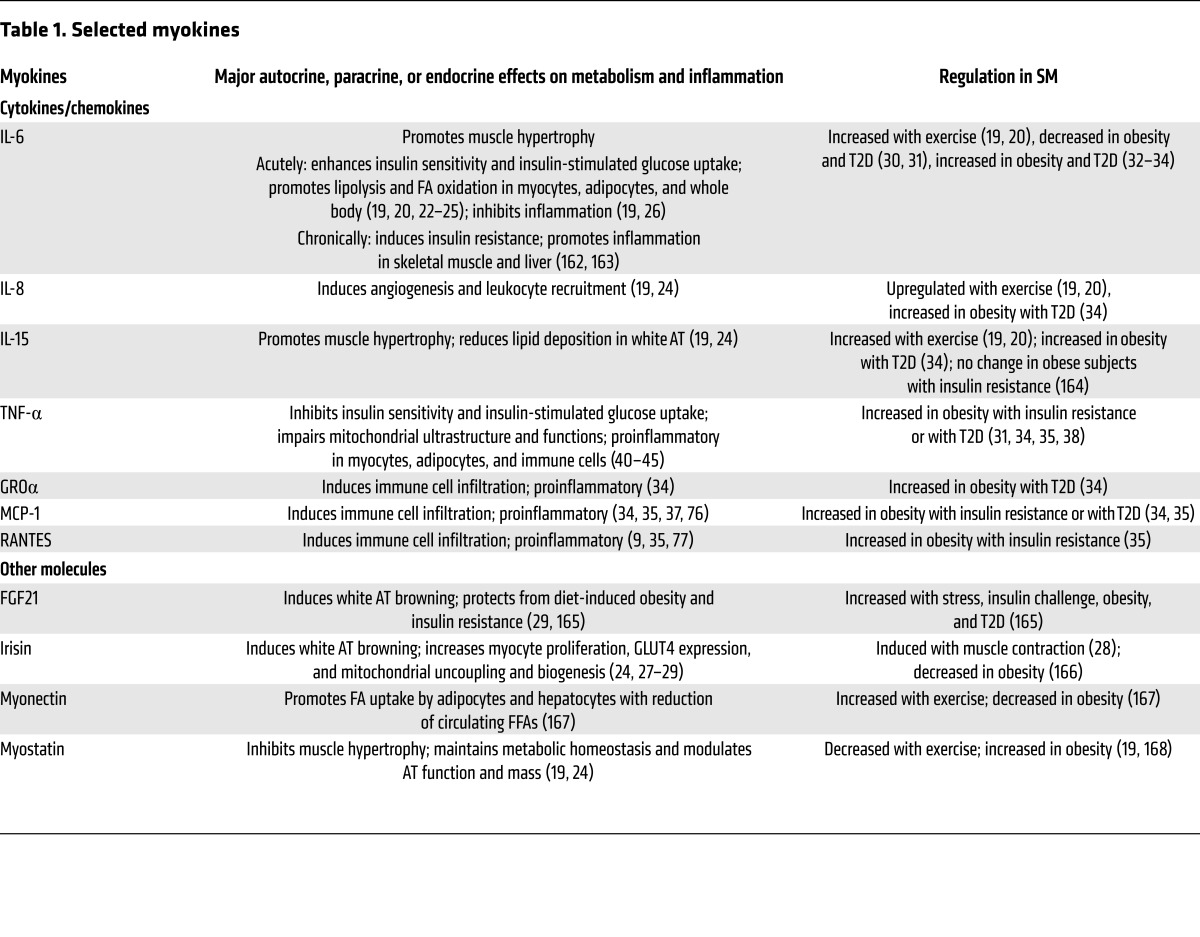
Similar to adipocytes, SM myocytes express and secrete numerous cytokines such as IL-6, IL-8, and IL-15 and other molecules such as FGF21, irisin, myonectin, and myostatin (known as myokines; see Table 1 and refs. 19, 20). Whereas most adipokines are proinflammatory, regulated by obesity, and involved in the development of obesity-linked metabolic dysfunction (4, 5, 11), most myokines are regulated mainly by exercise and muscle extraction, counteract the detrimental effects of adipokines, and have beneficial effects on glucose and lipid metabolism and inflammation (19, 20).
Table 1. Selected myokines.
Myokines may affect myocytes and immune cells locally via autocrine or paracrine actions and other cells such as adipocytes and hepatocytes via endocrine effects. IL-6 is the most well-studied myokine. Exercise and muscle extraction dramatically enhance IL-6 secretion from muscle and can increase plasma IL-6 levels up to 100-fold (19–21). Acute treatment of myocytes or intravenous infusion of healthy humans with IL-6 increases basal and insulin-stimulated glucose uptake by myocytes and improves whole-body insulin sensitivity (19, 22). IL-6 also increases lipolysis and fatty acid (FA) oxidation in myocytes and adipocytes; induces UCP1 expression in mouse white AT, which is indicative of browning (20, 22–25); and mediates antiinflammatory effects by inducing expression of antiinflammatory cytokines such as IL-10 and inhibiting expression of proinflammatory cytokines such as TNF-α (19, 26). However, IL-6 is generally considered proinflammatory and can induce insulin resistance, particularly under chronic conditions related to obesity (see below). Irisin, another myokine enhanced by muscle contraction, may increase glucose transporter 4 (GLUT4) expression and mitochondrial uncoupling and biogenesis in myocytes, and also induce browning of white AT (24, 27–29).
Studies on the effects of obesity on myokine expression are limited and have had inconsistent results. For example, obesity in rats decreased IL-6 and IL-15 expression in SM (30). Similarly, cultured myocytes from SM of obese subjects with impaired glucose tolerance or T2D expressed lower IL-6 levels than those from healthy controls (31). However, more studies found increased IL-6 expression or release in SM of obese subjects with impaired glucose tolerance or T2D compared with healthy controls (32–34). Differentiated myocytes can express numerous proinflammatory molecules (Table 1), particularly under stimulation of inflammatory cytokines and free FAs (FFAs) (32, 34–37). Differentiated cultured myocytes isolated from obese subjects with insulin resistance or T2D secrete more cytokines such as TNF-α and chemokines such as monocyte chemoattractant protein 1 (MCP-1) than myocytes from lean controls (31, 34, 38). Higher TNF-α levels were also observed in SM of rats fed a fructose-rich diet (39), and TNF-α can induce insulin resistance and mitochondrial dysfunction in myocytes (40–45). Thus, the obesity-linked increases in TNF-α secretion by myocytes may contribute to myocyte insulin resistance via autocrine effects. Therefore, obesity is associated with increased inflammation in myocytes, which may secrete elevated levels of proinflammatory molecules and contribute to muscle inflammation. Nevertheless, changes in myocyte secretion of cytokines do not appear to constitute the major component of SM inflammation in obesity (see below), and the role of various myokines in SM inflammation remains to be further investigated.
Infiltration of immune cells into SM.
Increased immune cell infiltration is the main characteristic of obesity-linked inflammation in AT (4, 5, 9, 11, 46–51). Growing evidence indicates that immune cells also accumulate in SM and may constitute the predominant inflammatory cells in SM in obesity (2, 11, 35, 52, 53). Increased macrophage and T cell levels have been reported in SM of obese humans with insulin resistance or T2D (35–37, 53–55). In fact, a short-term high-fat, high-calorie diet or overfeeding with induction of insulin resistance increased macrophage markers in SM in healthy subjects (56, 57). In mice, obesity and insulin resistance induced by a high-fat diet (HFD) was consistently associated with increased accumulation of immune cells including macrophages and T cells in SM (11, 35, 37, 47, 49, 53, 58–62). Similar to humans, mice fed a short-term HFD have increased macrophage content in SM (35, 53, 60). Mast cells and eosinophils were observed in mouse SM but showed no changes with obesity (53, 63). Changes in other immune cells including neutrophils, B cells, NK cells, and invariant NKT (iNKT) cells, which are found in visceral AT (2, 13, 64), have not been reported in SM in the setting of obesity.
Histologically, macrophages and T lymphocytes are primarily located in muscle AT between myocytes or surrounding the muscle, so-called intermyocellular/intermuscular AT (IMAT) or perimuscular AT (PMAT) (11, 35, 47, 49, 59). Both IMAT and PMAT are adjacent to myocytes and differ from subcutaneous AT (65). Both are extramyocellular fat that expands substantially in obesity and decreases following weight loss (66), and both depots are highly correlated with insulin resistance and expression of MCP-1 and C-reactive protein (65, 67–70). Macrophages and T cells within these adipose depots are markedly increased in obesity (35, 49, 53) and can form crown-like structures surrounding dead or dying adipocytes (35). Additionally, macrophages and T lymphocytes can be found in SM between myofibers at a lower frequency (35–37, 53). Obesity-linked changes in immune cells and inflammatory markers are much greater in muscle AT than in muscle (35), which may help explain the low levels of immune cells and inflammation in SM in human subjects with small-muscle biopsies (71), as well as why alterations in BMI or lifestyle intervention–induced weight loss do not alter macrophage numbers in SM in obese subjects in some studies (72, 73).
Similar to those in visceral AT, immune cells in SM tend to polarize into proinflammatory phenotypes in obesity. Most macrophages in SM are CD11c+ and display classically activated (M1-like) phenotypes (35, 37, 47, 53, 62). Both CD4+ and CD8+ T cells are increased in SM of obese mice. While the proportion of IFN-γ–expressing Th1 cells is increased, the proportion of Tregs is decreased in SM in mice with obesity (35). Accordingly, proinflammatory markers related to immune cell activation such as TNF-α, IL-1β, and IFN-γ are increased (32, 33, 35, 37, 47, 53, 58, 60, 61), while antiinflammatory markers such as IL-10 are reduced in SM in obesity (60). Although in vitro studies show capacity of differentiated myocytes to express proinflammatory molecules (32, 34–37), studies in mouse models of obesity indicate that levels of most proinflammatory markers are much higher and show greater obesity-linked changes in PMAT than in muscle (35, 37), suggesting that in vivo obesity-linked SM proinflammatory molecules may be mainly derived from immune cells in muscle adipose depots.
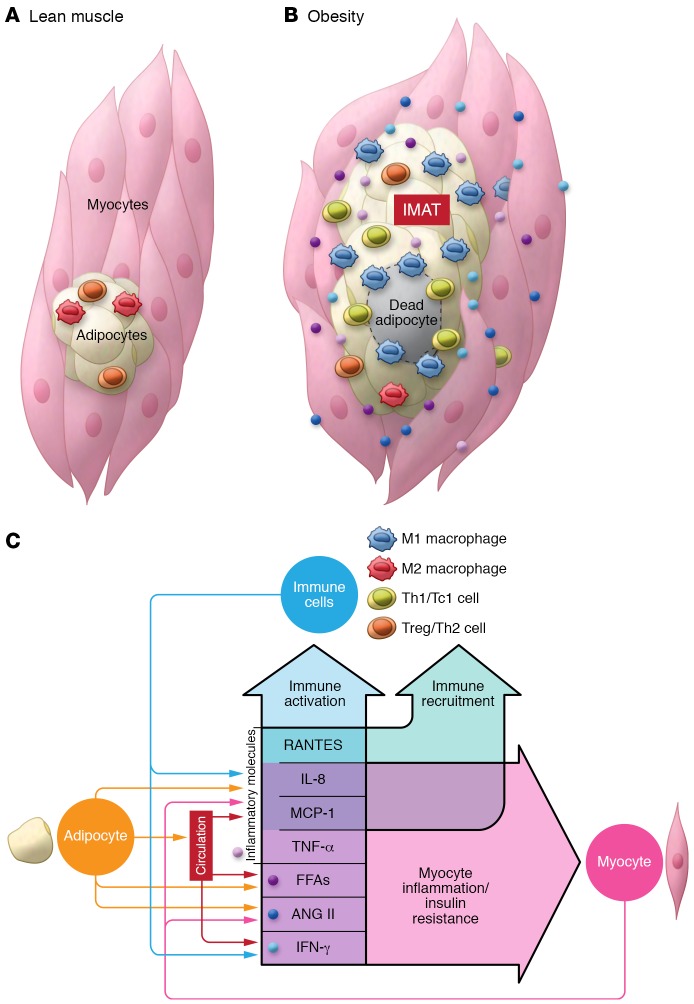
Taken together, compelling evidence supports the association of obesity with increased inflammation in SM in both humans and rodents. Myocytes have the capacity to express cytokines and may secrete more proinflammatory cytokines in obesity. However, increased SM inflammation in obesity may mainly result from increased infiltration of immune cells, particularly macrophages and T lymphocytes that are primarily localized in muscle adipose depots (IMAT/PMAT) and tend to polarize into proinflammatory phenotypes (Figure 1).
Figure 1. Inflammation in skeletal muscle in obesity.
(A) In lean conditions, few immune cells with resting or antiinflammatory phenotypes reside in skeletal muscle. (B) As obesity develops and progresses along with expansion of visceral and subcutaneous AT, adipose depots expand between muscle fibers or surrounding muscle, so-called IMAT/PMAT. In obesity, immune cells including macrophages and T cells infiltrate into IMAT/PMAT and polarize into proinflammatory phenotypes, leading to increased inflammation in skeletal muscle. At the same time, myocytes may become inflamed and express proinflammatory cytokines and chemokines. (C) Chemokines and cytokines secreted by myocytes, adipocytes, and immune cells, along with FFAs that are transferred into skeletal muscle and ANG II produced within skeletal muscle, may themselves further accelerate immune cell recruitment and activation and myocyte inflammation, forming a feed-forward loop of inflammation in skeletal muscle.
Regulation of inflammation in SM in obesity
Despite the evidence for increased SM inflammation in obesity, the underlying mechanisms remain largely unexamined. Below, we detail potential roles for various mediators in SM inflammation.
Chemokines, adhesion molecules, and immune cell infiltration.
Similar to what is observed in visceral AT (61, 74, 75), inflammation, including immune cell infiltration, starts early in SM during obesity development (35, 53, 56, 57, 60). Macrophage infiltration precedes T cell infiltration (35). Infiltration of leukocytes from the circulation into tissues requires attractant signals such as chemokines, and chemokines such as MCP-1 increase early in SM and visceral AT of mice fed a HFD. In visceral AT, the increase in MCP-1 appears to precede the increases in macrophages and the activation marker TNF-α (74, 75), suggesting that the initial increase in chemokines may derive from tissue-resident cells. Adipocytes and myocytes, the main resident cells in AT and SM, respectively, can express chemokines including MCP-1 (9, 32, 35–37, 61). Under stimulation with inflammatory molecules or FFAs or in obesity, adipocytes and myocytes secrete more chemokines (9, 32, 34–37, 61), which induce immune cell migration (9, 37, 61). Therefore, chemokines secreted by myocytes or adipocytes may play crucial roles in immune cell infiltration and inflammation in SM and visceral AT. MCP-1 overexpression in myocytes or adipocytes increases inflammation with enhanced immune cell infiltration in SM or visceral AT in mice (37, 76), while MCP-1 knockout prevents HFD-induced increases in muscle or AT macrophages (53). The RANTES/CCR5 pathway is also upregulated in SM and visceral AT in obesity (9, 35, 77) and may play a role in obesity-linked inflammation in visceral AT (77). The initiating signals that trigger SM or AT inflammation are not well known and may include FAs, particularly HFD-derived saturated FAs, which can induce expression of inflammatory molecules including chemokines in myocytes and adipocytes (34, 37). In addition to myocyte or adipocyte secretion of chemokines, as obesity progresses, recruited immune cells may also secrete chemokines, which may further increase inflammation in SM and AT.
The arachidonic acid–derived leukotriene LTB4, which is increased in SM, visceral AT, and liver of obese mice, also contributes to macrophage infiltration of visceral AT in obesity (78). Interactions of adhesion molecules on immune cells and their ligands on endothelial cells are crucial for immune cell migration. Lymphocyte function–associated antigen-1 (LFA-1), a β2 integrin mainly expressed on immune cells, plays an essential role in T cell accumulation and inflammation in SM and visceral AT of obese mice, likely by interacting with ICAM-1 on endothelial cells or antigen-presenting cells (35, 79).
While infiltration of circulating Ly-6Chi monocytes is important in obesity-linked inflammation and accumulation of proinflammatory CD11c+ macrophages in AT in mice (48, 80), the role of Ly-6Clo monocytes remains to be determined. In the circulation, Ly-6Clo, but not Ly-6Chi, monocytes express CD11c (81, 82). Circulating CD11c+/Ly-6Clo monocytes are increased with obesity and hyperlipidemia, infiltrate into atherosclerotic aortas, become CD11c+ macrophages/dendritic cells, and contribute to atherogenesis in mice (46, 81–83). Infiltration of CD11c+/Ly-6Clo monocytes likely also plays a role in CD11c+ macrophage accumulation and inflammation in visceral AT and SM in obesity. In addition, macrophages and T cells proliferate in visceral AT (79, 84, 85), and potential proliferation in SM warrants investigation.
Immune cell activation.
Macrophages and T lymphocytes not only are increased in number but also display proinflammatory phenotypes in SM and visceral AT in obesity. The tissue inflammatory milieu, including increased cytokines, macrophage/T cell interactions, and increased FFAs and metabolites, may play key roles in immune cell proinflammatory activation in obesity (Figure 1).
Cytokines and signaling pathways in immune cell activation.
Cytokines play central roles in immune cell activation. IFN-γ and TNF-α are crucial for macrophage polarization into M1 proinflammatory phenotypes, while IL-4, IL-13, and IL-10 are crucial for macrophage polarization into alternatively activated (M2) phenotypes (86). IL-12 is critical for T cell polarization to Th1, whereas IL-4 is critical for T cell polarization to Th2 phenotypes. TNF-α, the signature cytokine of M1-polarized macrophages, and IFN-γ, the signature cytokine of Th1, are both increased in SM and visceral AT in obesity and are involved in obesity-linked AT inflammation, including macrophage activation (35, 58, 87). These cytokines may also induce immune cell activation and play crucial roles in muscle inflammation. IL-10 is reduced in SM in obesity, and overexpression of IL-10 in SM attenuates obesity-induced macrophage activation in muscle (60).
TNF-α exerts proinflammatory effects mainly by activating IκB kinase/NF-κB (IKK/NF-κB) and JNK pathways. The IKK complex, which consists of the catalytic subunits IKKα and IKKβ and the regulatory subunit IKKγ, activates NF-κB transcription activity by phosphorylating and degrading the inhibitory protein IκB. Ablation of IKKβ in myeloid cells protects mice from obesity-induced inflammation (88). Activation of NF-κB in obesity also leads to increases in IKKε, a non-canonical IKK, in macrophages, adipocytes, and liver. Knockout or inhibition of IKKε in mice attenuates obesity-linked inflammation including reductions in accumulation and M1 polarization of macrophages in visceral AT and liver (89, 90).
Obesity increases JNK activity in muscle and AT (89, 91) and increases phosphorylated JNK levels in circulating monocytes (47). Ablation of JNK1 alone or both JNK1 and JNK2 in hematopoietic cells or myeloid cells dramatically decreases obesity-induced inflammation in mice (92, 93). Tissue culture studies support a crucial role of JNK in macrophage polarization to M1, but not M2, phenotypes (47, 92, 93).
IFN-γ exerts proinflammatory effects primarily through activating the JAK/STAT1 pathway. Upon binding its receptor, IFN-γ mainly activates JAK1 and JAK2, which phosphorylate and activate STAT1. STAT1 plays a pivotal role in M1 polarization and Th1 polarization (86). Short-term treatment of obese mice with a JAK1/JAK2 inhibitor decreases inflammation in SM (35), supporting an important role of the JAK/STAT pathway in obesity-linked muscle inflammation.
Cytokines may be the main mediators by which macrophages and T lymphocytes influence each other’s inflammatory status. For example, knockout of LFA-1 in mice reduces obesity-induced T cell infiltration and Th1 polarization, along with decreased IFN-γ levels, but does not change total macrophage content, in SM and visceral AT. However, macrophage expression of proinflammatory markers such as MCP-1 and TNF-α is decreased (35, 79), possibly because of reduced induction of macrophage activation by decreased Th1 cytokine in muscle.
T cells, particularly CD8+ memory T cells including those in AT, may become activated and proliferate under the stimulation of cytokines IL-12 and IL-18, which are mainly expressed by macrophages and dendritic cells and are increased in obesity (79). In addition, macrophages and dendritic cells can activate T cells through the MHC/antigen/TCR pathway. MHC-II and CD11c, which are mainly expressed on M1-like macrophages/dendritic cells, play important roles in macrophage/dendritic cell–induced T cell activation in obese AT (46, 84). Moreover, MHC-II is upregulated on obese adipocytes, which also contribute to T cell activation in obese AT (94). The potential role of these pathways in obesity-linked SM inflammation remains to be examined.
FFAs and signaling pathways in immune cell activation.
In addition to increased cytokines, increased influx of FFAs (derived from lipolysis in AT or from a HFD; see below) usually occurs in SM in obesity. FAs, particularly long-chain saturated FAs, have been consistently shown to induce inflammation, thereby also likely contributing to immune cell activation in SM in obesity. Palmitic acid or a mixture of long-chain FAs increases macrophage expression of proinflammatory molecules and induces M1 polarization, possibly via engagement of TLR2 and TLR4 and subsequent activation of NF-κB and JNK pathways (47, 92, 93, 95). In addition, palmitic acid and its metabolite ceramide activate the NLRP3 inflammasome, a cytosolic multiprotein complex that activates caspase-1, leading to maturation and secretion of the proinflammatory cytokines IL-1β and IL-18 (96). Consistently, in addition to NF-κB and JNK, TLR2/4 and the inflammasome play crucial roles in obesity-linked macrophage proinflammatory activation and inflammation (47, 95, 96).
Influx of FAs into SM and triglyceride-rich lipoproteins.
In obesity, elevated levels of circulating FFAs, mainly derived from lipolysis in adipocytes, lead to increased FA influx into SM, which not only induces inflammation in immune cells (see above) and myocytes in muscle, but also causes insulin resistance in myocytes (see below). In addition, obesity is usually associated with hypertriglyceridemia, with elevated levels of triglyceride-rich lipoproteins (TGRLs), including enterocyte-derived chylomicrons and hepatocyte-derived VLDLs, which may also release more FAs into SM and contribute to muscle inflammation and insulin resistance. Indeed, hypertriglyceridemia correlates with and may be a causal factor for insulin resistance and T2D (97). Diets enriched with saturated fat or carbohydrates tend to cause increased levels of TGRLs (98). Besides a diet high in saturated fat, a diet high in carbohydrates, particularly fructose, also induces inflammation in muscle (39, 99). In addition to the potential direct effect of high carbohydrates, elevated levels of TGRLs may contribute to muscle inflammation induced by a high-carbohydrate diet.
Under physiologic conditions, triglyceride in TGRLs is hydrolyzed by lipoprotein lipase (LPL) and releases FFAs, which are transferred into SM mainly as an energy source and into adipocytes, where they are re-esterified into triglyceride for storage (100). Increased blood TGRL levels (with no or modest changes in LPL activity; ref. 100) in obesity or increased LPL activity is expected to enhance TGRL-derived FA transfer into SM, leading to increased muscle lipid deposition and eliciting muscle inflammation. Indeed, obesity or muscle-specific overexpression of LPL increases muscle triglyceride content, with increased FA metabolites, including diacylglycerol (DAG) and ceramide, while muscle deletion of LPL decreases lipid content in SM (101–103). LPL-mediated lipid transfer appears to involve apolipoprotein E (apoE), as apoE deficiency impairs FA delivery, leading to less lipid content and decreased inflammation in muscle (58).
The renin-angiotensin system in immune cell activation.
In addition to cytokines and FAs, the renin-angiotensin system (RAS), which is activated locally in SM and AT and systemically in obesity (104, 105), has been involved in regulation of inflammation including immune cell inflammation (106–108). The classical RAS involves cleavage of angiotensinogen by renin in the circulation and formation of angiotensin I (ANG I). ANG I is converted to active ANG II by angiotensin-converting enzyme (ACE), which is mainly expressed on endothelial cells in pulmonary circulation. The nonclassical RAS involves generation of ANG 1–7 from ANG I or II by ACE2 (109, 110).
By interacting with ANG II receptors (ATRs), ANG II plays important roles in regulating blood pressure and fluid and electrolyte balance (109, 110). In addition, ANG II plays pathologic roles in fibrosis, oxidative stress, and inflammation, which all occur in obesity, via hemodynamic (blood flow reduction) or non-hemodynamic effects (109, 110). ANG II can induce activation of NF-κB, expression of MCP-1, TNF-α, and VCAM-1, and production of ROS (which activates p38 MAPK) in monocytes, endothelial cells, and cultured myocytes (106–108, 111–113). ACE inhibitors and ATR blockers (ARBs) reduce inflammation, including SM and AT inflammation induced by obesity or fructose feeding (39, 114), indicating a crucial role of ANG II in SM and AT inflammation in obesity. In contrast, ANG 1–7 exerts cellular effects mainly through the Mas receptor (109, 110) and has antiinflammatory effects including inhibition of macrophage infiltration and proinflammatory activation in AT induced by HFD or high-fructose diet (115, 116).
The impact of inflammation on insulin resistance in SM
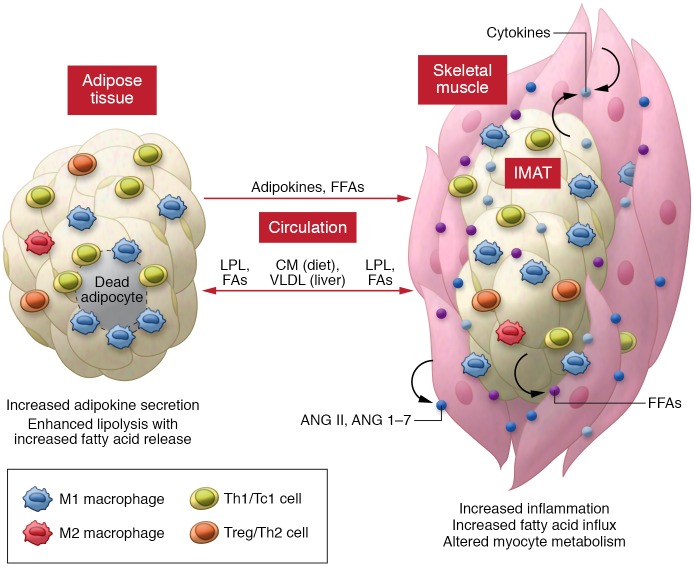
Local muscle inflammation may alter myocyte insulin sensitivity via paracrine or autocrine effects. TNF-α or conditioned medium from Th1 cells or activated macrophages decreases myocyte insulin sensitivity (35, 40, 47). In mice, local inflammation induced by muscle MCP-1 overexpression impairs muscle insulin signaling in some (37) but not all (76) studies, while local inflammation reduced by muscle-specific IL-10 overexpression improves obesity-induced muscle insulin resistance (60). In addition, inflammatory molecules from other tissues, particularly visceral AT, may influence SM metabolic functions through endocrine effects and adverse effects on adipocytes with increased release of FFAs into circulation. FFAs are then transferred from the circulation into muscle, where they induce myocyte inflammation and metabolic dysfunction. In obesity, elevated TGRLs release large amounts of FAs into SM and can also adversely affect myocyte metabolism. Moreover, activated RAS with local production of ANG II and ANG 1–7 may regulate insulin sensitivity in SM myocytes (Figure 2).
Figure 2. Inflammatory effects on myocytes in obesity.
In obesity, increased infiltration and activation of immune cells in skeletal muscle (mainly in IMAT/PMAT) and myocyte inflammation lead to increased secretion of proinflammatory cytokines, which negatively regulate myocyte metabolic functions through paracrine or autocrine effects. Inflammation in visceral AT, with increased secretion of inflammatory adipokines, may also adversely affect myocyte metabolic function through endocrine effects. In addition, inflammatory effects on adipocytes in visceral AT and IMAT/PMAT may accelerate FFA release and transfer into myocytes, resulting in myocyte inflammation and metabolic dysfunction. Furthermore, elevated levels of TGRLs, including diet/enterocyte-derived chylomicrons (CM) and liver-derived VLDL may undergo enhanced LPL-mediated triglyceride hydrolysis, increasing FA release and transfer into skeletal muscle (and AT) and contributing to myocyte inflammation and metabolic dysfunctions. Activation of the RAS in skeletal muscle with local production of ANG II and ANG 1–7 may also regulate myocyte inflammation and metabolic functions.
SM myocytes express TLR2 and TLR4 (52), which play essential roles in FA-induced effects. Individuals with obesity and T2D have increased TLR4 expression and signaling in SM (32). Inhibition or deletion of TLR4 protects against lipid-induced insulin resistance in cultured myocytes or mouse SM (117). ANG II may contribute to SM insulin resistance by reducing blood flow to SM and by directly impairing myocyte insulin signaling via increasing mitochondrial ROS production and activating inflammatory pathways (109, 110, 118, 119). ACE inhibitor or ARB treatment of human subjects with hypertension and insulin resistance is usually associated with improvement of insulin resistance (109, 118). Elevation of ANG II in animals increases oxidative stress and inflammation and decreases insulin sensitivity in SM while blockade of RAS reverses these effects (113). In contrast to ANG II, ANG 1–7 has beneficial effects on insulin sensitivity including direct insulin-sensitizing effects on SM (109, 110, 115, 120).
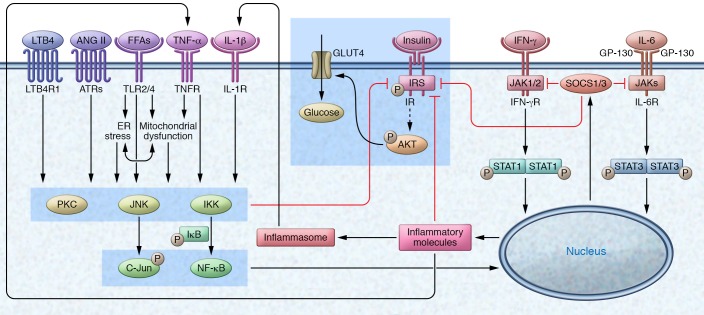
Below we summarize potential roles of various inflammatory pathways, which are depicted in Figure 3, in SM myocyte insulin resistance.
Figure 3. Inflammatory signaling mediates insulin resistance in myocytes.
Increased levels of cytokines such as TNF-α and IL-1β from M1-like macrophages, saturated FFAs derived from TGRL–triglyceride hydrolysis and adipocyte lipolysis, LTB4 derived from arachidonic acid metabolism, and ANG II derived from RAS activate the PKC, JNK, and IKK/NF-κB pathways in myocytes via interactions with their receptors on the cells. These inflammatory pathways can all impair insulin signaling by increasing serine or threonine phosphorylation and disrupting insulin-stimulated tyrosine phosphorylation of IR or IRS, or by downregulating molecules involved in insulin signaling. IFN-γ from Th1 cells and IL-6 activate JAK/STAT1/3 pathways, which may also impair insulin signaling in myocytes (possibly through SOCS proteins, particularly SOCS1 and SOCS3, which interrupt the interaction of IR with IRS-1 and IRS-2, inhibit IR tyrosine kinase activity, and interact with IRS-1 and IRS-2, leading to their ubiquitin-mediated degradation). PKCs, JNK, IKK/NF-κB, and STAT1/3 may also impair insulin signaling through other, undefined inflammatory pathways. SOCSs are involved in a negative feedback loop that leads to the termination of inflammatory effects of STAT through downregulation of JAK activity, which blocks further STAT phosphorylation.
The IKK/NF-κB pathway.
The main activators for the IKK/NF-κB pathway in obesity may include TNF-α, IL-1β, FAs, and ANG II. SM myocytes from obese subjects with T2D show enhanced activation of the IKK/NF-κB pathway (31). In cultured cells, overexpression or activation of IKKβ impairs insulin signaling (121), whereas inhibition of the IKK/NF-κB pathway prevents palmitic acid– or TNF-α–induced insulin resistance (117, 121, 122). In mice, inhibition or reduction of IKKβ prevents obesity- or lipid-induced insulin resistance in SM (121, 123), indicating a role of the IKK/NF-κB pathway in insulin resistance. The IKK/NF-κB pathway may cause insulin resistance via increased IKK-mediated serine phosphorylation of insulin receptor substrate 1 (IRS-1) or insulin receptor (IR), leading to impairment in insulin-induced tyrosine phosphorylation and subsequent inhibition of downstream insulin signaling, suppressed expression of molecules such as GLUT4 in the insulin signaling cascade, and induced expression of molecules such as inducible NOS, which may promote IRS-1 serine phosphorylation and induce nitration of IRS-1 tyrosine residues, leading to impaired insulin signaling (2, 122, 124).
Though the studies above establish signaling mechanisms by which the IKK/NF-κB pathway may influence obesity, the in vivo role of the muscle IKK/NF-κB pathway in obesity-linked insulin resistance remains unclear. Muscle-specific deletion or overexpression of IKKβ in mice does not impact muscle insulin sensitivity and systemic glucose tolerance (125, 126). Therefore, the beneficial effects of anti-IKK therapy on muscle insulin sensitivity are likely derived from its antiinflammatory effects on immune cells (121, 123), consistent with the observation that ablation of IKKβ in myeloid cells protects mice from obesity-induced insulin resistance (88).
JNKs and MAPKs.
JNKs are members of the MAPK family and can be activated by TNF-α, IL-1β, ER stress, saturated FAs, LTB4, and ANG II (78, 109, 127). JNK activity is increased in SM of obese mice and humans (89, 91, 128). In cultured myocytes, palmitate-induced insulin resistance is accompanied by increased JNK activity (129), whereas JNK knockdown attenuates palmitate-induced insulin resistance (130). JNKs also contribute to oxidative stress–induced insulin resistance in isolated SM (131). One study showed that muscle-specific deletion of JNK1 in mice selectively protected muscle against obesity-linked insulin resistance (132). However, another study showed no effects of muscle-specific overexpression or deletion of JNK1 on insulin sensitivity and glucose metabolism in mice (133). JNKs may cause insulin resistance directly by inducing serine and threonine phosphorylation of IRSs, thereby disrupting the interaction of IRSs with IR to impair downstream insulin signaling (93, 127).
Other MAPKs, particularly p38 MAPK, may also be involved in obesity-linked muscle insulin resistance. TNF-α, oxidative stress, or conditioned medium from palmitate-treated macrophages impairs insulin signaling and activates p38 MAPK in myocytes. Moreover, p38 MAPK inhibition or knockdown attenuates insulin resistance (45, 134, 135).
PKCs.
The roles of PKCs in metabolic functions have been reviewed in detail elsewhere (136). Studies of PKC effects on muscle insulin sensitivity have focused mainly on conventional PKCs (cPKCα, -βI, -βII, and -γ) and novel PKCs (nPKCδ, -ε, -η, and -θ), which both rely on DAG for full activation. In humans, raising plasma FA levels by lipid infusion with acute induction of muscle insulin resistance results in increased DAG content that is temporally associated with PKCθ, PKCδ, and PKCβII activation (137, 138). Obese subjects with T2D have increased PKCθ activity in SM (139). Rats fed a HFD show increased muscle expression and translocation of PKCθ and PKCε and decreased glucose disposal (140). Palmitate treatment of cultured myocytes, with induction of insulin resistance, increases PKCθ activation (122).
Most previous studies support important roles of PKCs in obesity- or lipid-induced muscle insulin resistance and inflammation. For example, inhibiting PKCθ activation prevents palmitate-induced insulin resistance and TNF-α expression in cultured myocytes (122). Dual inhibition or co-silencing of PKCθ and PKCε attenuates insulin resistance and inflammatory responses of myocytes to conditioned medium from palmitate-activated macrophages (141). Ablation of PKCθ in mice protects against lipid infusion–induced SM insulin resistance (142). Muscle PKCδ levels increase with age in mice, and muscle-specific deletion of PKCδ improves muscle insulin resistance and whole-body insulin sensitivity in aged mice (143). PKCs may induce insulin resistance by increasing serine or threonine phosphorylation of IR or IRS-1, with resultant impairment in downstream insulin signaling (136, 138, 142, 144).
JAK/STAT pathways.
Major activators of JAK/STAT pathways include IFNs, other cytokines, and growth factors. JAKs are the main upstream molecules that phosphorylate and activate STATs. IFN-γ mainly induces tyrosine phosphorylation and activation of STAT1 via JAK1 and JAK2. In addition, engagement of TLR2 and TLR4 can activate JNK and MAPK (47, 91, 145–147), which can also induce STAT1 phosphorylation via JAK1 (145, 148). STAT1 phosphorylation is increased in SM of obese mice; ablation of Th1 cells in αβT cell–deficient mice blunts this effect (35).
Treating myocytes with IFN-γ or Th1-conditioned medium increases STAT1 phosphorylation and decreases insulin sensitivity (35, 149), whereas pretreating myocytes with a JAK inhibitor attenuates Th1-conditioned medium–induced STAT1 phosphorylation and improves insulin sensitivity (35). Treatment of obese mice with a JAK1/JAK2 inhibitor improves systemic insulin resistance in conjunction with reduced SM inflammation (35). However, a potential in vivo role of the muscle JAK/STAT1 pathway in obesity-linked insulin resistance remains unconfirmed.
STAT3 phosphorylation is increased in SM of humans with T2D and positively correlated with FFA levels and measures of insulin sensitivity (150). IL-6 induces STAT3 phosphorylation and activation. Palmitate, which induces insulin resistance, also induces STAT3 phosphorylation in cultured myocytes; silencing STAT3 attenuates palmitate-induced insulin resistance, indicating a role for STAT3 in myocyte insulin resistance (150). However, muscle-specific deletion of STAT3 in mice does not appear to alter obesity-linked insulin resistance (151).
The mechanisms by which the JAK/STAT pathways contribute to insulin resistance remain unclear. One possibility is their regulation of suppressor of cytokine signaling (SOCS) proteins, particularly SOCS1 and SOCS3, which are downstream of STATs and are involved in a negative feedback loop that leads to termination of inflammatory effects by downregulating JAK activity, thereby blocking further STAT phosphorylation (152). Obesity with T2D increases SOCS1 and SOCS3 expression in muscle (150, 153, 154). Overexpression of SOCS1 or SOCS3 in cultured myocytes decreases insulin-stimulated glycogen synthesis (153). SOCS1 and SOCS3 may directly inhibit insulin signaling by interrupting the interaction of IR with IRSs, inhibiting IR tyrosine kinase activity, and interacting with IRSs to induce their ubiquitin-mediated degradation (152, 153, 155). Muscle-specific deletion of SOCS3 in mice protects against obesity-induced insulin resistance (156); overexpression of SOCS3 in muscle exacerbates obesity and insulin resistance in mice (157).
Because of the negative feedback roles of SOCSs in inflammation (152), direct inhibition or knockdown of SOCS1 and/or SOCS3 is expected to improve insulin resistance, but also to enhance inflammation, which may counteract beneficial effects on metabolic functions. Indeed, macrophage-specific ablation of SOCS1 or liver-specific deletion of SOCS3 in mice increases systemic inflammation and insulin resistance (158, 159). Therefore, targeting upstream molecules such as JAK or STAT may provide more therapeutic benefits on insulin sensitivity and inflammation.
The NLRP3 inflammasome may also play a role in the regulation of myocyte insulin sensitivity, likely by mediating IL-1β production. Overexpression of perilipin 2, a lipid droplet–associated protein, resulted in increased expression of NLRP3 and impaired insulin-stimulated glucose uptake in cultured myocytes. Knocking down NLRP3 reversed this effect (160). Further studies need to be carried out to confirm the potential role and examine the exact mechanisms by which the inflammasome participates in myocyte insulin resistance in obesity.
Conclusions and perspectives
Accumulating evidence indicates that obesity is associated with increased inflammation in SM, which is mainly manifested by enhanced immune cell infiltration in IMAT/PMAT and also includes increased myocyte inflammation. Increases in IMAT/PMAT are associated with obesity, aging, inflammation, and diabetes. The immune cells in IMAT/PMAT in obesity tend to polarize into proinflammatory phenotypes with increased expression of proinflammatory molecules, which, in conjunction with inflammatory molecules from other tissues, particularly from AT, may negatively regulate myocyte metabolic functions, contributing to insulin resistance locally in SM and systemically in the whole body. Targeting IMAT/PMAT may be a promising approach to prevent T2D. Exercise and weight loss have been shown to reduce both IMAT and visceral AT accompanied with improved physical performance; these effects were independent of the change in total fat (66). We postulate that exercise combined with modest weight loss leads to major effects on IMAT/PMAT — both the absolute amount and phenotype — with important benefits on muscle insulin/glucose metabolism that may help to explain the large reduction in new-onset diabetes observed with the Diabetes Prevention Program (161).
Based on the roles of chronic inflammation in obesity-linked metabolic dysfunction, antiinflammatory therapy has also been viewed as a promising strategy for obesity-linked metabolic disease. However, most effects demonstrated in tissue culture or animal models remain to be confirmed in humans. Moreover, current knowledge about the particular pathways that mediate obesity-linked inflammation is limited and remains an obstacle to the development of novel, specific “obesity-targeted” antiinflammatory approaches. Future study is needed to identify specific inflammatory pathways closely related to obesity, which could be therapeutically targeted to treat obesity-linked metabolic disease.
Acknowledgments
This work was supported by NIH grants to HW (R01 HL098839) and CMB (R01 DK078847), an American Heart Association award to HW (AHA16GRNT30410012), and an American Diabetes Award to HW (1-17-IBS-082). We thank Kerrie Jara for editorial assistance.
Footnotes
Conflict of interest: C.M. Ballantyne is a consultant for AstraZeneca, Boehringer Ingelheim, Merck, and Sanofi-Synthelabo, and has a provisional patent (no. 61721475) entitled “Biomarkers to improve prediction of heart failure risk,” filed by Baylor College of Medicine and Roche.
Reference information:J Clin Invest. 2017;127(1):43–54. doi:10.1172/JCI88880.
References
- 1.Poirier P, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Diabetes Public Health Resource. CDC Web site. http://www.cdc.gov/diabetes/statistics/prev/national/figadults.htm Updated December 1, 2015. Accessed October 20, 2016.
- 4.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 6.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 8.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 10.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 12.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Ballantyne CM. Inflammation versus host defense in obesity. Cell Metab. 2014;20(5):708–709. doi: 10.1016/j.cmet.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen KF, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(31):12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5A):11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci. 2008;86(14 suppl):E94–E104. doi: 10.2527/jas.2007-0462. [DOI] [PubMed] [Google Scholar]
- 17.Bouzakri K, Koistinen HA, Zierath JR. Molecular mechanisms of skeletal muscle insulin resistance in type 2 diabetes. Curr Diabetes Rev. 2005;1(2):167–174. doi: 10.2174/1573399054022785. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt K, Görgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57(6):1087–1099. doi: 10.1007/s00125-014-3224-x. [DOI] [PubMed] [Google Scholar]
- 21.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 22.Carey AL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55(10):2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20(12):3364–3375. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G. Cross-talk between adipokines myokines in fat browning. Acta Physiol (Oxf) doi: 10.1111/apha.12686. [published online ahead of print April 4, 2016]. doi: 10.1111/apha.12686. [DOI] [PubMed] [Google Scholar]
- 25.Wolsk E, Mygind H, Grøndahl TS, Pedersen BK, van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299(5):E832–E840. doi: 10.1152/ajpendo.00328.2010. [DOI] [PubMed] [Google Scholar]
- 26.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan RA, et al. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab. 2014;16(8):711–718. doi: 10.1111/dom.12268. [DOI] [PubMed] [Google Scholar]
- 28.Boström P, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin KO, et al. The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J Exerc Nutrition Biochem. 2015;19(2):91–98. doi: 10.5717/jenb.2015.15061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CJ, Pedersen M, Pedersen BK, Scheele C. Elevated NF-κB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes. 2011;60(11):2810–2819. doi: 10.2337/db11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyna SM, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57(10):2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corpeleijn E, Saris WH, Jansen EH, Roekaerts PM, Feskens EJ, Blaak EE. Postprandial interleukin-6 release from skeletal muscle in men with impaired glucose tolerance can be reduced by weight loss. J Clin Endocrinol Metab. 2005;90(10):5819–5824. doi: 10.1210/jc.2005-0668. [DOI] [PubMed] [Google Scholar]
- 34.Ciaraldi TP, Ryan AJ, Mudaliar SR, Henry RR. Altered myokine secretion is an intrinsic property of skeletal muscle in type 2 diabetes. PLoS One. 2016;11(7):e0158209. doi: 10.1371/journal.pone.0158209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan IM, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond) 2015;39(11):1607–1618. doi: 10.1038/ijo.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varma V, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296(6):E1300–E1310. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patsouris D, et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One. 2014;9(10):e110653. doi: 10.1371/journal.pone.0110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNFα by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97(4):1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togashi N, Ura N, Higashiura K, Murakami H, Shimamoto K. The contribution of skeletal muscle tumor necrosis factor-alpha to insulin resistance and hypertension in fructose-fed rats. J Hypertens. 2000;18(11):1605–1610. doi: 10.1097/00004872-200018110-00011. [DOI] [PubMed] [Google Scholar]
- 40.Austin RL, Rune A, Bouzakri K, Zierath JR, Krook A. siRNA-mediated reduction of inhibitor of nuclear factor-κB kinase prevents tumor necrosis factor-alpha-induced insulin resistance in human skeletal muscle. Diabetes. 2008;57(8):2066–2073. doi: 10.2337/db07-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieto-Vazquez I, Fernández-Veledo S, de Alvaro C, Rondinone CM, Valverde AM, Lorenzo M. Protein-tyrosine phosphatase 1B-deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-α-induced insulin resistance. Diabetes. 2007;56(2):404–413. doi: 10.2337/db06-0989. [DOI] [PubMed] [Google Scholar]
- 42.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276(5 pt 1):E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. MicroRNA-106b induces mitochondrial dysfunction and insulin resistance in C2C12 myotubes by targeting mitofusin-2. Mol Cell Endocrinol. 2013;381(1–2):230–240. doi: 10.1016/j.mce.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54(10):2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 45.de Alvaro C, Teruel T, Hernandez R, Lorenzo M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279(17):17070–17078. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2010;30(2):186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen MT, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 48.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R673–R680. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- 53.Fink LN, et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 2014;22(3):747–757. doi: 10.1002/oby.20615. [DOI] [PubMed] [Google Scholar]
- 54.Torres SH, De Sanctis JB, de L Briceño M, Hernández N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol. 2004;181(3):419–427. doi: 10.1677/joe.0.1810419. [DOI] [PubMed] [Google Scholar]
- 55.Fink LN, et al. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia. 2013;56(7):1623–1628. doi: 10.1007/s00125-013-2897-x. [DOI] [PubMed] [Google Scholar]
- 56.Boon MR, et al. Short-term high-fat diet increases macrophage markers in skeletal muscle accompanied by impaired insulin signalling in healthy male subjects. Clin Sci. 2015;128(2):143–151. doi: 10.1042/CS20140179. [DOI] [PubMed] [Google Scholar]
- 57.Tam CS, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab. 2014;99(5):1749–1757. doi: 10.1210/jc.2013-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, et al. ApoE and the role of very low density lipoproteins in adipose tissue inflammation. Atherosclerosis. 2012;223(2):342–349. doi: 10.1016/j.atherosclerosis.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong EG, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58(11):2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YS, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hevener AL, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altintas MM, et al. Leptin deficiency-induced obesity affects the density of mast cells in abdominal fat depots and lymph nodes in mice. Lipids Health Dis. 2012;11:21. doi: 10.1186/1476-511X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24(5):933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 66.Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn NW. Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging. 2015;19(9):913–921. doi: 10.1007/s12603-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albu JB, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 69.Haam JH, et al. Intermuscular adipose tissue is associated with monocyte chemoattractant protein-1, independent of visceral adipose tissue. Clin Biochem. 2016;49(6):439–443. doi: 10.1016/j.clinbiochem.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott D, et al. Associations of calf inter- and intra-muscular adipose tissue with cardiometabolic health and physical function in community-dwelling older adults. J Musculoskelet Neuronal Interact. 2015;15(4):350–357. [PMC free article] [PubMed] [Google Scholar]
- 71.Di Gregorio GB, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 72.Tam CS, Sparks LM, Johannsen DL, Covington JD, Church TS, Ravussin E. Low macrophage accumulation in skeletal muscle of obese type 2 diabetics and elderly subjects. Obesity (Silver Spring) 2012;20(7):1530–1533. doi: 10.1038/oby.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 74.Brake DK, Smith EO, Mersmann H, Smith CW, Robker RL. ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol. 2006;291(6):C1232–C1239. doi: 10.1152/ajpcell.00008.2006. [DOI] [PubMed] [Google Scholar]
- 75.Chen A, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13(8):1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 76.Evers-van Gogh IJ, et al. Muscle-specific inflammation induced by MCP-1 overexpression does not affect whole-body insulin sensitivity in mice. Diabetologia. 2016;59(3):624–633. doi: 10.1007/s00125-015-3822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitade H, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61(7):1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li P, et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21(3):239–247. doi: 10.1038/nm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang E, et al. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol. 2014;34(1):34–43. doi: 10.1161/ATVBAHA.113.302077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119(20):2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu L, et al. Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35(8):1787–1797. doi: 10.1161/ATVBAHA.115.305609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris DL, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62(8):2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amano SU, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rocha VZ, et al. Interferon-γ, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103(5):467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arkan MC, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 89.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138(5):961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reilly SM, et al. An inhibitor of the protein kinases TBK1 and IKK-ε improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 92.Han MS, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 94.Deng T, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17(3):411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li N, Fu J, Koonen DP, Kuivenhoven JA, Snieder H, Hofker MH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233(1):130–138. doi: 10.1016/j.atherosclerosis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 98.Furtado JD, et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87(6):1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benetti E, et al. High sugar intake and development of skeletal muscle insulin resistance and inflammation in mice: a protective role for PPAR-δ agonism. Mediators Inflamm. 2013;2013:509502. doi: 10.1155/2013/509502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297(2):E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes. 2001;50(5):1064–1068. doi: 10.2337/diabetes.50.5.1064. [DOI] [PubMed] [Google Scholar]
- 102.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98(13):7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58(1):116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goossens GH, Blaak EE, Arner P, Saris WH, van Baak MA. Angiotensin II: a hormone that affects lipid metabolism in adipose tissue. Int J Obes (Lond) 2007;31(2):382–384. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- 105.Kalupahana NS, et al. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity (Silver Spring) 2012;20(1):48–56. doi: 10.1038/oby.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hahn AW, Jonas U, Bühler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347(2–3):178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- 107.Tummala PE, et al. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100(11):1223–1229. doi: 10.1161/01.CIR.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 108.Lastra G, Sowers JR. Obesity and cardiovascular disease: role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm Mol Biol Clin Investig. 2013;15(2):49–57. doi: 10.1515/hmbci-2013-0025. [DOI] [PubMed] [Google Scholar]
- 109.Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. 2015;35(3):437–463. doi: 10.1002/med.21343. [DOI] [PubMed] [Google Scholar]
- 110.Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalupahana NS, Voy BH, Saxton AM, Moustaid-Moussa N. Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity (Silver Spring) 2011;19(2):245–254. doi: 10.1038/oby.2010.196. [DOI] [PubMed] [Google Scholar]
- 112.Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21(1):20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 113.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294(2):E345–E351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 114.Premaratna SD, et al. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int J Obes (Lond) 2012;36(2):233–243. doi: 10.1038/ijo.2011.95. [DOI] [PubMed] [Google Scholar]
- 115.Marcus Y, et al. Angiotensin 1-7 as means to prevent the metabolic syndrome: lessons from the fructose-fed rat model. Diabetes. 2013;62(4):1121–1130. doi: 10.2337/db12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel VB, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65(1):85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Radin MS, Sinha S, Bhatt BA, Dedousis N, O’Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51(2):336–346. doi: 10.1007/s00125-007-0861-3. [DOI] [PubMed] [Google Scholar]
- 118.Henriksen EJ, Prasannarong M. The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol. 2013;378(1–2):15–22. doi: 10.1016/j.mce.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 119.Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89(6):2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- 120.Williams IM, Otero YF, Bracy DP, Wasserman DH, Biaggioni I, Arnold AC. Chronic angiotensin-(1-7) improves insulin sensitivity in high-fat fed mice independent of blood pressure. Hypertension. 2016;67(5):983–991. doi: 10.1161/HYPERTENSIONAHA.115.06935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 122.Jové M, Planavila A, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-κB activation. Endocrinology. 2006;147(1):552–561. doi: 10.1210/en.2005-0440. [DOI] [PubMed] [Google Scholar]
- 123.Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108(3):437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao Z, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem. 2002;277(50):48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 125.Röhl M, et al. Conditional disruption of IκB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest. 2004;113(3):474–481. doi: 10.1172/JCI18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai D, et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 127.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35(9):490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54(8):2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 129.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281(37):26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 130.Vijayvargia R, Mann K, Weiss HR, Pownall HJ, Ruan H. JNK deficiency enhances fatty acid utilization and diverts glucose from oxidation to glycogen storage in cultured myotubes. Obesity (Silver Spring) 2010;18(9):1701–1709. doi: 10.1038/oby.2009.501. [DOI] [PubMed] [Google Scholar]
- 131.Santos FR, Diamond-Stanic MK, Prasannarong M, Henriksen EJ. Contribution of the serine kinase c-Jun N-terminal kinase (JNK) to oxidant-induced insulin resistance in isolated rat skeletal muscle. Arch Physiol Biochem. 2012;118(5):231–236. doi: 10.3109/13813455.2012.713366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sabio G, et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30(1):106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pal M, Wunderlich CM, Spohn G, Brönneke HS, Schmidt-Supprian M, Wunderlich FT. Alteration of JNK-1 signaling in skeletal muscle fails to affect glucose homeostasis and obesity-associated insulin resistance in mice. PLoS One. 2013;8(1):e54247. doi: 10.1371/journal.pone.0054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Talbot NA, Wheeler-Jones CP, Cleasby ME. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol Cell Endocrinol. 2014;393(1–2):129–142. doi: 10.1016/j.mce.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diamond-Stanic MK, Marchionne EM, Teachey MK, Durazo DE, Kim JS, Henriksen EJ. Critical role of the transient activation of p38 MAPK in the etiology of skeletal muscle insulin resistance induced by low-level in vitro oxidant stress. Biochem Biophys Res Commun. 2011;405(3):439–444. doi: 10.1016/j.bbrc.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmitz-Peiffer C, Biden TJ. Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes. 2008;57(7):1774–1783. doi: 10.2337/db07-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 138.Szendroedi J, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111(26):9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Itani SI, Pories WJ, Macdonald KG, Dohm GL. Increased protein kinase C θ in skeletal muscle of diabetic patients. Metab Clin Exp. 2001;50(5):553–557. doi: 10.1053/meta.2001.22512. [DOI] [PubMed] [Google Scholar]
- 140.Schmitz-Peiffer C, et al. Alterations in the expression and cellular localization of protein kinase C isozymes ε and θ are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46(2):169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 141.Kewalramani G, Fink LN, Asadi F, Klip A. Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C θ and ε. PLoS One. 2011;6(10):e26947. doi: 10.1371/journal.pone.0026947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim JK, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114(6):823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li M, Vienberg SG, Bezy O, O’Neill BT, Kahn CR. Role of PKCδ in insulin sensitivity and skeletal muscle metabolism. Diabetes. 2015;64(12):4023–4032. doi: 10.2337/db14-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Y, et al. Protein kinase C θ inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279(44):45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 145.Xiao L, et al. Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int J Obes (Lond) 2016;40(1):112–120. doi: 10.1038/ijo.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rhee SH, Jones BW, Toshchakov V, Vogel SN, Fenton MJ. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J Biol Chem. 2003;278(25):22506–22512. doi: 10.1074/jbc.M208633200. [DOI] [PubMed] [Google Scholar]
- 147.Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59(2):242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 149.Grzelkowska-Kowalczyk K, Wieteska-Skrzeczyńska W. Treatment with IFN-γ prevents insulin-dependent PKB, p70S6k phosphorylation and protein synthesis in mouse C2C12 myogenic cells. Cell Biol Int. 2009;34(1):117–124. doi: 10.1042/CBI20090135. [DOI] [PubMed] [Google Scholar]
- 150.Mashili F, Chibalin AV, Krook A, Zierath JR. Constitutive STAT3 phosphorylation contributes to skeletal muscle insulin resistance in type 2 diabetes. Diabetes. 2013;62(2):457–465. doi: 10.2337/db12-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.White AT, LaBarge SA, McCurdy CE, Schenk S. Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance. Mol Metab. 2015;4(8):569–575. doi: 10.1016/j.molmet.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne) 2012;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24(12):5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rieusset J, et al. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53(9):2232–2241. doi: 10.2337/diabetes.53.9.2232. [DOI] [PubMed] [Google Scholar]
- 155.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277(44):42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 156.Jorgensen SB, et al. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes. 2013;62(1):56–64. doi: 10.2337/db12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lebrun P, et al. Constitutive expression of suppressor of cytokine signalling-3 in skeletal muscle leads to reduced mobility and overweight in mice. Diabetologia. 2009;52(10):2201–2212. doi: 10.1007/s00125-009-1474-9. [DOI] [PubMed] [Google Scholar]
- 158.Sachithanandan N, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60(8):2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sachithanandan N, et al. Liver-specific suppressor of cytokine signaling-3 deletion in mice enhances hepatic insulin sensitivity and lipogenesis resulting in fatty liver and obesity. Hepatology. 2010;52(5):1632–1642. doi: 10.1002/hep.23861. [DOI] [PubMed] [Google Scholar]
- 160.Cho KA, Kang PB. PLIN2 inhibits insulin-induced glucose uptake in myoblasts through the activation of the NLRP3 inflammasome. Int J Mol Med. 2015;36(3):839–844. doi: 10.3892/ijmm.2015.2276. [DOI] [PubMed] [Google Scholar]
- 161.Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54(5):1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Franckhauser S, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51(7):1306–1316. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 163.Kim HJ, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53(4):1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 164.Pierce JR, Maples JM, Hickner RC. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: local effects of IL-15 on adipose tissue lipolysis. Am J Physiol Endocrinol Metab. 2015;308(12):E1131–E1139. doi: 10.1152/ajpendo.00575.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Itoh N. FGF21 as a hepatokine, adipokine, and myokine in metabolism and diseases. Front Endocrinol (Lausanne) 2014;5:107. doi: 10.3389/fendo.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moreno-Navarrete JM, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 167.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287(15):11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58(1):30–38. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]