Abstract
Key points
The gastrointestinal epithelial enterochromaffin (EC) cell synthesizes the vast majority of the body's serotonin. As a specialized mechanosensor, the EC cell releases this serotonin in response to mechanical forces. However, the molecular mechanism of EC cell mechanotransduction is unknown.
In the present study, we show, for the first time, that the mechanosensitive ion channel Piezo2 is specifically expressed by the human and mouse EC cells.
Activation of Piezo2 by mechanical forces results in a characteristic ionic current, the release of serotonin and stimulation of gastrointestinal secretion.
Piezo2 inhibition by drugs or molecular knockdown decreases mechanosensitive currents, serotonin release and downstream physiological effects.
The results of the present study suggest that the mechanosensitive ion channel Piezo2 is specifically expressed by the EC cells of the human and mouse small bowel and that it is important for EC cell mechanotransduction.
Abstract
The enterochromaffin (EC) cell in the gastrointestinal (GI) epithelium is the source of nearly all systemic serotonin (5‐hydroxytryptamine; 5‐HT), which is an important neurotransmitter and endocrine, autocrine and paracrine hormone. The EC cell is a specialized mechanosensor, and it is well known that it releases 5‐HT in response to mechanical forces. However, the EC cell mechanotransduction mechanism is unknown. The present study aimed to determine whether Piezo2 is involved in EC cell mechanosensation. Piezo2 mRNA was expressed in human jejunum and mouse mucosa from all segments of the small bowel. Piezo2 immunoreactivity localized specifically within EC cells of human and mouse small bowel epithelium. The EC cell model released 5‐HT in response to stretch, and had Piezo2 mRNA and protein, as well as a mechanically‐sensitive inward non‐selective cation current characteristic of Piezo2. Both inward currents and 5‐HT release were inhibited by Piezo2 small interfering RNA and antagonists (Gd3+ and D‐GsMTx4). Jejunum mucosal pressure increased 5‐HT release and short‐circuit current via submucosal 5‐HT3 and 5‐HT4 receptors. Pressure‐induced secretion was inhibited by the mechanosensitive ion channel antagonists gadolinium, ruthenium red and D‐GsMTx4. We conclude that the EC cells in the human and mouse small bowel GI epithelium selectively express the mechanosensitive ion channel Piezo2, and also that activation of Piezo2 by force leads to inward currents, 5‐HT release and an increase in mucosal secretion. Therefore, Piezo2 is critical to EC cell mechanosensitivity and downstream physiological effects.
Keywords: enterochromaffin cell, mechanosensitive ion channel, mechanotransduction, serotonin
Key points
The gastrointestinal epithelial enterochromaffin (EC) cell synthesizes the vast majority of the body's serotonin. As a specialized mechanosensor, the EC cell releases this serotonin in response to mechanical forces. However, the molecular mechanism of EC cell mechanotransduction is unknown.
In the present study, we show, for the first time, that the mechanosensitive ion channel Piezo2 is specifically expressed by the human and mouse EC cells.
Activation of Piezo2 by mechanical forces results in a characteristic ionic current, the release of serotonin and stimulation of gastrointestinal secretion.
Piezo2 inhibition by drugs or molecular knockdown decreases mechanosensitive currents, serotonin release and downstream physiological effects.
The results of the present study suggest that the mechanosensitive ion channel Piezo2 is specifically expressed by the EC cells of the human and mouse small bowel and that it is important for EC cell mechanotransduction.
Abbreviations
- CFP
cyan fluorescent protein
- DAPI
4′,6‐diamidino‐2‐phenylindole
- EC
enterochromaffin
- EP50
half‐point of the pressure–stimulus relationship
- Gd3+
gadolinium
- GI
gastrointestinal
- GR
GR 113808
- Gt
tissue conductance
- 5‐HT
5‐hydroxytryptamine
- IHC
immunohistochemsity
- Isc
short‐circuit current
- PB
phosphate buffer
- PFA
paraformaldehyde
- siRNA
small interfering RNA
- TPH‐1
tryptophan hydroxylase 1
Introduction
In the 1930s, Vittorio Erspamer derived a biologically active amine, now known as serotonin (5‐hydroxytryptamine; 5‐HT), from the gut mucosa (Erspamer, 1957). He also showed that a unique chromium stained (hence enterochromaffin; EC) cell in the gut epithelium was solely responsible for production and presumably secretion of 5‐HT into the peripheral circulation. 5‐HT is now established as a neurotransmitter and an important autocrine, paracrine and endocrine molecule (Mawe & Hoffman, 2013). 5‐HT controls gastrointestinal (GI) secretory (Brown, 1996; Hoffman et al. 2012), motor (Heredia et al. 2009; Hoffman et al. 2012; Heredia et al. 2013) and sensory (Zhu et al. 2001; Hoffman et al. 2012) functions. 5‐HT is also taken up by platelets and becomes circulating 5‐HT with important functions at a distance from the GI tract (Berger et al. 2009), such as platelet aggregation (Carneiro et al. 2008), vascular tone (Launay et al. 2002), skin sensation (Press et al. 2010), and even bone (Yadav et al. 2010) and metabolic health (Crane et al. 2015).
The EC cell is a part of a vast enteroendocrine sensory network that responds to chemical stimuli by hormone secretion. In 1959, Edith Bulbring's seminal work showed that small bowel mucosal pressure led to 5‐HT release from the EC cell (Bulbring & Crema, 1959). The EC cell is now established as a primary mechanosensor of the gastrointestinal epithelium, meaning that the EC cell response to force defines the GI epithelium response to mechanical stimuli (Bulbring & Lin, 1958). Although we know that mechanical forces are strong stimuli for 5‐HT release (Bertrand, 2004), the molecular mechanism of EC cell mechanotransduction remains unknown. Previous studies have implicated several molecules in EC cell mechanosensitivity, including G‐protein coupled receptors (e.g. Gαq) (Kim et al. 2001) and purine receptors P2X and P2Y (Christofi, 2008; Chin et al. 2012; Linan‐Rico et al. 2013). However, these molecules are probably downstream of the primary mechanosensor (Linan‐Rico et al. 2013).
Mechanical force activates the EC cell within milliseconds (Bertrand, 2004). It is well known that fast cellular mechanosensation relies on mechanically gated ion channels, which serve as primary mechanosensors by converting mechanical energy into electrical signals (Arnadottir & Chalfie, 2010). Recent studies have shown that the mechanosensitive ion channel Piezo2 is the primary mechanosensor in several specialized mechanosensory cells, including proprioceptive (Woo et al. 2015) and light‐touch dorsal root ganglion neurons (Ranade et al. 2014), as well as Merkel cells in the skin (Ikeda et al. 2014; Ranade et al. 2014; Woo et al. 2014). Merkel and EC cells share important functional and developmental properties. Both are specialized epithelial mechanosensors (Raybould et al. 2004; Nakatani et al. 2014), with a genetic lineage that depends on the transcription factor Atoh1 (Math1) (Yang et al. 2001; Li et al. 2011; Roach et al. 2013; Wright et al. 2015), and secrete neurotransmitters in response to mechanical forces (Forsberg & Miller, 1983; Tachibana et al. 2005), and contact neurons (Haeberle et al. 2004; Bohorquez et al. 2015).
Piezo2 (encoded by PIEZO2 or FAM38B) is a transmembrane protein that forms a cation selective mechanosensitive ion channel (Coste et al. 2010; Coste et al. 2012). In the Merkel cell, force activates Piezo2 mechanosensitive inward cation currents (Woo et al. 2014), leading to depolarization and activation of an L‐type calcium channel (Ikeda et al. 2014), resulting in further increase of intracellular Ca2+, presumably leading to exocytosis (Haeberle et al. 2004). Similarly, force applied to the EC cell leads to a Ca2+ influx via depolarization and subsequent activation of a voltage‐gated L‐type calcium channel (Raghupathi et al. 2013), which is important for 5‐HT release.
Given the similarities between EC and Merkel cells, we investigated whether Piezo2 is involved in EC cell mechanosensation. In the present study, we found that Piezo2 is specifically expressed in human and mouse EC cells throughout the length of the small bowel and also that it is required for coupling of mechanical forces to 5‐HT release and downstream GI physiological functions.
Methods
Ethical approval
All experimental procedures were approved by the Institutional Review Board and Institutional Animal Care and Use Committee (A34414) of the Mayo Clinic.
Drugs
Fluoxetine and gadolinium (Gd3+) were made as stock solution (10 mm in water). Ruthenium red was prepared fresh daily. All drugs were obtained from Sigma‐Aldrich (St Louis, MO, USA). Working extracellular solutions were prepared from stock on the day of experiments. D‐GsMTx4 was made as a working solution on the day of experiments by dissolving directly into the relevant extracellular solution.
Cell culture
QGP‐1 cells
QGP‐1 (passages 17–20) (a kind gift from Dr Valeria Giandomenico, Uppsala University, Uppsala, Sweden) were cultured in modified RPMI 1640 with 10% fetal bovine serum, 1% penicillin–streptomycin and 1% l‐glutamine. Cells were grown to 50–60% confluence in either T25 flasks or laminin coated flexible membranes (Flexcell International, Burlington, NC, USA). When noted, Piezo2 was knocked down using 50 nm small interfering RNA (siRNA) (L‐013925‐02‐0005; Dharmacon, Lafayette, CO, USA) for 48 h with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) transfection. Cells for electrophysiology were co‐transfected with siGLO Green Transfection Indicator (D‐001630‐01‐05; Dharmacon). On the day of experiments, cells for electrophysiology experiments were lifted with trypsin‐EDTA (0.5%) and plated onto the test chamber.
Molecular biology
Real time RT‐PCR
Tissue was collected from human jejunum and mouse mucosa of duodenum, jejunum and ileum. QGP‐1 cells were trypsinized and collected. Collected tissues and cells were frozen in liquid nitrogen. Total RNA was isolated using RNA‐bee (Tel‐Test Inc., Friendswood, TX, USA) and cleaned using a RNeasy Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. Reverse transcription of the RNA was completed using a SuperScript VILO cDNA Synthesis Kit (Invitrogen) and a PCR reaction that included annealing the RNA for 10 min at 25°C, and also a 60 min cycle at 42°C and 5 min at 85°C to denature the enzyme. cDNA was diluted 10‐fold and analysed for Piezo2 (primer from Origene, Rockville, MD, USA) by quantitative real‐time PCR in accordance with the instructions of the manufacturer of the LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland). The mRNA expression of Piezo2 was normalized to species‐dependent β‐actin expression. Error bars indicate the SE.
Immunohistochemistry (IHC)
IHC protocols
Human jejunum tissues were obtained from patients undergoing Roux‐en‐Y gastric bypass surgery. Tissues were placed in ice‐cold F12 medium (Invitrogen). A piece of tissue (2 × 2 cm) was dissected, pinned out in 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB) overnight, washed in 0.1 m PBS and then transferred into 30% sucrose in PBS overnight, and then frozen in OCT embedding compound (Sakura Finetek, Torrance, CA, USA) at −80°C until sectioning. Mouse tissues comprised flat sheets (1 × 0.5 cm) or tubes (length 0.5 cm) from duodenum, jejunum and ileum of TPH1‐CFP mice. Tissues were fixed in 4% PFA‐PB for 4 h separately, then washed in PBS and moved into 30% sucrose in PBS overnight before being frozen in OCT embedding compound (Sakura Finetek) at −80°C until sectioned. Tissues were cut into 12 μm thick sections, rinsed with PBS twice for 5 min and then blocked with 200 μl per slide of 1% BSA/PBS/0.3% Triton X/10% normal donkey serum in a humidity chamber. Primary antibodies (Piezo2 mouse antibody a kind gift from Dr Ardem Patapoutian) were added in 200 μl per slide of BSA/PBS/0.3% Triton/10% normal donkey serum and were incubated at 4°C overnight in a humidity chamber. Slides were then rinsed five times for 3 min in PBS. Secondary antibody was incubated for 1 h in the dark. Slides were mounted in slowfade gold with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Life Technologies, Grand Island, NY, USA) mounting buffer.
Cell quantification
5‐HT and/or Piezo2 positive cells were quantified in the epithelium only, and were defined by DAPI‐staining as the luminal facing layer of the mucosa. Imaging was performed using Olympus BX51W1 (40×) and Olympus FV1000 confocal (20×, 0.95 NA and 60×, 1.2 NA, z‐res 0.91 μm) microscopes (Olympus Corporation, Tokyo, Japan). We counted EC cells (TPH1‐CFP+) from three mice across all small bowel segments, each with three sections separated by z‐distance >50 μm (n = 3 mice, n = 97 ± 23 fields per mouse, n = 217 ± 30 cells per mouse). Error bars indicate the SE.
Single cell electrophysiology
Solutions
The extracellular solution contained (in mm): 150 Na+, 5 K+, 2.5 Ca2+, 1 Mg2+,160 Cl−, 10 Hepes and 5.5 glucose (pH 7.35), 300 mmol kg−1; the intracellular solution contained (in mm): 140 Cs+, 150 Cl−, 4 Mg2+, 2 Ca2+, 10 Hepes and 5 EGTA (pH 7.3), 300 mmol kg–1.
Data acquisition
Standard whole cell voltage clamp was used as described previously (Strege et al. 2003; Saito et al. 2009; Strege et al. 2012). Electrodes (Kimble KG12 glass) were pulled using a Sutter P97 puller (Sutter Instruments, Novato, CA, USA), coated with R6101 (Dow Corning, Auburn, MI, USA) and fire polished to 2–5 MΩ. Stimulation and data acquisition were performed using an an Axopatch 200B patch clamp amplifier, CyberAmp 320 signal conditioner, Digidata 1550A and pClamp, version 10.5 (Molecular Devices, Sunnyvale, CA, USA). Once the cells were voltage clamped, mechanical stimulation was applied via fire‐polished glass microelectrodes (10–25% of cell size) driven by a piezo‐transducer P‐621.1CD attached to an E‐625.CR controller (Physik Instrumente, Karlsruhe, Germany) (Coste et al. 2010; Maksimovic et al. 2014). Cells were subjected to a series of mechanical steps of 0.3 μm increments.
Data analysis
Whole cell patch clamp data were analysed in pClamp, version 10.5 (Molecular Devices). The peak currents within 10 ms of stimulus start were selected for analysis. pClamp (Molecular Devices) and Origin 2016 (OriginLab Co., Northampton, MA, USA) were used for electrophysiology data analysis. Current–voltage relationships were fit with a linear function, V = A + B*I, where I is current, V is voltage, A is the y‐intercept and B is the slope. Displacement‐current curves were fit in using a Boltzmann function I = A 2 + (A 1 – A 2)/(1 + exp[(x – x 0)/dx]), where I is current, A 1 is the y‐intercept, A 2 is peak, x is displacement, x 0 is half‐point displacement and dx is slope displacement. Error bars indicate the SE. P < 0.05 was considered statistically significant (ANOVA with Bonferroni post test when appropriate) using GraphPad Prism, version 6; GraphPad Software, San Diego, CA, USA).
Cell stretch on flexible substrates
QGP‐1 cells or QGP‐1 cells with Piezo2 siRNA on Flexcell plates were incubated in 3 ml of serum free media containing 2 μm fluoxetine and either vehicle or drug for 30 min. The substrate stretch protocol was 30 cycles min–1 at 25% strain with 1:2 ratio of relax:stretch using a validated custom instrument (Stroetz et al. 2001). At each time point (rest = 0 min and 1 min), samples were collected and analysed by a 5‐HT enzyme‐linked immunosorbent assay in accordance with the manufacturer's instructions (BA E‐5900; Rocky Mountain Diagnostics, Colorado Springs, CO, USA).
Ussing chamber
Solutions
Krebs‐Ringer (in mm): 120 NaCl, 5.9 KCl, 15 NaH2CO3, 1.6 NaH2PO4, 1.3 CaCl2, 2.4 MgCl2 (pH 7.4), gassed with a 95:5 mixture of O2/CO2. Glucose (10 mm) was added to the serosa side bath and mannitol (10 mm) was added to the mucosa bath to maintain osmotic balance.
Tissue preparation
Segments of jejunum (4 cm) were cut along the mesenteric border and the luminal contents were gently removed. Tissue was cut into 2 cm segments. During preparation, the tissues were bathed in ice‐cold Krebs‐Ringer solution.
Short‐circuit current measurements
The full thickness preparations of mouse jejunum with a cross‐sectional area of 0.3 cm2 were mounted in 4 ml Ussing chambers (Physiologic Instruments, San Diego, CA, USA). The trans‐epithelial potential difference was measured using paired Ag‐AgCl electrodes via 3% agar with 3 m KCl bridges and clamped at 0 mV using another pair of Ag‐AgCl electrodes. The mucosal and serosal surfaces of the tissue were bathed with 4 ml of Krebs‐Ringer solution with mannitol and glucose, respectively, maintained at 37°C during the course of the experiments. Tissue was allowed to equilibrate to attain a stable basal short‐circuit current (I sc) and tissue conductance (Gt) for 30 min before conducting the experiment. Hydrostatic pressure was applied using a DPM‐1 pneumatic transducer (Bio‐Tek Instruments, Burlington, VT, USA) in a sealed mucosal chamber. From a resting applied pressure of 0 mmHg, pressure stimuli were applied until the peak response in I sc current was achieved (1 min). Prior to these experiments, we determined the half‐point of the pressure–stimulus relationship (EP50) value using current responses (ΔI sc) to graded pressure stimuli (data not shown). To test the effect of drugs on pressure‐induced responses, three separate stimulations to the EP50 value (22 mmHg) were delivered to the mucosal side, with 15 min allowed between stimulations for recovery. After complete recovery to baseline following the third stimulus, drug or vehicle was added to the mucosal chamber and allowed to equilibrate for 20 min. In the presence of the drug, three separate stimuli were applied again. At baseline, recordings were made at 0 mmHg (atmospheric pressure). To ensure tissue viability, at the onset and conclusion of the experiment, ACh (100 μm) was applied to the serosal side to determine tissue viability and the response to stimulation (data not shown). Tissue was not used if there was no response to ACh either before or after the application of pressure. Data were recorded using Acquire and Analyse 2.3 (Physiologic Instruments).
Data analysis
Raw data were exported into text format and uploaded into Clampfit, version 10.5 (Molecular Devices). Pressure‐induced peak I sc was measured as the mean of three measurements ΔI sc = I sc_peak – I sc_baseline.
Statistical analysis
All values represent the mean ± SE. All electrophysiology data and flex cell data were analysed using ANOVA with Bonferroni correction. A two‐tailed paired t test was used in the Ussing chamber data analysis. P < 0.05 was considered statistically significant.
Results
Human and mouse jejunum epithelium expresses PIEZO2 mRNA
Piezo2 mRNA was expressed in human small bowel (jejunum; n = 3) and in mouse small bowel mucosa (n = 3) from duodenum, jejunum and ileum (Fig. 1).
Figure 1. Piezo2 mRNA present in human jejunum and mouse small bowel mucosa.
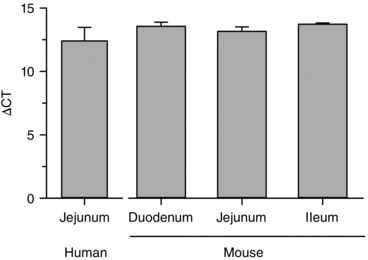
Piezo2 mRNA is found in human jejunum (n = 3) and mouse mucosa duodenum, jejunum and ileum (n = 3).
Piezo2 is distributed specifically in the 5‐HT positive EC cells in human jejunum epithelium
We then used IHC to determine the presence and distribution of Piezo2 protein in human jejunum epithelium (Fig. 2). Strikingly, Piezo2 was found only in a well defined population of epithelial cells morphologically similar to enteroendocrine cells. We immunolabelled both 5‐HT (Fig. 2 A) and Piezo2 (Fig. 2 B) in human jejunum. We found that Piezo2 specifically labelled 91.2 ± 4.5% of 5‐HT positive cells (Fig. 2 C). Controls lacking Piezo2 or 5‐HT primary antibodies or containing Piezo2 blocking peptide were appropriately negative (data not shown). Therefore, our data showed that Piezo2 was specifically localized within EC cells in human jejunum epithelium.
Figure 2. Piezo2 is a specific marker of the human small bowel EC cells.
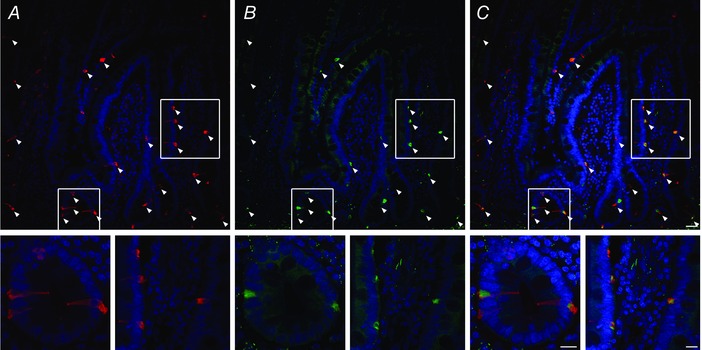
High resolution laser confocal microscopy of (A) 5‐HT immunolabelling showing EC cells in red and DAPI labelling nuclei in blue, (B) Piezo2 immunolabelling in green and DAPI in blue and (C) overlap of 5‐HT (red) and Piezo2 (green) and DAPI (blue). Arrowheads indicate EC cells and insets are enlarged sections from the areas enclosed by the white boxes. Scale bars = 30 μm (large field) and 15 μm (gland and villus insets).
Piezo2 is distributed specifically in mouse small bowel EC cells
Next, we examined the murine small bowel. Tryptophan hydroxylase 1 (TPH‐1) is the rate limiting enzyme found specifically within EC cells that converts tryptophan to 5‐HT (Cote et al. 2003). We used a transgenic mouse model TPH1‐CFP in which cyan fluorescent protein (CFP) expression was genetically encoded to express in EC cells (Li et al. 2014). The EC cell CFP fluorescence was easily observed (n = 3) (Fig. 3 A) and Piezo2 immunofluorescence, using a different antibody from the one used above for human tissues (Ranade et al. 2014; Woo et al. 2014; Woo et al. 2015), revealed that Piezo2 positive cells were confined to the epithelium (n = 3) (Fig. 3 B) and that they co‐localized specifically with TPH1‐CFP EC cells (n = 3) (Fig. 3 C). Controls lacking Piezo2 primary antibody were appropriately negative (data not shown). Our data for the TPH1‐CFP mouse model were consistent with those for the human small bowel, where Piezo2 was specifically expressed by EC cells.
Figure 3. Piezo2 is selectively expressed in mouse small bowel 5‐HT EC cells.
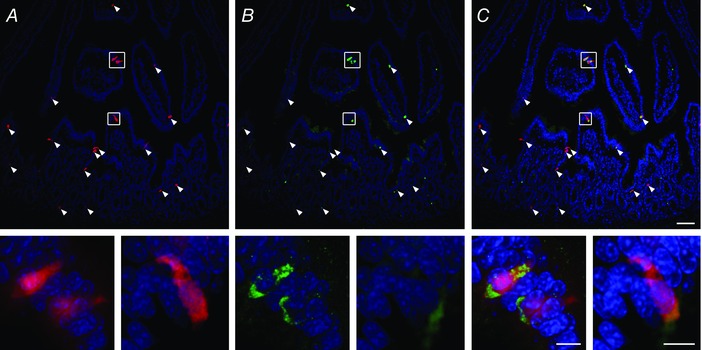
High resolution laser confocal microscopy of jejunum from TPH1‐CFP mouse showing (A) CFP positive (pseudocoloured red) EC cells and DAPI, (B) Piezo2 immunolabelling (green) and DAPI and (C) co‐localization of EC cell (red), Piezo2 (green) and DAPI. Arrowheads indicate EC cells and insets are enlarged sections from the areas enclosed by the white boxes. Scale bars = 50 μm (large field) and 10 μm (insets).
We then quantified Piezo2 distribution in the EC cells of duodenum, jejunum and ileum. Using the TPH1‐CFP mouse model, we found that Piezo2 along the small intestine was exclusively localized in EC cells: duodenum (86.5 ± 14.3%, n = 3 animals, n = 244 cells), jejunum (91.3 ± 7.3%, n = 3 animals, n = 264 cells) and ileum (88.7 ± 8.1%, n = 3 animals, n = 143 cells) (Fig. 4). Overall, our mouse small bowel immunohistochemistry data showed that Piezo2 was specifically located within the EC cells.
Figure 4. Piezo2 localizes specifically within EC cells in mouse small bowel.
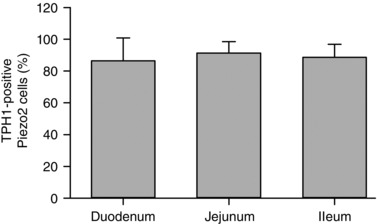
Quantification of Piezo2 positive cells that are TPH1 positive shows overlap in 86.5 ± 14.3% cells in duodenum (n = 3 animals, n = 244 cells), 91.3 ± 7.3% cells in jejunum (n = 3 animals, n = 264 cells) and 88.7 ± 8.1% cells in ileum (n = 3 animals, n = 143 cells).
Piezo2 mediates a mechanosensitive cation current in an EC cell model
Piezo2 encodes a mechanosensitive cation selective ion channel (Coste et al. 2010; Coste et al. 2012), and so we next examined whether Piezo2 is functional in EC cells. We used a validated EC cell model (QGP‐1) that produces and releases 5‐HT (Doihara et al. 2009; Kojima et al. 2014; Schulze et al. 2014) and expresses Piezo2 (data not shown). Whole cell voltage clamped cells had a variety of baseline inward currents and a fast pressure‐induced inward current −8.3 ± 1.6 pA pF–1 (n = 15 out of 23 cells examined) (Fig. 5 A) that inactivated with a mono‐exponential time constant τi = 7.0 ± 1.0 ms at −60 mV (Fig. 5 A), which was consistent with the published Piezo2 inactivation rates (6.2–7.3 ms) (Coste et al. 2010; Coste et al. 2012). The inactivation rate was also similar to that found for Piezo2 heterologously expressed in HEK‐293 cells (data not shown). We observed an increase in current with an increasing stimulus, fit well by a two state Boltzmann function with a mid‐point sensitivity of 3.30 ± 0.23 μm and slope of 0.67 ± 0.14 μm (n = 8) (Fig. 5 B), consistent with Piezo2 (Schrenk‐Siemens et al. 2015). Finally, mechanically induced currents had a linear current–voltage relationship with an x‐intercept of −2.5±1.6 mV (n = 3) (Fig. 5 C), suggestive of Piezo2 non‐selective cation currents (Coste et al. 2010).
Figure 5. Membrane force leads to a fast non‐rectifying inward current in QGP‐1 EC cell model.
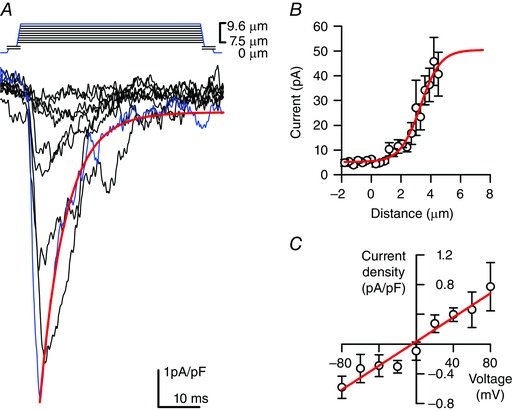
A, stepwise (0.3 μm step–1) increase in cell membrane deformation resulted in a typical set of rapidly activating and inactivating pressure induced inward currents with blue traces highlighting current in response to max deformation. Red line is a mono‐exponential fit of the blue curve (τi = 5.8 ms). B, peak current–deformation relationship (n = 8) fit by a two‐state Boltzmann function (red line) with mid‐point (x 0) 3.30 ± 0.23 μm and slope (dx) 0.67 ± 0.14 μm. C, peak current–voltage relationship (n = 3) fit by a linear function (red line) with x‐intercept of −4.1 mV.
We then tested whether Piezo2 antagonists and siRNA inhibited these mechanosensitive currents. Compared to no stretch controls (−8.3 ± 1.6 pA pF–1, n = 15), the non‐selective mechanosensitive ion channel blocker Gd3+ (30 μm) (Coste et al. 2010) inhibited the pressure‐induced current (Fig. 6 A) by 64% (−3.1 ± 0.8 pA pF–1 Gd3+, n = 7, P < 0.05) (Fig. 6 B) and the specific Piezo mechanosensitive ion channel antagonist D‐GsMTx4 (5 μm) (Suchyna et al. 2000; Bae et al. 2011; Lee et al. 2014) inhibited Piezo2 currents in transfected HEK‐293 cells (data not shown) and mechanosensitive inward currents in QGP‐1 cells (Fig. 6 A) by 89% (−1.0 ± 0.3 pA pF–1 GsMTx‐4, n = 6, P < 0.05) (Fig. 6 B). Importantly, Piezo2 siRNA knocked down Piezo2 mRNA by 70 ± 15% (n = 3, data not shown) and inhibited pressure‐induced current (Fig. 6 A) by 96% (−0.37 ± 0.38 pA/pF Piezo2 siRNA, n = 6, P < 0.05) compared to non‐targeted (NT) siRNA (−6.5±1.6 pA pF–1 NT, n = 4, P > 0.05) (Fig. 6 B). NT siRNA was not different from the stretch controls (P > 0.05). Therefore, our data showed that an EC cell model had mechanosensitive currents with Piezo2 biophysical properties and inhibited by Piezo2 siRNA and pharmacological antagonists.
Figure 6. Piezo2 blockade inhibits mechanosensitive ion current in QGP‐1 cells.
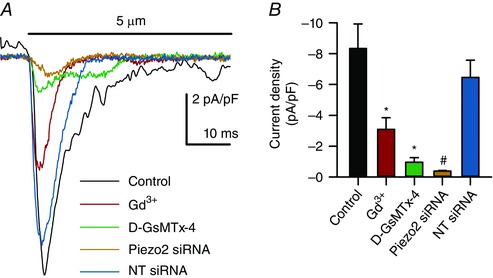
A, superimposition of membrane force resulted in a fast inward current which was blocked by Gd3+, GsMTx‐4 and Piezo2 siRNA but not by NT siRNA. B, compared to the peak current in controls (n = 15), there were significant decreases in peak current when Piezo2 was inhibited by Gd3+ (n = 7, * P < 0.05, ANOVA with Bonferroni correction) and D‐GsMTx4 (n = 6, * P < 0.05, ANOVA with Bonferroni correction) and knocked down by Piezo2 siRNA (n = 6, # P < 0.05 compared to NT siRNA, ANOVA) but not with NT siRNA (n = 4, P > 0.05 compared to controls, ANOVA with Bonferroni correction).
Piezo2 is necessary for mechanically induced release of 5‐HT from EC cells
Next, to determine how mechanical activation of Piezo2 couples to 5‐HT release, we stretched QGP‐1 cells grown on laminin‐coated flexible substrates and measured the released 5‐HT. Compared to no stretch controls (Δ5‐HT = 0.10 ± 0.10 ng ml−1, n = 4) (Fig. 7), depolarization by KCl and cyclic stretch (30 Hz for 1 min) significantly increased 5‐HT release (Δ5‐HT) by 0.91 ± 0.35 ng ml−1 (n = 4, P < 0.05) and by 0.62 ± 0.04 ng ml−1 (n = 4, P < 0.05), respectively (Fig. 7). Piezo2 pharmacologic inhibitors and Piezo2 siRNA inhibited stretch‐induced 5‐HT release compared to no stretch (Fig. 7): 30 μm Gd3+ (Δ5‐HT = 0.090 ± 0.10 ng ml−1, n = 4, P < 0.05), 30 μm ruthenium red (Δ5‐HT = 0.18 ± 0.11 ng ml−1, P < 0.05) and 5 μm D‐GsMTx4 (Δ5‐HT = 0.012 ± 0.046 ng ml−1, n = 4, P < 0.05). Importantly, Piezo2 siRNA inhibited pressure‐induced 5‐HT release (Δ5‐HT = 0.073 ± 0.12 ng ml−1, n = 7, P < 0.05) compared to NT siRNA (Δ5‐HT = 0.50 ± 0.16 ng ml−1, n = 7). NT siRNA did not significantly alter pressure‐induced 5‐HT release compared to stretch controls (P > 0.05). Therefore, our data show that Piezo2 is important for 5‐HT release in response to force.
Figure 7. Piezo2 blockade inhibits stretch induced 5‐HT release in QGP‐1 cells.
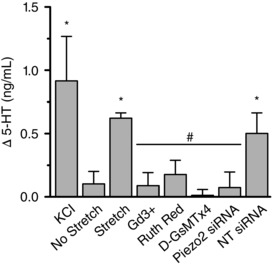
QGP‐1 cells grown on laminin‐coated flexible substrates released 5‐HT in response to 50 mm KCl (n = 4, * P < 0.05 compared to no stretch, ANOVA with Bonferroni correction) and cyclic tensile stretch (n = 4, * P < 0.05 compared to no stretch, ANOVA with Bonferroni correction). Stretch‐dependent 5‐HT release was inhibited by Piezo2 blockers Gd3+, ruthenium red and GsMTx‐4 (n = 4 for each, all # P < 0.05 compared to stretch, ANOVA with Bonferroni correction). Piezo2 siRNA inhibited stretch‐dependent 5‐HT release, whereas NT siRNA did not (n = 7 for each, all # P < 0.05 compared to stretch, ANOVA with Bonferroni correction).
Piezo2 is critical for the regulation of 5‐HT mediated mucosal secretion in mouse small bowel epithelium
We wanted to understand how Piezo2 contributes to the physiological control of GI function. Previous studies showed that pressure applied to small bowel mucosa increases secretion, probably via 5‐HT (Bulbring & Lin, 1958; Bulbring & Crema, 1959). To determine the involvement of Piezo2 in the mechanism of pressure‐induced secretion from mouse jejunum mucosa, we modified our Ussing chamber to allow transient hydrostatic pressure application to mouse jejunum mucosa. Pressure stimulus‐dependently increased I sc with a pressure EP50 = 22 mmHg (data not shown), and so we used this pressure for the subsequent experiments. Transient mucosal pressure increased I sc as shown in a typical trace (Fig. 8 A) from 5.3 ± 16.0 μA to 59.0 ± 13.4 μA (n = 12, P < 0.05) (Fig. 8 B) and simultaneously increased 5‐HT levels at the mucosal side from 1.25 ± 0.07 ng ml−1 at rest to 1.43 ± 0.07 ng ml−1 (n = 12, P < 0.05) (Fig. 8 C).
Figure 8. Mucosal pressure leads to an increase in secretion and release of 5‐HT.
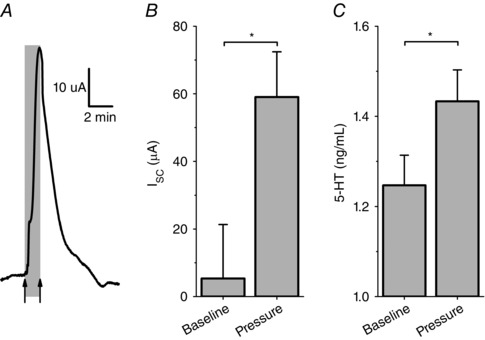
A, typical trace of mouse jejunum in Ussing chamber with +22 mmHg of mucosal hydrostatic pressure resulted in a significant (B) Increase in I sc (n = 12, * P < 0.05) and (C) in the same tissues mucosal side 5‐HT increase as measured by ELISA (n = 12, * P < 0.05).
To determine whether 5‐HT receptors and EC cell Piezo2 affect mechanically induced mucosal secretion, we designed a protocol in which three discrete control pressure steps were followed by three pressure steps in the presence of vehicle or drug (Fig. 9 A). Addition of each drug at the testing concentrations without pressure did not alter the I sc (data not shown). Because 5‐HT stimulates mucosal secretion via 5‐HT3 and 5‐HT4 receptors (Vanner & Macnaughton, 2004), we blocked them with 1 μm ondansetron and 30 nm GR 113808 (GR), respectively. We tested these inhibitors at the mucosal and then basolateral sides of the tissue. When applied to the mucosal side, we found that these blockers (ondansetron + GR) did not affect pressure‐induced short circuit increase (ΔI sc from 39.6 ± 9.4 μA to 39.6 ± 9.5 μA, n = 3, P > 0.05) (Fig. 9 B). By contrast, when ondansetron + GR were applied to the basolateral side, there was a 51% decrease in the short circuit response to pressure (ΔI sc from 63.0 ± 11.2 to 30.8 ± 3.4 μA, n = 4, P < 0.05) (Fig. 9 B), which is consistent with secretion block via the established submucosal 5‐HT circuit (Vanner & Macnaughton, 2004).
Figure 9. Pressure‐induced increase in mucosal secretion is via 5‐HT3/4 receptors and activation of Piezo2 mechanosensitive ion channels.
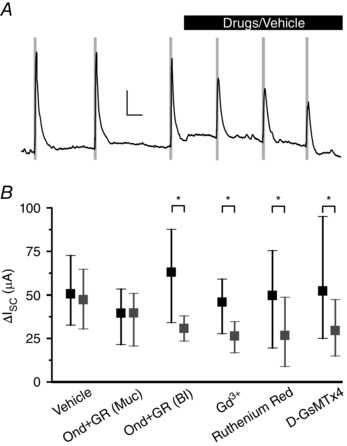
A, typical Ussing experiment showing an increase in short‐circuit current with pressure in the first three pressure applications (grey bars) and a block by mucosal side Gd3+ (black bar) for the subsequent three steps. Scale bars = 25 μA and 5 min. B, rise in ΔI sc mean of three pressure steps without (black) and with the drug (grey) was unchanged for vehicle (n = 4) and ondansetron (1 μm) and GR 113808 (30 nm) on mucosal (Muc) side (n = 3, P ≥ 0.05) but blocked by basolateral (bl) side ondansetron (1 μm) and GR 113808 (30 nm) (n = 4, * P < 0.05), Gd3+ (100 μm, n = 5, * P < 0.05), ruthenium red (100 μm, n = 4, * P < 0.05) and D‐GsMTx4 (5 μm, n = 4, * P < 0.05).
We then investigated whether EC cell Piezo2 plays a role in pressure‐induced increase in short circuit current. To specifically target the epithelium, we added drugs only to the mucosal side. Piezo2 blockers Gd3+ and ruthenium red (Coste et al. 2010; Coste et al. 2012) reduced ΔI sc in response to pressure by 42% for 100 μm Gd3+ (ΔI sc from 45.8 ± 5.9 μA to 26.4 ± 3.1 μA, n = 5, P < 0.05) (Fig. 9 B) and by 46% for 100 μm ruthenium red (ΔI sc from 49.6 ± 11.5 μA to 26.7 ± 10.3 μA, n = 4, P < 0.05) (Fig. 9 B). We then used 5 μm D‐GsMTx4, a specific blocker of mechanosensitive ion channels (Suchyna et al. 2000; Bae et al. 2011), and particularly Piezo2 (data not shown), and found that it inhibited the ΔI sc response to pressure by 43% (ΔI sc from 52.2 ± 15.1 μA to 29.6 ± 6.9 μA, n = 4, P < 0.05) (Fig. 9 B). Therefore, our Ussing chamber results showed that mucosal pressure results in an EC cell Piezo2‐dependent 5‐HT mediated increase in mucosal secretion.
Discussion
Mechanical force applied to the GI mucosa leads to EC cell activation and the release of 5‐HT(Bulbring & Crema, 1959), which plays important roles in GI and systemic physiology (Mawe & Hoffman, 2013). The EC cell is established as the specialized mechanosensor of the GI mucosa, whereas the identity of the EC cell primary mechanosensory molecule remained unknown. Interestingly, the skin Merkel cells are specialized mechanosensors (Ikeda & Gu, 2014; Maksimovic et al. 2014; Ranade et al. 2014; Woo et al. 2014) with important functional (Haeberle et al. 2004) and developmental similarities to EC cells (Yang et al. 2001; Wright et al. 2015). Mechanosensitive ion channel Piezo2 is fundamental for Merkel cell mechanosensitivity (Ikeda & Gu, 2014; Ranade et al. 2014; Woo et al. 2014).The similarities between Merkel and EC cell prompted us to examine whether Piezo2 was involved in EC cell mechanosensitivity.
We identified Piezo2 mRNA in human jejunum and all segments of mouse small bowel mucosa (Fig. 1) and, using immunolabelling with 2 different Piezo2 antibodies in human and mouse small bowel epithelium (Fig. 2 and 3), we found strong Piezo2 protein expression specifically within EC cells (Fig. 4).
We examined Piezo2 function using an established EC cell model (Doihara et al. 2009; Schulze et al. 2013; Kojima et al. 2014; Schulze et al. 2014) that has Piezo2 and releases 5‐HT (Doihara et al. 2009; Schulze et al. 2013; Schulze et al. 2014). Local force stimulated inward mechanosensitive currents with Piezo2 biophysical characteristics that were inhibited by Piezo2 siRNA and pharmacologic antagonists (Fig. 5 and 6). Therefore, mechanical activation of Piezo2 in EC cells results in an inward current that would depolarize the cell membrane, which is similar to other specialized mechanosensory cells, such as Merkel cells (Ikeda & Gu, 2014; Ranade et al. 2014; Woo et al. 2014), as well as proprioceptive (Woo et al. 2015) and touch sensitive (Ranade et al. 2014) neurons. In the EC cell, membrane depolarization leads to 5‐HT release by exocytosis (Racke & Schworer, 1993; Lomax et al. 1999; Raghupathi et al. 2013). Indeed, we found that Piezo2 activation by stretch led to an increase in 5‐HT release because Piezo2 siRNA and pharmacological antagonists diminished stretch‐induced 5‐HT release (Fig. 7). Therefore, our findings strongly suggest that Piezo2 activation by force is coupled to 5‐HT release. Previous studies exploring EC cell mechanosensitivity suggest that other associated and downstream molecules are involved in EC cell mechanotransduction. For example, cholesterol‐rich lipid rafts were found to be important (Kim et al. 2007) and recent studies suggest that Piezo2 sensitivity is indeed tuned by lipid rafts (Qi et al. 2015). It is also interesting that ATP release and purine receptors are often involved in cellular mechanotransduction and specifically EC cell mechanosensation (Chin et al. 2012; Linan‐Rico et al. 2013). This is also consistent with our findings because, in bladder epithelium, ATP release was found downstream of Piezo1 activation (Miyamoto et al. 2014). Piezo1 is also found in the gut epithelial cells, although it is currently unknown whether Piezo1 contributes to the EC cell mechanosensitive response. The use of an EC cell model in the present study was advantageous because it allowed siRNA Piezo2 knockdown in pure cultures but was limited in terms of determining the detailed mechanism of EC cell mechanotransduction. Future work that aims to determine the coupling of Piezo2 to downstream signalling, including the source of intracellular Ca2+, requires primary cultures and Piezo2 knockouts.
To examine the role of EC cell Piezo2 in mechanically stimulated GI epithelium secretion, we adapted Ussing chambers to pressure and voltage clamp mouse jejunum epithelium. Mucosal pressure acutely and reproducibly increased EC cell 5‐HT release and I SC (Fig. 8) via 5‐HT acting on submucosal 5‐HT3 and 5‐HT4 receptors (Fig. 9), which are known to be important for 5‐HT mediated secretomotor responses (Vanner & Macnaughton, 2004). EC cell Piezo2 was important for pressure‐induced increase in secretion because Piezo2 pharmacological antagonists applied only to the mucosal side reduced the pressure‐induced I SC increase by ∼50% (Fig. 9). However, given the residual response, our data also suggest that another mechanism may be involved. This may include an additional EC cell mechanosensitive mechanism or mechanical activation of submucosa projecting neurons (Frieling et al. 1992; Vanner & Macnaughton, 2004), such as the intrinsic primary afferent neurons, which are well known for being mechanosensitive (Kunze et al. 2000).
Our results have important implications for future studies in GI mechanobiology. First, previous studies investigating how mechanically induced 5‐HT release from EC cells affects GI motility and sensation have relied on the manipulation of 5‐HT levels (Heredia et al. 2013) or its functional circuits (Spencer et al. 2011) and the results have been mixed, in part because no effective strategies exist to specifically inhibit EC cell mechanosensitivity. Our work presents Piezo2 as the first specific EC cell mechanotransducer, and so future studies can focus on Piezo2 to determine the specific downstream effects of EC cell activation by force. Second, EC cell 5‐HT is an important hormone that is released in response to mechanical and chemical stimuli. Recent studies implicate EC cell 5‐HT in a wide range of physiological processes outside the GI tract (Yadav et al. 2010; Crane et al. 2015). It would be interesting to determine the differences between the physiological effects as a result of EC cell mechano‐ vs. chemo‐sensation.
In conclusion, in the present study conducted in the human and mouse small bowel GI epithelium, we show that Piezo2 is expressed specifically in EC cells, that mechanical forces result in ionic currents consistent with Piezo2 mechanosensitive ion channels and Piezo2‐dependent 5‐HT release, and that mucosal force results in a Piezo2‐dependent increase in mucosal secretion via the 5‐HT pathway. Our findings have significant implications for understanding how mechanical forces in the GI tract are coupled to downstream physiological effects.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
FW, DRL, SJG, PK, MG, RO, PAG, HJL, ABL, GF and AB were involved in the study conception or design and also conducted the study. FW, KK, CA and AB were involved in the acquisition, analysis or interpretation of data. FW, KK, CA, DRL, SJG, PK, MG, RO, PAG, HJL, ABL, GF and AB drafted the work or revised it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and those who qualify for authorship are listed.
Funding
The work was supported by NIH K08 to AB (DK106456), a Pilot and Feasibility Grant from Mayo Clinic Center for Cell Signalling in Gastroenterology to AB (NIH P30DK084567), a 2015 American Gastroenterological Association Research Scholar Award to (AGA RSA) AB and NIH R01 (DK52766) to GF. FW was supported by China Scholarship Council #201406260139, MG by NIH K23 DK 103911, PK by K08 DK100638.
Acknowledgements
We thank Mrs Jennifer Rud for administrative assistance, Mr Gary Stoltz for assistance with the tissue dissociation, Mrs Cheryl Bernard for assistance with the cell culture and Mr Peter Strege for assistance with the preparation of the artwork.
Linked articles This article is highlighted by a Perspective by Galligan. To read this Perspective, visit http://dx.doi.org/10.1113/JP273041.
This is an Editor's Choice article from the 1 January 2017 issue.
References
- Arnadottir J & Chalfie M (2010). Eukaryotic mechanosensitive channels. Annu Rev Biophys 39, 111–137. [DOI] [PubMed] [Google Scholar]
- Bae C, Sachs F & Gottlieb PA (2011). The Mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry (Mosc) 50, 6295–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA & Roth BL (2009). The expanded biology of serotonin. Ann Rev Med 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP (2004). Real‐time detection of serotonin release from enterochromaffin cells of the guinea‐pig ileum. Neurogastroenterol Motil 16, 511–514. [DOI] [PubMed] [Google Scholar]
- Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F & Liddle RA (2015). Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR (1996). Mucosal protection through active intestinal secretion: neural and paracrine modulation by 5‐hydroxytryptamine. Behav Brain Res 73, 193–197. [DOI] [PubMed] [Google Scholar]
- Bulbring E & Crema A (1959). The release of 5‐hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol 146, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E & Lin RC (1958). The effect of intraluminal application of 5‐hydroxytryptamine and 5‐hydroxytryptophan on peristalsis; the local production of 5‐HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140, 381–407. [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL & Blakely RD (2008). Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest 118, 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM & Kidd M (2012). The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol 302, G397–G405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL (2008). Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal 4, 213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M & Patapoutian A (2012). Piezo proteins are pore‐forming subunits of mechanically activated channels. Nature 483, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J & Vodjdani G (2003). Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA 100, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blumer RM, Fullerton MD, Yabut JM, Kim JJ, Ghia JE, Hamza SM, Morrison KM, Schertzer JD, Dyck JR, Khan WI & Steinberg GR (2015). Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 21, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doihara H, Nozawa K, Kojima R, Kawabata‐Shoda E, Yokoyama T & Ito H (2009). QGP‐1 cells release 5‐HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem 331, 239–245. [DOI] [PubMed] [Google Scholar]
- Erspamer V (1957). Occurrence and distribution of 5‐hydroxytryptamine (enteramine) in the living organism. Z Vitam Horm Fermentforsch 9, 74–96. [PubMed] [Google Scholar]
- Forsberg EJ & Miller RJ (1983). Regulation of serotonin release from rabbit intestinal enterochromaffin cells. J Pharmacol Exp Ther 227, 755–766. [PubMed] [Google Scholar]
- Frieling T, Wood JD & Cooke HJ (1992). Submucosal reflexes: distension‐evoked ion transport in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 263, G91–G96. [DOI] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J & Lumpkin EA (2004). Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci USA 101, 14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW & Smith TK (2009). Localized release of serotonin (5‐hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136, 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T & Smith TK (2013). Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591, 5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood‐Van Meerveld B & Mawe GM (2012). Activation of colonic mucosal 5‐HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142, 844–854 e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D & Gu JG (2014). Merkel cells transduce and encode tactile stimuli to drive abeta‐afferent impulses. Cell 157, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R & Gu JG (2014). Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci Lett 583, 210–215. [DOI] [PubMed] [Google Scholar]
- Kim M, Christofi FL, Xue J, Robinson JM & Cooke HJ (2007). Mechanically evoked 5‐hydroxytryptamine release is mediated by caveolin‐associated cholesterol rich membrane domains. Neurogastroenterol Motil 19, 309–317. [DOI] [PubMed] [Google Scholar]
- Kim M, Javed NH, Yu JG, Christofi F & Cooke HJ (2001). Mechanical stimulation activates Galphaq signaling pathways and 5‐hydroxytryptamine release from human carcinoid BON cells. J Clin Invest 108, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T & Itou H (2014). Effects of novel TRPA1 receptor agonist ASP7663 in models of drug‐induced constipation and visceral pain. Eur J Pharmacol 723, 288–293. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB & Gola M (2000). The soma and neurites of primary afferent neurons in the guinea‐pig intestine respond differentially to deformation. J Physiol 526, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G & Maroteaux L (2002). Function of the serotonin 5‐hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
- Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F & Liedtke WB (2014). Synergy between Piezo1 and Piezo2 channels confers high‐strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA 111, E5114–E5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Johnston B, Aiello D, Caffrey DR, Giel‐Moloney M, Rindi G & Leiter AB (2014). Distinct cellular origins for serotonin‐expressing and enterochromaffin‐like cells in the gastric corpus. Gastroenterology 146, 754–764 e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Ray SK, Singh NK, Johnston B & Leiter AB (2011). Basic helix‐loop‐helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab 13 (Suppl 1), 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linan‐Rico A, Wunderlich JE, Grants IS, Frankel WL, Xue J, Williams KC, Harzman AE, Enneking JT, Cooke HJ & Christofi FL (2013). Purinergic autocrine regulation of mechanosensitivity and serotonin release in a human EC model: ATP‐gated P2X3 channels in EC are downregulated in ulcerative colitis. Inflamm Bowel Dis 19, 2366–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax RB, Gallego S, Novalbos J, Garcia AG & Warhurst G (1999). L‐Type calcium channels in enterochromaffin cells from guinea pig and human duodenal crypts: an in situ study. Gastroenterology 117, 1363–1369. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A & Lumpkin EA (2014). Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM & Hoffman JM (2013). Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M & Tominaga M (2014). Functional role for Piezo1 in stretch‐evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 289, 16565–16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M, Maksimovic S, Baba Y & Lumpkin EA (2014). Mechanotransduction in epidermal Merkel cells. Pflügers Arch 467, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press D, Mutlu S & Guclu B (2010). Evidence of fast serotonin transmission in frog slowly adapting type 1 responses. Somatosens Mot Res 27, 174–185. [DOI] [PubMed] [Google Scholar]
- Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M & Hu J (2015). Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun 6, 8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke K & Schworer H (1993). Characterization of the role of calcium and sodium channels in the stimulus secretion coupling of 5‐hydroxytryptamine release from porcine enterochromaffin cells. Naunyn Schmiedeberg's Arch Pharmacol 347, 1–8. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJ, Sia TC, Wattchow DA, Spencer NJ & Keating DJ (2013). Identification of unique release kinetics of serotonin from guinea‐pig and human enterochromaffin cells. J Physiol 591, 5959–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR & Patapoutian A (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Cooke HJ & Christofi FL (2004). Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 16 (Suppl 1), 60–63. [DOI] [PubMed] [Google Scholar]
- Roach G, Heath Wallace R, Cameron A, Emrah Ozel R, Hongay CF, Baral R, Andreescu S & Wallace KN (2013). Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility. Dev Biol 376, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito YA, Strege PR, Tester DJ, Locke GR, Talley NJ 3rd, Bernard CE, Rae JL, Makielski JC, Ackerman MJ & Farrugia G (2009). Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 296, G211–G218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk‐Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, Utikal J, Lewin GR, Lechner SG & Siemens J (2015). PIEZO2 is required for mechanotransduction in human stem cell‐derived touch receptors. Nat Neurosci 18, 10–16. [DOI] [PubMed] [Google Scholar]
- Schulze A, Hartung P, Schaefer M & Hill K (2014). Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine‐type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium 55, 200–207. [DOI] [PubMed] [Google Scholar]
- Schulze A, Oehler B, Urban N, Schaefer M & Hill K (2013). Apomorphine is a bimodal modulator of TRPA1 channels. Mol Pharmacol 83, 542–551. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP & Keating DJ (2011). Mechanisms underlying distension‐evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301, G519–G527. [DOI] [PubMed] [Google Scholar]
- Strege P, Beyder A, Bernard C, Crespo‐Diaz R, Behfar A, Terzic A, Ackerman M & Farrugia G (2012). Ranolazine inhibits shear sensitivity of endogenous Na (+) current and spontaneous action potentials in HL‐1 cells. Channels (Austin) 6, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG & Farrugia G (2003). Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 285, G1111–G1121. [DOI] [PubMed] [Google Scholar]
- Stroetz RW, Vlahakis NE, Walters BJ, Schroeder MA & Hubmayr RD (2001). Validation of a new live cell strain system: characterization of plasma membrane stress failure. J Appl Physiol 90, 2361–2370. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM & Sachs F (2000). Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation‐selective stretch‐activated channels. J Gen Physioogy 115, 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Endoh M, Fujiwara N & Nawa T (2005). Receptors and transporter for serotonin in Merkel cell‐nerve endings in the rat sinus hair follicle. An immunohistochemical study. Arch Histol Cytol 68, 19–28. [DOI] [PubMed] [Google Scholar]
- Vanner S & Macnaughton WK (2004). Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil 16 (Suppl 1), 39–43. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA & Patapoutian A (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A (2014). Piezo2 is required for Merkel‐cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Reed‐Geaghan EG, Bolock AM, Fujiyama T, Hoshino M & Maricich SM (2015). Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J Cell Biol 208, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G & Ducy P (2010). Pharmacological inhibition of gut‐derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med 16, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ & Zoghbi HY (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C & Li Y (2001). Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 530, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


