Abstract
Key points
Experiments on neonatal rodent spinal cord showed that serotonin (5‐HT), acting via 5‐HT7 receptors, is required for initiation of locomotion and for controlling the action of interneurons responsible for inter‐ and intralimb coordination, but the importance of the 5‐HT system in adult locomotion is not clear.
Blockade of spinal 5‐HT7 receptors interfered with voluntary locomotion in adult rats and fictive locomotion in paralysed decerebrate rats with no afferent feedback, consistent with a requirement for activation of descending 5‐HT neurons for production of locomotion.
The direct control of coordinating interneurons by 5‐HT7 receptors observed in neonatal animals was not found during fictive locomotion, revealing a developmental shift from direct control of locomotor interneurons in neonates to control of afferent input from the moving limb in adults.
An understanding of the afferents controlled by 5‐HT during locomotion is required for optimal use of rehabilitation therapies involving the use of serotonergic drugs.
Abstract
Serotonergic pathways to the spinal cord are implicated in the control of locomotion based on studies using serotonin type 7 (5‐HT7) receptor agonists and antagonists and 5‐HT7 receptor knockout mice. Blockade of these receptors is thought to interfere with the activity of coordinating interneurons, a conclusion derived primarily from in vitro studies on isolated spinal cord of neonatal rats and mice. Developmental changes in the effects of serotonin (5‐HT) on spinal neurons have recently been described, and there is increasing data on control of sensory input by 5‐HT7 receptors on dorsal root ganglion cells and/or dorsal horn neurons, leading us to determine the effects of 5‐HT7 receptor blockade on voluntary overground locomotion and on locomotion without afferent input from the moving limb (fictive locomotion) in adult animals. Intrathecal injections of the selective 5‐HT7 antagonist SB269970 in adult intact rats suppressed locomotion by partial paralysis of hindlimbs. This occurred without a direct effect on motoneurons as revealed by an investigation of reflex activity. The antagonist disrupted intra‐ and interlimb coordination during locomotion in all intact animals but not during fictive locomotion induced by stimulation of the mesencephalic locomotor region (MLR). MLR‐evoked fictive locomotion was transiently blocked, then the amplitude and frequency of rhythmic activity were reduced by SB269970, consistent with the notion that the MLR activates 5‐HT neurons, leading to excitation of central pattern generator neurons with 5‐HT7 receptors. Effects on coordination in adults required the presence of afferent input, suggesting a switch to 5‐HT7 receptor‐mediated control of sensory pathways during development.
Keywords: fictive locomotion, serotonin, voluntary locomotion
Key points
Experiments on neonatal rodent spinal cord showed that serotonin (5‐HT), acting via 5‐HT7 receptors, is required for initiation of locomotion and for controlling the action of interneurons responsible for inter‐ and intralimb coordination, but the importance of the 5‐HT system in adult locomotion is not clear.
Blockade of spinal 5‐HT7 receptors interfered with voluntary locomotion in adult rats and fictive locomotion in paralysed decerebrate rats with no afferent feedback, consistent with a requirement for activation of descending 5‐HT neurons for production of locomotion.
The direct control of coordinating interneurons by 5‐HT7 receptors observed in neonatal animals was not found during fictive locomotion, revealing a developmental shift from direct control of locomotor interneurons in neonates to control of afferent input from the moving limb in adults.
An understanding of the afferents controlled by 5‐HT during locomotion is required for optimal use of rehabilitation therapies involving the use of serotonergic drugs.
Abbreviations
- 5‐HT
serotonin (5‐hydroxytryptamine)
- 5‐HT7
serotonin type 7
- Add
adductor longus muscle
- CIN
commissural interneuron
- CP
common peroneal nerve (lCP/rCP, left/right)
- CPG
central pattern generator
- EMG
electromyography
- ENG
electroneurogram
- F‐E
flexor–extensor
- IN
interneuron
- MLR
mesencephalic locomotor region
- MTP
metatarsophalangeal joint
- Sol
soleus muscle (lSol/rSol, left/right)
- Tib
tibial nerve (lTib/rTib, left/right)
- Tri
triceps brachii muscle (lTri/rTri, left/right)
- TA
tibialis anterior muscle (rTA, right)
Introduction
Serotonin (5‐hydroxytryptamine; 5‐HT) receptor agonists are sufficient to activate the spinal central pattern generator (CPG) for locomotion in the isolated neonatal rat spinal cord preparation, an effect that can be blocked by 5‐HT antagonists (Cazalets et al. 1992; Cowley & Schmidt, 1994). Subsequent work showed that 5‐HT2A and 5‐HT7 receptors are important for this action of 5‐HT (reviewed in Schmidt & Jordan, 2000; Hochman et al. 2001; Sławińska et al. 2014 b; Gackiere & Vinay, 2014). These receptors also play a role in recovery of locomotion when fetal brainstem tissue is transplanted below a spinal cord transection (Sławińska et al. 2013), and they are abundant on spinal neurons that are active during locomotion (Jordan & Schmidt, 2002; Noga et al. 2009). But despite many publications on the role of 5‐HT in locomotion in neonatal preparations and after spinal cord injury, the role played by 5‐HT in controlling locomotion in adult animals is not clear. According to Vinay and colleagues (Pearlstein et al. 2005), ‘the injection of 5‐HT receptor antagonists should be tested in vivo to answer definitively the question of the contribution of 5‐HT to rhythm generation.’ Here we use intrathecal application of SB269970 (a specific 5‐HT7 receptor antagonist) during overground locomotion to test the hypothesis that 5‐HT7 receptor activation is required for voluntary locomotion in the adult rat, and we compared these results to those obtained using fictive locomotion in adult rats to test whether the 5‐HT7 receptor action involves control of afferent feedback from the moving limb.
Fictive locomotion evoked in neonatal rats in vitro by stimulation of 5‐HT neurons with spinal projections in the parapyramidal region of the medulla is blocked by the 5‐HT7 receptor antagonist SB269970 (Liu & Jordan, 2005). SB269970 did not perturb locomotion in neonatal or adult mice lacking 5‐HT7 receptors (Liu et al. 2009), showing that 5‐HT7 receptors are necessary for this drug to be effective. In neonatal rat and mouse in vitro preparations (Madriaga et al. 2004; Liu & Jordan, 2005; Pearlstein et al. 2005) as well as in adult mouse preparations (Liu et al. 2009), SB269970 interferes with hindlimb intra‐ and interlimb coordination. Here we test the hypothesis that hindlimb coordination is controlled by 5‐HT7 receptors in the intact adult rat and during fictive locomotion produced by mesencephalic locomotor region (MLR) stimulation, a condition much more readily obtained in the adult rat than in adult mouse preparations (Meehan et al. 2012). Our previous reports on 5‐HT's effects during fictive locomotion were carried out using isolated neonatal rodent spinal cord preparations (Liu & Jordan, 2005; Liu et al. 2009), but it is now clear that 5‐HT's effects can be different in neonatal and older rodents (Abbinanti et al. 2012; Husch et al. 2015). The absence of afferent feedback during fictive locomotion allowed us to test the hypothesis that 5‐HT7 receptor‐mediated control of afferent input contributes to locomotion in the adult rat. Serotonin is involved in the control of sensory feedback to spinal neurons (Jankowska et al. 2000; Chopek et al. 2013), and some effects on primary afferents and their actions are due to 5‐HT7 receptors (Cardenas et al. 1999, 2001; Garraway & Hochman, 2001 b; Millan, 2002). Results obtained in neonatal animals have led to the conclusion that 5‐HT controls inter‐ and intralimb coordination via direct actions on coordinating interneurons (Liu et al. 2009; Gackiere & Vinay, 2014). If this were the case in the adult, coordination would be similarly affected by 5‐HT7 receptor blockade during fictive locomotion in adult rats.
The use of MLR‐evoked fictive locomotion also allowed us to test the hypothesis that MLR activation of the descending medullary 5‐HT neurons contributes to the production of locomotion. We previously suggested that 5‐HT7 receptors are involved in MLR‐evoked fictive locomotion in decerebrate cats (Schmidt & Jordan, 2000). We also tested the hypothesis that 5‐HT7 receptors on motoneurons (Doly et al. 2005) contribute to the effects of SB269970. These results provide new insight into the neuromodulatory control of locomotion in adult animals and suggest a new framework for understanding the actions of 5‐HT in adult animals. A portion of the data has been presented in abstract form (Sławińska et al. 2012; Cabaj et al. 2014).
Methods
Animals
The experiments with chronic intrathecal drug administrations were performed on six female Wistar rats, aged 3 months at the beginning of the experiments, weighing 250–300 g. The animals were kept in separate cages in a room with 12:12 h dark–light cycles. All procedures were conducted with the approval of the First Ethics Committee for Animal Experimentation in Poland, according to the principles of experimental conditions and laboratory animal care of the European Union and the Polish Law on Animal Protection.
Investigation of the effects of SB269970 was carried out during fictive locomotion induced by MLR electrical stimulation on 6 of 7 Sprague–Dawley female rats aged 3–6 months, weighing 250–300 g. In one experiment the locomotion was not consistent enough during the control period for results from this animal to be included in the analysis of the SB269970 effects. These experiments were conducted with the approval of the University of Manitoba Animal Care Committee in Canada in accordance with the guidelines of the Canadian Council on Animal Care.
Our experimental procedures of chronic and acute experiments also comply with policies set out by The Journal of Physiology (Grundy, 2015).
Intact freely moving rats
Intrathecal cannula implantation
The procedure for intrathecal cannula implantation was similar to that used by us previously (Majczyński et al. 2005). The implantation was performed in aseptic conditions under anaesthesia using Equithesin without chloral hydrate (Deacon & Rawlins, 1996), formulated as follows: MgSO4 (1.06 g in 5 ml sterile H2O), pentobarbital sodium salt (0.485 g in 5 ml sterile H2O), 95% ethanol (5 ml), propylene glycol (25.6 ml), and sterile H2O to make a solution of 50 ml total volume. This formulation was administered i.p. using doses of 0.35–0.6 ml (100 g b.w.)–1 (34–58 mg kg−1 pentobarbital sodium). A polyethylene cannula (PE‐10) was inserted into the subarachnoid space through a small opening made at the Th10/11 vertebral level and was pushed caudally aiming to reach the L5/L6 root entrance to the spinal cord. The cannula was fixed by sewing it to the Th10 spinous process and stabilized by suturing the overlying back muscles in place. The other end of the cannula was guided under the skin to reach the skull and connected to a custom‐made adaptor cemented to the bone. After surgery, the animals received a non‐steroidal anti‐inflammatory and analgesic treatment (Tolfedine, 4 mg kg–1 s.c.) and antibiotic (Baytril, 5 mg kg–1 s.c.). Two to five days after surgery, demonstration of the proper placement of the intrathecal cannula was accomplished in each case by injecting lidocaine (10–15 μl of 2%), followed by 15 μl of sterile saline (sodium chloride 0.9%), which produced temporary paralysis for 10–15 min. The exact location of the tip of the cannula was determined by post mortem inspection. In all cases reported here the cannula tip was located at the level of the L4/L6 spinal segments. Every intrathecal application of drug was performed as a single bolus of 30 μl of drug dissolved in sterile saline that was followed by a bolus of about 15 μl of sterile saline to wash the drug from the cannula. The procedure of drug injection lasted approximately 30 s. The experiments with intrathecal injections were always separated by an interval of at least 72 h. A similar volume of sterile 0.9% saline was used as a control injection.
Implantation of EMG electrodes
For the electromyography (EMG) recordings, bipolar electrodes were implanted in extensor muscles (active during the stance phase of the step cycle) and flexor muscles (active during the swing phase of the step cycle) of each limb. In forelimbs, the triceps brachii medial head (Tri) was implanted (extensor); in hindlimb muscles, adductor longus (Add, extensor), soleus (Sol, extensor) and tibialis anterior (TA, flexor) were implanted under anaesthesia with Equithesin without chloral hydrate (0.35–0.6 ml (100 g b.w.)–1 administered i.p.). The electrodes were made of Teflon‐coated stainless steel wire (0.24 mm in diameter; AS633, Cooner Wire Co., Chatsworth, CA, USA). The tips of the electrodes with 1–1.5 mm of the insulation removed were pulled through a cutaneous incision on the back of the animal, and each of the hook electrode was inserted into the appropriate muscle and secured by a suture (Sławińska et al. 2000). The distance between the electrode tips was 1–2 mm. The ground electrode was placed under the skin on the back of the animal some distance from the hindlimb muscles. The connector with the other ends of the wires fixed to it, covered with dental cement (Spofa Dental, Prague, Czech Republic) and silicone (3140 RTV, Dow Corning), was secured to the back of the animal. After surgery, the animals received a non‐steroidal anti‐inflammatory and analgesic treatment (Tolfedine, 4 mg kg–1 s.c.) and antibiotic (Baytril, 5 mg kg–1 s.c.).
Testing the effects of intrathecal drug applications on locomotor performance
Locomotion along the horizontal runway was always tested with simultaneous EMG recordings. The procedure for the examination of runway locomotion of freely moving rats was based on a shelter‐seeking paradigm. Rats were pre‐trained to walk on a horizontal runway 2.5 m long, 12 cm wide with an illuminated start platform at one end and a darkened goal box at the other (for details see Majczyński et al. 2007). Only runs with stable, rhythmic locomotor movements with at least 10 regular steps were chosen for further analysis.
Following a pre‐drug trial, the effects of 5‐HT7 receptor antagonist (SB269970; (2R)‐1‐[(3‐hydroxyphenyl) sulfonyl]‐2‐[2‐(4‐methyl‐1‐piperidinyl)ethyl]pyrrolidine hydrochloride, 150 μg in 30 μl of saline; Tocris) on hindlimb locomotor performance was tested immediately after drug intrathecal injection (i.e. when the first effect of the drug administration was usually observed). In intact freely moving rats we needed to use a higher dose of the antagonist than the dose used in acute experiments because in freely moving rats the lower dose produced a rather short and limited effect. To reduce any discomfort during intrathecal drug application, before each session all the animals received Butomidor (0.5 mg kg–1, s.c.; Richter Pharma, Wels, Austria) premedication. Butomidor contains 10 mg ml−1 butorphanol, a synthetic opioid agonist/antagonist with potent analgesic and moderate sedative properties. In control experiments we showed that Butomidor application by itself did not alter the locomotor performance of the experimental rats on the runway or on the treadmill. In the first experiments we administered SB269970 without Butomidor and successfully blocked locomotion, but the animals were made uncomfortable by the drug injection, as demonstrated by vocalizations and agitation. Pretreatment with Butomidor eliminated the signs of discomfort without affecting the motor response. This was consistent with our previous experiments using intact adult mice (Liu et al. 2009), where we used Butomidor to prevent pain from the surgery.
EMG recordings and EMG analysis recorded during locomotion on a horizontal runway
EMG activity recorded in freely moving rats was filtered (0.1–1 kHz band pass), digitized and stored on a computer (2 kHz sampling frequency) using the Winnipeg Spinal Cord Research Centre data capture system. The locomotor EMG pattern was analysed using custom software (http://www.scrc.umanitoba.ca/doc/). The coordination between flexor and extensor muscles on the two sides, as well as left–right coordination among the muscles of the left and right sides was determined using polar plot analysis (Zar, 2010). In the polar plots the phase position of the vector at 0 or 360 deg reflects synchrony of analysed EMG burst onsets, whereas 180 deg is equivalent to alternation. The length of the vector (r, ranging from 0 to 1) indicates the concentration of phase values around the mean and can be interpreted as the strength of coordination between analysed muscle burst onsets. When the onsets of EMG bursts of the same muscle of left and right hindlimbs are strongly coupled, the phase values should be highly concentrated around the mean phase. Conversely, when bursting in analysed muscle activities is independent and there is no coupling, the distribution of phases should show some degree of dispersion, with wide distribution on polar plot circles. In our analysis, Rayleigh's circular statistical test was applied to determine whether the inter‐ and intralimb coordination r values were concentrated, suggesting coupling of burst activity, or dispersed, indicating no coordination. We considered the inter‐ or intralimb coordination to be phase‐related if r was greater than critical Rayleigh's value (cR) for a given P value (Zar, 2010). When describing these results we present the r value for each polar plot together with its statistical significance (P). The relationships between the EMG burst duration and step cycle duration were determined using the regression line method.
Kinematic analysis was performed from video recordings during locomotion on a treadmill
Rat locomotor ability was tested on a treadmill with various speeds of the moving belt (10 or 20 cm s−1) in order to perform kinematic analysis of hindlimb stepping. During testing the rat locomotor performance on the treadmill before and after the drug administration the EMG activity was synchronized with simultaneous recordings of video images. Before each recording session, the hindquarters of the rat were shaved and six markers were set on the skin of the left hindlimb at the bone landmarks: ilium, hip, knee, ankle and the metatarsophalangeal joint (MTP) and the tip of the third toe. The centre of the treadmill was viewed with a high speed camera (TEMA Camera) with a capture rate of 250 frame s−1. Video data were stored in computer memory and were analysed using Image System AB software (Linköping, Sweden). Kinematic parameters of the stepping movements, such as swing and stance durations and joint angles, were measured from data files created by the image system (www.imagesystems.se/image-systems-motion-analysis). The video images were digitized and the x–y coordinates of different joint markers were obtained at the frequency of 250 frames s−1 by de‐interlacing each video frame. These coordinates were used to calculate angular joint movements and could be displayed as continuous angular displacements or stick diagrams of one step cycle.
Reflexes
Reflex responses to dorsi‐ and plantar‐flexion of the ankle (Figs 5 and 6) were obtained as previously described (Sławińska & Kasicki, 2002) in an attempt to determine whether the 5‐HT7 receptor antagonist has a direct effect on motoneuron excitability.
Figure 5. 5‐HT7 receptor blockade has no direct effect on motoneuron excitability, but alters sensory input to flexor motoneurons produced by plantar flexion of the hindlimb at the ankle joint.
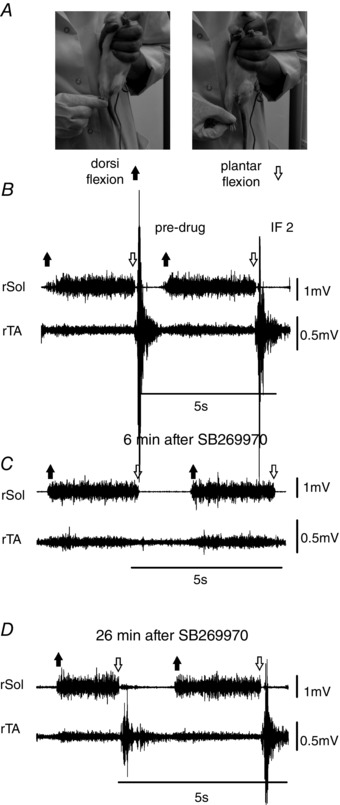
A, the procedure for eliciting reflex EMG discharges in Sol and TA muscles (described in detail in the text). B–D, after SB269970 the activity produced during dorsi‐flexion of both Sol and TA was relatively unaffected by the drug (compare B, C and D), showing the absence of a direct effect of the drug on motoneuron excitability, whereas the reflex response in TA to plantar flexion was reversibly abolished (compare B, C and D). Abbreviations: rSol, right soleus muscle; rTA, right tibialis anterior muscle.
Figure 6. EMG amplitudes in reflex responses were reduced in TA (plantar flexion) but not in Sol muscles (dorsiflexion) after SB269970 (n = 6).
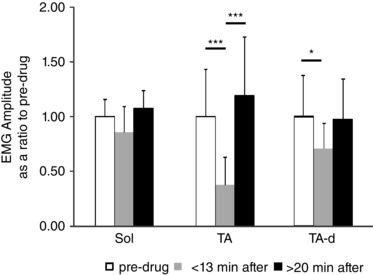
TA response to dorsiflexion (TA‐d) was slightly decreased. There was no significant decrease in the Sol reflex response in comparison to the pre‐drug response. Abbreviations: Sol, soleus muscle; TA, tibialis anterior muscle. The significance of the results was tested using Friedman's non‐parametric test followed by Dunn's multiple comparison (* P < 0.05, *** P < 0.001). The data are presented as means ± SD.
Statistics
Statistical analysis was performed using Prism 6 (GraphPad Software, La Jolla, CA, USA). For analysis of changes in the cycle duration and of changes in the EMG amplitude expressed as a ratio of the control values induced by drug application during locomotion and during reflexes, the non‐parametric Friedman's test with Dunn's multiple comparison test was used. Statistical analyses of circular data was carried out using the Watson–Williams test, a circular analogue of the one‐factor ANOVA (IGOR Pro software; Wavemetrics, Lake Oswego, OR, USA). Repeated measures MANOVA with the Bonferroni post hoc test was used for multiple comparisons of the strength of intra‐ and interlimb coordination. The data are presented throughout as means ± standard deviation (SD).
Fictive locomotion induced by MLR electrical stimulation
Our procedures for the use of this preparation have been previously described (MacDonell et al. 2015). For the initial surgery the rats were anaesthetised in an induction chamber with 5% isoflurane with oxygen. Before the surgery started the animals were given a bolus intraperitoneal injection of 2 ml saline containing 0.05 mg kg−1 atropine and 100 mg dextrose. Anaesthesia was maintained throughout the surgical procedures (2.5% isoflurane and O2). The trachea was intubated, and artificial ventilation was maintained throughout the experiment (56–62 bpm, volume 2.0–2.5 ml) with CO2 in the expired gas at 3.4–3.8 % by adjusting tidal volume and/or ventilation rate. The left carotid artery was isolated and a catheter inserted for monitoring blood pressure and infusion of fluids. During the course of the entire experiment an infusion of saline or bicarbonate buffer (0.1 m NaHCO3 and 0.28 m dextrose in sterile water) was given at a rate of 0.9 ml h−1. After the carotid catheterization dexamethasone was given (2–4 mg kg−1 i.v.) to decrease brain swelling. The blood pressure was maintained between 80 and 95 mmHg.
Dissection of the tibial (innervating extensor muscles) and peroneal nerves (innervating flexor muscles) was carried out by making an incision on the posterior aspect of both hindlimbs and dissecting the nerves away from musculature. When the animal was moved to the stereotaxic apparatus the muscles together with the skin were used to construct a natural bath filled with mineral oil to allow recording electroneurograms (ENGs) from the isolated nerves mounted on silver bipolar electrodes.
A laminectomy at the Th13/L1 vertebra level was performed to record a cord dorsum potential. A laminectomy at the L3/L4 vertebra was also performed to implant an intrathecal cannula for drug application. The procedure for intrathecal cannula implantation was similar to that used previously (Majczyński et al. 2005), except in this case the approach was to insert the cannula from the caudal end of the vertebral canal to allow placement of a recording electrode on the spinal cord at the Th13/L1 vertebral level. A polyethylene cannula (PE‐10) was inserted into the subarachnoid space through a small opening made at the L3 vertebral level and was pushed rostrally to the L1 vertebra level. The cannula was fixed by sewing it to the L4 spinous process and stabilized by suturing the overlying back muscles in place.
The rat was then moved to a stereotaxic frame where the head, thoracic and lumbar vertebrae, and both feet were immobilized. A precollicular decerebration was performed using slow suction after the cortex was removed. The skull was then packed with surgical absorbable haemostat and saline soaked cotton. After decerebration the rats were removed from isoflurane anaesthesia and pancuronium bromide (0.2–0.3 mg kg−1 i.v.) was given intravenously every hour to ensure no movement. Two vertebral clamps (vertebral levels Th11 and L5/6) secured the stability of the spine and spinal cord. The skin flaps around the exposed areas of the spinal cord and both hindlimbs were retracted and stabilized within the stereotactic frame to form pools that were filled with warm paraffin oil.
The rats were ventilated with ambient air enriched with humidified 100% O2 throughout the experiment. The heart rate and O2 saturation were monitored using a pulse oximeter placed on the foot.
The terminal experiments consisted of investigation of the effectiveness of the MLR electrical stimulation (10–100 μA, 10–40 Hz) to induce fictive locomotion, which was then altered by intrathecal application of 5‐HT7 receptor antagonist (SB269970; 60 μg/20 μl saline). The effects on hindlimb locomotor performance were tested immediately after intrathecal injection, with subsequent tests every 3–4 min.
Results
Intrathecal SB269970 in freely moving animals
General observations
Locomotion of the rats in the control condition, prior to drug administration, was identical to that observed in our previous studies under similar conditions (Majczyński et al. 2005; Sławińska et al. 2012, 2014 a). After control trials on the treadmill or on the runway, SB269970 (150 μg in 30 μl of saline) was applied intrathecally, with the tip of the intrathecal cannula placed in the lumbar region over L4/L6 segments. This facilitated exposure to the drug in most if not all of the lumbar spinal cord, because drug injected through the cannula tended to emerge from the tip and diffuse rostrally along the cannula. A paralysis‐like state (Fig. 1) was evident within 30 s after SB269970 application in all cases (n = 6). The animals depended upon their forelimbs for forward progression. EMG activity in forelimb muscles increased accordingly. During the initial paralysis‐like period, the animals were dragging their hindlimbs behind them in a passively abducted position with the medial surface of the paws touching the runway surface (Fig. 1). There was no plantar stepping of the hindlimbs, but movements about the ankle joint persisted, and rhythmic EMG activity in the hindlimb muscles was always present. Sustained passive extension at the hip was observed shortly after the drug administration. Examples from two rats at 3 min after SB269970 (Figs 1 and 2) show limited movements in the extended hip despite continued rhythmic EMG activity in all muscles. The sustained hip extension was within the normal range of movement at this joint (Fig. 2 Bc and Cc). The hyperextension observed in the adult mouse after SB269970 (Liu et al. 2009) was never observed in adult rats. Kinematic analysis (Fig. 2, joint angle plots and stick figures) revealed that during impairment of plantar stepping the hip and knee joint angles hardly varied at all, while movement about the ankle joint continued to predominate. Knee movement was markedly reduced, and the knee joint maintained a flexed position. The main movement was at the ankle joint, but with the reduced movement at the hip and knee there was a lack of proper placement of the paw, resulting in ankle movements that caused the medial surface of the hind paw to brush along the surface of the substrate without producing any weight support (‘crawling’ or ‘swimming‐like’ movements).
Figure 1. Blockade of 5‐HT7 receptors in the lumbar spinal cord produces hindlimb locomotor impairment in adult freely moving rats.
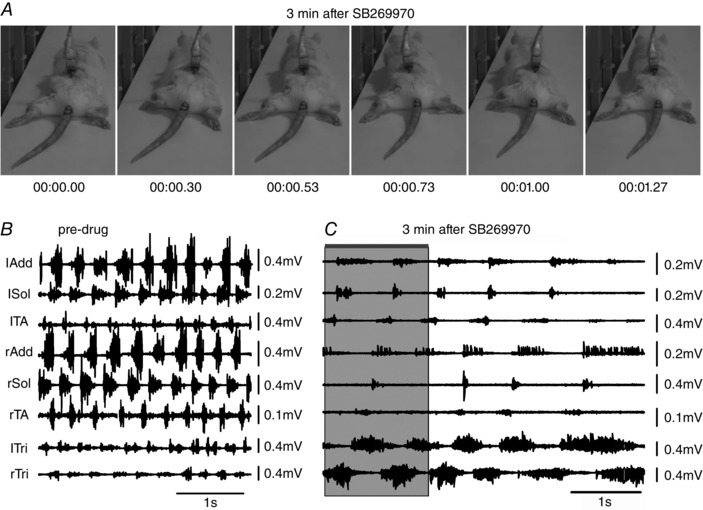
A, the antagonist SB269970 was applied intrathecally (150 μg in 30 μl of saline) while the rat was walking on a runway. Plantar stepping and weight support was lost at 3 min after drug administration, despite the persistence of EMG activity. B, EMG activity during a period immediately prior to drug administration. C, 3 min after drug administration the hindlimb EMG was greatly reduced in amplitude and frequency. The shaded area in C indicates the time during which the frames in A were obtained (0.00 to 1.27 s). The increase in forelimb EMG (lTri and rTri) reflects the increased use of the forelimbs for progression. This behaviour resembles ‘crawling’ in neonatal animals. See text for a detailed description. Abbreviations: lAdd/rAdd, left/right adductor muscle; lSol/rSol, left/right soleus muscle; lTA/rTA, left/right tibialis anterior muscle; lTri/rTri, left/right triceps brachii muscle.
Figure 2. Locomotor impairment of treadmill locomotion due to blockade of 5‐HT7 receptors is accompanied by coordination and kinematic changes.
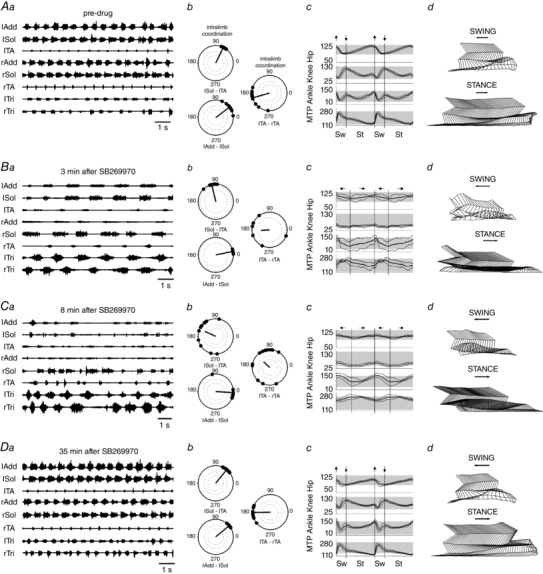
A polar plots, joint angle recordings and stick figures from the pre‐drug condition show the normal locomotor activity. B, the initial effect of the drug (3 min after intrathecal application SB269970, a–d) was a disruption of left–right coordination, and increased cycle duration (b). Movements at the hip and knee joints were reduced (c and d), while movements about the ankle and MTP persisted, but became irregular. C, at 8 min after SB269970 (a–d) the Sol EMG burst was reduced in duration on the left side, and became variable on the right. Both inter‐ and intralimb coordination were disrupted (b), and movement was limited to the ankle and MTP joints (c–d). In B and C the terms swing and stance refer to forward and backward movements about the ankle joint, but without weight support during the stance phase, when the limb was dragging on the treadmill surface. D, recovery was obvious at 35 min after the drug was administered. See text for further details. Abbreviations: lAdd/rAdd, left/right adductor muscle; lSol/rSol, left/right soleus muscle; lTA/rTA, left/right tibialis anterior muscle; lTri/rTri, left/right triceps brachii muscle.
The sequential video frames in Fig. 1 show that plantar stepping was lost shortly after SB269970 administration, despite the persistence of considerable EMG activity. Intra‐ and interlimb coordination were disrupted (Figs 1, 2, 3), and the EMG activity was reduced in amplitude (Figs 1, 2 and 4). In Fig. 1, for example, there were two movements in the left hindlimb while only one movement occurred in the right. The simultaneous EMG recordings show that this occurs because of the deletion of both Sol (ankle extensor) and TA (ankle flexor) activity on the right while two bursts occur on the left side. On the right side, EMG activity continued in the Add muscle (hip muscle active during the stance phase of the step cycle in the normal condition) during this deletion of activity in the ankle muscles. These effects persisted until recovery (see Fig. 2). Some recovery of stepping was observed within 10–15 min after the drug injection, with little body weight support. Within 30 to 40 min all of the rats had recovered to the extent that they were walking in a qualitatively normal‐looking manner. Control injections of vehicle (10–30 μl) were without effect. It is clear from these results that our hypothesis that 5‐HT7 receptor activation is necessary for voluntary locomotion in the adult rat is confirmed.
Figure 3. Intra‐ and interlimb coordination was altered in all 6 animals examined during runway locomotion.
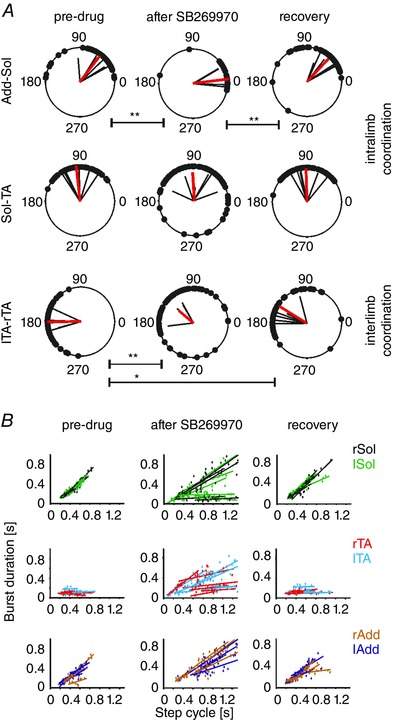
A, polar plots showing intralimb and interlimb coordination changes from all 6 animals (black vectors are the means for each animal, mean vector for all animals in red). B, regression analysis showed that the drug increased cycle duration and altered the normal relationship between cycle duration and burst duration in both flexor and extensor muscles. The drug caused the occasional switch from the normal extensor‐dominated rhythm to a flexor‐dominated one (see text). Abbreviations: Add, adductor muscle; Sol, soleus muscle; lTA/rTA, left/right tibialis anterior muscle; lTib‐rTib, polar plot analysis of interlimb coordination between burst activity recorded in both (left and right) TA muscles; Add‐Sol, polar plot analysis of intralimb coordination between burst activity recorded in left Add and left Sol muscles; Sol‐TA, polar plot analysis of intralimb coordination between burst activity recorded in left Sol and left TA muscles. The significance of the results of circular data was tested using the Watson–Williams test (* P < 0.05, ** P < 0.005).
Figure 4. EMG amplitude during voluntary runway locomotion was significantly reduced by 5‐HT7 receptor blockade in TA and Add muscles, which came back to pre‐drug level within 30 min after drug application.
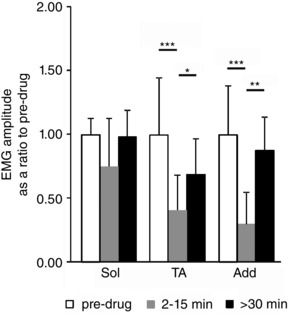
In the case of Sol muscles the decrement induced by SB269970 application was not statistically significant (P > 0.05). Abbreviations: Add, adductor muscle; Sol, soleus muscle; TA, tibialis anterior muscle. The significance of the results was tested using Friedman's non‐parametric test followed by Dunn's multiple comparison (* P < 0.05, ** P < 0.01, *** P < 0.001). The data are presented as means ± SD.
SB269970 alters cycle duration
Cycle duration was increased by the drug in all the rats tested (Figs 1, 2, 3). The increase in cycle duration after the drug in several cases is shown in Fig. 3 B. The cycle duration during the pre‐drug period ranged from a minimum of 280.3 to a maximum of 549.3 ms (mean, 413.0 ± 108.8 ms), while after the drug (2–15 min) the range was 418.3–1657.4 ms (mean, 799.7 ± 453.9 ms). This change was significant (P < 0.02, n = 6, Friedman's non‐parametric test followed by Dunn's multiple comparison test). The mean cycle duration after recovery was 452.5 ± 136.2 ms. This is consistent with the observations in the neonatal rat preparations and in neonatal and adult mouse preparations (Liu & Jordan, 2005; Liu et al. 2009).
SB269970 alters coordination
We then tested the hypothesis that intra‐ and interlimb coordination are disrupted by SB269970 in the adult rat, as they are in the neonatal rat and mouse fictive locomotion preparations and in the adult mouse. Polar plot analysis was used to determine the changes in coordination that might underlie the alterations in stepping movements, as was previously suggested on the basis of our experiments in neonatal rats (Liu & Jordan, 2005) and in 5‐HT7 receptor knockout mice (Liu et al. 2009). As illustrated in Fig. 2, left–right coordination was first disrupted (3 min after SB269970), with deterioration of flexor/extensor coordination evident at 8 min after drug administration. Inter‐ and intralimb coordination were altered (Fig. 2 Cb), even though rhythmic EMG activity persisted.
A detailed analysis of inter‐ and intralimb coordination obtained from EMG data was performed on six rats (Fig. 3). In the polar plots the mean vectors represent the relative timing of the onset of activity in the two muscles examined for each experiment (black vectors), along with the population mean (red vector). The Watson–Williams test was used to show that there was a significant decrease in the phase shift between the times of onset of ipsilateral Sol and Add. The phase shift between ipsilateral TA and Sol bursts (Watson–Williams test, P > 0.05) was not significantly changed, but the strength of the coordination between these muscles was diminished, as shown by a significantly reduced r value (repeated measurements MANOVA with Bonferroni post hoc test, P < 0.05). The left and right TA times of onset were altered significantly by the drug (Watson–Williams test, P < 0.005), so that their periods of activity were no longer alternating. Recovery from these changes occurred within 40 min after administering the drug. The drug disrupted the alternation between ipsilateral flexor and extensor muscle activity and between left and right sides in all cases.
Regression analysis (Fig. 3 B) of the burst duration plotted against cycle duration showed that blockage of 5‐HT7 receptors decreased the soleus burst duration in several cases, and reduced the normal positive correlation of extensor burst duration with cycle duration. These results are consistent with 5‐HT7 receptor‐mediated excitation of excitatory interneurons in a pathway controlling extensor motoneurons. Therefore, an effect of the drug is to impair extensor muscle activity so that weight support can no longer be accomplished throughout the stance phase. At the same time, the TA burst duration became prolonged, and it tended to increase with increasing cycle duration (a flexor dominated pattern, see Yakovenko et al. 2005).
SB269970 reduces hindlimb muscle EMG amplitude during locomotion
As is readily apparent in the examples shown in Figs 1 and 2, the amplitudes of EMGs were decreased after 5‐HT7 receptor blockade. Figure 4 shows that EMG amplitude significantly decreased in TA (to 41 ± 28%; P < 0.001) and in Add (to 30 ± 25%; P < 0.0001) muscles with no significant change in the Sol muscle (to 75 ± 38%; P = 0.13) after SB269970 application (Friedman's non‐parametric test followed by Dunn's multiple comparison test). These results show a reduction in motoneuron output due to 5‐HT7 receptor blockade during voluntary locomotion in adult rats.
The changes in EMG amplitude during locomotion could be interpreted as the result of decreased excitability of the motoneurons themselves due to reduced direct 5‐HT effects. There is evidence for 5‐HT7 receptors on some spinal motoneurons (Doly et al. 2005). Alternatively, the effect of 5‐HT7 receptor blockade may be at the level of premotor CPG neurons with consequent decreased excitatory drive to motoneurons. In order to distinguish between these two possibilities we used reflex activation of Sol and TA as a test of any direct effect on motoneurons.
Effect of 5‐HT7 receptor blockade on reflexes
Figure 5 A shows the procedure used to induce reflexes at the ankle joint in awake rats. This technique was previously described (Sławińska & Kasicki, 2002). Reflex discharges of the Sol muscle were produced by a sustained dorsiflexion at the ankle joint, a movement that stretches the triceps surae muscles, including Sol. TA discharge could be induced by rapid plantar flexion to stretch the pretibial flexor muscles, including TA. The dorsiflexion‐induced effects in Sol were accompanied by a low amplitude response in TA as well (Fig. 5 B and D). Heckman and co‐workers (Frigon et al. 2011) previously reported the presence of a similar burst of activity in the TA muscle during stretch of the triceps surae.
SB269970 administration produced no reduction (to 85.4 ± 34.6%; P = 0.17) in the tonic stretch reflex in Sol muscles (Figs 5 and 6). In contrast, the drug almost completely suppressed (to 37.5 ± 27.4%; P = 0.0007) the response in TA to phasic plantar flexion of the ankle (Figs 5 C and 6). At the same time, the reflex response seen in TA (TA‐d) to dorsiflexion of the ankle (reduced to 71.4 ± 22%; P = 0.03) was altered (Fig. 5 C, rTA, and Fig. 6), but not to the degree that SB269970 altered TA EMG amplitude during locomotion. The statistical significance was determined using Friedman's non‐parametric test followed by Dunn's multiple comparison test. The relative sparing of the discharge in Sol and TA with stretch of the triceps surae suggests that the effect of SB269970 to reduce motoneuron output during locomotion (Fig. 4) is not due to a direct action on 5‐HT7 receptors in either flexor or extensor motoneurons.
Fictive locomotion in the adult rat
General observations
Figure 7 shows an example of the electrode tract and the MLR stimulus site in a decerebrate rat in which the effect of intrathecal SB269970 was tested. This and other sites verified in our experimental animals reveal that the effective stimulus site was in the vicinity of the nucleus cuneiformis (Fig. 7 A and B), previously shown to be effective in the decerebrate rat (Skinner & Garcia‐Rill, 1984; Coles et al. 1989) and cat (Shik et al. 1967; Steeves & Jordan, 1984; Mori et al. 2001; Takakusaki et al. 2008; Takakusaki et al. 2016). Once a low threshold site for MLR‐evoked locomotion was identified, repeated stimuli over a prolonged period in the experiments described here produced consistent responses (Figs 8 and 9). The effective MLR stimulus strengths used to produce sustained locomotion during the control and drug trials were 20–100 μA.
Figure 7. An example of an effective MLR stimulus site.
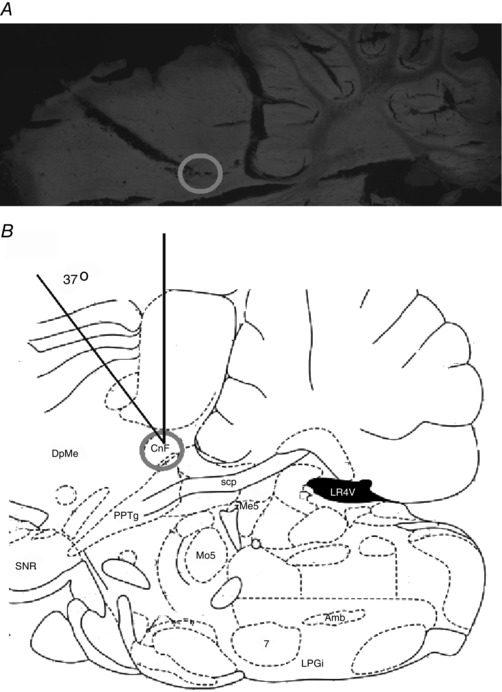
A, sagittal section of the brainstem showing the position of an electrolytic lesion placed at the effective MLR site (right side) in this experiment. B, a diagram of a sagittal slice (from Paxinos & Watson, 2007) shows the tip of the electrode (grey circle) located in the vicinity of the nucleus cuneiformis (CnF). The electrode was positioned at a 37 deg angle and inserted 1.5 mm lateral to the midline at a depth of 3.0 mm below the surface of the superior colliculus. The fictive locomotion produced by stimulation at this site in this animal is illustrated in Fig. 9. Abbreviations: 7, facial nucleus; Amb, ambiguous nucleus; CnF, nucleus cuneiformis; DpMe, deep mesencephalic nucleus; LPGi, lateral paragigantocellular nicleus; LR4V, lateral recess of the 4th ventricle; Mo5, motor trigeminal nucleus; Me5, mesencephalic trigeminal nucleus; PPTg, pedunculopontine tegmental nucleus; scp, superior cerebellar peduncule; SNR, substantia nigra, reticular part.
Figure 8. Fictive locomotion evoked by MLR stimulation recruits a serotonergic pathway acting at spinal 5‐HT7 receptors.
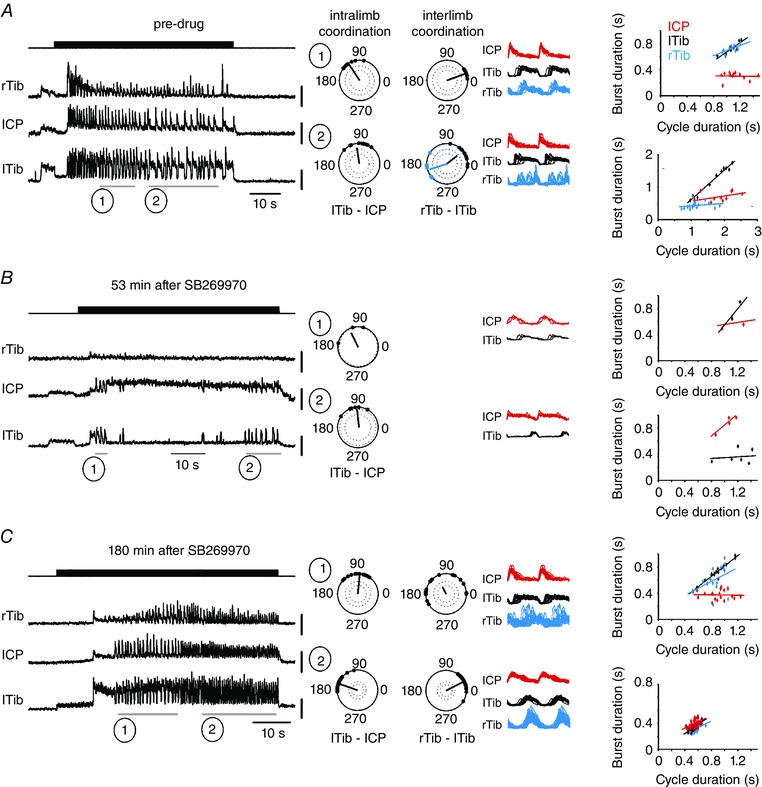
Fictive locomotion was attempted at 4 min intervals. The effective stimulus was 40 μA at a frequency of 20 Hz, pulse duration 0.5 ms. These stimulus parameters were maintained throughout the pre‐drug period (A), during the period after intrathecal application of the 5‐HT7 receptor antagonist SB269970 (B), and after recovery (C). A record of the stimulus is provided in the top traces of A–C. The remaining 3 traces are rectified and filtered ENG records of fictive locomotion recorded in the indicated peripheral nerves. Right common peroneal nerve (rCP) was silent throughout this run of fictive locomotion. Polar plots, linear envelopes and regression lines are provided for the periods of locomotor activity marked by numbered horizontal lines below the traces. Dots on the polar plots show the timing of each right tibial nerve (rTib) burst onset after the onset of activity in lTib in interlimb coordination analysis and the timing of each lTib burst onset after the onset of activity in lCP in intralimb coordination analysis. The dark lines represent the average vector, with the strength of the relationship shown as the length of the vector. The dotted lines in polar plots show the P < 0.001 (outer circle) and P < 0.05 (inner circle) significance levels of the r vector using a critical Rayleigh's value calculated for a given P value. Plots with only one dotted circle represent only P < 0.05. Linear envelopes show overlays of the rectified and filtered bursts during each period (lCP, red; lTib, black; rTib, blue) plotted in relation to the onset of lCP activity. The colour of the regression lines is coded according to the colour of the linear envelopes. Reciprocal activity in the flexor and extensor nerves on the left side was maintained after the drug suppressed locomotor activity (B) and during recovery (C), while left–right coordination could not be evaluated because the activity in rTib ceased during the drug trial. During the pre‐drug period left–right coordination was synchronous (1) or displayed double bursting on the right (2). During recovery (3 h after SB269970 application) left–right coupling was disorganized (C, top right polar plot) or synchronous (C, bottom right polar plot). The regression lines show that the mode of locomotion could be extensor dominated (A, top diagram of regression lines) or flexor dominated (right side) (A, bottom diagram of regression lines) in the control situation, and this variability was sustained through the drug trial (B), where both modes occurred, extensor domination (1) and flexor domination (2). During the recovery period, 3 h after drug administration (C), extensor domination (1) or nearly symmetrical modes of activity (2) was observed. Abbreviations: lCP/rCP, left/right common peroneal nerve; lTib/rTib, left/right tibial nerve; rTib‐lTib, polar plot analysis of interlimb coordination between burst activity recorded in both (left and right) tibial nerves; lTib‐lCP, polar plot analysis of intralimb coordination between burst activity recorded in left tibial and left common peroneal nerves.
Figure 9. Synchronous interlimb coupling observed during fictive locomotion was disrupted by 5‐HT7 receptor blockade.
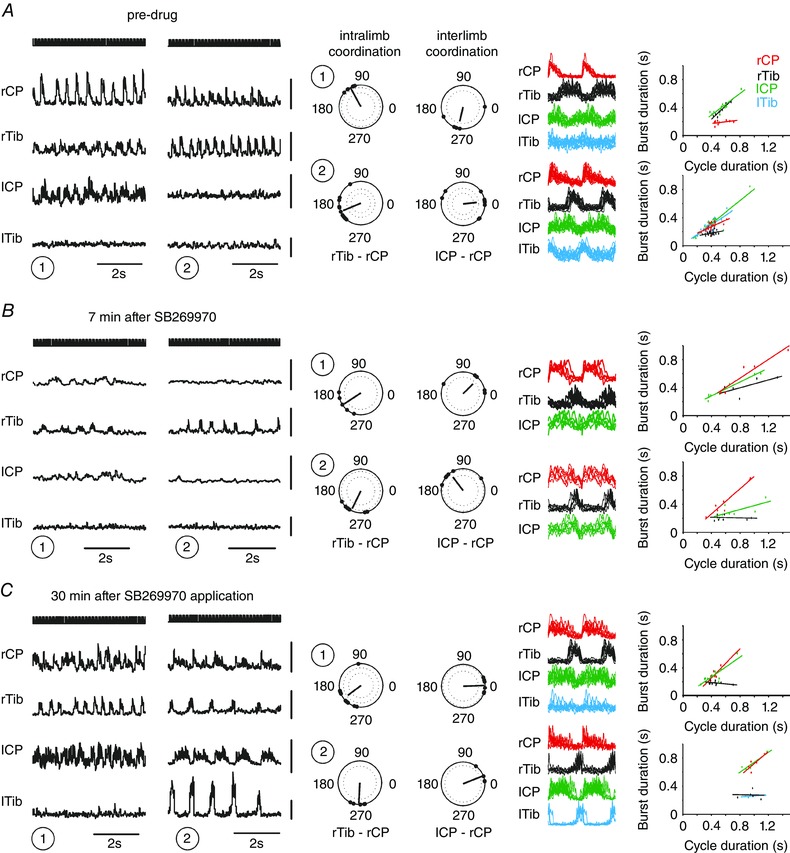
Different modes of locomotor activity sampled from 60 s periods of left MLR stimulation at 30 μA at a depth of 2.5 mm are illustrated (1 and 2 in A, B and C). These 60 s periods of stimulation were applied every 2–3 min and locomotion was reliably elicited in each case during the control and after recovery. Approximately 10 min after SB269970 application locomotion ceased completely, but at 7 min, later prior to cessation (1 and 2), it was possible to analyse the effect of the drug on intra‐ and interlimb coordination. During the pre‐drug condition the interlimb coordination was either chaotic (right top polar plot) or synchronous (right bottom polar plot), as shown in the polar plots and linear envelopes. Intralimb coordination was characterized by strict alternation between flexor and extensor nerves regardless of the mode of locomotor activity. The records in B show that ENG amplitude and frequency were decreased by the drug prior to complete blockade of locomotion, but intralimb coordination (polar plots) was unchanged from that during the flexor‐domination mode observed in A (2). Interlimb coordination became chaotic (right polar plots), and recovered to synchrony (C, right top polar plots). The regression lines show rhythms that are symmetrical, extensor dominated, and flexor domination, without any clear effect of 5‐HT7 receptor blockade on these modes of activity. Dots on the polar plots show the timing of each lCP burst onset after the onset of activity in rCP in interlimb coordination analysis and the timing of each rTib burst onset after the onset of activity in rCP in intralimb coordination analysis. The dark lines represent the average vector, with the strength of the relationship shown as the length of the vector. The dotted lines in polar plots show the P < 0.001 (outer circle) and P < 0.05 (inner circle) significance levels of the r vector using a critical Rayleigh's value calculated for a given P value. Plots with only one dotted circle represent only P < 0.05. Linear envelopes show overlays of the rectified and filtered bursts during each period (rCP, red; rTib, black; lCP, green; lTib, blue) plotted in relation to the onset of rCP activity. The colour of the regression lines is coded according to the colour of the linear envelopes. Abbreviations: lCP/rCP, left/right common peroneal nerve; lTib/rTib, left/right tibial nerve; lCP‐rCP, polar plot analysis of interlimb coordination between burst activity recorded in both (left and right) common peroneal nerves; rTib‐rCP, polar plot analysis of intralimb coordination between burst activity recorded in right tibial and right common peroneal nerve.
We report data from six rats in which we were able to evoke hindlimb fictive locomotion and to successfully test the actions of SB269970. Stable responses could be observed for hours in these preparations, as illustrated for locomotor trials over a period of up to 3 h (Fig. 8 C). Such consistent responses to the MLR stimulus are a necessary feature of the preparation in order for reliable results to be obtained with intrathecal drug treatment effects followed by recovery over repeated trials. The mode of locomotion could be flexor dominated or extensor dominated (Bem et al. 1993; Iles & Nicolopoulos‐Stournaras, 1996; Canu et al. 2001; Juvin et al. 2007; Yakovenko et al. 2005). In each of the experiments robust intralimb coordination consisting of temporal correspondence of the onset of activity in the common peroneal nerve (innervates ankle flexors) and termination of activity in the ipsilateral tibial nerve (innervates ankle extensors) was observed. This is evident in each of the cases illustrated in Figs 8 and 9.
In contrast, variable modes of interlimb coordination occur in these preparations (Figs 8 and 9). In many cases the CPGs on the left and right sides could operate at different frequencies. A second mode of interlimb coordination was near‐synchronous activity (Figs 8 A and C, and 9 A and C) similar to galloping. The coupling between the left and right sides could change from one mode to another spontaneously. Double bursts were occasionally observed on one side in comparison to the other as well (Fig. 8 A). We recorded 183 episodes of MLR‐induced fictive locomotion in seven rats (6 tested with SB269970 and 1 control animal) prior to drug administration. In 84 episodes (46%) the nerves of both sides were rhythmically active. In 61% of these we observed left–right synchrony. In 13% there were different frequencies on the two sides, including multiple bursts on one side, and in 25% the two sides were not coordinated in any detectable way. In one episode of locomotion there was alternation of the left and right sides. The variability seen during fictive locomotion in comparison to spontaneous overground locomotion leads to the conclusion that afferent input contributes substantially to the coordination of the two hindlimbs, and the CPGs that are activated during fictive locomotion are loosely coupled, with neurons involved in synchrony predominant. A role for the control of contralateral afferent input by ipsilateral afferent feedback from stance‐phase force detectors has been demonstrated (Hayes et al. 2012).
As shown in Figs 8 and 9, the response to MLR stimulation at a low threshold site was remarkably stable and often lasted for several hours (the evoked activity in Fig. 8 C was recorded over 3 h after the drug was administered). Although the response during the course of a trial of MLR‐induced fictive locomotion could be quite variable, the patterns of activity in repeated trials were consistent, so that reliable assessment of the drug effects and recovery was possible. In Fig. 8 A and C spontaneous changes in locomotor frequency despite maintained MLR stimulation are evident.
SB269970 suppresses fictive locomotion
Intrathecal application of SB269970 consistently suppressed both spontaneous fictive locomotion and fictive locomotion evoked by MLR stimulation. The effect was apparent within 5 min of the drug administration, and lasted for over 60 min in some cases. The precise onset and termination of the effect could not be determined with accuracy, because the stimuli to the MLR were applied every 3–4 min. This suppression of locomotion was observed in each of six experiments, and differs from the observations in intact animals, where EMG activity was not completely suppressed. Figure 8 shows an example of MLR‐evoked fictive locomotion (30 μA) that was blocked by intrathecal infusion of SB269970 (60 μg in 20 μl saline). The initial response (data not shown) was a total suppression of locomotor activity that began to recover by 53 min after the drug administration. Recovery occurred in all cases described, with recovery clearly evident as early as 30 min (Fig. 9) or as late as 1 h after the drug administration. A reduction in ENG amplitude as well as slowing of the evoked rhythm was also consistently observed (Figs 8 and 9). A quantitative assessment of these effects was not possible, because the episodes of fictive locomotion that persisted in the presence of SB269970 were rare. The primary outcome of the drug application was the cessation of locomotor activity, with rather abrupt recovery and little in the way of a transition from blocked to recovery.
Effects on fictive locomotion required smaller doses of SB269970 (60 μg/20 μl) than in the intact rat (150 μg/30 μl), and the recovery time was usually much longer, so the intact rat appears to have ways of overcoming the effects of the drug that the fictive preparation does not possess. The attempts made by the conscious rat to overcome the deficits appear to have resulted in the drug's effects being obscured at lower doses.
Effects of SB269970 on coordination
To our surprise, the dramatic effects of SB269970 on coordination observed in the freely moving rats (see above) and in isolated brainstem–spinal cord preparations (Madriaga et al. 2004; Liu & Jordan, 2005; Pearlstein et al. 2005; Liu et al. 2009) were not observed when the drug was tested on MLR‐evoked fictive locomotion. The examples in Fig. 9 A, B and C show that intralimb coordination is hardly altered by the drug. In every case examined the bursts in the common peroneal nerve alternate with the burst in the tibial nerve. This occurred whether the mode of locomotion was extensor dominated (Fig. 9 A (1)) or flexor dominated (Fig. 9 A (2), B and C). Thus our hypothesis that the disruption of locomotor activity produced by SB269970 is due to actions on the coordinating interneurons responsible for flexor–extensor alternation was not confirmed.
The linear envelopes in Figs 8 and 9 provide a demonstration that regardless of whether there is extensor domination or flexor domination, either sustained or changing during the drug trial, these factors do not alter the consistent reciprocal intralimb coordination. Furthermore, no obvious change in interlimb coordination was observed after the drug, when, despite the dramatic reduction in amplitude, the alternating activity persisted on one side, with much weaker or absent locomotor activity on the other (Figs 8 and 9). Also, the frequently observed near left–right synchrony typical of galloping or bounding behaviour (Bellardita & Kiehn, 2015) was not consistently altered by SB269970 (Fig. 9).
Discussion
5‐HT7 receptor activation is required for voluntary and fictive locomotion in the adult rat
Our data provide clear confirmation of the fact that recruitment of descending 5‐HT neurons is required for voluntary locomotion in rodents, and that activation of 5‐HT7 receptors is necessary for this effect (Hochman et al. 2001; Madriaga et al. 2004; Liu & Jordan, 2005; Pearlstein et al. 2005; Gordon & Whelan, 2006; Landry et al. 2006; Jordan et al. 2008; Lapointe et al. 2008; Liu et al. 2009; Noga et al. 2009; Dunbar et al. 2010; Sławińska et al. 2013, 2014 a,b). In addition, our results show that recruitment of descending 5‐HT neurons acting through spinal 5‐HT7 receptors is necessary for fictive locomotion evoked from the MLR. The interneurons responsible for producing the locomotor rhythm are thus controlled by 5‐HT via 5‐HT7 receptors in adult as well as neonatal animals. We ruled out the possibility that constitutively active 5‐HT7 receptors account for our results by conducting preliminary experiments using adult rats with complete spinal transections, where SB269970, in the absence of descending 5‐HT fibres, had minimal effects on spinal locomotor activity (Sławińska et al. 2010).
The presence of 5‐HT7 receptors may be a defining feature of the interneurons responsible for the initiation of locomotion and control of motoneuron excitability. The likely candidates include V2a interneurons, characterized by the Chx10 transcription factor, which are capable of inducing the locomotor rhythm in neonatal mouse (Crone et al. 2008). V2a interneurons are excited by 5‐HT in mouse spinal cord, with changes in the response to 5‐HT in older animals, where 5‐HT‐induced bistable properties appear (Husch et al. 2015). 5‐HT enhancement of interneuron bistability could be the basis for the ability of SB269970 to block the initiation of locomotor activity, to reduce motoneuron output in overground and fictive locomotion, and to shorten the extensor burst in intact rats. It will be of interest to determine whether 5‐HT7 receptors are located on any of these candidates for the rhythm generating kernel producing locomotion, such as Shox2 (Dougherty et al. 2013) and Hb9 interneurons (Wilson et al. 2005). The observed increase in cycle duration suggests that the excitability of rhythm generating interneurons (McCrea & Rybak, 2008) is controlled by 5‐HT7 receptor activation during locomotion.
Influence of 5‐HT7 receptor blockade on motoneuron output
The amplitude of motoneuron discharges during overground and fictive locomotion is reduced by SB269970, and 5‐HT7 receptor protein and mRNA have been found in some motoneurons (Doly et al. 2005; Chopek et al. 2015). The reduction in EMG amplitude that we observed is clearly not due to blockade of 5‐HT7 receptors on motoneurons, however. This was demonstrated by our tests of the effects of SB269970 on reflexes, showing that motoneuron excitability is not altered by SB269970. These experiments also demonstrate that 5‐HT7 receptor blockade can prevent transmission in a reflex pathway (the response to plantar flexion in TA, Fig. 5), consistent with the suggestion that these receptors control the effects of afferent feedback in adult rats. The components of the reflex in TA that are suppressed by blockade of 5‐HT7 receptors must be produced by 5‐HT7 receptors on the afferents activated by plantar flexion of the ankle or on polysynaptic pathways from the afferents in question rather than by a change in TA motoneuron excitability. The interneurons in this pathway are unknown, but they may possess 5‐HT7 receptors. The absence of a significant reduction in the Sol reflex shows that motoneurons to this muscle are not controlled by 5‐HT7 receptors. These results suggest that the actions of SB269970 are due to effects on the spinal networks for locomotion, not to antagonism of any direct effects on motoneurons mediated by 5‐HT7 receptors. Decreased activity in motoneurons is likely to be due to reduced excitability of CPG interneurons with 5‐HT7 receptors, including those implicated in the initiation of locomotion.
A consistent feature of overground locomotion is that changes in the duration of the locomotor cycle are associated with prolongation of the extensor burst (Grillner & Dubuc, 1988; Frigon & Gossard, 2009) with little or no change in the flexor burst duration (an extensor‐dominated rhythm). However, either flexor‐dominated or extensor‐dominated rhythms are produced during fictive locomotion in rats (Bem et al. 1993; Iles & Nicolopoulos‐Stournaras, 1996; Juvin et al. 2007). The flexor‐dominated rhythms can occur spontaneously, even in the same run of MLR evoked locomotion. According to Juvin et al. (2007), ‘sensory inputs are normally responsible for imposing extensor biasing on otherwise symmetrically alternating extensor/flexor oscillators.’ Iles & Nicolopoulos‐Stournaras (1996) reported that the flexor‐dominated pattern was far more frequent in the rat. The shortening of the extensor burst produced by SB269970 during voluntary locomotion is consistent with afferent control by 5‐HT7 receptors. The extensor burst is not consistently shortened by the drug during fictive locomotion. Thus our findings show that the pattern of motoneuron activity during locomotion in adult rats is partly under the control of interneurons in sensory pathways or afferents with 5‐HT7 receptors.
The ability of 5‐HT7 receptor blockade to produce a flexor‐dominated rhythm even during overground locomotion (Fig. 3 B) suggests that the 5‐HT system controls flexor and extensor CPG output differentially. It is not clear what might account for the prolonged activity of flexor muscles compared to control, but if 5‐HT7 receptors are present on the inhibitory interneurons responsible for reciprocal inhibition of motoneurons during locomotion, the prolongation produced by SB269970 could be due to the absence of inhibitory input to flexor motoneurons or last‐order interneurons (INs) to flexors during the time when extensor activity is shortened. These findings suggest that the normal relationship between burst duration and cycle duration for both flexor and extensor muscles at the ankle is partly under the control of neural elements with 5‐HT7 receptors. The Add EMG recordings at the same time did not reveal similar changes in the burst duration–cycle duration plots due to SB269970 (Fig. 3 B), but the normal delay of Add discharge after the onset of the Sol burst during locomotion (Fig. 2 and 3 A) appears to be determined by 5‐HT7 receptors. This is an example of 5‐HT control of intralimb coordination (sometimes referred to as ‘intra‐segmental coordination’) other than merely flexor–extensor alternation. This delay has been previously described during MLR‐induced locomotion with limb movement in decerebrate rats (Nicolopoulos‐Stournaras & Iles, 1984). The delay may normally allow the extension of the ankle to proceed before hip extension, to assure proper placement of the paw on the plantar surface so weight support at the ankle is possible during the extension at the hip.
Are interneurons responsible for intra‐ and interlimb coordination controlled by 5‐HT7 receptors in the adult rat?
Disruption of coordination by 5‐HT7 receptor blockade was observed during overground locomotion in the adult rat, but not during fictive locomotion. Removal of afferent feedback during fictive locomotion is accompanied by persistent activity in the circuits controlling F‐E coordination, while these circuits are impaired by the drug during overground locomotion. This suggests that during overground voluntary locomotion the drug disrupts afferent feedback controlling the activation of F‐E coordinating interneurons. Our hypothesis that 5‐HT7 receptors control the activity of interneurons for intralimb coordination, suggested by the effects of SB269970 in neonatal animals (Liu & Jordan, 2005; Liu et al. 2009), was not supported by these results. Thus, these neurons may to be directly controlled by 5‐HT7 receptors in neonatal animals but not in adults.
The cells responsible for reciprocal inhibition of motoneurons during locomotion in mice, V1 and V2b interneurons (Zhang et al. 2014), including Ia inhibitory interneurons (Goulding et al. 2014), appear to be under the control of 5‐HT7 receptors in neonatal animals, but not in adult animals. Our results show that interneurons responsible for reciprocal inhibition during locomotion are always recruited when the CPG is activated, even in the absence of afferent feedback. It is important to determine whether these inhibitory interneurons possess 5‐HT7 receptors, as previously suggested (Jordan & Sławińska, 2011; Gackiere & Vinay, 2014) in neonatal but not in adult rats.
There are problems with basing interpretation of data obtained in the rat upon knowledge gained from mouse preparations, however. Although 5‐HT7 receptor blockade interferes with voluntary locomotion in adult mice (Liu et al. 2009), some differences between the species might be expected. The observation in mouse preparations of hyperextension produced by SB269970 (Liu et al. 2009) was not confirmed in the adult rat. Thus our previous suggestion that the presence of 5‐HT7 receptors might control the neurons or afferents involved in the onset of the flexion phase of locomotion (Liu et al. 2009) was not confirmed. There is evidence that the adult mouse recovers spontaneous stepping more readily than the adult rat (Leblond et al. 2003) after spinal cord injury, and pharmacological activation of the locomotor CPG in functionally mature mice requires dopamine and NMDA in addition to 5‐HT (Jiang et al. 1999), again suggestive of a species difference. In our experience, inducing fictive locomotion with MLR stimulation in adult mice is much more difficult than in adult rats. Furthermore, the pattern of intralimb coordination during chemically induced fictive locomotion in the adult mouse does not correspond to the pattern observed in the intact mouse. During fictive locomotion in the mouse, a brief flexor burst is followed immediately by a brief extensor burst, with a long period of inactivity between flexor bursts (Meehan et al. 2012). This was not observed during fictive locomotion in the rat, where extensor ENG activity was maintained throughout the cycle, similar to the case in overground locomotion. During treadmill locomotion in the decerebrate mouse, with intact afferent feedback, intralimb coordination is characterized by alternating bursts of activity between ankle flexor and extensor muscle groups with a mean phase of 194.8 deg (Nakanishi and Whelan, 2012), suggesting that afferents control extensor burst duration in the mouse to a greater degree than in the rat. It is also remarkable that interlimb alternation persists during fictive locomotion in the adult mouse (Meehan et al. 2012), while it is rare during adult rat fictive locomotion.
The absence of left–right alternation during fictive locomotion in the rat suggests that the interneurons responsible for such coordination are not controlled by the CPG, but rather require afferent feedback for determining the synergy that they produce. However, commissural interneurons (CINs) are known to be activated by 5‐HT (Hammar et al. 2004; Carlin et al. 2006; Abbinanti et al. 2012). It is noteworthy that the effect of 5‐HT on some CINs is developmentally regulated, with 5‐HT‐induced plateau properties appearing only in mature animals (Abbinanti et al. 2012).
During fictive locomotion in the adult rat, our data suggest that a synchrony circuit is still active despite the removal of afferent feedback. Left–right alternation is not consistently observed after the removal of afferent feedback. This is unlike the neonatal rat and mouse, where left–right alternation is accepted as a necessary component of fictive locomotion. This implies a role for afferent feedback in the recruitment of coordinating interneurons controlling left–right alternation in adult but not neonatal rats. V0D CINs are implicated in control of alternating left–right coordination at slow locomotor speeds, and ipsilateral V2a INs are thought to be necessary for left–right alternation at higher speeds in mice (Shevtsova et al. 2015). If this model is correct, then V0D and V2a INs are strongly driven by afferent input during locomotion in the adult rat, but are not strongly recruited during locomotion in the absence of sensory feedback. There appears to be a persistence of activity in CINs responsible for synchrony, so they seem to receive a more reliable input from the CPG. These interneurons are likely to include the V3 class of interneurons, which are implicated in left–right synchrony (Rabe Bernhardt et al. 2012). Any role for 5‐HT7 receptors on CINs of any kind in the adult is not revealed in our results, however.
5‐HT7 receptor‐mediated control of afferent input contributes to locomotion in the adult rat
5‐HT is known to control afferent input (Meuser et al. 2002; Chopek et al. 2013; Viguier et al. 2013; Garcia‐Ramirez et al. 2014), in addition to its ability to facilitate the induction of locomotion. There are 5‐HT7 receptors on medium size and small dorsal root ganglion cells (Pierce et al. 1996; Meuser et al. 2002; Doly et al. 2005) and on afferent terminals in the dorsal horn (Doly et al. 2005), as well as on neurons in the dorsal horn (Garraway & Hochman, 2001 a; Brenchat et al. 2010), so that presynaptic or postsynaptic inhibitory control of afferent input during locomotion by 5‐HT7 receptors does occur. The discomfort displayed by intact rats after intrathecal SB269970 in our initial experiments (see Methods) is consistent with an antinociceptive action mediated by 5‐HT7 receptors. 5‐HT control of muscle (Riddell et al. 1993; Quevedo et al. 1995; Dougherty et al. 2005) and cutaneous afferents (Meuser et al. 2002) is well documented. Gackiere and Vinay (2014) suggested that there may be 5‐HT7 receptor‐mediated control of GABAergic interneurons in the spinal cord, and this is consistent with the finding that 5‐HT7 receptors are localized on GABAergic interneurons in the spinal cord (Doly et al. 2005; Viguier et al. 2012; Lin et al. 2015), perhaps implicating 5‐HT7 receptors in presynaptic inhibition. Our previous work on 5‐HT's effects during fictive locomotion was carried out using isolated neonatal rodent spinal cord preparations (Liu & Jordan, 2005; Liu et al. 2009), but it is now clear that 5‐HT's effects can be different in neonatal and older rodents (Abbinanti et al. 2012; Husch et al. 2015).
A major difference between fictive and overground locomotion was an unexpected influence of afferent input on the control of F‐E coordination. In the intact animals (Figs 2 and 3) SB269970 impaired intralimb coordination, consistent with previous effects observed with 5‐HT7 receptor blockade in isolated neonatal rat spinal cord and adult mouse (Hochman et al. 2001; Madriaga et al. 2004; Liu & Jordan, 2005; Pearlstein et al. 2005; Landry et al. 2006; Jordan et al. 2008; Liu et al. 2009; Dunbar et al. 2010; Gackiere & Vinay, 2014). Furthermore, these data show a switch from direct neuromodulatory control of coordinating interneurons within the CPG by 5‐HT7 receptors in neonatal animals to 5‐HT7 receptor control of afferent feedback in adults. Further research is necessary to determine the nature of the afferent control by 5‐HT7 receptors during locomotion, as well as the relative contributions of 5‐HT7 receptor‐mediated control of CPG neurons and afferent feedback from the moving limb in developing and adult animals.
Additional information
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
A.M.C., H.M., U.S. and L.M.J. conceived and designed the experiments. A.M.C. coordinated and participated in experiments conducted in Warsaw; L.M.J. supervised and participated in experiments conducted in Winnipeg. Implantation of EMG electrodes and locomotor and drug trials on awake freely moving rats were performed in Warsaw by A.C.M. and H.M., who also performed EMG data analysis. Experiments in Canada were performed by U.S., L.M.J., E.C., K.S. and P.G. U.S., E.C. and L.M.J. analysed the data from fictive locomotion. All authors participated in discussion of results and wrote the paper. All authors approved the final version of the manuscript, all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by grants from the Polish National Science Centre (N N404 318040) to H.M. and by the European Union within the European Regional Development Fund POIG 01.01.02‐00‐109/09‐00 and from the Nencki Institute statutory donation to U.S. and grants from the Canadian Institutes of Health Research to L.M.J. (CIHR 115147) and P.F.G. (CIHR 84250).
Acknowledgements
The authors thank Kalan R. Gardiner, Christopher W. MacDonell, Zhicheng Zhou, Gilles Detillieux, Matt Ellis, Marek Bekisz and Aleksandra Straus for their contributions to these experiments.
Contributor Information
Anna M. Cabaj, Email: a.cabaj@nencki.gov.pl
Larry M. Jordan, Email: larry@scrc.umanitoba.ca.
References
- Abbinanti MD, Zhong G & Harris‐Warrick RM (2012). Postnatal emergence of serotonin‐induced plateau potentials in commissural interneurons of the mouse spinal cord. J Neurophysiol 108, 2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellardita C & Kiehn O (2015). Phenotypic characterization of speed‐associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25, 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem T, Orsal D & Cabelguen JM (1993). Fictive locomotion in the adult thalamic rat. Exp Brain Res 97, 301–304. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sanchez‐Arroyos R, Portillo‐Salido E, Pujol M, Montero A, Codony X, Burgueno J, Zamanillo D, Hamon M, Maldonado R & Vela JM (2010). Pharmacological activation of 5‐HT7 receptors reduces nerve injury‐induced mechanical and thermal hypersensitivity. Pain 149, 483–494. [DOI] [PubMed] [Google Scholar]
- Cabaj AM, Sławińska U, Jordan LM & Majczyński H (2014). Blocking 5‐HT2a and 5‐HT7 but not 5‐HT2c receptors impairs locomotion in intact rats. 2014 Meeting of the Australasian Neuroscience Society, # 157 Adelaide, 28–31 January 2014.
- Canu MH, Falempin M & Orsal D (2001). Fictive motor activity in rat after 14 days of hindlimb unloading. Exp Brain Res 139, 30–38. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ & Scroggs RS (1999). Serotonergic modulation of hyperpolarization‐activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol 518, 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG & Scroggs RS (2001). 5HT increases excitability of nociceptor‐like rat dorsal root ganglion neurons via cAMP‐coupled TTX‐resistant Na+ channels. J Neurophysiol 86, 241–248. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Dai Y & Jordan LM (2006). Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco‐lumbar spinal cord of the neonatal mouse. J Neurophysiol 95, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli‐Houssaini Y & Clarac F (1992). Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455, 187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopek JW, MacDonell CW, Power KE, Gardiner K & Gardiner PF (2013). Removal of supraspinal input reveals a difference in the flexor and extensor monosynaptic reflex response to quipazine independent of motoneuron excitation. J Neurophysiol 109, 2056–2063. [DOI] [PubMed] [Google Scholar]
- Chopek JW, Sheppard PC, Gardiner K & Gardiner PF (2015). Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J Neurophysiol 113, 1369–1376. [DOI] [PubMed] [Google Scholar]
- Coles SK, Iles JF & Nicolopoulos‐Stournaras S (1989). The mesencephalic centre controlling locomotion in the rat. Neuroscience 28, 149–157. [DOI] [PubMed] [Google Scholar]
- Cowley KC & Schmidt BJ (1994). A comparison of motor patterns induced by N‐methyl‐D‐aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171, 147–150. [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O & Sharma K (2008). Genetic ablation of V2a ipsilateral interneurons disrupts left‐right locomotor coordination in mammalian spinal cord. Neuron 60, 70–83. [DOI] [PubMed] [Google Scholar]
- Deacon RM & Rawlins JN (1996). Equithesin without chloral hydrate as an anaesthetic for rats. Psychopharmacology 124, 288–290. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Verge D & Conrath M (2005). Pre‐ and postsynaptic localization of the 5‐HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol 490, 256–269. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Bannatyne BA, Jankowska E, Krutki P & Maxwell DJ (2005). Membrane receptors involved in modulation of responses of spinal dorsal horn interneurons evoked by feline group II muscle afferents. J Neurosci 25, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM & Kiehn O (2013). Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80, 920–933. [DOI] [PubMed] [Google Scholar]
- Dunbar MJ, Tran MA & Whelan PJ (2010). Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol 588, 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A & Gossard JP (2009). Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat. J Physiol 587, 4617–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Johnson MD & Heckman CJ (2011). Altered activation patterns by triceps surae stretch reflex pathways in acute and chronic spinal cord injury. J Neurophysiol 106, 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackiere F & Vinay L (2014). Serotonergic modulation of post‐synaptic inhibition and locomotor alternating pattern in the spinal cord. Front Neural Circuits 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Ramirez DL, Calvo JR, Hochman S & Quevedo JN (2014). Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9, e89999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway SM & Hochman S (2001. a). Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol 86, 2183–2194. [DOI] [PubMed] [Google Scholar]
- Garraway SM & Hochman S (2001. b). Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br J Pharmacol 132, 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IT & Whelan PJ (2006). Monoaminergic control of cauda‐equina‐evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol 96, 3122–3129. [DOI] [PubMed] [Google Scholar]
- Goulding M, Bourane S, Garcia‐Campmany L, Dalet A & Koch S (2014). Inhibition downunder: an update from the spinal cord. Curr Opin Neurobiol 26, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S & Dubuc R (1988). Control of locomotion in vertebrates: spinal and supraspinal mechanisms. Adv Neurol 47, 425–453. [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA & Jankowska E (2004). The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci 19, 1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chang YH & Hochman S (2012). Stance‐phase force on the opposite limb dictates swing‐phase afferent presynaptic inhibition during locomotion. J Neurophysiol 107, 3168–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW & Shay BL (2001). 5‐HT receptors and the neuromodulatory control of spinal cord function In Motor Neurobiology of the Spinal Cord, ed. Cope TC, pp 48–87. CRC Press, New York. [Google Scholar]
- Husch A, Dietz SB, Hong DN & Harris‐Warrick RM (2015). Adult spinal V2a interneurons show increased excitability and serotonin‐dependent bistability. J Neurophysiol 113, 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF & Nicolopoulos‐Stournaras S (1996). Fictive locomotion in the adult decerebrate rat. Exp Brain Res 109, 393–398. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B & Heden CH (2000). Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci 12, 701–714. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP & Brownstone RM (1999). An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816, 493–499. [DOI] [PubMed] [Google Scholar]
- Jordan LM & Schmidt BJ (2002). Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res 137, 125–139. [DOI] [PubMed] [Google Scholar]
- Jordan LM & Sławińska U (2011). Chapter 12–modulation of rhythmic movement: control of coordination. Prog Brain Res 188, 181–195. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T & Pearson KG (2008). Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57, 183–191. [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J & Morin D (2007). Locomotor rhythmogenesis in the isolated rat spinal cord: a phase‐coupled set of symmetrical flexion extension oscillators. J Physiol 583, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe NP & Guertin PA (2008). Synergistic effects of D1/5 and 5‐HT1A/7 receptor agonists on locomotor movement induction in complete spinal cord‐transected mice. J Neurophysiol 100, 160–168. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB & Guertin PA (2006). Contribution of spinal 5‐HT1A and 5‐HT7 receptors to locomotor‐like movement induced by 8‐OH‐DPAT in spinal cord‐transected mice. Eur J Neurosci 24, 535–546. [DOI] [PubMed] [Google Scholar]
- Leblond H, L'Esperance M, Orsal D & Rossignol S (2003). Treadmill locomotion in the intact and spinal mouse. J Neurosci 23, 11411–11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Heo BH, Kim WM, Kim YC & Yoon MH (2015). Antiallodynic effect of tianeptine via modulation of the 5‐HT7 receptor of GABAergic interneurons in the spinal cord of neuropathic rats. Neurosci Lett 598, 91–95. [DOI] [PubMed] [Google Scholar]
- Liu J & Jordan LM (2005). Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor‐like activity involving spinal 5‐HT7 and 5‐HT2A receptors. J Neurophysiol 94, 1392–1404. [DOI] [PubMed] [Google Scholar]
- Liu J, Akay T, Hedlund PB, Pearson KG & Jordan LM (2009). Spinal 5‐HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5‐HT7 receptor knockout mice. J Neurophysiol 102, 337–348. [DOI] [PubMed] [Google Scholar]
- MacDonell CW, Power KE, Chopek JW, Gardiner KR & Gardiner PF (2015). Extensor motoneurone properties are altered immediately before and during fictive locomotion in the adult decerebrate rat. J Physiol 593, 2327–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ & Whelan PJ (2004). Modulation of locomotor activity by multiple 5‐HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92, 1566–1576. [DOI] [PubMed] [Google Scholar]
- Majczyński H, Cabaj A & Górska T (2005). Intrathecal application of cyproheptadine impairs locomotion in intact rats. Neurosci Lett 381, 16–20. [DOI] [PubMed] [Google Scholar]
- Majczyński H, Maleszak K, Górska T & Sławińska U (2007). Comparison of two methods for quantitative assessment of unrestrained locomotion in the rat. J Neurosci Methods 163, 197–207. [DOI] [PubMed] [Google Scholar]
- McCrea DA & Rybak IA (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CF, Grondahl L, Nielsen JB & Hultborn H (2012). Fictive locomotion in the adult decerebrate and spinal mouse in vivo. J Physiol 590, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Gabriel A, Xie GX, Lim KJ & Pierce Palmer P (2002). 5‐HT7 receptors are involved in mediating 5‐HT‐induced activation of rat primary afferent neurons. Life Sci 71, 2279–2289. [DOI] [PubMed] [Google Scholar]
- Millan MJ (2002). Descending control of pain. Prog Neurobiol 66, 355–474. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F & Nakajima K (2001). Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol 87, 25–40. [PubMed] [Google Scholar]
- Nakanishi ST & Whelan PJ (2012). A decerebrate adult mouse model for examining the sensorimotor control of locomotion. J Neurophysiol 107, 500–515. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos‐Stournaras S & Iles JF (1984). Hindlimb muscle activity during locomotion in the rat (Rattus norvegicus) (Rodentia: Murydae). J Zool (Lond) 203, 427–440. [Google Scholar]
- Noga BR, Johnson DM, Riesgo MI & Pinzon A (2009). Locomotor‐activated neurons of the cat. I. Serotonergic innervation and co‐localization of 5‐HT7, 5‐HT2A, and 5‐HT1A receptors in the thoraco‐lumbar spinal cord. J Neurophysiol 102, 1560–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C (2007). The Rat Brain in Stereotaxic Coordinates. Academic Press, Elsevier, San Diego. [Google Scholar]
- Pearlstein E, Ben Mabrouk F, Pflieger JF & Vinay L (2005). Serotonin refines the locomotor‐related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci 21, 1338–1346. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD & Peroutka SJ (1996). 5‐Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience 70, 553–559. [DOI] [PubMed] [Google Scholar]
- Pratt CA & Jordan LM (1987). Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol 57, 56–71. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Jimenez I & Rudomin P (1995). Raphe magnus and reticulospinal actions on primary afferent depolarization of group I muscle afferents in the cat. J Physiol 482, 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe Bernhardt N, Memic F, Gezelius H, Thiebes AL, Vallstedt A & Kullander K (2012). DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev Biol 366, 279–289. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E & Eide E (1993). Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol 461, 723–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ & Jordan LM (2000). The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53, 689–710. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Talpalar AE, Markin SN, Harris‐Warrick RM, Kiehn O & Rybak IA (2015). Organization of left–right coordination of neuronal activity in the mammalian spinal cord: Insights from computational modelling. J Physiol 593, 2403–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV & Orlovskii GN (1967). [Structures of the brain stem responsible for evoked locomotion]. Fiziol Zh SSSR Im I M Sechenova 53, 1125–1132. [PubMed] [Google Scholar]
- Skinner RD & Garcia‐Rill E (1984). The mesencephalic locomotor region (MLR) in the rat. Brain Res 323, 385–389. [DOI] [PubMed] [Google Scholar]
- Sławińska U, Jordan LM, Cabaj AM, Burger B, Fabczak H & Majczyński H (2012). Locomotion of intact adult rats is controlled by 5‐HT2A and 5‐HT7 but not 5‐HT2C receptors. Programme No. 85.09, Neuroscience 2012 Meeting Planner. Society for Neuroscience, Washington.
- Sławińska U & Kasicki S (2002). Altered electromyographic activity pattern of rat soleus muscle transposed into the bed of antagonist muscle. J Neurosci 22, 5808–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska U, Majczyński H & Djavadian R (2000). Recovery of hindlimb motor functions after spinal cord transection is enhanced by grafts of the embryonic raphe nuclei. Exp Brain Res 132, 27–38. [DOI] [PubMed] [Google Scholar]
- Sławińska U, Majczyński H, Kisielnicka E & Jordan LM (2010). Upright bipedal locomotion in rat: A suitable model for investigation of interventions after SCI? Programme No. 259.17, Neuroscience 2010 Meeting Planner. Society for Neuroscience, Washington.
- Sławińska U, Miazga K & Jordan LM (2014. a). The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol Exp (Wars) 74, 172–187. [DOI] [PubMed] [Google Scholar]
- Sławińska U, Miazga K & Jordan LM (2014. b). 5‐HT2 and 5‐HT7 receptor agonists facilitate plantar stepping in chronic spinal rats through actions on different populations of spinal neurons. Front Neural Circuits 8, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska U, Majczyński H, Dai Y & Jordan LM (2012). The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol 590, 1721–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska U, Miazga K, Cabaj AM, Leszczyńska AN, Majczynski H, Nagy JI & Jordan LM (2013). Grafting of fetal brainstem 5‐HT neurons into the sublesional spinal cord of paraplegic rats restores coordinated hindlimb locomotion. Exp Neurol 247, 572–581. [DOI] [PubMed] [Google Scholar]
- Steeves JD & Jordan LM (1984). Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res 307, 263–276. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Chiba R, Nozu T & Okumura T (2016). Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm (Vienna) 123, 695–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Tomita N & Yano M (2008). Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol 255 Suppl 4, 19–29. [DOI] [PubMed] [Google Scholar]
- Viguier F, Michot B, Hamon M & Bourgoin S (2013). Multiple roles of serotonin in pain control mechanisms–implications of 5‐HT7 and other 5‐HT receptor types. Eur J Pharmacol 716, 8–16. [DOI] [PubMed] [Google Scholar]
- Viguier F, Michot B, Kayser V, Bernard JF, Vela JM, Hamon M & Bourgoin S (2012). GABA, but not opioids, mediates the anti‐hyperalgesic effects of 5‐HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology 63, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM & Brownstone RM (2005). Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 25, 5710–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S, McCrea DA, Stecina K & Prochazka A (2005). Control of locomotor cycle durations. J Neurophysiol 94, 1057–1065. [DOI] [PubMed] [Google Scholar]
- Zar JH (2010). Biostatistical Analysis, 5th edn, chapters 26, 27 pp. 605–668. Prentice Hall, Upper Saddle River. [Google Scholar]
- Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E & Goulding M (2014). V1 and V2b interneurons secure the alternating flexor‐extensor motor activity mice require for limbed locomotion. Neuron 82, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]


