Abstract
Objectives
To determine if weight extremes impact clinical outcomes in pediatric ARDS (PARDS).
Design
Post-hoc analysis of a cohort created by combining 5 multicenter PARDS studies
Setting
43 academic pediatric intensive care units worldwide
Patients
711 subjects prospectively diagnosed with PARDS
Intervention
Subjects >2 years were included and categorized by CDC BMI z-score criteria: underweight (<−1.89), normal weight (−1.89 to +1.04), overweight (+1.05 to +1.64), and obese (≥+1.65). Subjects were stratified by direct vs. indirect lung injury leading to PARDS. The primary outcome was in-hospital mortality. In survivors, secondary analyses included duration of mechanical ventilation (DMV) and ICU length of stay (LOS).
Measurements and Main Results
331 patients met inclusion criteria; 12% were underweight, 50% normal weight, 11% overweight, and 27% obese. Overall mortality was 20%. By multivariate analysis, BMI category was independently associated with mortality (p=0.004). When stratified by lung injury type, there was no mortality difference between BMI groups with direct lung injury; however, in the indirect lung injury group, the odds of mortality in the obese were significantly lower than normal weight subjects (OR=0.11, 95%CI 0.02–0.84). Survivors with direct lung injury had no difference in DMV or ICU LOS; however, those with indirect lung injury, the overweight required longer DMV than other groups (p<0.001).
Conclusions
These data support the obesity paradox in PARDS. Obese children with indirect lung injury PARDS have a lower risk of mortality. Importantly, among survivors the overweight with indirect lung injury require longer DMV. Our data require prospective validation to further elucidate the pathobiology of this phenomenon.
Keywords: pediatric acute respiratory distress syndrome, acute lung injury, obesity, pediatric
Introduction
In the United States, 3.7% of children and adolescents are underweight (1) and 17% are obese(2). Because both represent states of malnutrition and are associated with multiple comorbidities, it is unsurprising that the prevalence of both underweight and obesity among hospitalized children remains high, at approximately 20% each(3). In studies of adult critically ill patients, the underweight have high rates of mortality, and while obese individuals require longer intensive care unit (ICU LOS) and hospital stays, they appear to have the lowest risk of in-hospital mortality (4–8). This is known as the “obesity paradox”.
Acute Respiratory Distress Syndrome (ARDS) represents a small subset of patients cared for in the ICU but is associated with mortality rates of approximately 22% to 35% in the pediatric population(9–11). Several studies have found that the obesity paradox persists in adults with ARDS(12–14). No studies have been published regarding the effects of weight extremes on clinical outcomes in pediatric ARDS (PARDS).
The objective of this study was to determine whether weight extremes impact clinical outcomes of PARDS patients. We hypothesize that children with PARDS who are either underweight or obese will have higher risk of in-hospital mortality and longer durations of mechanical ventilation and ICU LOS. If this hypothesis is correct, it will be imperative to understand the fundamental pathophysiologic differences between the different weight groups so that medical management can be adapted to reduce rates of mortality and morbidity in this population.
Methods and Materials
Design, Setting and Patients
Our cohort was developed from the combination of 5 prospective PARDS studies (2 clinical trials and 3 observational studies) that were completed between 2000 and 2010 via the collaboration of 43 academic hospitals affiliated with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. In these studies, pediatric ARDS (PARDS) and pediatric acute lung injury (ALI) were identified based on the 1994 North American-European Consensus Conference criteria: a PaO2 to FiO2 ratio (PF ratio) of <300, bilateral infiltrates on chest radiograph, and no evidence of left atrial hypertension(15). The term ALI has since been replaced with the term mild ARDS(11) and any patient meeting the above criteria was said to have PARDS and was included in the analyzed cohort. Details of the 5 studies are provided in an online data supplement. Any patient enrolled in more than one of the studies was included once in the combined cohort.
Within the combined cohort, patients 2 to 18 years old were eligible and were included in the analysis. Subjects were excluded if there was no documented admission height and weight, PF ratio at disease onset, or patient outcome. Subjects younger than 2 years were excluded because BMI is not a validated growth parameter in this age group. Children with an uncorrected congenital cardiac disease were excluded. This study received IRB exemption from the UCSF Benioff Children’s Hospital, Oakland.
Measurements and Data Collection
BMI z-scores were calculated using admission height and weight and corrected for age and gender based on Center for Disease Control and Prevention (CDC) formulations. Each subject was categorized into one of four CDC defined BMI groups(16): underweight (z-score <−1.89, <3rd percentile), normal weight (z-score −1.89 to +1.04, 3rd–84th percentile), overweight (z-score +1.05 to +1.65, 85th–95th percentile), and obese (z-score ≥ +1.65, >95th percentile). For all analyses, the reference group was those of normal weight. The cohort was further stratified by PARDS secondary to a direct lung injury (pneumonia, aspiration, near drowning, pulmonary contusion, pulmonary hemorrhage) vs. indirect lung injury (sepsis, trauma, or transfusion related acute lung injury, complete congenital cardiac repair, vasculitis)(15, 17). Other data collected included age, gender, race, ethnicity, prior pulmonary disease, immuno-compromised status, and PRISM-III score (pediatric risk of mortality score)(18).
Outcomes Measures and Statistical Analysis
The primary outcome was in-hospital mortality, and secondary outcomes were duration of mechanical ventilation and ICU LOS among survivors. We completed unadjusted analyses by comparing the outcome between BMI groups. Associations between categorical variables and mortality were analyzed by χ2 test. Normally distributed continuous data are reported as mean and standard deviation, and were compared by Student’s t-test. Non-normally distributed continuous data are reported as median and interquartile range, and were compared by Wilcoxon rank-sum test. For multivariate logistic regression analysis, the model included demographic data (age, African American vs. non-African American race), PF ratio at PARDS onset, PRISM-III score, immuno-compromised status, and original study group. This model was stratified by direct vs. indirect lung injury. In the direct lung injury stratum, the reference group was the normal weight with direct lung injury group; similarly, in the indirect lung injury stratum, the reference group was the normal weight with indirect lung injury group. The Wald test was used to test the significance of the 4-category BMI variable.
Secondary analysis was performed on the survivors. Duration of mechanical ventilation up to 28 days from disease onset was compared between the BMI groups. We used Cox Proportional Hazard Ratios to compare the rates of extubation between BMI groups. For each subject we determined the duration of mechanical ventilation with day 0 being the day of initial intubation, and the day of extubation being the first day the patient tolerated 24 hours without invasive mechanical ventilation. Any patient requiring mechanical ventilation for >28 days was deemed to have not been extubated within the observed period. Rates of extubation lower than that of the normal weight (reference) group correspond to longer periods of mechanical ventilation than the reference group.
A similar method was used to compare PICU LOS up to 60 days from disease onset. Any patient remaining in the PICU >60 days was deemed to not have been discharged from the PICU within the observed period. Rates of discharge were compared, where a rate less than the normal weight (reference) group correlated to longer PICU stays for that group. Both the mechanical ventilation duration and PICU LOS models were adjusted for the same variables as the primary analysis and stratified by direct vs. indirect lung injury. A p-value of < 0.05 was accepted as being statistically significant in all analyses. All analyses were performed using STATA software, version 13.1 (StataCorp, College Station, Texas).
Results
After combining the 5 pediatric PARDS studies, a cohort of 711 patients was created. We excluded 288 who were <2 years old, 68 with insufficient data to calculate a BMI, and 24 for missing diagnosis or outcome data. Six remaining patients were concurrently enrolled in two of the original studies and were only included into the final cohort once. This left 331 patients for analysis (figure 1); 40 (12%) were underweight, 165 (50%) were normal weight, 35 (11%) were overweight, and 91 (27%) were obese. The overall in-hospital mortality was 20%. The median PRISM-III score was 11 (IQR 6,16) and median PF ratio at disease onset was 124 (IQR 84, 167), consistent with PARDS of moderate severity.
Figure 1. Included and Excluded Patients in Study Cohort.
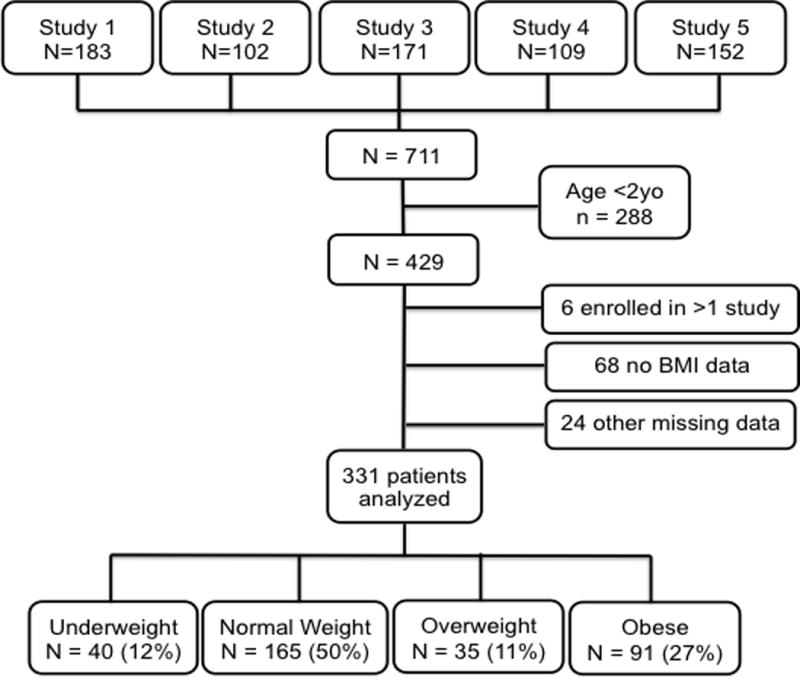
Flow of included and excluded subjects in our cohort. (BMI indicates body mass index)
Table 1 compares patient characteristics between the BMI groups. There were statistically significant differences between BMI groups with regard to age, prior pulmonary disease, PARDS etiology and, therefore, proportion of direct lung injury. The underweight group was younger and had a greater prevalence of pneumonia when compared to the other BMI groups. There were no differences between BMI groups by race, ethnicity, immuno-compromised status, PRISM-III score, PF ratio or original study.
Table 1.
Characteristics of Patients by BMI Group
| Whole Cohort n = 331 |
Underweight n = 40 |
Normal Weight n = 165 |
Overweight n = 35 |
Obese n = 91 |
p-value | |
|---|---|---|---|---|---|---|
| Mean Age, years (SD) | 9.6 (5.1) | 7.1 (4.3) | 10.3 (5.2) | 9.8 (5) | 9.3 (5) | 0.003b |
| Male, n (%) | 182 (55) | 23 (58) | 89 (54) | 17 (49) | 53 (58) | 0.77c |
| Race/Ethnicity, n (%) | 0.08c | |||||
| Caucasian | 185 (56) | 20 (50) | 88 (53) | 21 (60) | 56 (61.5) | |
| African American | 60 (18) | 5 (12.5) | 22 (20) | 9 (26) | 13 (14) | |
| Asian/Pacific Islander | 14 (4) | 5 (12.5) | 8 (5) | 0 (0) | 1 (1) | |
| Hispanic/Latino | 48 (14.5) | 5 (12.5) | 22 (13) | 4 (11) | 17 (19) | |
| Other | 24 (7) | 5 (12.5) | 14 (8.5) | 1 (3) | 4 (4) | |
| Any Past Medical History, n (%) | 194 (59) | 27 (67.5) | 95 (58) | 14 (40) | 58 (64) | 0.085c |
| Prior Pulmonary Disease, n (%)a | 34 (10) | 6 (15) | 8 (5) | 3 (9) | 17 (19) | 0.004c |
| Immunocompromised, n (%)a | 69 (21) | 5 (12.5) | 39 (24) | 4 (11) | 21 (23) | 0.2c |
| Indirect Lung Injury, n (%) | 81 (24) | 4 (10.5) | 52 (34) | 7 (20) | 18 (21) | 0.03c |
| ARDS Etiology, n (%) | 0.013c | |||||
| Pneumonia | 149 (45) | 24 (60) | 68 (41) | 14 (40) | 43 (47) | |
| Sepsis | 43 (13) | 0 (0) | 30 (18) | 1 (3) | 12 (13) | |
| Aspiration | 38 (11.5) | 5 (12.5) | 13 (8) | 5 (14) | 15 (16.5) | |
| Trauma | 21 (6) | 1 (2.5) | 12 (7) | 6 (17) | 2 (2) | |
| Near Drowning | 16 (5) | 1 (2.5) | 6 (4) | 4 (11) | 5 (5.5) | |
| TRALI | 13 (4) | 3 (7.5) | 8 (5) | 0 (0) | 2 (2) | |
| Pulmonary Hemorrhage | 13 (4) | 2 (5) | 6 (4) | 2 (6) | 3 (3) | |
| Other | 38 (11.5) | 4 (10) | 22 (13) | 3 (9) | 9 (10) | |
| Median PF Ratio at Diagnosis (IQR) | 123.6 (84, 167) | 118.5 (77, 165) | 116 (61, 165) | 152 (115, 202) | 126 (79, 160) | 0.06b |
| Median PRISM-III Score (IQR) | 11 (6, 16) | 12 (6, 17) | 10 (5, 16) | 10 (5, 16) | 11 (6, 16) | 0.53b |
| Study, n (%) | 0.93c | |||||
| 1 | 88 (27) | 10 (1) | 49 (56) | 10 (11) | 19 (22) | |
| 2 | 40 (12) | 5 (12.5) | 19 (47.5) | 4 (10) | 12 (30) | |
| 3 | 55 (17) | 6 (11) | 24 (44) | 8 (15) | 17 (31) | |
| 4 | 52 (16) | 8 (25) | 25 (48) | 6 (11.5) | 13 (25) | |
| 5 | 96 (29) | 11 (11.5) | 48 (50) | 7 (7) | 30 (30) | |
| Died, n (%) | ||||||
| Indirect Lung Injury | 21 (26) | 2 (50) | 16 (31) | 1 (14) | 2 (11) | 0.2c |
| Direct lung Injury | 38 (16) | 6 (18) | 17 (17) | 3 (11) | 12 (18) | 0.9c |
| Total | 65 (20) | 8 (20) | 38 (23) | 4 (11) | 15 (16) | 0.4c |
May be less than expected as entity was an exclusion criteria for one or more studies combined to form this cohort
p values obtained using Kruskal-Wallis test or ANOVA with Bonferroni
p values obtained using chi-square comparisons
p values obtained using Student’s t-test
Abbreviations: SD=standard deviation; IQR=interquartile range
There were no significant differences in the percent of deaths when comparing BMI groups to each other; 20% (n = 8) of the underweight, 23% (n = 38) of the normal weight, 11% (n = 4) of the overweight, and 16%(n = 15) of the obese died (p = 0.38). Fifty two percent (n = 34) of the immuno-compromised died compared to only 13% (n = 35) of the non-immuno-compromised (p<0.001). Those who died had a higher PRISM-III score (median of 14, IQR: 10, 23) compared to those who survived (median of 10, IQR: 5, 15, p<0.001). PF ratio at PARDS onset, age and prior pulmonary disease were not associated with in-hospital mortality.
With multivariate logistic regression, BMI category was independently associated with odds of in-hospital mortality (p < 0.01), as were immuno-compromised status (OR 6.0, 95%CI: 4.1, 8.8), and PRISM-III score (9.1% increased odds of death for every 1 unit increase in PRISM-III score, 95%CI: 5.9%, 12.9%). Table 2 shows the adjusted odds of death in the BMI groups when stratified by direct vs. indirect lung injury. Overall, those who are overweight and obese appear to have lower adjusted odds of in-hospital mortality than the control group, this being most notable in the obese group with indirect lung injury.
Table 2.
Logistic Regression Model for In-hospital Mortality, Stratified by Direct vs. Indirect Lung Injury
| Variable | Whole Cohort | Direct Lung Injury | Indirect Lung Injury | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| BMI Category | 0.004 | 0.7 | < 0.001 | |||
| Underweight vs. normal weighta | 1.05 (0.5–2) | 1.16 (0.5–2.7) | 1.38 (0.69–2.73) | |||
| Overweight vs. normal weighta | 0.56 (0.2–1.4) | 0.57 (0.13–2.5) | 0.86 (0.18–4.2) | |||
| Obese vs. normal weighta | 0.61 (0.3–1.3) | 0.99 (0.39–2.5) | 0.11 (0.01–0.8) | |||
| PRISM-III | 1.09 (1.06–1.1) | < 0.001 | 1.09 (1.02–1.16) | 0.01 | 1.13 (1.08–1.18) | < 0.001 |
| PF Ratio at ARDS Onset | 0.99 (0.99–1.0) | 0.2 | 0.99 (0.98–1.0) | 0.09 | 1.0 (0.99–1.0) | 0.08 |
| African American (vs. not African American) | 0.69 (0.29–1.6) | 0.4 | 0.79 (0.47–1.3) | 0.4 | 0.35 (0.04–3.2) | 0.3 |
| Immuno-compromised | 6.0 (4.1–8.8) | < 0.001 | 6.3 (3.0–13.1) | < 0.001 | 6.0 (3.16–11.3) | <0.001 |
| Age (years) | 0.99 (0.96–1.0) | 0.8 | 0.98 (0.92–1.06) | 0.7 | 1.0 (0.9–1.14) | 0.9 |
The reference group for those with direct lung injury are those with direct lung injury of normal weight. The reference group for those with indirect lung injury are those of normal weight with indirect lung injury. For BMI category, a p-value <0.05 indicates there is a statically significant difference between the BMI groups when compared to those of normal weight within the same strata
Median duration of mechanically assisted ventilation was 11 days (IQR 6, 28) and median ICU LOS was 13 days (IQR 8, 21). In univariate analyses of the survivors, longer duration of mechanical ventilation and longer ICU stays were associated with higher PRISM-III scores (p<0.001) and with any chronic illness reported (p = 0.05). Additionally, those with lower PF ratio also required longer duration of mechanical ventilation (p = 0.03). There were no differences between the BMI groups with regard to duration of mechanical ventilation (p = 0.14) or ICU LOS (p = 0.62) in the unadjusted analyses.
After adjusting for age, African American race, PRISM-III, PF ratio, and immuno-compromised state and stratifying by direct vs. indirect lung injury, there remains no significant difference in risk of prolonged mechanical ventilation between BMI groups for those with direct lung injury (p = 0.6); however, in the indirect lung injury group, the overweight required longer durations of mechanical ventilation with a rate of extubation being 66% lower than that of normal weight (HR of extubation 0.34, 95%CI 0.13 to 0.89). In this indirect lung injury group, BMI was independently associated with duration of mechanical ventilation, p<0.001 (figure 2). The only other covariate with a statistically significant impact on duration of mechanical ventilation was PRISM-III score, with a hazard ratio of extubation of 0.98 (95%CI 0.97 to 0.998) correlating to a 2% reduction in rate of extubation for every 1 unit increase in PRISM-III score.
Figure 2. Duration of Mechanical Ventilation to Day 28 by BMI Group Stratified by Direct vs. Indirect Lung Injury.

Adjusting for age, African American race, Pediatric Risk of Mortality-III score, PaO2:FiO2 at ARDS onset. P=0.6 between BMI groups with direct lung injury (a) and p<0.001 between BMI groups with indirect lung injury (b). Calculated using proportional hazards regression accounting for original study as a cluster variable.
When evaluating PICU LOS of the whole cohort, BMI group was independently associated with PICU LOS (p <0.001) with underweight, overweight and obese groups all exhibiting lower HR of discharge from the PICU (and therefore increased risk of longer PICU stays) than the normal weight group. While not significant, stratification by direct vs. indirect lung injury shows that all weight groups with indirect lung injury require longer PICU stays than the normal weight group; this is not the case in those with direct lung injury (figure 3).
Figure 3. ICU Length of Stay to Day 60 by BMI Group Stratified by Direct vs. Indirect Lung Injury.
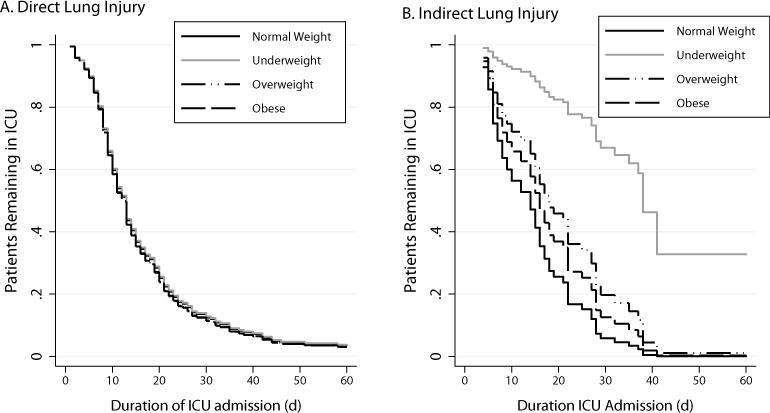
Adjusting for age, African American race, Pediatric Risk of Mortality-III score, PaO2:FiO2 at ARDS onset. P=0.98 between BMI groups with direct lung injury (a) and p = 0.63 between BMI groups with indirect lung injury (b). Calculated using proportional hazards regression accounting for original study as a cluster variable.
Discussion
In this large cohort of prospectively identified PARDS patients, BMI category is independently associated with risk of in-hospital mortality. There is no significant difference in odds of in-hospital mortality between the underweight and normal weight PARDS patients. Interestingly, the lowest adjusted odds of in-hospital mortality is seen in the obese patients with indirect lung injury PARDS, i.e. sepsis, trauma or transfusion related acute lung injury. While there were no differences in duration of mechanical ventilation between BMI groups with direct lung injury PARDS, those who were overweight with indirect lung injury required longer duration of mechanical ventilator support than the normal weight group. A similar trend was seen in the obese, but statistical significance was not achieved. There is an association between ICU LOS and BMI group when evaluating the cohort as a whole; with underweight, overweight, and obese groups all exhibiting increased risk of longer ICU stays compared to those of normal weight. While not statistically significant, stratification by direct vs. indirect lung injury shows distinct differences in ICU LOS between BMI groups with indirect lung injury and almost no difference among the BMI groups with direct lung injury.
Obesity induces a chronic pro-inflammatory state that has been implicated in many diseases(19). Additionally, obese adults have reduced expiratory reserve volume and diffusion capacity on pulmonary function testing(20) and utilize a five-fold greater percentage of oxygen consumption for respiratory work at rest(21). Furthermore, mechanical ventilation is hampered by reduced chest wall and lung compliance and an increase in airway resistance(22). Together, these factors lead one to impart a survival disadvantage for critically ill obese individuals.
Surprisingly, data on the relationship between BMI and clinical outcomes in critically ill adults indicate either unchanged or reduced mortality in the overweight or obese(4–8); this has become known as the “obesity paradox”. ARDS is characterized by an immense inflammatory reaction in the lungs in response to either a local injury or a systemic inflammatory illness(25). Several studies reveal that the obesity paradox likely persists in adults with ARDS(12–14). In order to elucidate the pathobiology behind this phenomenon, Stapleton et al. evaluated the plasma biomarkers of adult ARDS subjects and found that the pro-inflammatory cytokines TNF-α, IL-6, and IL-8 which are known to be elevated with chronic obesity and in ARDS, were decreased in the obese ARDS patients when compared to normal weight ARDS patients(26). This suggests that obese patients, who exhibit a chronically inflamed state, have an attenuation of this inflammation with the added stress of acute critical illness, signifying a potentially protective response. Similar results have been seen in obese, leptin resistant mice with acute lung injury(27, 28). This premise prompted us to evaluate for differences in outcome between those with a local injury leading to PARDS (direct lung injury) and those with PARDS secondary to a systemic inflammatory illness (indirect lung injury); a novel approach in both pediatric and adult studies. Interestingly in our analysis we found that in those with an indirect cause of PARDS, the obese had an almost 90% reduction in adjusted odds of in-hospital mortality compared to normal weight individuals. Our research refocuses and repurposes the imperative to better understand how adiposity modulates the acute inflammatory response in critically ill children.
We are aware of only two studies published regarding pediatric weight extremes and PICU outcomes. Goh et al. evaluated the effects of obesity on mechanically ventilated pediatric patients and found mortality risk, duration of mechanical ventilation and PICU stay in the obese similar to those of normal weight(30). On close inspection, the adjusted odds of ICU mortality was lowest in their obese group and increasing in a U-shape fashion with underweight and severely overweight individuals having the highest adjusted odds of death; a pattern very similar to adult studies. These findings may be non-statistically significant, in part, due to the very heterogenous patient population incorporated when evaluating patients requiring mechanical ventilation for all causes, including non-respiratory disease indications. Numa et al. showed that PICU patients with admission weights at the extremes had increased mortality(31). The population studied, however, was over-represented by underweight subjects and was not accurately representative of the population. Both of these studies defined obesity by weight percentile or z-score, a measurement less validated than BMI in appropriately defining childhood obesity(16).
In 2000, the ARDS Network showed a great reduction in mortality and duration of mechanical ventilation in patients who received low tidal volume mechanical ventilation, defined as 6mL/kg of predicted body weight(32). Since then both pediatric and adult intensivists strive to utilize this strategy in ARDS and PARDS patients. Our cohort had a mean tidal volume of 7.5mL/kg of admission weight (± 2.5ml/kg) on both days 0 and 1 of PARDS, suggesting that the 38% who were overweight or obese, and whose admit weight is likely greater than their ideal body weight, were quite possibly being exposed to higher tidal volumes and thus higher risk of volutrauma, although given the limited data on mechanical ventilation in this cohort, this has not been determined. This emphasizes the importance of differentiating patients based on their BMI classification and improving adherence to protective mechanical ventilation strategies.
Our study is not without limitations. We performed a secondary analysis of existing data not initially designed to test the influence of weight on PARDS outcomes and, as such, confounding by unmeasured covariates may have occurred. Additionally, our conclusion is dependent on the completeness of the data collected. We removed 68 individuals without BMI data and 24 without data on diagnosis or outcome, although demographic characteristics between those included and those excluded were not significantly different. Further, our cohort was created by the combination of five different PARDS studies, with no unified ventilation strategy and all having their own inclusion and exclusion criteria. Most notably, one or more of the studies excluded patients of greatest risk for in-hospital mortality, specifically the immuno-compromised (bone marrow or solid organ transplantation) or patients deemed too hemodynamically unstable for the intervention being evaluated. Given that there was no difference in number of immuno-compromised between BMI groups and that our mortality incidence is similar to that previously published (9, 11), this limitation would most likely lead to a bias toward the null hypothesis, if it has any impact at all. Possible inaccuracy of weight measurements in critically ill children presents an additional limitation. Weight measurement may be greatly affected by resuscitative efforts or by measurement error and may lead to misclassification of patients by BMI category. To reduce inaccuracy, we used the earliest weight measurement; that being the weight measured upon admission to the emergency department or PICU.
In conclusion, BMI category is independently associated with in-hospital mortality risk in children with PARDS with highest risk in the underweight and normal weight and lowest risk in the obese, implying that the obesity paradox may exist in children with PARDS. This effect appears to be primarily seen in the indirect lung injury cohort, suggesting a possible link between increased adiposity and an attenuated systemic inflammatory response to critical illness. Despite having lower mortality risk, children with indirect lung injury who are overweight and survive require longer periods of assisted ventilation. Although not statistically significant, this trend is noted in the obese with indirect lung injury as well. A prospective investigation that focuses on the influence of body mass at the extremes in children with PARDS and systemic inflammatory illnesses is needed to confirm our findings with particular attention to respiratory indices, metabolic distinctions, mechanical ventilation strategies and inflammatory indicators. Understanding the pathophysiologic differences and therapeutic implications between patients because of varying body habitus and baseline metabolic function is a vital step to develop new therapeutic approaches for critically ill children.
Supplementary Material
Acknowledgments
We thank the members of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network for their contribution to one or more of the original studies used to create the combined PARDS cohort.
Supported by National Institutes of Health grants 5T32HD049303-08 (Ward), UL1 RR024131 (Gildengorin), Charlotte Coleman Frey Fellowship Fund (Ward)
Copyright form disclosures: Dr. Ward received support for article research from the National Institutes of Health (NIH). His institution received funding from NIH grants 5T32HD049303-08 (Ward); NIH grants UL1 RR024131 (Gildengorin); and the Charlotte Coleman Frey Fellowship Fund (Ward), payment made to UCSF Benioff Children’s Hospital, Oakland. Dr. Sapru received support from the NIH and received support for article research from the NIH. Dr. Curley disclosed other support (YesNational Institute of Nursing Research Prone positioning in pediatric acute lung injury RO1 NR05336-01 2001-2006 Principal Investigator: M.A.Q. Curley) and received support for article research from the NIH. Dr. Thomas received funding from Discovery Labs, Therabron, and the FDA. Dr. Flori received support for article research from the NIH.
Footnotes
Author Contributions: Drs. Ward and Flori conceived of and designed the study. Drs. Ward, Valentine, Sapru, Curley, Willson, Thomas, and Flori participated in data acquisition and interpretation. Drs. Ward, Gildengorin and Flori conducted the data analysis. All authors contributed to the interpretation of the analysis results. Dr. Ward prepared the first draft of the manuscript, and all authors revised the draft critically. All authors have approved the final manuscript for publication.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Fryar, MSPH Cheryl D, Ogden, PhD Cynthia L. D of H and NE, Surveys: Prevalence of Underweight Among Adults Aged 20 Years and Over: United States, 2007–2008. 2010 [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Woo JG, Zeller MH, Wilson K, et al. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatr. 2009;154:327–31. doi: 10.1016/j.jpeds.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieracci FM, Hydo L, Pomp A, et al. The relationship between body mass index and postoperative mortality from critical illness. Obes Surg. 2008;18:501–7. doi: 10.1007/s11695-007-9395-5. [DOI] [PubMed] [Google Scholar]
- 5.Newell Ma, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007;204:1056–61. doi: 10.1016/j.jamcollsurg.2006.12.042. discussion 1062–4. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123:1202–7. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]
- 7.Ray DE, Matchett SC, Baker K, et al. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127:2125–31. doi: 10.1378/chest.127.6.2125. [DOI] [PubMed] [Google Scholar]
- 8.Akinnusi ME, Pineda La, El Solh Aa. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 9.Flori H, Glidden D, Rutherford G, et al. Pediatric acute lung injury prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 10.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand—A prospective, multicenter, observational study*. Pediatr Crit Care Med. 2007;8 doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 11.Khemani R, Smith LS, Zimmerman JJ, et al. Pediatric Acute Respiratory Distress Syndrome: Consensus Recommendations From the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16 doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien JM, Phillips GS, Ali Na, et al. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34:738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris AE, Stapleton RD, Rubenfeld GD, et al. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131:342–8. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 14.Gong MN, Bajwa EK, Thompson BT, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Committee on ARDS: Definitions, Mechanisms, Relevant Outcomes, and Clinical Trial Coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Barlow SE. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity : Summary Report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L, Pelosi P, Suter PM, et al. Acute Respiratory Distress Syndrome Caused by Pulmonary and Extrapulmonary Disease. Different Syndromes? Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 18.Pollack MM, Patel KM, Ruttimann UE. The pediatric risk of mortality III— Acute physiology score (PRISM III-APS): A method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante A. Obesity-induced inflammation : a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 20.Ray CS, Sue DY, Bray G, et al. Effects of Obesity on Respiratory Function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 21.Kress JP, Pohlman AS, Alverdy J, et al. The Impact of Morbid Obesity on Oxygen Cost of Breathing (V˙o 2RESP) at Rest. Am J Respir Crit Care Med. 1999;160:883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 22.Pelosi P, Croci M, Ravagnan I, et al. TOtal respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 23.Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988;84:1053–1060. doi: 10.1016/0002-9343(88)90310-5. [DOI] [PubMed] [Google Scholar]
- 24.Stokholm KH, Brøchner-Mortensen J, Hoilund-Carlsen PF. Increased glomerular filtration rate and adrenocortical function in obese women. Int J Obes. 1980;4:57–63. [PubMed] [Google Scholar]
- 25.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 26.Stapleton RD, Dixon AE, Parsons PE, et al. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–77. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellmeyer A, Martino JM, Chandel NS, et al. Leptin Resistance Protects Mice from Hyperoxia-induced Acute Lung Injury. Am J Respir Crit Care Med. 2007;175:587–594. doi: 10.1164/rccm.200603-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain M, Budinger GRS, Lo A, et al. Leptin Promotes Fibroproliferative Acute Respiratory Distress Syndrome by Inhibiting Peroxisome Proliferator – activated Receptor-gamma. Am J Respir Crit Care Med. 2011;183:1490–1498. doi: 10.1164/rccm.201009-1409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bechard L, Rothpletz-Puglia P, Touger-Decker R, et al. Influence of obesity on clinical outcomes in hospitalized children: A systematic review. JAMA Pediatr. 2013;167:476–482. doi: 10.1001/jamapediatrics.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goh VL, Wakeham MK, Brazauskas R, et al. Obesity is not associated with increased mortality and morbidity in critically ill children. JPEN J Parenter Enter Nutr. 2013;37:102–8. doi: 10.1177/0148607112441801. [DOI] [PubMed] [Google Scholar]
- 31.Numa A, McAweeney J, Williams G, et al. Extremes of weight centile are associated with increased risk of mortality in pediatric intensive care. Crit Care. 2011;15:R106. doi: 10.1186/cc10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Acute Respiratory Distress Syndrome Network. Ventilation With Lower Tidal Volumes As Compared With Tradtional Tidal Volumes For Acute Lung Injury And The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


