Abstract
As a nutritionally essential metal ion, zinc (Zn) not only constitutes a structural element for more than 3000 proteins but also plays important regulatory functions in cellular signal transduction. Zn homeostasis is tightly controlled by regulating the flux of Zn across cell membranes through specific transporters, i.e. ZnT and ZIP family proteins. Zn deficiency and malfunction of Zn transporters have been associated with many chronic diseases including cancer. However, the mechanisms underlying Zn regulatory functions in cellular signaling and their impact on the pathogenesis and progression of cancers remain largely unknown. In addition to these acknowledged multifunctions, Zn modulates a wide range of ion channels that in turn may also play an important role in cancer biology. The goal of this review is to propose how zinc deficiency, through modified Zn homeostasis, transporter activity and the putative regulatory function of Zn can influence ion channel activity, and thereby contribute to carcinogenesis and tumorigenesis. This review intends to stimulate interest in, and support for research into the understanding of Zn-modulated channels in cancers, and to search for novel biomarkers facilitating effective clinical stratification of high risk cancer patients as well as improved prevention and therapy in this emerging field.
Keywords: Zn Homeostasis, ZnT, ZIP, TRP Channels, Store-operated Ca2+ Entry, Orai Channels, STIM, Prostate Cancer, Breast Cancer, Esophageal Cancer, Review
2. INTRODUCTION
The essential micronutrient zinc (Zn) is the second most abundant and essential trace element in the human body after iron. It conducts multifunctional biological roles, and up to 10% of all proteins in mammalian cells require Zn for their folding, conformational change or activity (1). Zn is required for the activity of over 300 enzymes, and as such it participates in many enzymatic and metabolic functions in the body (more detailed reviews can be found elsewhere) (2–5). Zn is also known to be an essential component for DNA-binding proteins with Zn fingers, as well as copper/Zn superoxide dismutase and various proteins involved in DNA repair (6, 7). Thus, Zn plays a critical role in transcription factor function, antioxidant defense and DNA repair.
In the human body, Zn is absorbed across the intestinal mucosa and excreted mostly through pancreatic acinar cells into the small intestine, thus maintaining body zinc homeostasis. Intracellular Zn homeostasis is also tightly controlled by the regulation of Zn fluxes across membranes, the buffering of free Zn by metallothionein, and the storage of Zn in subcellular organelles such as vesicles. As Zn cannot freely cross cellular membranes, a number of proteins such as Zn permeable channels and transporters fulfill this function. Among them, Zrt, Irt-like protein (ZIP) also called Solute Carrier family 39A (SLC39A) (8, 9) and Zn transporter (ZnT, SLC30A) (10–12) are the two most important transporter families. There is increasing evidence that dysregulation and/or mutations in ZIP and ZnT transporter genes may result in functional disorders (13). For example, ZnT8 with a single-nucleotide polymorphism (SNP) has been shown to be associated with increased risk of both type 1 and type 2 diabetes mellitus (14–19) and a ZIP4 mutation results in acrodermatitis enteropathica (20–24). The roles of ZnTs and ZIPs in diseases are the current focus of much research and clinical attention.
Dietary Zn deficiency is a global health problem, especially in developing countries, and about two billion people are thought to be affected. Since it was first reported by Prasad et al, in 1961 (25), epidemiological studies indicate that Zn deficient populations have increased susceptibility to numerous diseases (26–28). The broad spectrum of Zn-deficient related diseases includes growth retardation, diarrhea in children, immune system dysfunction (29), neurological disorders (30), diabetes and cardiovascular disorders (31–33). More recently, the association between Zn deficiency and increased risk of cancer has been suggested (11, 27, 34). The mechanisms underlying the association are attributed to Zn deficiency induced single- and double-strand DNA breaks, increased oxidative stress and/or impaired immune function, however, other mechanisms may very likely co-exist. In particular, Zn is able to modulate a number of transporters and ion channels, such as TRP channels and K+ channels; Zn as a secondary signaling mediator is able to cross-talk to other cellular signaling pathways, e.g. Ca2+ signaling. Given the importance of these channels and signaling pathways in cell proliferation, migration and metastasis, appreciating the impact of Zn deficiency on the functions of these channels in cancer cells is an exigent priority. This review presents the case that Zn homeostasis in cancers and its potential regulatory function on various ion channels may contribute to carcinogenesis and tumorigenesis. This review also discusses the possible translational value of new information on Zn deficiency, Zn and Zn modulated channels in cancers, such as novel biomarkers, for effective clinical assessment to stratify high risk patients.
3. ZN HOMEOSTASIS IN HUMAN CELLS
3.1. Plasma Zn and intracellular Zn
The human body mass contains 2–3 g of Zn, and 57% is in skeletal muscle, 29%, 6% and 5% in bone, liver and skin, respectively (35, 36). There is only about 0.1% of total Zn circulating in plasma, and yet this tiny fraction of total Zn is important to maintain Zn homeostasis at a systemic level. Plasma Zn turns over rapidly to meet tissue needs and has to be replenished daily from diet. Once getting into plasma, the dietary Zn is further delivered to peripheral tissues. The systemic level of Zn distribution is tightly regulated and the physiological concentration of plasma Zn is maintained within the range of 10–20 mM (37). The plasma Zn concentration is normally around 15 mM in Zn adequate adults and around 11.0–13.5 mM in Zn adequate children (2, 38). Greater than 70% of plasma Zn is weakly bound to albumin and is mostly coming from intestinal absorption with a destination of the liver and soft tissues.
After Zn enters the cells, it is further distributed to different cellular organelles: about 30–40% to the nucleus, 10% to membranes and more than 50% to the cytoplasm. The intracellular Zn exists in three pools: 1) tightly bound to metalloenzymes, metalloproteins, and nucleoproteins; 2) loosely bound with various protein and amino acid ligands; 3) unbound as the free Zn2+ ion, albeit at very low concentrations. While the total cellular Zn concentration is estimated to be in mM range, the cytosolic concentration of free Zn2+ is estimated to be in the nM-pM range. The accurate determination of free Zn2+ concentration is of importance to understand many Zn-mediated cellular events, yet the reliable measurement of free intracellular Zn2+ and visualization of its dynamic changes remain a technical challenge. This is partially a consequence of the selectivity and sensitivity of Zn probes, which is strongly influenced by the concentrations of Zn and other cations in living biological systems. Attempts have been made to develop fluorescent probes for Zn using benzothiazole, fluorescein and quinolone (39, 40). Some commonly used Zn fluorescent indicators include N-(6-methoxy-8-quinolyl)-p-toluenesulfonamide (TSQ) (41, 42), FluoZin-2 and FluoZin-3 (43, 44). Additionally, fluorescent resonance energy transfer (FRET)-based engineered fluorescent proteins have also been developed as Zn biosensors (45, 46). Many of these Zn probes can be used under various physiological conditions, but they suffer from one or more problems such as inadequate selectivity, insufficient sensitivity, and dependence of fluorescence upon the dye concentration, slow kinetics and so forth. It will continue to be an active research area to develop brighter and highly selective Zn indicators with different Kds to suit measurement at different physiological conditions.
3.2. ZIPs and ZnTs
We now know that maintenance of intracellular Zn homeostasis is mainly dependent on two families of Zn transporters (47–49). In particular, ZIP family proteins function in the uptake of Zn into the cytoplasm of the cell from the extracellular space or from intracellular compartments, such as endoplasmic reticulum (ER), Golgi apparatus, and mitochondria; ZnT proteins function in the efflux of Zn from the cytoplasm to the extracellular space or to intracellular compartments. There are at least 14 ZIP transporters and 10 ZnT proteins in the human body with differential tissue-specific expression (50). Depending on their degree of sequence conservation, the 14 ZIP proteins can be further classified into 4 subgroups: the ZIP subfamily I, the ZIP subfamily II, the gufA subfamily, and the LIV-1 subfamily (51). While the functions of the ZIP subfamily II and the LIV-1 subfamily have been extensively investigated, the ZIP subfamily I (ZIP9) and the gufA subfamily (ZIP11) are less well characterized. The structural homology and differences among these Zn transporters as well as their tissue distributions and functions has been the subject of numerous excellent reviews (13, 49, 50). This review will focus on recent advances in the pathophysiology roles of the Zn transporters in cancers in section 4.3.
3.3. Zn permeable channels
Long before the first mammalian Zn transporter, i.e., ZnT1 was discovered two decades ago, Zn was thought to cross membranes through certain ion channels (52, 53). Because of the similar divalent cationic properties of Ca2+ and Zn2+, some Ca2 + permeable channels were suspected to be Zn permeable. The first identified Zn-permeable channel was the voltage-dependent Ca2+ channel (VDCC) in neurons. The supporting evidence includes that Zn influx or Zn influx-mediated action potentials could be blocked by VDCC inhibitors, such as Co2+, La3+ and verapamil (54, 55). Subsequent studies indicated that VDCCs were permeable to Zn2+ in other cells as well, such as cardiomyocytes, pancreatic β cells and chromaffin cells (56, 57). The different subunits of VDCCs may have different Zn2+ permeability and/or properties (58). For example, both Cav1.2 and Cav1.3 isoforms of VDCCs permeate Zn2+ using direct recording of zinc currents, while Cav1.3 Zn currents are much more sensitive to extracellular acidification than Cav1.2 currents (59).
In addition to VDCCs, another group of Ca2+ permeable channels may conduct Zn influx in neurons. Imaging and neurotoxicity studies demonstrated that Zn2+ can enter neurons through NMDA receptors and Ca2+-permeable AMPA/kainate (Ca-A/K) channels (55, 60).
The third group of Zn permeable channels is the Transient Receptor Potential (TRP) channel group (61). The TRP superfamily has been extensively studied and nearly thirty genes have been identified. These genes are further classified into seven subfamilies, i.e. TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), TRPA (ankyrin transmembrane protein) and TRPN (NompC-like) (62). TRP channels have widespread distribution in the human body and play important roles in a wide range of physiological and pathophysiological processes, such as cell proliferation, migration, adhesion, differentiation, apoptosis and necroptosis. Based on patch clamp and fluorescent Zn2+ imaging data, several members of this family are Zn2+-permeable, such as TRPC6, TRPV6, TRPM3, TRPM6/7, TRPML1 and TRPA1 (53). TRPM6 and TRPM7 are two unique TRP channels in that they contain both ion conducting pore and kinase domains (63). They are non-selective, cationic channels permeable to various divalent cations including Zn2+ and Mg2+. Indeed, the permeation profile for TRPM7 revealed a permeability sequence of Zn2+ >> Mg2+ > Ca2+ indicating that TRPM7 may mainly function as a Zn permeable channel and could potentially play a major role in Zn-related neurotoxicity in the brain during ischemia (64, 65). TRPM3 channels, endogenously expressed in pancreatic β cells, are also highly permeable for Zn ions (66). Using FluoZin3 to image changes of the intracellular Zn concentration, pancreatic β cells were found to incorporate Zn through TRPM3 channels even when extracellular Zn concentrations were low and physiological levels of Ca2+ and Mg2+ were present. Activation of TRPM3 channels caused plasma membrane depolarization and additional Zn influx through VDCCs in pancreatic b cells. In addition to Zn permeating through plasma membrane, TRPs are also likely involved in Zn transport between cytosol and intracellular organelles. TRPC6 is associated with Zn accumulation in the nucleus and TRPML1, also known as mucolipin-1 localized in endosomes and lysosomes, is responsible for Zn entering these intracellular organelles (67).
4. ZN IN HUMAN HEALTH AND CANCERS
4.1. Zn homeostasis and human health
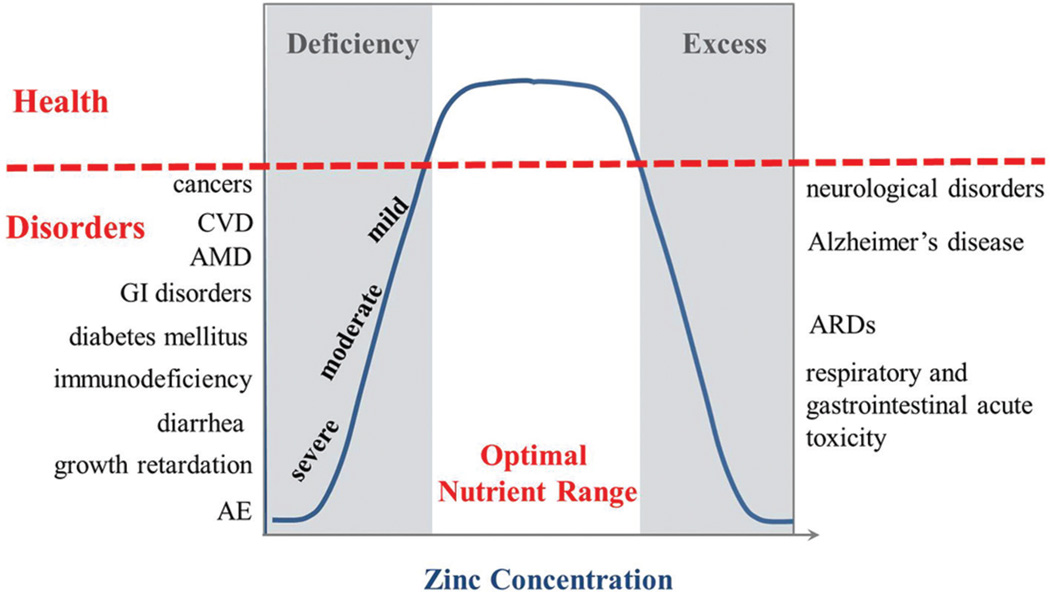
The optimal amount of nutritional Zn and maintenance of systemic and cellular Zn homeostasis are essential for human health. Both “too much” and “too little” are associated with various diseases (Figure 1). Excess Zn is known to be toxic to cells, especially to neurons. Acute toxicity from Zn poisoning affects the respiratory and gastrointestinal systems and is characterized by nausea, vomiting, loss of appetite, abdominal cramps and headaches (68). Excess Zn in neuronal and glial cells is associated with a variety of excitotoxicity conditions, such as epilepsy, ischemia, brain trauma, neuro-degenerations in Alzheimer’s disease. However, the evidence for significant toxic effects of Zn in humans is limited to accidental exposure to compounds of high Zn concentration and these cases are rather rare.
Figure 1.
Zn homeostasis and human disorders. There is an optimal nutrient range for human body. Too much or too little is detrimental to health. Dependent upon severity of Zn deficiency, one can suffer from a school of disorders. AE, acrodermatitis enteropathica; AMD, age-related macular degeneration; ARDS, adult respiratory distress syndrome; CVD, cardiovascular disorders; GI, gastrointestinal tract.
On the other hand, Zn deficiency is widespread and remains a major public health issue. Nutritional deficiency of Zn is common in countries with a high consumption of cereal-based foods low in Zn and high in phytic acid (69), a potent inhibitor of intestinal Zn absorption. Zn deficiency has also been reported in developed countries such as the USA (34). Depending on the severity of Zn deficiency, the patients can suffer from a wide range of symptoms and diseases (70) (Figure 1). There are many signs of acute or severe Zn deficiency including parakeratosis, hair loss, poor growth, impaired wound healing and teratogenesis. More moderate or mild Zn deficiency adversely affects, for example, immune function, cognitive function and cardiovascular health. Immunodeficiency syndromes have been reported in patients with Zn deficiency, leading to increased susceptibility to infections. We previously suggested that Zn deficiency may be a risk factor for atherosclerosis (70) and demonstrated evidence for this using a mouse model of atherosclerosis (71). A progressive age-related reduction of plasma Zn concentration has been observed in the elderly and age-related macular degeneration (AMD) has been related to Zn deficiency. Low concentration of Zn could lead to oxidative stress and retinal damage (72). The number of diseases associated with Zn deficiency is growing and a more comprehensive list can be found in other recent reviews (13, 73, 74).
4.2. Zn deficiency and cancers
As a co-factor for more than 300 enzymes, Zn participates in the function of enzymes including key kinases, proteases and phosphatases. Zn also stabilizes the structure of proteins, DNA, RNA and ribosomes, and regulates gene expression through 3000 zinc finger transcription factors, which are all highly relevant to cancers. In addition, Zn protects against inflammatory and oxidative stress. Therefore, the idea that Zn has a tumor suppressing role and an association between Zn deficiency and cancer can be hypothesized. However, the specific evidence and mechanisms underlying for this association remain largely undefined. Although serum or plasma Zn is not a good biomarker for Zn deficiency, there is compelling evidence that dysregulated Zn homeostasis is indeed associated with many cancers. Multiple studies show that serum Zn levels are generally low in patients with certain cancers, including esophageal squamous cell carcinoma (ESCC) (75–78), malignant prostate cancer (79, 80), breast cancer (79), ovarian cancer, and so on (81–83). Normal physiological concentrations of Zn inhibit cancer cell proliferation and migration, maintain balanced metabolism and promote apoptosis in cancer cells. Thus, Zn deficiency appears to be involved in every aspect of cancer cell generation and growth.
The anti-cancer function of Zn has been extensively studied in prostate cancer. The normal human prostate stores the highest content of Zn of all soft tissues in the body, with a typical Zn content more than 1000 µg/g dry tissue (84). The accumulation of Zn by prostate glandular epithelial cells is essential for the specialized function of these cells: to produce and secrete enormously high levels of citrate. During the transformation from prostatic epithelial hyperplasia to carcinoma, a reduction in Zn concentration occurs at a relative early stage and continues throughout tumor progression towards the androgen-resistant stage in prostate cancer patients. Zn contents in the prostate at the malignant stage can decline to as low as 150 µg/g dry tissue, which in turn dramatically decreases the production of citrate in the prostate. Concomitantly, the plasma Zn levels in patients with prostate carcinoma were much lower than those in healthy subjects or in patients with benign prostatic hyperplasia (80). A number of recent studies suggested that Zn deficiency is more than a bystander or “passenger” during the carcinogenesis and tumorigenesis. For example, the Akt-Mdm2-p53 signaling axis in human normal prostate epithelial cells PrEC cells was activated as response to Zn deficiency while the Akt-p21 signaling axis was stimulated in malignant prostate LNCaP cells under the same condition (85). As a result, LNCaP cells but not PrEC cells survived better and progressed through the G0/G1 phase of the cell cycle at Zn deficient conditions (85). In a study with combined gene profiling and protein analysis, nuclear p53 protein expression was increased with Zn deficiency along with an increase in the binding activity of transcription factors involved in regulating cell proliferation and apoptosis (86). Thus, this study supported the contention that Zn deficiency may compromise DNA integrity in the prostate by impairing the function of zinc-containing proteins. In androgen-independent PC-3 and DU-145 prostate cancer cells, Golovine et al, showed that physiological levels of Zn suppressed NF- kB activity and reduced expression of pro-angiogenic and pro-metastatic cytokines VEGF, IL-6, IL-8, and MMP-9 associated with negative prognostic features in prostate cancer (87). Selective Zn deficiency induced by the Zn chelator N,N,N’,N’-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) increased activation of NF- kB and up-regulated expression of the NF- kB controlled pro-angiogenic and pro-metastatic cytokines VEGF, IL-6 and IL-8. This study supports the contention that Zn deficiency may contribute to tumor progression via increased expression of the NF-kB-dependent pro-tumorigenic cytokines (87).
The “driver” function of Zn deficiency in carcinogenesis has been best documented in a series of in vivo studies in carcinogen-induced esophageal cancer animal models. Epidemiological studies suggest that ESCC, a major form of esophageal cancer, is strongly related with environmental factors. Among them, Zn deficiency is well-known to enhance esophageal tumor induction (88–90). Fong et al, have conducted serial studies using N-nitrosomethylbenzylamine (NMBA)-induced esophageal cancer rat or mouse models to understand the impact of dietary Zn deficiency on carcinogenesis and tumor development (88, 91–101). In these studies, the animals were fed with either Zn-deficient or Zn-sufficient diet. The data demonstrated that the tumor incidence increased from 6% in the Zn-sufficient group to 100% in the Zn-deficient group at 11 weeks after NMBA treatment (96). Using in vivo bromodeoxyuridine (BrDU) labeling followed by immunohistochemical detection of cells in S-phase, they further showed that Zn deficiency significantly increased cell proliferation in esophageal epithelial cells, with shortened lag time for tumor induction. In cultured esophageal cancer cells, reports from a number of groups suggested that replenishment of Zn inhibits proliferation and induces apoptosis. In another words, the replenishment of Zn in the diet could reduce cell proliferation and induce apoptosis in esophageal epithelia and thus greatly reduce both tumor incidence and tumor size in the esophagi and forestomach of NMBA-treated animals (88, 90, 92). Recent genomic profiling established the deregulation of genes associated with Zn homeostasis in ESCC (102).
Studies regarding the association between serum Zn and breast cancer are controversial. A recent statistical study conducted a systematic literature search and identified 14 reports presenting data for serum Zn concentrations in breast cancer patients (103). Based on a random effects model, the meta-analysis revealed no difference in serum Zn levels between breast cancer patients and controls (SMD (95%CI): −0.65[−1.42, 0.13]). However, the hair Zn levels were lower in women with breast cancer compared with those of control subjects (SMD (95%CI): −1.99[−3.46, −0.52]). Using a Zn deficient mouse model, Bostanci et al, demonstrated that marginal Zn intake created a toxic microenvironment in the mammary gland impairing breast development, which could increase the risk for breast disease and cancer (104).
It is worthwhile noting that breast biopsies from breast cancer patients contain significantly higher Zn levels compared with normal breast tissue (105). Epidemiological studies have established a relationship between high breast tissue Zn levels and development of breast cancer (106). It seems paradoxical since breast cancer patients have low Zn levels in hair or serum and Zn deficiency is believed to contribute to the development of breast cancer. These studies suggest that the dysregulated Zn homeostasis is complicated, and relates not only to Zn concentrations but also to its distribution as well as its temporal pattern (107). The high Zn content in breast tumor tissues implies that cancer cells selectively increase Zn uptake or decrease Zn efflux via regulating one or more Zn transporters.
4.3. Zn transporters in cancers
There are a growing number of studies that report the involvement of ZnTs and ZIPs in a wide variety of cancers. Although no mutation or SNP variant of Zn transporter genes has been reported so far to be associated with a particular cancer, a common scenario is that these Zn transporters have either altered expression levels or abnormal activity, which in turn contributes to Zn homeostasis dysregulation in cancer cells or tumor tissues. As summarized in Table 1, at least 5 ZnTs and almost all ZIP transporters are involved in a number of cancers, such as prostate, pancreatic, breast and esophageal cancers.
Table 1.
Zn transporters in cancers
| Zn transporter | Cancer type | Changes | Reference |
|---|---|---|---|
| ZnT1 | Prostate cancer | Decreased mRNA in tumor tissues | (108) |
| lymphoblastic leukemia | Increased mRNA in gallium-resistant CCRF-CEM cells | (170) | |
| ZnT2 | Breast cancer | Increased in luminal but not basal tumors | (107) |
| ZnT3 | Prostate cancer | Increased mRNA in low- androgen-sensitive LNCaP cells | (171) |
| ZnT4 | Prostate cancer | Decreased malignant vs. benign | (110) |
| ZnT7 | Esophageal cancer | Increased mRNA | (102) |
| ZIP1 | Prostate cancer | Decreased in malignant tissue | (112) |
| ZIP2 | Prostate cancer | Decreased in malignant tissue | (113) |
| ZIP3 | Prostate cancer | Decreased in malignant tissue | (113) |
| Pancreatic cancer | Decreased in adenocarcinoma tissue | (115,117,172) | |
| ZIP4 | Hepatocellular cancer | Increased in tumor tissue | (173) |
| Glioma | Increased in higher grade | (174,175) | |
| Pancreatic cancer | Increased pancreatic ductal adenocarcinoma | (116,176,177) | |
| Prostate cancer | Decreased | (114) | |
| ZIP5 | Esophageal cancer | Increased in ESCC tissue | (178) |
| ZIP6 LIV-1 | Breast cancer | Increased in ER-positive invasive ductal carcinoma tissue | (179–181) |
| Prostate cancer | Increased | (182) | |
| Pancreatic cancer | Increased | (183) | |
| Cervical cancer | Increased | (184) | |
| Hepatic cancer | Increased in liver carcinoma tissues | (185) | |
| Esophageal cancer | Increased in ESSC tissues | (186) | |
| ZIP7 HKE4 | Breast cancer | Increased in Tamoxifen-resistant MCF-7 cells | (121,187,188) |
| ZIP9 | Prostate cancer | Increased in malignant tissues | (189) |
| Breast cancer | Increased in malignant breast tissues | (189) | |
| ZIP10 | Kidney cancer | Increased mRNA in tumor | (190) |
| Breast cancer | Increased mRNA in lymph-node metastasis-positive tumor tissues | (191) | |
| ZIP11 | Glioma | Decreased in higher grade glioma | (175) |
| Bladder cancer, kidney cancer | SNPs are associated with the risks | (192) | |
| ZIP14 | Hepatocellular cancer | Decreased in hepatoma tissues | (193) |
ZnT1 can transport cytosolic Zn to the extracellular space and thus make the cells resistant to Zn toxicity (52). The mRNA expression level of ZnT1 is significantly lower in human prostate cancer tissues compared to that in benign prostate hyperplasia tissues (108), while extracellular Zn treatment increases the expression level of ZnT1 protein in cultured human prostate cancer cell lines, i.e. LNCaP and PC-3 cells. Abundant ZnT2 was observed in Luminal breast tumors but not in Basal tumors and adjacent non-malignant tissues (107). ZnT4 was found in cytoplasmic vesicles, Golgi apparatus, and the plasma membrane (109, 110). From immunohistochemistry data with normal, hyperplastic and malignant prostate tissues, low expression levels of ZnT4 were associated with more advanced cancer stages (110). In esophageal cancer, ZnT7 gene expression was up-regulated compared to esophageal nonmalignant or dysplastic tissue samples (102).
Compared with ZnT transporters, there are much more data on the association between ZIP members and cancers. As mentioned before, Zn concentrations in prostate gland decrease dramatically during tumorigenesis (111). Four Zn influx transporters, i.e. ZIP1–4, were reported to be down-regulated in prostate cancer tissues, which may result in the reduction of Zn concentration in prostate gland (111–114). Both ZIP3 and ZIP4 were also found to be altered in pancreatic cancer in the opposite manner, namely a reduction of ZIP3 and elevation of ZIP4 in tumor tissues (115, 116). According to in situ Zn staining images of human pancreatic tissues, the Zn concentration in pancreatic adenocarcinoma is lower than that in normal pancreatic ductal and acinar epithelium (115). These changes in Zn concentration and ZIP3 expression level are believed to be an early event in the development of pancreatic cancer, which likely makes the malignant cells resistant to Zn cytotoxic effects (117, 118). As regards ZIP4, it is difficult to understand why its expression level is increased in pancreatic cancer cells and even positively correlated with tumor progression in a xenograft animal model (116, 119). A recent study provided evidence to show that ZIP4 could render pancreatic cancer cells resistant to Zn deficiency-induced apoptosis (120), however, the exact mechanism remains elusive.
In addition to involvement in cancer cell proliferation and apoptosis, Zn transporters also contribute to drug-resistant properties in cancer cells. Taylor et al. reported that TamR cells, a MCF7-derived tamoxifen resistant breast cancer cell line had increased levels of Zn and ZIP7. Using siRNA specifically targeting the ZIP7 gene to reduce the expression level of ZIP7, they further demonstrated that TamR cells recovered tamoxifen sensitivity by reducing intracellular Zn levels and destroying EGFR/IGF-I receptor/Src signaling (121).
Despite accumulating evidence to reveal dysregulated Zn transporters in cancers, whether the malfunction of Zn transporter is a “driver” or a “passenger” for carcinogenesis or tumorigenesis is still unclear. It is also unclear whether the dysregulated Zn transporters per se or the consequent changes in Zn status exert these effects. The data summarized in Table 1 appear to reveal a trend toward ZIP transporters upregulation in most of cancers except prostate cancer. It may indicate an increased necessity of cellular Zn uptake to meet the needs of increased rate of proliferation and metabolism. Since Zn transporters are also subject to regulation by Zn status itself, hormones, growth factors as well as cellular redox state, multiple mechanisms underlying the involvement of Zn transporters may co-exist in cancers, which require further investigation.
5. ZN AS A CHANNEL MODULATOR
The well-understood multifunctions of Zn include roles in reducing oxidative stress and in cell signaling. Labile Zn ions, which are free or loosely bound to small molecules or proteins, can signal directly and are able to cross talk to other signaling pathways, such as Ca2+ signaling and participate in a redox signaling network (122–124). Since these functions have been summarized in other reviews (124, 125), we will not reiterate them here but rather discuss another hitherto less-appreciated roles of Zn in cancers: Zn as a modulator for a multitude of Ca2+ channels and voltage-gated K channels. It is beyond the scope of this brief review to cover all the channels regulated by Zn; again, we will just focus on a few representative channels that may play an important role in cancers.
5.1. Zn modulated-ion channels
Zn-regulated channels have been investigated to some extent in the central nervous system (CNS) (126). Many channels in CNS excitable cells are regulated by Zn, such as NMDA and GABA receptors, glycine receptors (glyR) and serotonin receptors (5-HT3). The purinergic receptors are adenosine triphosphate (ATP) gated cation channels at the plasma membrane, with wide cell-type expression including neurons, cancer and immune cells. The physiological concentrations of Zn can strongly inhibit human purinergic receptors P2×2 (hP2×2), while Zn enhances current activated by 5 µM ATP in a voltage independent manner for P2×4 (127, 128). Acid-sensing ion channel 3 (ASIC3) is a proton-gated, voltage-insensitive Na(+) channel that plays an important role in pain perception, particularly as a pH sensor following cardiac ischemia. Zinc is reported to be “an important regulator of ASIC3 at physiological concentrations”, and it inhibits ASIC3 in a pH- and Ca2+-independent manner. The inhibition of ASIC3 currents is dependent upon the interaction of zinc with extracellular domain(s) of ASIC3 (129).
For large-conductance voltage- and Ca2+-activated Slo1 K (BK) channels, intracellular Zn potently and reversibly activates the channel through His(365) in the RCK1 (regulator of conductance for K+) domain of the channel (130). Extracellular Zn activates Slo1 BK channels when they are coexpressed with Zn-permeable TRPM7. The study suggests that Zn can positively and directly regulate BK channels and shape the overall intracellular signaling.
Zn is also able to modulate a number of subunits of Ca2+ channels, including Cav1.2, Cav1.3, Cav3.1, Cav3.2 and Cav3.3 (126). In cultured cells exogenously expressing recombinant T-type Ca2+ channels (transient opening Ca2+ channels), i.e. Cav3.1, Cav3.2 and Cav3.3, the Cav3.2 current is significantly more sensitive to Zn than that of Cav3.1 and Cav3.3 (131). Zn can cause a significant increase in Cav3.3 current in action potential clamp experiments while Cav3.1 and Cav3.2 currents are significantly reduced, indicating Zn exhibits differential modulatory effects on T-type Ca2+ channels. In addition to voltage-gated Ca2+ channels, other Ca2+ channels can be modulated by Zn in general, such as TRP channels. In whole-cell patch-clamp recordings, extracellular application of Zn inhibited TRPM5 currents (132). Using mutagenesis approach, it was further demonstrated that inhibition by 30 µM ZnCl2 was impaired in TRPM5 mutants in which His at 896, and Glu at 926 and/or Glu at 939 in the outer pore loop were replaced with Gln. These data suggest that extracellular Zn inhibits TRPM5 channels through its interaction with the extracellular pore loop domain. Another Zn-modulated unique TRP family member is TRPA1 (133, 134). In this case, the activation of TRPA1 firstly requires Zn influx through the TRPA1 channel which subsequenctly activates the channel itself. Several intracellular cysteine and histidine residues are required for the Zn activation. TRPA1 is highly sensitive to intracellular Zn and nM concetrations are sufficient to activate the channel.
5.2. Dysregulated channels in cancer cells
Recently, the role of ion channels in driving the cell cycle phases (135), resistance to apoptosis (136), cell invasiveness (137), and angiogenesis (138) has been proved. Ion transport is implicated in these cell functions in many ways, including the classic mechanisms relating membrane potential to Ca2+ homeostasis, control of pH, cell volume, and interaction with the extracellular matrix. A major function of ion channels is to mediate the cell interaction with its environment. For these reasons, ion channels have now become a promising player for the development of novel cancer biomarkers and anticancer therapies.
Interestingly, many of these carcinogenesis or tumorigenesis involved channels either have been reported or theoretically can be regulated by Zn. Here, we will overview the recent studies on channels involved carcinogenesis and tumor progression, such as K+, TRP and Orai channels.
5.3. K channels
Multiple studies have reported dysregulated K+ channel expression in cancers. Among the four classes of K+ channels, voltage-gated K+ channels (Kv) and Ca2+-activated K+ channels (KCa) are the most studied in cancer (139–142). For example, Kv10.1 is overexpressed in almost all solid tumors compared to the matched normal tissue, which makes it a differential diagnostic factor and a prognostic factor often correlating with bad clinical outcomes (143, 144). The intermediate Ca2+-activated K+ channel, (KCa3.1) has been also reported to be overexpressed in several cancer types including lung and breast cancers (145, 146). Recently, Schwab’s team demonstrated that KCa3.1 channel gene (KCNN4) promoter is hypomethylated in an aggressive non-small cell lung carcinoma cell line and in patient samples (145). The loss of DNA methylation of the KCNN4 promoter was associated with increased KCa3.1 channel expression and function; both findings are strong indicators of poor prognosis in lung cancer.
Many studies have highlighted the importance of K+ channels in cancer cell proliferation, survival, migration, and invasion (147). In breast cancer, both Kv10.1 and KCa3.1 play a dual role in cancer cells according to the tumor phenotype. Kv10.1 is involved in both agonist and serum-induced membrane hyperpolarization that leads to Ca2+ entry, cell cycle progression by upregulating both cyclins D1 and E expression, and therefore cell proliferation in the non-invasive MCF-7 cells (148, 149). In contrast, in the invasive MDA-MB-231 breast cancer cell line, Kv10.1 does not affect proliferation but regulates migration through two mechanisms: the regulation of the Ca2+ influx and the formation of a complex with β-integrin and focal adhesion kinase (FAK) (150). In concordance with this notion, Kv10.1 participates in the acquisition of a malignant phenotype in lung tumor cells (151). Additionally, KCa3.1 regulates both cancer cell proliferation and migration (142, 152). In breast cancer, KCa3.1 regulates the G1 phase and G1/S transition of the cell cycle (153). According to the “potential membrane model,” activation of K+ channels amplifies the Ca2+ signals by hyperpolarizing the membrane potential, thus increasing the driving force for Ca2+ influx and this, in turn, activates Ca2+-dependent transcriptional factors leading to the expression of G1 regulating cyclins and CDK proteins. Both TRPC1 and TRPV6 co-localize with KCa3.1 and may be the major provider of passive Ca2+ influx in response to the hyperpolarization associated with KCa3.1 channel activation in cancer cells. In invasive MDA-MB231-breast cancer cells, KCa3.1 is also co-localized with TRPC1 and controls cell migration. Moreover, silencing KCa3.1 or TRPC1 reduced metastasis in vivo (unpublished data).
5.4. TRP channels
During the past 20 years, numerous studies have indicated that the expression and/or the activity of TRP channels are altered in cancers. In particular, the presence of the TRPC, TRPM, and TRPV subfamilies correlates with malignant growth and cancer progression (154, 155). As such, the expression of TRP channels has been proposed as a tool for diagnosis or predicting prognosis in several diseases (156), and targeting TRP channels has been suggested as a novel therapeutic strategy. For example, TRPV6 and TRPM8 have been proposed as markers of prostate cancer progression (157) and TRPC6 as a novel therapeutic target for esophageal carcinoma (158). In breast cancer, TRPC6, TRPM7, TRPM8, and TRPV6 are overexpressed, and their expression profiles are associated with pathologic parameters, suggesting their use as prognostic markers (155, 159). TRPM8 is considered a good prognostic marker for non-invasive well-differentiated estrogen-positive breast cancer tumors (ER+). While the expression profile of TRPM7 depends on both invasive and hormonal status (in non-invasive ER+ cells, TRPM7 as a proliferative marker of poorly differentiated tumors by regulating cell proliferation through the calcium influx (160)), these same channels may be proposed as a marker of poor prognosis in aggressive estrogen-negative cancers (ER−) by regulating cell migration through an interaction with cytoskeleton proteins.
5.5. Orai channels
To date, three Orai channels isoforms have been identified (Orai1, Orai2, and Orai3) (161–163). Of these isoforms, Orai1 was the first reported to play a crucial role in store operated calcium entry (SOCE) in breast cancer migration and metastasis. In this process, Orai1 is associated to Stim1 that senses the Ca2+ store depletion and triggers the SOCE (164). Orai1 is reported to promote MDA-MB-231 breast cancer cell migration through the promotion of a high rate of focal adhesion turnover, while its knockdown reduces the spread of tumor cells in xenografted mice. We also reported that elevated Orai1 was associated with poor prognosis in esophageal squamous cell carcinoma patients and inhibition of the Orai1 channels either by the pharmacological compounds or the knocking-down of Orai1 expression could block cancer cell proliferation and migration in vitro as well as tumor growth in vivo (165). Moreover, Orai1 is reported to be associated to Kv10.1 and SK3 K+ channels in regulating breast cancer migration (150, 166).
Orai3 is overexpressed in breast cancer tissues and MCF-7 and T47D cell lines as compared to adjacent normal tissues and non-cancerous MCF-10A cell line, respectively (167). Orai3 is involved in cell proliferation, cell cycle progression, and cell survival through regulating the expression of the G1 phase and G1/S transition regulatory proteins. Indeed, silencing Orai3 leads to significant down regulation of cell proliferation and stops the cell cycle at the G1 phase along with an up regulation of cell apoptosis. Moreover, knockdown of Orai3 results in a decrease of CDKs 4/2 (cyclin-dependent kinases) and cyclins D1/E expression concurrently with an up-regulation of p21Waf1/Cip1 (a cyclin-dependent kinase inhibitor) and p53 expression. It also increases Bax/Bcl-2 ratio, a measure of apoptotic susceptibility by the ratio between the major pro-apoptotic protein Bax and the major anti-apoptotic protein Bcl-2 in breast cancer cells (167). Interestingly, these effects are exclusively observed in cancer cells but not in a non-cancerous MCF-10A cell line. The above mentioned involvement of Orai3 in cell proliferation, survival and cell cycle progression is, in part, related to Ca2+ influx through this same channel as illustrated by the significant decrease in cell proliferation subsequent to decreasing extracellular Ca2+ concentration to 0.2 mM (167). The Orai3 mediated Ca2+ entry is reported to affect the proto-oncogenes NFAT and C-myc through the MAP kinase pathway (168, 169). This finding will undoubtedly help in determining the downstream events whereby Orai3 ultimately controls cell proliferation, the cell cycle progression, cell survival, and cell invasion of ERα+ breast cancer cells (168, 169).
6. CONCLUSIONS AND PERSPECTIVES
It is becoming evident that Zn deficiency and Zn transporters are involved in many types of cancer. Dysregulated Zn homeostasis may play a role more like a “driver” than a “passenger” in carcinogenesis or tumorigenesis. Traditionally, the diseases caused by inherited mutations that alter ion channel biophysical properties are called “channelopathies”. Currently, certain diseases caused by dysregulated ion channel expression, membrane trafficking, and/or posttranslational modifications are included in this group. In fact, some electrical and episodic disorders classified as channelopathies, have no defects in the ion channel itself but instead have a dysfunctional regulatory protein of the ion channel (or transporter). Since many cancers may be caused by the dysregulation of ion channel expression and/or activity, the cancers caused by Zn-dysregulated channel may be classified within the channelopathy family. Investigation of the pathophysiological roles of ZnTs and ZIPs in various cancers is an emerging research field. Further investigation may help to determine the mechanistic basis of the association between Zn-modulated channels and cancers. Novel biomarker discovery for the effective clinical identification of high risk in cancer patients with Zn deficiency are required, as are improved preventive measures and therapy.
Acknowledgments
The authors would like to acknowledge financial support from NIH-NCI (R01-CA185055), UMDNJ Foundation (62-09) and Pelotonia to ZP, the Rural and Environment Science and Analytical Services of the Scottish Government to JHB, the Region Hauts-de-France to IK and the contribution of the COST Action TD1304.
REFERENCES
- 1.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5(1):196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 2.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 4.McCall KA, Huang C, Fierke CA. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130(5S Suppl):1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 5.Andreini C, Bertini I. A bioinformatics view of zinc enzymes. J Inorg Biochem. 2012;111:150–156. doi: 10.1016/j.jinorgbio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983;306(5940):284–287. doi: 10.1038/306284a0. [DOI] [PubMed] [Google Scholar]
- 7.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci U S A. 2002;99(26):16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med. 2013;34(2–3):548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med. 2013;34(2–3):612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweigel-Rontgen M. The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. Curr Top Membr. 2014;73:321–355. doi: 10.1016/B978-0-12-800223-0.00009-8. [DOI] [PubMed] [Google Scholar]
- 11.Kambe T, Hashimoto A, Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci. 2014;71(17):3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golan Y, Berman B, Assaraf YG. Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation. J Biol Chem. 2015;290(14):9050–9063. doi: 10.1074/jbc.M114.617332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 14.Sorgjerd EP, Skorpen F, Kvaloy K, Midthjell K, Grill V. Prevalence of ZnT8 antibody in relation to phenotype and SLC30A8 polymorphism in adult autoimmune diabetes: results from the HUNT study, Norway. Autoimmunity. 2013;46(1):74–79. doi: 10.3109/08916934.2012.732132. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Wang J, Chen B. SLC30A8 (ZnT8) variations and type 2 diabetes in the Chinese Han population. Genet Mol Res. 2012;11(2):1592–1598. doi: 10.4238/2012.May.24.1. [DOI] [PubMed] [Google Scholar]
- 16.Howson JM, Krause S, Stevens H, Smyth DJ, Wenzlau JM, Bonifacio E, Hutton J, Ziegler AG, Todd JA, Achenbach P. Genetic association of zinc transporter 8 (ZnT8) autoantibodies in type 1 diabetes cases. Diabetologia. 2012;55(7):1978–1984. doi: 10.1007/s00125-012-2540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki E. ZnT8 and type 1 diabetes. Endocr J. 2012;59(7):531–537. doi: 10.1507/endocrj.ej12-0069. [DOI] [PubMed] [Google Scholar]
- 18.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58(9):2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57(10):2693–2697. doi: 10.2337/db08-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiser J, Venken KJ, De Lisle RC, Andrews GK. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 2012;8(6):e1002766. doi: 10.1371/journal.pgen.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem Soc Trans. 2008;36(Pt 6):1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambe T, Andrews GK. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol Cell Biol. 2009;29(1):129–139. doi: 10.1128/MCB.00963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet. 2007;16(12):1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 24.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278(35):33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 25.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 26.Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate cancer. Epidemiol Rev. 2001;23(1):93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- 27.Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002;21(3–4):291–295. doi: 10.1023/a:1021215111729. [DOI] [PubMed] [Google Scholar]
- 28.Stocks P, Davies RI. Zinc and Copper Content of Soils Associated with the Incidence of Cancer of the Stomach and Other Organs. Br J Cancer. 1964;18:14–24. doi: 10.1038/bjc.1964.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622(1–2):84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci. 2013;5:33. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen J, Karges W, Rink L. Zinc and diabetes--clinical links and molecular mechanisms. J Nutr Biochem. 2009;20(6):399–417. doi: 10.1016/j.jnutbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Huang G, Xiang Y, Pan L, Li X, Luo S, Zhou Z. Zinc transporter 8 autoantibody (ZnT8A) could help differentiate latent autoimmune diabetes in adults (LADA) from phenotypic type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013;29(5):363–368. doi: 10.1002/dmrr.2396. [DOI] [PubMed] [Google Scholar]
- 33.Haglund B, Ryckenberg K, Selinus O, Dahlquist G. Evidence of a relationship between childhood-onset type I diabetes and low groundwater concentration of zinc. Diabetes Care. 1996;19(8):873–875. doi: 10.2337/diacare.19.8.873. [DOI] [PubMed] [Google Scholar]
- 34.Prasad AS. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130(5S Suppl):1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 36.Jackson MJ. Physiology of Zinc: General Aspects. In: Mills CF, editor. Zinc in Human Biology. London: Springer London; 1989. [Google Scholar]
- 37.Meunier N, Feillet-Coudray C, Rambeau M, Andriollo-Sanchez M, Brandolini-Bunlon M, Coulter SJ, Cashman KD, Mazur A, Coudray C. Impact of micronutrient dietary intake and status on intestinal zinc absorption in late middle-aged men: the ZENITH study. Eur J Clin Nutr. 2005;59(Suppl 2):S48–S52. doi: 10.1038/sj.ejcn.1602298. [DOI] [PubMed] [Google Scholar]
- 38.Rukgauer M, Klein J, Kruse-Jarres JD. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol. 1997;11(2):92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 39.Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ. Fluorescent sensors for Zn(2+) based on a fluorescein platform: synthesis, properties and intracellular distribution. J Am Chem Soc. 2001;123(32):7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 40.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292(5526):2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 41.Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987;20(2):91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- 42.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321(2):517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 43.Gibon J, Tu P, Frazzini V, Sensi SL, Bouron A. The thiol-modifying agent N-ethylmaleimide elevates the cytosolic concentration of free Zn(2+) but not of Ca(2+) in murine cortical neurons. Cell Calcium. 2010;48(1):37–43. doi: 10.1016/j.ceca.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Chevallet M, Jarvis L, Harel A, Luche S, Degot S, Chapuis V, Boulay G, Rabilloud T, Bouron A. Functional consequences of the over-expression of TRPC6 channels in HEK cells: impact on the homeostasis of zinc. Metallomics. 2014;6(7):1269–1276. doi: 10.1039/c4mt00028e. [DOI] [PubMed] [Google Scholar]
- 45.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6(10):737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284(24):16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukunaka A, Kambe T. Mechanism of zinc transport by zinc transporters, ZnT and ZIP. Seikagaku. 2010;82(1):30–34. [PubMed] [Google Scholar]
- 48.Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem. 2011;75(6):1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- 49.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 50.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 51.Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14(3–4):251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- 52.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14(4):639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue K, O’Bryant Z, Xiong ZG. Zinc-permeable ion channels: effects on intracellular zinc dynamics and potential physiological/pathophysiological significance. Curr Med Chem. 2015;22(10):1248–1257. doi: 10.2174/0929867322666150209153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawa K. Zinc-dependent action potentials in giant neurons of the snail, Euhadra quaestia. J Membr Biol. 1979;49(4):325–344. doi: 10.1007/BF01868990. [DOI] [PubMed] [Google Scholar]
- 55.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17(24):9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J Biol Chem. 2006;281(14):9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki S, Hasegawa A, Hojyo S, Ohashi W, Fukada T, Nishida K, Hirano T. A novel role of the L-type calcium channel alpha1D subunit as a gatekeeper for intracellular zinc signaling: zinc wave. PLoS One. 2012;7(6):e39654. doi: 10.1371/journal.pone.0039654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouron A, Oberwinkler J. Contribution of calcium-conducting channels to the transport of zinc ions. Pflugers Arch. 2014;466(3):381–387. doi: 10.1007/s00424-013-1295-z. [DOI] [PubMed] [Google Scholar]
- 59.Park SJ, Min SH, Kang HW, Lee JH. Differential zinc permeation and blockade of L-type Ca2+ channel isoforms Cav1.2 and Cav1.3. Biochim Biophys Acta. 2015;1848(10 Pt A):2092–2100. doi: 10.1016/j.bbamem.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol. 2002;543(Pt 1):35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85(5):661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 62.Gautier M, Dhennin-Duthille I, Ay AS, Rybarczyk P, Korichneva I, Ouadid- Ahidouch H. New insights into pharmacological tools to TR(i)P cancer up. Br J Pharmacol. 2014;171(10):2582–2592. doi: 10.1111/bph.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291(5506):1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 64.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121(1):49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285(10):7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner TF, Drews A, Loch S, Mohr F, Philipp SE, Lambert S, Oberwinkler J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch. 2010;460(4):755–765. doi: 10.1007/s00424-010-0838-9. [DOI] [PubMed] [Google Scholar]
- 67.Kiselyov K, Colletti GA, Terwilliger A, Ketchum K, Lyons CW, Quinn J, Muallem S. TRPML: transporters of metals in lysosomes essential for cell survival? Cell Calcium. 2011;50(3):288–294. doi: 10.1016/j.ceca.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. 1994;102(Suppl 2):5–46. doi: 10.1289/ehp.941025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brnic M, Wegmuller R, Melse-Boonstra A, Stomph T, Zeder C, Tay FM, Hurrell RF. Zinc Absorption by Adults Is Similar from Intrinsically Labeled Zinc-Biofortified Rice and from Rice Fortified with Labeled Zinc Sulfate. J Nutr. 2016;146(1):76–80. doi: 10.3945/jn.115.213421. [DOI] [PubMed] [Google Scholar]
- 70.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91(2):177–181. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 71.Beattie JH, Gordon MJ, Duthie SJ, McNeil CJ, Horgan GW, Nixon GF, Feldmann J, Kwun IS. Suboptimal dietary zinc intake promotes vascular inflammation and atherogenesis in a mouse model of atherosclerosis. Mol Nutr Food Res. 2012;56(7):1097–1105. doi: 10.1002/mnfr.201100776. [DOI] [PubMed] [Google Scholar]
- 72.Jampol LM, Ferris FL., 3rd Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA. 2001;286(19):2466–2468. [PubMed] [Google Scholar]
- 73.Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol. 2014;28(4):357–363. doi: 10.1016/j.jtemb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40(8 Pt 1):2633–2644. [PubMed] [Google Scholar]
- 76.Jaskiewicz K, Marasas WF, Rossouw JE, Van Niekerk FE, Heine Tech EW. Selenium and other mineral elements in populations at risk for esophageal cancer. Cancer. 1988;62(12):2635–2639. doi: 10.1002/1097-0142(19881215)62:12<2635::aid-cncr2820621232>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 77.Barch DH. Esophageal cancer and microelements. J Am Coll Nutr. 1989;8(2):99–107. doi: 10.1080/07315724.1989.10720284. [DOI] [PubMed] [Google Scholar]
- 78.Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97(4):301–306. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 79.Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, Hrabeta J, Eckschlager T, Stiborova M, Kizek R. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr Med Chem. 2011;18(33):5041–5051. doi: 10.2174/092986711797636126. [DOI] [PubMed] [Google Scholar]
- 80.Christudoss P, Selvakumar R, Fleming JJ, Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol. 2011;27(1):14–18. doi: 10.4103/0970-1591.78405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Issell BF, MacFadyen BV, Gum ET, Valdivieso M, Dudrick SJ, Bodey GP. Serum zinc levels in lung cancer patients. Cancer. 1981;47(7):1845–1848. doi: 10.1002/1097-0142(19810401)47:7<1845::aid-cncr2820470721>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 82.Buntzel J, Bruns F, Glatzel M, Garayev A, Mucke R, Kisters K, Schafer U, Schonekaes K, Micke O. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res. 2007;27(4A):1941–1943. [PubMed] [Google Scholar]
- 83.Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4(8):875–903. doi: 10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29(5):565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 85.Han CT, Schoene NW, Lei KY. Influence of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in normal and malignant human prostate cells. Am J Physiol Cell Physiol. 2009;297(5):C1188–C1199. doi: 10.1152/ajpcell.00042.2009. [DOI] [PubMed] [Google Scholar]
- 86.Yan M, Song Y, Wong CP, Hardin K, Ho E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr. 2008;138(4):667–673. doi: 10.1093/jn/138.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golovine K, Uzzo RG, Makhov P, Crispen PL, Kunkle D, Kolenko VM. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-kappaB-dependent pathway. Prostate. 2008;68(13):1443–1449. doi: 10.1002/pros.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93(20):1525–1533. doi: 10.1093/jnci/93.20.1525. [DOI] [PubMed] [Google Scholar]
- 89.Lipman TO, Diamond A, Mellow MH, Patterson KY. Esophageal zinc content in human squamous esophageal cancer. J Am Coll Nutr. 1987;6(1):41–46. doi: 10.1080/07315724.1987.10720164. [DOI] [PubMed] [Google Scholar]
- 90.Fong LY, Sivak A, Newberne PM. Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. J Natl Cancer Inst. 1978;61(1):145–150. doi: 10.1093/jnci/61.1.145. [DOI] [PubMed] [Google Scholar]
- 91.Barch DH, Kuemmerle SC, Hollenberg PF, Iannaccone PM. Esophageal microsomal metabolism of N-nitrosomethylbenzylamine in the zinc-deficient rat. Cancer Res. 1984;44(12 Pt 1):5629–5633. [PubMed] [Google Scholar]
- 92.Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis. 1998;19(9):1591–1596. doi: 10.1093/carcin/19.9.1591. [DOI] [PubMed] [Google Scholar]
- 93.Fong LY, Feith DJ, Pegg AE. Antizyme overexpression in transgenic mice reduces cell proliferation, increases apoptosis, and reduces N-nitrosomethylbenzylamine-induced forestomach carcinogenesis. Cancer Res. 2003;63(14):3945–3954. [PubMed] [Google Scholar]
- 94.Fong LY, Ishii H, Nguyen VT, Vecchione A, Farber JL, Croce CM, Huebner K. p53 deficiency accelerates induction and progression of esophageal and forestomach tumors in zinc-deficient mice. Cancer Res. 2003;63(1):186–195. [PubMed] [Google Scholar]
- 95.Fong LY, Lau KM, Huebner K, Magee PN. Induction of esophageal tumors in zinc-deficient rats by single low doses of N-nitrosomethylbenzylamine (NMBA): analysis of cell proliferation, and mutations in H-ras and p53 genes. Carcinogenesis. 1997;18(8):1477–1484. doi: 10.1093/carcin/18.8.1477. [DOI] [PubMed] [Google Scholar]
- 96.Fong LY, Li JX, Farber JL, Magee PN. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis. 1996;17(9):1841–1848. doi: 10.1093/carcin/17.9.1841. [DOI] [PubMed] [Google Scholar]
- 97.Fong LY, Magee PN. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 1999;143(1):63–69. doi: 10.1016/s0304-3835(99)00191-3. [DOI] [PubMed] [Google Scholar]
- 98.Fong LY, Mancini R, Nakagawa H, Rustgi AK, Huebner K. Combined cyclin D1 overexpression and zinc deficiency disrupts cell cycle and accelerates mouse forestomach carcinogenesis. Cancer Res. 2003;63(14):4244–4252. [PubMed] [Google Scholar]
- 99.Fong LY, Nguyen VT, Farber JL, Huebner K, Magee PN. Early deregulation of the the p16ink4a-cyclin D1/cyclin-dependent kinase 4-retinoblastoma pathway in cell proliferation-driven esophageal tumorigenesis in zinc-deficient rats. Cancer Res. 2000;60(16):4589–4595. [PubMed] [Google Scholar]
- 100.Fong LY, Pegg AE, Magee PN. Alpha-difluoromethylornithine inhibits N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in zinc-deficient rats: effects on esophageal cell proliferation and apoptosis. Cancer Res. 1998;58(23):5380–5388. [PubMed] [Google Scholar]
- 101.Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, Farber JL, Croce CM, Fong LY. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2012;31(42):4550–4558. doi: 10.1038/onc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar A, Chatopadhyay T, Raziuddin M, Ralhan R. Discovery of deregulation of zinc homeostasis and its associated genes in esophageal squamous cell carcinoma using cDNA microarray. Int J Cancer. 2007;120(2):230–242. doi: 10.1002/ijc.22246. [DOI] [PubMed] [Google Scholar]
- 103.Wu X, Tang J, Xie M. Serum and hair zinc levels in breast cancer: a meta-analysis. Sci Rep. 2015;5:12249. doi: 10.1038/srep12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bostanci Z, Mack RP, Jr, Lee S, Soybel DI, Kelleher SL. Paradoxical zinc toxicity and oxidative stress in the mammary gland during marginal dietary zinc deficiency. Reprod Toxicol. 2015;54:84–92. doi: 10.1016/j.reprotox.2014.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52(5):868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 106.Cui Y, Vogt S, Olson N, Glass AG, Rohan TE. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1682–1685. doi: 10.1158/1055-9965.EPI-07-0187. [DOI] [PubMed] [Google Scholar]
- 107.Chandler P, Kochupurakkal BS, Alam S, Richardson AL, Soybel DI, Kelleher SL. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol Cancer. 2016;15(1):2. doi: 10.1186/s12943-015-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200(2):187–195. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 109.McCormick NH, Kelleher SL. ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells. Am J Physiol Cell Physiol. 2012;303(3):C291–C297. doi: 10.1152/ajpcell.00443.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG, Quinn DI, Turner JJ, Delprado W, Lee CS, Golovsky D, Brenner PC, O’Neill GF, Kooner R, Stricker PD, Grygiel JJ, Mack DH, Sutherland RL. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene. 2003;22(38):6005–6012. doi: 10.1038/sj.onc.1206797. [DOI] [PubMed] [Google Scholar]
- 111.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52(4):316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol. 2012;30(6):906–911. doi: 10.1016/j.urolonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Costello LC, Levy BA, Desouki MM, Zou J, Bagasra O, Johnson LA, Hanna N, Franklin RB. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12(4):297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104(47):18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Franklin RB, Zou J, Costello LC. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol Ther. 2014;15(10):1431–1437. doi: 10.4161/cbt.29927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Costello LC, Franklin RB. A Review of the Current Status and Concept of the Emerging Implications of Zinc and Zinc Transporters in the Development of Pancreatic Cancer. Pancreat Disord Ther. 2013;(Suppl 4) doi: 10.4172/2165-7092.S4-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15(19):5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cui X, Zhang Y, Yang J, Sun X, Hagan JP, Guha S, Li M. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle. 2014;13(7):1180–1186. doi: 10.4161/cc.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology. 2008;149(10):4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 122.Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011;24(3):411–418. doi: 10.1007/s10534-010-9406-1. [DOI] [PubMed] [Google Scholar]
- 123.Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010;26(11–12):1050–1057. doi: 10.1016/j.nut.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 124.Korichneva I. Zinc dynamics in the myocardial redox signaling network. Antioxid Redox Signal. 2006;8(9–10):1707–1721. doi: 10.1089/ars.2006.8.1707. [DOI] [PubMed] [Google Scholar]
- 125.Hartwig A. Metal interaction with redox regulation: an integrating concept in metal carcinogenesis? Free Radic Biol Med. 2013;55:63–72. doi: 10.1016/j.freeradbiomed.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Marger L, Schubert CR, Bertrand D. Zinc: an underappreciated modulatory factor of brain function. Biochem Pharmacol. 2014;91(4):426–435. doi: 10.1016/j.bcp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Punthambaker S, Blum JA, Hume RI. High potency zinc modulation of human P2X2 receptors and low potency zinc modulation of rat P2X2 receptors share a common molecular mechanism. J Biol Chem. 2012;287(26):22099–22111. doi: 10.1074/jbc.M112.369157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81(5):2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Q, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience. 2010;169(2):574–583. doi: 10.1016/j.neuroscience.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 130.Hou S, Vigeland LE, Zhang G, Xu R, Li M, Heinemann SH, Hoshi T. Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J Biol Chem. 2010;285(9):6434–6442. doi: 10.1074/jbc.M109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Traboulsie A, Chemin J, Chevalier M, Quignard JF, Nargeot J, Lory P. Subunit-specific modulation of T-type calcium channels by zinc. J Physiol. 2007;578(Pt 1):159–171. doi: 10.1113/jphysiol.2006.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uchida K, Tominaga M. Extracellular zinc ion regulates transient receptor potential melastatin 5 (TRPM5) channel activation through its interaction with a pore loop domain. J Biol Chem. 2013;288(36):25950–25955. doi: 10.1074/jbc.M113.470138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Banke TG, Wickenden AD. Intracellular zinc irritates TRPA1. Nat Chem Biol. 2009;5(3):141–142. doi: 10.1038/nchembio0309-141. [DOI] [PubMed] [Google Scholar]
- 134.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5(3):183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ouadid-Ahidouch H, Ahidouch A. K(+) channels and cell cycle progression in tumor cells. Front Physiol. 2013;4:220. doi: 10.3389/fphys.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lehen’kyi V, Shapovalov G, Skryma R, Prevarskaya N. Ion channnels and transporters in cancer. 5. Ion channels in control of cancer and cell apoptosis. Am J Physiol Cell Physiol. 2011;301(6):C1281–C1289. doi: 10.1152/ajpcell.00249.2011. [DOI] [PubMed] [Google Scholar]
- 137.Becchetti A, Arcangeli A. Integrins and ion channels in cell migration: implications for neuronal development, wound healing and metastatic spread. Adv Exp Med Biol. 2010;674:107–123. doi: 10.1007/978-1-4419-6066-5_10. [DOI] [PubMed] [Google Scholar]
- 138.Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am J Physiol Cell Physiol. 2012;302(1):C9–C15. doi: 10.1152/ajpcell.00280.2011. [DOI] [PubMed] [Google Scholar]
- 139.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205(3):115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 140.Comes N, Serrano-Albarras A, Capera J, Serrano-Novillo C, Condom E, Ramon YCS, Ferreres JC, Felipe A. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta. 2015;1848(10 Pt B):2477–2492. doi: 10.1016/j.bbamem.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 141.Gueguinou M, Chantome A, Fromont G, Bougnoux P, Vandier C, Potier-Cartereau M. KCa and Ca(2+) channels: the complex thought. Biochim Biophys Acta. 2014;1843(10):2322–2333. doi: 10.1016/j.bbamcr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 142.Catacuzzeno L, Fioretti B, Franciolini F. Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma. J Signal Transduct. 2012;2012:421564. doi: 10.1155/2012/421564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodriguez-Rasgado JA, Acuna-Macias I, Camacho J. Eag1 channels as potential cancer biomarkers. Sensors (Basel) 2012;12(5):5986–5995. doi: 10.3390/s120505986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ouadid-Ahidouch H, Ahidouch A, Pardo LA. Kv10.1 K channel: from physiology to cancer. Pflugers Arch. 2016 doi: 10.1007/s00424-015-1784-3. [DOI] [PubMed] [Google Scholar]
- 145.Bulk E, Ay AS, Hammadi M, Ouadid-Ahidouch H, Schelhaas S, Hascher A, Rohde C, Thoennissen NH, Wiewrodt R, Schmidt E, Marra A, Hillejan L, Jacobs AH, Klein HU, Dugas M, Berdel WE, Muller-Tidow C, Schwab A. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int J Cancer. 2015;137(6):1306–1317. doi: 10.1002/ijc.29490. [DOI] [PubMed] [Google Scholar]
- 146.Haren N, Khorsi H, Faouzi M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. Intermediate conductance Ca2+ activated K+ channels are expressed and functional in breast adenocarcinomas: correlation with tumour grade and metastasis status. Histol Histopathol. 2010;25(10):1247–1255. doi: 10.14670/HH-25.1247. [DOI] [PubMed] [Google Scholar]
- 147.Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14(1):39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 148.Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. Journal of Membrane Biology. 2008;221(1):1–6. doi: 10.1007/s00232-007-9080-6. [DOI] [PubMed] [Google Scholar]
- 149.Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: Possible involvement of a h-ether.a-gogo K+ channel. Recept. Channels. 2001;7(5):345–356. [PubMed] [Google Scholar]
- 150.Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H, Ouadid-Ahidouch H. Human ether a-gogo K+ channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol. 2012;227(12):3837–3846. doi: 10.1002/jcp.24095. [DOI] [PubMed] [Google Scholar]
- 151.Restrepo-Angulo I, Sanchez-Torres C, Camacho J. Human EAG1 potassium channels in the epithelial-to-mesenchymal transition in lung cancer cells. Anticancer Res. 2011;31(4):1265–1270. [PubMed] [Google Scholar]
- 152.Faouzi M, Chopin V, Ahidouch A, Ouadid-Ahidouch H. Intermediate Ca2+- sensitive K+ channels are necessary for prolactin-induced proliferation in breast cancer cells. J Membr Biol. 2010;234(1):47–56. doi: 10.1007/s00232-010-9238-5. [DOI] [PubMed] [Google Scholar]
- 153.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287(1):C125–C134. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 154.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772(8):937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19(2):117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 156.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772(8):805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 157.Gkika D, Prevarskaya N. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl. 2011;13(5):673–676. doi: 10.1038/aja.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ding X, He Z, Shi Y, Wang Q, Wang Y. Targeting TRPC6 channels in oesophageal carcinoma growth. Expert Opin Ther Targets. 2010;14(5):513–527. doi: 10.1517/14728221003733602. [DOI] [PubMed] [Google Scholar]
- 159.Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28(5):813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 160.Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297(3):C493–C502. doi: 10.1152/ajpcell.00624.2008. DOI: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 161.Cai X. Molecular evolution and structural analysis of the Ca(2+) release-activated Ca(2+) channel subunit, Orai. J Mol Biol. 2007;368(5):1284–1291. doi: 10.1016/j.jmb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 162.Amcheslavsky A, Wood ML, Yeromin AV, Parker I, Freites JA, Tobias DJ, Cahalan MD. Molecular biophysics of Orai store-operated Ca2+ channels. Biophys J. 2015;108(2):237–246. doi: 10.1016/j.bpj.2014.11.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95(4):1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 165.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5(11):3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clarysse L, Gueguinou M, Potier-Cartereau M, Vandecasteele G, Bougnoux P, Chevalier S, Chantome A, Vandier C. cAMP-PKA inhibition of SK3 channel reduced both Ca2+ entry and cancer cell migration by regulation of SK3-Orai1 complex. Pflugers Arch. 2014;466(10):1921–1932. doi: 10.1007/s00424-013-1435-5. [DOI] [PubMed] [Google Scholar]
- 167.Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol. 2011;226(2):542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 168.Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, Penner R, Ouadid-Ahidouch H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim Biophys Acta. 2013;1833(3):752–760. doi: 10.1016/j.bbamcr.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 169.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor alpha-regulated Ca(2)(+) channel that promotes tumorigenesis. Faseb j. 2013;27(1):63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang M, Kroft SH, Chitambar CR. Gene expression analysis of gallium-resistant and gallium-sensitive lymphoma cells reveals a role for metal-responsive transcription factor-1, metallothionein-2A, and zinc transporter-1 in modulating the antineoplastic activity of gallium nitrate. Mol Cancer Ther. 2007;6(2):633–643. doi: 10.1158/1535-7163.MCT-06-0557. [DOI] [PubMed] [Google Scholar]
- 171.Iguchi K, Ishii K, Nakano T, Otsuka T, Usui S, Sugimura Y, Hirano K. Isolation and characterization of LNCaP sublines differing in hormone sensitivity. J Androl. 2007;28(5):670–678. doi: 10.2164/jandrol.107.002675. [DOI] [PubMed] [Google Scholar]
- 172.Costello LC, Zou J, Desouki MM, Franklin RB. Evidence for changes in RREB-1, ZIP3, and Zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer. 2012;43(4):570–578. doi: 10.1007/s12029-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu X, Guo HJ, Xie HY, Li J, Zhuang RZ, Ling Q, Zhou L, Wei XY, Liu ZK, Ding SM, Chen KJ, Xu ZY, Zheng SS. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J Biol Sci. 2014;10(3):245–256. doi: 10.7150/ijbs.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, Cui X, Chen L, Yan W, Jiang T, Hergenroeder GW, Fletcher SA, Levine JM, Kim DH, Tandon N, Zhu JJ, Li M. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 2013;15(8):1008–1016. doi: 10.1093/neuonc/not042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kang X, Chen R, Zhang J, Li G, Dai PG, Chen C, Wang HJ. Expression Profile Analysis of Zinc Transporters (ZIP4, ZIP9, ZIP11, ZnT9) in Gliomas and their Correlation with IDH1 Mutation Status. Asian Pac J Cancer Prev. 2015;16(8):3355–3360. doi: 10.7314/apjcp.2015.16.8.3355. [DOI] [PubMed] [Google Scholar]
- 176.Xu C, Wallace MB, Yang J, Jiang L, Zhai Q, Zhang Y, Hong C, Chen Y, Frank TS, Stauffer JA, Asbun HJ, Raimondo M, Woodward TA, Li Z, Guha S, Zheng L, Li M. ZIP4 is a novel diagnostic and prognostic marker in human pancreatic cancer: a systemic comparison between EUS-FNA and surgical specimens. Curr Mol Med. 2014;14(3):309–315. doi: 10.2174/1566524013666131217112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, Sun S, Yang G. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330(2):163–169. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 178.Jin J, Li Z, Liu J, Wu Y, Gao X, He Y. Knockdown of zinc transporter ZIP5 (SLC39A5) expression significantly inhibits human esophageal cancer progression. Oncol Rep. 2015;34(3):1431–1439. doi: 10.3892/or.2015.4097. [DOI] [PubMed] [Google Scholar]
- 179.Schneider J, Ruschhaupt M, Buness A, Asslaber M, Regitnig P, Zatloukal K, Schippinger W, Ploner F, Poustka A, Sultmann H. Identification and meta-analysis of a small gene expression signature for the diagnosis of estrogen receptor status in invasive ductal breast cancer. Int J Cancer. 2006;119(12):2974–2979. doi: 10.1002/ijc.22234. [DOI] [PubMed] [Google Scholar]
- 180.Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, Cohen P, Lidereau R, Bieche I. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription- PCR approach. Endocr Relat Cancer. 2006;13(4):1109–1120. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 181.Kasper G, Weiser AA, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castanos-Velez E, Lehmann K. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J Cancer. 2005;117(6):961–973. doi: 10.1002/ijc.21235. [DOI] [PubMed] [Google Scholar]
- 182.Lue HW, Yang X, Wang R, Qian W, Xu RZ, Lyles R, Osunkoya AO, Zhou BP, Vessella RL, Zayzafoon M, Liu ZR, Zhau HE, Chung LW. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PLoS One. 2011;6(11):e27720. doi: 10.1371/journal.pone.0027720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Unno J, Satoh K, Hirota M, Kanno A, Hamada S, Ito H, Masamune A, Tsukamoto N, Motoi F, Egawa S, Unno M, Horii A, Shimosegawa T. LIV-1 enhances the aggressive phenotype through the induction of epithelial to mesenchymal transition in human pancreatic carcinoma cells. Int J Oncol. 2009;35(4):813–821. doi: 10.3892/ijo_00000394. [DOI] [PubMed] [Google Scholar]
- 184.Zhao L, Chen W, Taylor KM, Cai B, Li X. LIV-1 suppression inhibits HeLa cell invasion by targeting ERK1/2-Snail/Slug pathway. Biochem Biophys Res Commun. 2007;363(1):82–88. doi: 10.1016/j.bbrc.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 185.Shen R, Xie F, Shen H, liu Q, Zheng T, Kou X, Wang D, Yang J. Negative correlation of LIV-1 and E-cadherin expression in hepatocellular carcinoma cells. PLoS One. 2013;8(2):e56542. doi: 10.1371/journal.pone.0056542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu C, Li D, Jia W, Hu Z, Zhou Y, Yu D, Tong T, Wang M, Lin D, Qiao Y, Chang J, Zhai K, Wei L, Tan W, Shen H, Zeng Y. Genome-wide association study identifies common variants in SLC39A6 associated with length of survival in esophageal squamous-cell carcinoma. Nat Genet. 2013;45(6):632–638. doi: 10.1038/ng.2638. [DOI] [PubMed] [Google Scholar]
- 187.Taylor KM. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem Soc Trans. 2008;36(Pt 6):1247–1251. doi: 10.1042/BST0361247. [DOI] [PubMed] [Google Scholar]
- 188.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13(7–8):396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thomas P, Pang Y, Dong J, Berg AH. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology. 2014;155(11):4250–4265. doi: 10.1210/en.2014-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pal D, Sharma U, Singh SK, Prasad R. Association between ZIP10 gene expression and tumor aggressiveness in renal cell carcinoma. Gene. 2014;552(1):195–198. doi: 10.1016/j.gene.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 191.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98(5):692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu L, Chaffee KG, Parker AS, Sicotte H, Petersen GM. Zinc transporter genes and urological cancers: integrated analysis suggests a role for ZIP11 in bladder cancer. Tumour Biol. 2015;36(10):7431–7437. doi: 10.1007/s13277-015-3459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Franklin RB, Levy BA, Zou J, Hanna N, Desouki MM, Bagasra O, Johnson LA, Costello LC. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer. 2012;43(2):249–257. doi: 10.1007/s12029-011-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]