Abstract
Key points
Physical inactivity, which drastically increases with advancing age, is associated with numerous chronic diseases.
The nucleus accumbens (the pleasure and reward ‘hub’ in the brain) influences wheel running behaviour in rodents.
RNA‐sequencing and subsequent bioinformatics analysis led us to hypothesize a potential relationship between the regulation of dendritic spine density, the molecules involved in synaptic transmission, and age‐related reductions in wheel running. Upon completion of follow‐up studies, we developed the working model that synaptic plasticity in the nucleus accumbens is central to age‐related changes in voluntary running.
Testing this hypothesis, inhibition of Cdk5 (comprising a molecule central to the processes described above) in the nucleus accumbens reduced wheel running.
The results of the present study show that reductions in synaptic transmission and Cdk5 function are related to decreases in voluntary running behaviour and provide guidance for understanding the neural mechanisms that underlie age‐dependent reductions in the motivation to be physically active.
Abstract
Increases in age are often associated with reduced levels of physical activity, which, in turn, associates with the development of numerous chronic diseases. We aimed to assess molecular differences in the nucleus accumbens (NAc) (a specific brain nucleus postulated to influence rewarding behaviour) with respect to wheel running and sedentary female Wistar rats at 8 and 14 weeks of age. RNA‐sequencing was used to interrogate transcriptomic changes between 8‐ and 14‐week‐old wheel running rats, and select transcripts were later analysed by quantitative RT‐PCR in age‐matched sedentary rats. Voluntary wheel running was greatest at 8 weeks and had significantly decreased by 12 weeks. From 619 differentially expressed mRNAs, bioinformatics suggested that cAMP‐mediated signalling, dopamine‐ and cAMP‐regulated neuronal phosphoprotein of 32 kDa feedback, and synaptic plasticity were greater in 8‐ vs. 14‐week‐old rats. In depth analysis of these networks showed significant (∼20–30%; P < 0.05) decreases in cell adhesion molecule (Cadm)4 and p39 mRNAs, as well as their proteins from 8 to 14 weeks of age in running and sedentary rats. Furthermore, Cadm4, cyclin‐dependent kinase 5 (Cdk5) and p39 mRNAs were significantly correlated with voluntary running distance. Analysis of dendritic spine density in the NAc showed that wheel access increased spine density (P < 0.001), whereas spine density was lower in 14‐ vs. 8‐week‐old sedentary rats (P = 0.03). Intriguingly, intra‐NAc injection of the Cdk5 inhibitor roscovitine, dose‐dependently decreased wheel running. Collectively, these experiments suggest that an age‐dependent loss in synaptic function and Cdk5/p39 activity in the NAc may be partially responsible for age‐related declines in voluntary running behaviour.
Keywords: Cdk5, nucleus accumbens, physical activity, reward, synapse, wheel running
Key points
Physical inactivity, which drastically increases with advancing age, is associated with numerous chronic diseases.
The nucleus accumbens (the pleasure and reward ‘hub’ in the brain) influences wheel running behaviour in rodents.
RNA‐sequencing and subsequent bioinformatics analysis led us to hypothesize a potential relationship between the regulation of dendritic spine density, the molecules involved in synaptic transmission, and age‐related reductions in wheel running. Upon completion of follow‐up studies, we developed the working model that synaptic plasticity in the nucleus accumbens is central to age‐related changes in voluntary running.
Testing this hypothesis, inhibition of Cdk5 (comprising a molecule central to the processes described above) in the nucleus accumbens reduced wheel running.
The results of the present study show that reductions in synaptic transmission and Cdk5 function are related to decreases in voluntary running behaviour and provide guidance for understanding the neural mechanisms that underlie age‐dependent reductions in the motivation to be physically active.
Abbreviations
- Cadm
cell adhesion molecule
- Cdk5
cyclin‐dependent kinase 5
- Darpp‐32
dopamine‐ and cAMP‐regulated neuronal phosphoprotein of 32 kDa
- Gad
glutamic acid decarboxylase
- GO
Gene Ontology
- IPA
ingenuity pathway analysis
- MSN
medium spiny neuron
- Nac
nucleus accumbens
- RPKM
reads per kilobase per million mapped reads
- RNA‐seq
RNA‐ sequencing
- TBST
Tris‐buffered saline with Tween 20
Introduction
Physical inactivity has reached pandemic levels in the USA and rest of the developed world. Strong evidence shows that physical inactivity is associated with 35 chronic diseases, including major non‐communicable diseases, such as type 2 diabetes and coronary heart disease, as well as premature mortality (Booth et al. 2012). Strikingly, the World Health Organization has declared physical inactivity as the fourth leading risk factor for death worldwide, being responsible for ∼6% of the deaths worldwide in 2008 (Organization, 2010; Lee et al. 2012).
Several studies demonstrate that physical activity levels first fall at young ages. Troiano et al. (2008) reported a ∼34% decline in physical activity between 6–11 and 12–17 years of age. A similar report observed a 40% decline in time spent performing moderate–vigorous physical activity in grade school children (Trost et al. 2002). In one analysis of 3700 US youths from the age of 6 to 11 years old, the minutes of physical activity in girls and boys dropped by ∼67% and 60%, respectively (Wolff‐Hughes et al. 2014). Taken together, these reports show that by ∼9–11 years of age, 50% of US youths are not undertaking sufficient daily physical activities for health according to US Physical Activity Guidelines.
Findings from rodent studies provide similar results. Jakubczak (1969) demonstrated that declines in free wheel running activity begin early in the life of rats (66 days of age). In addition, our laboratory has shown voluntary wheel running initially declines at ∼8 weeks of age (Ruegsegger et al. 2016). Interestingly, increases in age are associated with declining levels of physical activity in Caenorhabditis elegans (Herndon et al. 2002; Kirkwood & Finch, 2002), flies (Marden et al. 2003) and dogs (Siwak et al. 2003), suggesting that the decrease in physical activity with ageing is fundamental to many species. Thus, it is imperative to understand the neuromolecular mechanisms that control the maturation‐associated decline in physical activity motivation.
Studies in animals and humans estimate the genetic component for physical inactivity to be between 20% and 80% (Festing, 1977; Kaprio et al. 1981; Lauderdale et al. 1997; Lerman et al. 2002; Lightfoot et al. 2004; Lightfoot et al. 2008) and analysis from twins suggests that 31% of sedentary behaviour is heritable (den Hoed et al. 2013).
Substantial evidence suggests the mesolimbic dopaminergic pathway, specifically the nucleus accumbens (NAc), plays a significant role in determining voluntary running behaviour (Knab et al. 2009; Knab & Lightfoot, 2010; Knab et al. 2012). Subsequent to its characterization as the neural interface between the limbic ‘motivation’ and motor systems (Mogenson et al. 1980), the NAc, as well as its associated circuitry, has been shown to mediate many motivating and rewarding behaviours. Furthermore, a loss of dopaminergic action has been postulated to drive age‐related declines in physical activity in humans (Ingram, 2000).
In the present study, we aimed to examine the NAc transcriptome of wheel running rats at 8 and 14 weeks of age to identify the transcripts associated with the initial age‐related decline in voluntary physical activity. This ‘omics’ approach was used to generate testable hypotheses by which possible NAc features may be contributing to running motivation. Based on RNA‐sequencing (RNA‐seq) and bioinformatics analysis suggesting that NAc neuronal function decreases from 8 to 14 weeks, follow‐up studies in sedentary and wheel running rats were conducted to test this hypothesis. Additionally, in light of our RNA‐seq and initial follow‐up experiments, the Cdk5 inhibitor roscovitine was infused into the NAc to assess the role that it may play in determining wheel running motivation.
Methods
Experimental animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Missouri and complied with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The investigators understand the ethical principles under which the Journal of Physiology operates and the work presented complies with the animal ethics checklist. Female, Wistar rats were used in this study (Charles River, Raleigh, NC, USA). Thus, a limitation of the present study is the inability to analyse sex differences. We employed female rats because females usually run further than males (Pitts, 1984; Jones et al. 1990). Further rationale for the use of female rats is their body mass plateau, minimizing the effect of continued body growth in male rats, and our use of female rats balances the predominance of literature utilizing male rats.
Rats were maintained under a 12:12 h light/dark cycle at 21–22°C, with food (Formulab Diet 5008, Purina) and water provided ad libitum throughout all experiments.
Experiment 1: Analysis of voluntary running behaviour
Female, Wistar rats were weaned at 21 days of age, and were provided with access to a voluntary‐running wheel beginning at 5 weeks of age (circumference of 1.062 m) (Tecniplast 2154; Tecniplast, Buguggiate, Italy), and running distance and time were monitored using Sigma Sport BC 800 bicycle computers (Cherry Creek Cyclery, Foster Falls, VA, USA). Evidence from our laboratory suggested that voluntary wheel running peaks at 8 weeks of age (Ruegsegger et al. 2016). Therefore, to assess the hypothesized neuromolecular‐reshaping that may regulate the decrease in wheel running behaviour at this age, we killed rats at either: (i) 8 weeks of age (8 weeks; n = 7) or (ii) 14 weeks of age (14 weeks; n = 5). We selected 14 weeks of age, after we noted a significant drop in running distance at 12 weeks of age. NAc samples from these rats were submitted for RNA‐seq and protein analysis. Given that rats in the 14‐week‐old group ran for 6 weeks more than rats in the 8‐week‐old group, it is just as probable that differences between 8 and 14 weeks are a result of duration‐dependent effects of chronic exercise rather than age or nightly running distance. However, our objective was to associate molecular differences at the time when wheel running first declines during the life course; thus, approaches to match exercise duration were not feasible. As a result of financial limitations and because our primary objective was to identify mRNAs associated with changes in wheel running behaviour, we could only perform RNA‐seq on wheel running rats. Additionally, separate groups of sedentary rats were killed at 8 weeks (n = 8) and 14 weeks (n = 8) to assess mRNA and protein levels in target molecules identified by RNA‐seq independent of voluntary wheel running. Thus, a limitation of the present study is that data for many comparisons were obtained from different methods and thus are not statistically compared.
At each experimental time point, rats were killed between 17.00 and 19.00 h, which is up to 2 h prior to the dark cycle, with carbon dioxide asphyxiation. This point of death was chosen as a basal observational time‐point to avoid acute running‐induced differences in mRNA expression that probably exist upon the onset of the dark cycle. Similarly, an interesting characteristic of females is the presence of the 4‐day oestrous cycle influencing voluntary running distance, with peak running in the 4‐day running rhythm occurring at pro‐oestrus (Anantharaman‐Barr & Decombaz, 1989). Therefore, to minimize the effects of cycling hormones, rats were killed on the night of pro‐oestrus as determined by the peak running night in the 4‐day running rhythm or vaginal cytology.
NAc RNA isolation and cDNA library preparation for RNA‐seq
At the time of death, brains were quickly removed and NAc tissue was extracted using a 2 mm punch tool and brain sectioning apparatus (Braintree Scientific, Braintree, MA, USA). Tissue plugs from 2 mm thick coronal brain slices, which were identified as NAc per a rat brain atlas (Paxinos & Watson, 1998), were stored at −80°C until processing. An objective of the present study was to compare the RNA‐seq results with a previous RNA‐seq experiment analysing the NAc of rats selectively bred for high and low motivation for wheel running (Roberts et al. 2014), which analysed both the NAc shell and core. Thus, a limitation of our RNA‐seq data is the lack of an explicit distinction between the NAc shell and core. During tissue processing, samples were lysed in NucleoSpin XS lysis (Macherey‐Nagel, Bethlehem, PA, USA) buffer using a high‐speed shaking apparatus (Tissuelyser, LT; Quiagen, Valencia, CA, USA) with RNase‐free stainless steel beads. RNA was subsequently extracted using the NucleoSpin XS RNA extraction kit in accordance with the manufacturer's instructions (Macherey‐Nagel, Bethlehem, PA, USA). RNA was eluted in 40 μl of RNase‐free water. The integrity of each sample was confirmed using BioAnalyzer 2100 automated electrophoresis system (Bio‐Rad, Hercules, CA, USA) prior to cDNA library construction.
cDNA library preparation was performed at the University of Missouri DNA Core in accordance with the manufacturer's instructions and reagents supplied in the TruSeq RNA sample preparation kit v2 (Illumina, Inc., San Diego, CA, USA), as described previously (Roberts et al. 2014).
Illumina sequencing and statistical analysis of RNA‐seq data
The University of Missouri DNA Core performed all RNA‐seq procedures, as described in detail elsewhere (Rustemeyer et al. 2011). Briefly, following cDNA library construction, samples were loaded into flow cells where clusters of each oligo were replicated. Following this procedure, flow cells were placed in the sequencer and fluorescence labelled bases were attached to the complementary bases of each sequence. The Illumina Genome Analyzer recorded 50 bp reads. Reads were trimmed to ensure adaptor sequence removal and tiled to a custom reference library that had been modified and updated from the publically available rattus norvigecus NCBI library (Roberts et al. 2014) using NextGENe, version 2.4.1 (SoftGenetics, State College, PA, USA).
mRNA expression patterns were analysed for annotated genes using reads per kilobase per million mapped reads (RPKM) values. To examine mRNA expression, differences associated with the decreased wheel running behaviour, our analysis compared the transcriptomes of 8‐ and 14‐week‐old wheel running rats. Data processing and statistical analyses were performed using Excel, version 2013 (Microsoft Corp., Redmond, WA, USA). We implemented the following filters based on previous studies (Heruth et al. 2012; Song et al. 2012; Zhang et al. 2012; Roberts et al. 2013): unique mRNA sequences with counts per million reads minus 2 SD > 0, RPKM >1, fold‐change value of > ±1.2‐fold, and a false discovery rate <0.05. Additionally, mRNAs input into bioinformatics software had a Student's t test (P < 0.05). Although a ±1.2‐fold cut‐off may be considered liberal compared to other recent RNA‐seq publications, which have used a 1.5–2.0 fold‐change cut‐off (Heruth et al. 2012; Song et al. 2012; Zhang et al. 2012), we contend that subtle mRNA differences within the NAc of 8‐ and 14‐week‐old rats exist given that (i) our model is a physiological, polygenic, in vivo model whereby rats are being observed in an unperturbed state and (ii) only 67 and three transcripts differed between groups at ±1.5 and ±2.0‐fold change thresholds, respectively. An overview of our filtering process is shown in Fig. 1 A.
Figure 1. Characteristics of RNA‐seq dataset and transcript filtering.
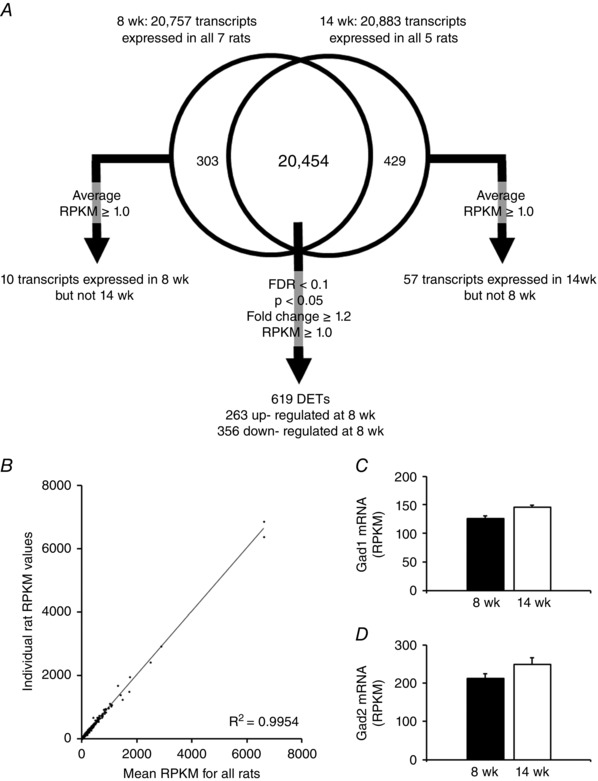
A, overview of the filtering process used to generate differentially expressed transcripts between 8‐ and 14‐week‐old wheel running rats. For each group, a transcript was omitted if it was not a known transcript. B, RNA‐seq data were highly reliable as demonstrated by the high correlation (r = 0.995) of RPKM values from a single rat compared to RPKM average values from all rats. Both 8‐ and 14‐week‐old groups expressed high amounts of Grd1 (C) and Grd2 (D), which is indicative of NAc MSNs. FDR, false discovery rate; DET, differentially expressed transcripts.
Our approach in the present study was to identify differentially expressed transcripts at time points that were at and after the observed peak lifetime, nightly voluntary wheel running distance, aiming to determine initial transcriptomic changes that may contribute to decreases in running phenotype during the early life course. Therefore, all transcripts were correlated with running distance to further explore whether a given gene was associated with running behaviour (r > ±0.70). Although correlation does not represent causation, a high correlation between the expression of a transcript and running distance could signify: (i) the transcript of interest being a contributor to running motivation or (ii) the transcript of interest being altered by attenuated running volumes, with such indications warranting future interrogation.
Bioinformatics analysis of RNA‐seq data
Ingenuity Pathway Analysis (IPA; Ingenuity Systems Inc., Redwood, CA, USA) was used to examine gene networks and physiological system functions in the NAc that differed between 8‐ and 14‐week‐old wheel running rats. In addition, the Gene Ontology (GO) Consortium database (http://www.geneontology.org) was used to examine biological, cellular, and molecular functions differing between the 8‐ and 14‐week‐old wheel running rats.
qRT‐PCR for RNA‐seq validation and follow‐up analysis in sedentary rats
Gene primers were constructed using PrimerExpress, version 3.0 (Applied Biosystems) and efficiency curves were produced for all primers. Primer efficiencies ranged between 90% and 110% for all genes. Twenty‐five nanograms of cDNA from each sample were assayed in duplicate for each of the target genes shown in Table 1 using SYBR Green Mastermix (Applied Biosystems, Carlsbad, CA, USA). mRNA expression values were quantified using the 2ΔΔCt method, whereby ΔCT = 18S Ct–gene of interest Ct.
Table 1.
Primer sequences for gene expression analysed by qRT‐PCR
| Gene | Forward (5′‐ to 3′) | Reverse (5′‐ to 3′) | Accession number |
|---|---|---|---|
| 18S | GCCGCTAGAGGTGAAATTCTTG | CATTCTTGGCAAATGCTTTCG | NR_046237.1 |
| Adcy1 | GAAGTCCACCGACTGCTGAA | TAGCATCTCTCCCTTGCCCT | NM_001107239.1 |
| Birc5 | TGAAGGGAGGGTTGTGCAAG | ACCAACACCTACACATGGGC | NM_022274.1 |
| Cadm4 | CTGAGATCACCTGCCGTCTG | GGCTGGGTTCTGAATGACGA | NM_001047107.1 |
| Cdk5 | TCTGTCACAGCCGTAACGTG | CAGCGGACTGGGATACCAAA | NM_080885.1 |
| Cdk5r2 (p39) | CCGCGGTGTCTGGATAAACT | CAGACGGAAAGGGTGAACGA | NM_001109309.1 |
| Znf238 | GATGATGACCCCAGAGAGCG | CACAGGGGGCACATGAAGAT | NM_022678.1 |
Western blotting confirmation of RNA‐seq targets up‐regulated in 8‐ vs. 14‐week‐old rats
From the same rats in which samples were submitted for RNA‐seq (n = 5–7 per group), and age‐matched sedentary rats (n = 8 per group), ∼5–10 mg of NAc tissue was homogenized on ice in RIPA buffer [50 mm Tris‐HCl (pH 8.0), 150 mm NaCl, 1% NP‐40, 0.5% sodium deoxycholate, 1% SDS, 1 × protease inhibitor cocktail] using a Tissuelyser at 25 Hz for 1 min. The homogenate was centrifuged at 12,000 g for 10 min and the resultant supernatant was obtained. The BCA assay (Pierce Biotechnology, Rockford, IL) was used to determine protein concentrations. Next, 20 μg of protein in loading buffer was loaded onto 10 or 18% SDS‐PAGE gels. Proteins were transferred onto nitrocellulose membranes and incubated with Ponceau S (Sigma, St Louis, MO, USA) to confirm equal loading in all lanes. Primary antibodies [rabbit polyclonal for Cadm4 (dilution 1:1000; Abcam, Cambridge, MA, USA), p39 (dilution 1:1000; Cell Signaling Technology, Beverly, MA, USA), p35/25 (dlution 1:1000; Cell Signaling), and dopamine‐ and cAMP‐regulated neuronal phosphoprotein of 32 kDa (Darpp‐32) (dilution 1:1000; Cell Signaling)] were diluted in Tris‐buffered saline with Tween 20 (TBST) with 5% BSA and applied to membranes overnight at 4°C. HRP‐conjugated secondary antibody (dilution 1:1000; Cell Signaling) was then applied to membranes for 1 h at room temperature in TBST with 5% non‐fat milk. Prior to exposure, ECL substrate (Pierce Biotechnology, Rockford, IL, USA) was applied for 2 min. A Kodak 4000R Imager and Molecular Imagery Software (Kodak Molecular Imaging Systems, New Haven, CT, USA) were used to obtain band densitometry.
Golgi staining for medium spiny neuron (MSN) dendritic spine determination
For rapid Golgi staining, 8‐ and 14‐week‐old sedentary and wheel running rats (n = 6 per group) were killed using carbon dioxide asphyxiation and brains were rapidly removed from the skull and briefly rinsed in distilled water. Golgi staining was performed using the FD rapid GolgiStain kit (FD NeuroTechnologies, Inc., Columbia, MD, USA) in accordance with the manufacturer's instructions. Coronal sections (200 μm) were cut with a vibrotome (Vibratome 3000; Vibratome Company Inc., St Louis, MO, USA). Slices were mounted on 3% gelatin‐coated microscope slides (Fisher Scientific Co., Pittsburgh, PA, USA). Spine density was assessed on 10–12 dendritic segments (sampled from the entire medial NAc shell and similar rostrocaudal levels of the NAc core) per rat, sampled equally between hemispheres, resulting in the analysis of 70–72 segments per treatment condition. These dendritic segments were collected from two to three neurons per hemisphere. We restricted our study to dendritic segments of second‐order branches, and spine density was assessed by manual labelling spines ∼50 μm from the soma. Spine density was counted along the entire length of the branch and the results were expressed as spine/10 μm. Mean spine numbers per dendritic length were calculated from measurements on two‐dimensional digital images, and thus probably represent underestimates. Distinct spine morphologies were manually scored as thin, stubby or mushroom types on dendrite segments as defined previously (Peters & Kaiserman‐Abramof, 1970). For counting, images were coded and counted by a blinded individual. Sections were visualized using a BX60 photomicroscope (Olympus, Melville, NY, USA) at an overall magnification of 1000× and photographed with a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). ImageJ Fiji software (National Institutes of Health, Bethesda, MD, USA) was used to assess dendritic spine density.
Experiment 2: Follow‐up intra‐accumbens cannula injection
Based upon the results of the Experiment 1, an additional set of female, Wistar rats were used to determine whether intra‐accumbens injection of the Cdk5 inhibitor roscovitine (APExBIO, Houston, TX, USA) influences voluntary running behaviour. Rats were weaned at 21 days of age, and provided access to voluntary running wheels beginning at 10 weeks of age. After acclimating to the running wheels, brain cannula were surgically inserted at 13 weeks of age, and injections began at ∼14 weeks of age. This age was chosen based on preliminary data suggesting running behaviour is level between 14 and 18 weeks of age (data not shown), allowing for a ‘cleaner’ experimental interpretation free of natural age‐related declines in voluntary running. Additionally, although the results from Experiment 1 suggest that mRNAs and proteins central to Cdk5 function decrease from 8 to 14 weeks, expression levels are still robust at 14–15 weeks, allowing for pharmacological inhibition of Cdk5 effects on running to be determined.
Surgery and injection protocol
On the day of the surgery, animals (250–275 g) were anaesthetized with an i.p. injection of a ketamine (87 mg kg−1) and xylazine (13 mg kg−1) mixture. The surgical procedures used have been described previously (Roberts et al. 2012; Ruegsegger et al. 2015). Briefly, 10 mm, 23 gauge guide cannulae were bilaterally positioned 2.5 mm above the NAc core using the coordinates (in mm relative to Bregma): anteroposterior 1.30, mediolateral ±1.85 mm and dorsoventral –4.63 mm (Whishaw et al. 1977). We chose to target the NAc core given previous findings suggesting that the core (and not shell) is associated with wheel running behaviour (Werme et al. 2002). Following surgery, animals were warmed on a 32°C heating pad for 2 h and topical Neosporin was applied around the surgical area. Following recovery from surgery, rats were placed back into their home cages with running wheels and monitored for 7 days to ensure that running patterns returned to pre‐surgical values. Note that the running pattern of each rat was monitored prior to surgery to determine a voluntary running periodicity and to establish anticipated high running nights for drug injections.
The injection protocol is shown in Fig. 2 A. Because of the cyclic effects of the oestrous cycle on running behaviour, we chose to take measurements during the night of pro‐oestrus, which is the night of peak running distance (Anantharaman‐Barr & Decombaz, 1989). At around 1 week after NAc core cannulation, bilateral intra‐NAc infusion of 0.5 μl of vehicle (PBS/50% DMSO) was performed to acclimatize rats to the injection protocol. An additional vehicle injection was performed 4 days later. This injection served as a baseline for all rats. Four days after baseline injection, rats were bilaterally injected with either vehicle (n = 8), or roscovitine: 40 nmol/0.5 μl (n = 8) or 80 nmol/0.5 μl (n = 9). Drug concentrations were determined from previous studies suggesting that intra‐NAc roscovitine injection influences locomotor activity (Taylor et al. 2007; Massart et al. 2015). This injection scheme was maintained for 5 days. On the ‘baseline’ and first and fifth day of continuous vehicle or roscovitine injection, running distance (km) was recorded in 30 min increments up to 120 min post‐injection. Injections took place immediately prior to the onset of the dark cycle. We chose this timeframe because running patterns are more homogeneous during this period compared to later time periods. Unpublished observations (GN Ruegsegger, RG Toedebusch, TE Childs, KB Grigsby & FW Booth) suggest that rats immediately begin voluntary wheel running upon the start of the dark cycle compared to an intermittent and random running pattern later in the dark cycle.
Figure 2. NAc roscovitine injection.
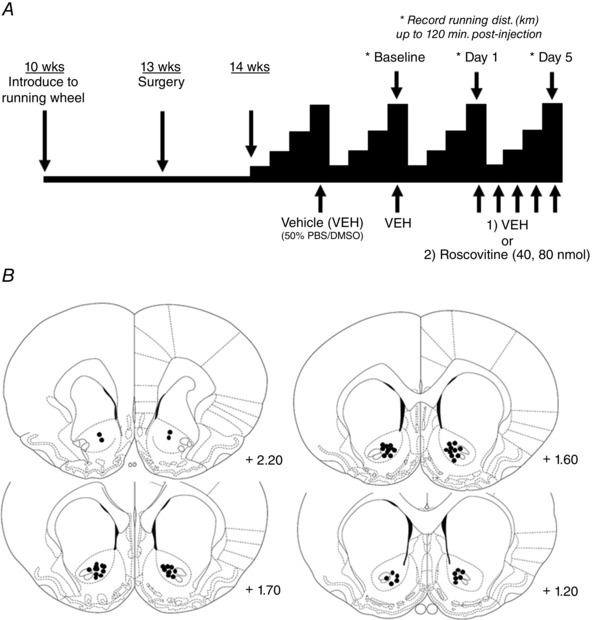
A, overview of the study design for intra‐NAc vehicle or roscovitine injection. The schematic illustration of ascending distances of voluntary running to the peak fourth night mimics the 4‐day running cycles of female rats employed in the experiment. Rats were introduced to running wheels at 10 weeks of age, had cannulae surgically implanted bilaterally in the NAc core at 13 weeks of age, and began the injection protocol at ∼14 weeks of age (wheel running was monitored for 8 days post‐surgery to ensure that surgery did not disrupt running pattern). Baseline vehicle (VEH) injection was performed in all rats. Rats were then randomly administered VEH, 40 nmol roscovitine or 80 nmol roscovitine for 5 continuous days ∼10–15 min before the beginning of the dark cycle. Running distance was monitored for 120‐min post‐injection for the baseline injection and on the first and fifth night of VEH or roscovitine injection. B, coronal section of rat brain, in accordance with a rat brain atlas (Paxinos & Watson, 1998), which shows the cannulae location as determined by cresyl violet staining (with the position of each section given in mm relative to Bregma). Black dots indicate the location of injector tips.
To perform the injections, rats were gently hand‐restrained for 90 s and 10 mm Hamilton syringes were mounted to an infusion pump (Harvard Apparatus, Holliston, MA, USA). Next, 12.5 mm, 30 gauge injector cannulae were connected to Hamilton syringes with PE‐10 tubing that were used to deliver vehicle or roscovitine at a rate of 0.32 μl min−1. The injectors remained in place for 60 s after completion of injection to ensure that vehicle/drug was properly infused and, upon completion of the injections, rats were returned to their home cages to monitor nightly wheel running.
Verification of cannulae placement
The methods of Parker et al. (2010) were used to determine cannulae placement. In brief, sections containing tracks from injectors were mounted on charged microscope slides, stained with cresyl violet, and examined using a light microscope to determine whether correct cannulae placement had been made. The endpoint of each injector was mapped on a rat brain atlas (Paxinos & Watson, 1998) as presented in Fig. 2 B.
Statistical analysis
All analytical procedures were performed using SigmaPlot, version 12.0 (Systat Software, Inc., Chicago, IL, USA). All values are reported as the mean ± SE. Significance for all analyses was set with an α value of 0.05. Student's t test was used to assess between group differences in mRNA expression. Statistical analyses on band densities and dendritic spine measurements were conducted using a two‐way ANOVA [Age (8 vs. 14 weeks) × Treatment (Sed vs. Wheel)] with Holm–Sidak post hoc analyses when appropriate. One‐way repeated‐measures ANOVA was used to assess age‐related changes in wheel running. Two‐way repeated‐measures ANOVA using treatment and time as the repeated variables followed by Holm–Sidak post hoc analyses, when necessary, was used to assess the influence of roscovitine on running distance, and percentage running distance compared to baseline injection. Deviations from these statistical analyses are reported when appropriate.
Results
Voluntary wheel running peaks at 8 weeks of age
One‐way repeated‐measures ANOVA showed a significant effect of age on running distance (F 8,46 = 14.14, P < 0.001) (Fig. 3 A). Post hoc analysis revealed running distance was lower at 6 and 7 weeks (P < 0.001), and at 12, 13 and 14 weeks of age (P < 0.05), compared to 8 weeks. Similar results were obtained for running time (F 8,46 = 22.95, P < 0.001) (Fig. 3 B). Repeated‐measures ANOVA also showed a significant effect of running velocity (F 8,46 = 4.21, P < 0.001); however, post hoc analysis revealed differences in velocity from 8 weeks only at 6 weeks (P < 0.01) (Fig. 3 C).
Figure 3. Voluntary wheel running characteristics.
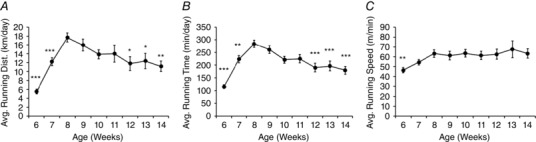
Average nightly running (A) distance (km), (B) time (min) and (C) velocity (m min–1) over the course of the study. * P < 0.05, ** P < 0.01, *** P < 0.001.
Characterization of RNA‐seq data from 8‐ and 14‐week‐old wheel running rats
A summary of the total number of reads as well as the percentage of reads aligned to the reference genome is presented in Table 2. We observed a high correlation (r = 0.995) between RPKM values from an individual rat vs. the mean RPKM values from all 12 rats assessed, demonstrating a high reliability of transcript detection using our RNA‐seq methods (Fig. 1 B). High enrichment of glutamic acid decarboxylase 1 (Gad1) and Gad2 transcripts (RPKM, log2 values of 7–8) has been associated with NAc MSNs following laser capture microdissection (Chen et al. 2011). Our data showed high enrichment, based on RPKM values, of Gad1 and Gad2, suggesting that NAc MSNs were present in our assayed brain tissue (Fig. 1 C and D). Additionally, differently expressed transcripts of interest were confirmed by qRT‐PCR (Table 3).
Table 2.
Summary of RNA‐seq reads mapped to reference library
| Group | Average total reads | Reads aligned to reference library | Total annotated transcripts expressed* |
|---|---|---|---|
| 8 weeks | 45,040,967 | 84.3% | 25,898 |
| 14 weeks | 41,524,614 | 83.7% | 26,088 |
According to Chen et al. (2011), 20–30 million total reads per sample in the nucleus accumbens are sufficient to provide gene expression data that correlate well with microarray and qRT‐PCR data. To ensure uniform tiling across the reference genome, tiled reads from each sample were analysed using NextGENe, version 2.4 (SoftGenetics, State College, PA, USA). *RPKM >0 in all animals within each group, as performed by Song et al. (2012).
Table 3.
qRT‐PCR validation of select RNA‐seq transcripts
| RNA‐seq | qRT‐PCR | |||
|---|---|---|---|---|
| Transcript | Fold change (8 weeks/14 weeks) | P value | Fold change (8 weeks/14 weeks) | P value |
| Adcy1 | 1.64 | 0.006 | 1.78 | 0.005 |
| Birc5 | 1.62 | 0.03 | 1.50 | 0.007 |
| Cadm4 | 1.23 | 0.01 | 1.16 | 0.06 |
| Cdk5r2 (p39) | 1.28 | 0.004 | 1.42 | 0.03 |
| Znf238 | 1.68 | 0.03 | 1.54 | 0.02 |
mRNA differences in 8‐ and 14‐week‐old wheel running rats
Using the aforementioned thresholds (8 weeks/14 weeks ±1.2‐fold, P < 0.05), we determined that 619 NAc mRNAs were differentially expressed between 8‐ and 14‐week‐old wheel running rats, of which 263 were up‐regulated at 8 weeks and 356 down‐regulated at 8 weeks (Fig. 1 A). Of these mRNAs, the top 10 up‐ and down‐regulated at 8 weeks/14 weeks are presented in Table 4. Of these transcripts, Znf238 (Diotel et al. 2015), Birc5 (Survivin) (Jiang et al. 2005), Tnfaip6 (Bhattacherjee et al. 2007) and Vamp7 (Bal et al. 2013) have been associated with adult neurogenesis, brain regeneration, neural repair, survival and development, and neurotransmission.
Table 4.
Top ten up‐ and down‐regulated NAc transcripts between 8 weeks/14 weeks
| Transcript | 8‐week RPKM | 14‐week RPKM | Fold change (8 weeks/14 weeks) | P value | Run distance r value |
|---|---|---|---|---|---|
| Up‐regulated in 8 weeks/14 weeks | |||||
| Titin cap protein (Tcap) | 2.16 | 1.23 | 1.75 | 0.04 | 0.75 |
| Zinc finger protein 238 (Znf238) | 28.09 | 16.67 | 1.68 | 0.03 | 0.62 |
| Adenylate cyclase 1 (brain) (Adcy1) | 74.38 | 45.21 | 1.65 | 0.001 | 0.68 |
| Receptor (G protein‐coupled) activity modifying protein 3 (Ramp3) | 5.22 | 3.19 | 1.63 | 0.02 | 0.68 |
| Calcium binding protein 1, transcript variant 3 (Cabp1) | 34.63 | 21.41 | 1.62 | 0.02 | 0.75 |
| Baculovirus IAP repeat containing 5 (Birc5) | 1.80 | 1.12 | 1.62 | 0.03 | 0.31 |
| Tumour necrosis factor, α‐induced protein 6 (Tnfaip6) | 2.71 | 1.68 | 1.61 | 0.03 | 0.76 |
| Vesicle‐associated membrane protein 7 (Vamp7) | 6.57 | 4.08 | 1.61 | 0.01 | 0.78 |
| Trophoblast glycoprotein (Tpbg) | 9.10 | 5.74 | 1.58 | 0.01 | 0.57 |
| Sterile α motif domain containing 9 (Samd9) | 7.61 | 4.87 | 1.56 | 0.02 | 0.69 |
| Down‐regulated in 8 weeks/14 weeks | |||||
| Keratin 77 (Krt77) | 1.21 | 2.65 | −2.19 | 0.009 | −0.63 |
| BAI1‐associated protein 3 (Baiap3) | 7.06 | 14.82 | −2.10 | 0.01 | −0.49 |
| von Willebrand factor A domain containing 5A (Vwa5a) | 3.97 | 8.00 | −2.01 | 0.005 | −0.61 |
| Fibronectin type III domain containing 9 (Fndc9) | 1.52 | 3.03 | −1.99 | 0.01 | −0.59 |
| Delta‐like 1 homologue (Drosophila) (Dlk1) | 5.16 | 10.28 | −1.99 | 0.02 | −0.56 |
| Major histocompatibility complex, class I, A (Hla‐a) | 7.29 | 13.88 | −1.90 | 0.001 | −0.81 |
| Huntingtin‐associated protein 1 (Hap1) | 2.93 | 5.48 | −1.87 | 0.003 | −0.59 |
| Glutathione peroxidase 3 (Gpx3) | 6.06 | 11.22 | −1.85 | 0.01 | −0.57 |
| Neuropeptide Y receptor Y2 (Npy2r) | 1.25 | 2.23 | −1.85 | 0.006 | −0.57 |
| Family with sequence similarity 70, member A (Fam70a) | 8.13 | 14.93 | −1.84 | 0.02 | −0.40 |
These transcripts were the top ten up‐ and down‐regulated NAc transcripts from the 619 transcripts that were differentially expressed between animals killed at 8 and 14 weeks of age. Run distance r value is the Pearson correlation coefficient between distance ran in the 8‐ and 14‐week‐old rats vs. the RPKM value of each gene.
Transcripts meeting our filtering criteria and strongly positively or negatively correlated (r > ±0.70) with running distance during the week of death are presented in Table 5. Of note, the top positively correlated transcripts, Cadm2 and Cadm4, and other cell adhesion molecule family proteins, are associated with synaptic plasticity and motivation‐based behaviours (Biederer et al. 2002; Robbins et al. 2010), Fa2h is essential for proper spatial learning, memory and myelination (Potter et al. 2011), and Vamp7, which also strongly up‐regulated at 8 weeks, directs synaptic transmission (Bal et al. 2013).
Table 5.
Top NAc transcripts correlated with running distance between 8 weeks and 14 weeks
| Transcript | Run distance r value | P value | Fold change (8 weeks/14 weeks) |
|---|---|---|---|
| Positively correlated | |||
| Cell adhesion molecule 2 (Cadm2) | 0.87 | <0.001 | 1.42 |
| Cell adhesion molecule 4 (Cadm4) | 0.84 | <0.001 | 1.22 |
| Fatty acid 2‐hydroxylase (Fa2h) | 0.83 | <0.001 | 1.27 |
| UDP glycosyltransferase 8 (Ugt8) | 0.82 | <0.01 | 1.33 |
| Deiodinase, iodothyronine, type II (Dio2) | 0.82 | <0.01 | 1.32 |
| Ras viral oncogene homologue 2 (Rras2) | 0.81 | <0.01 | 1.22 |
| Calcium/calmodulin‐dependent protein kinase II inhibitor 1 (Camk2n1) | 0.79 | <0.01 | 1.34 |
| Proteolipid protein 1 (Plp1) | 0.78 | <0.01 | 1.21 |
| Vesicle‐associated membrane protein 7 (Vamp7) | 0.78 | <0.01 | 1.61 |
| Rab11 family interacting protein 2 (class I) (Ran11fip2) | 0.77 | <0.01 | 1.26 |
| Negatively correlated | |||
| Threonine synthase‐like 2 (Thnsl2) | −0.90 | <0.001 | −1.24 |
| Erythrocyte membrane protein band 4.1 like 4b (Epb41l4b) | −0.87 | <0.001 | −1.31 |
| Major histocompatibility complex, class I, H (pseudogene) (Hla‐h) | −0.84 | <0.001 | –1.31 |
| dCMP deaminase (Dctd) | −0.83 | <0.01 | –1.27 |
| Kelch domain containing 8b (Klhdc8b) | −0.82 | <0.01 | –1.26 |
| Major histocompatibility complex, class I, A (Hla‐a) | −0.81 | <0.01 | –1.90 |
| Monooxygenase, DBH‐like 1 (Moxd1) | −0.80 | <0.01 | –1.24 |
| Filamin C, γ (Flnc) | −0.80 | <0.01 | –1.28 |
| Major histocompatibility complex, class I, C (Hla‐c) | −0.79 | <0.01 | –1.78 |
| Pygopus homologue 1 (Drosophila) (Pygo1) | −0.79 | <0.01 | –1.21 |
These transcripts were the top ten positively and negatively correlated NAc transcripts from the 619 transcripts that were differentially expressed between animals killed at 8 and 14 weeks of age. Run distance r value and P value determined Pearson correlation coefficient between distance ran in the 8‐ and 14‐week‐old rats vs. the RPKM value of each gene.
Bioinformatics reveals up‐regulation of networks pertaining to nervous system function in 8‐ vs. 14‐week‐old running rats
IPA was used to examine pathways and networks different between 8‐ and 14‐week‐old running rats. Of the top associated gene networks defined by IPA, the following networks were up‐regulated at 8 vs 14 weeks: (i) ‘nervous system and function, organ morphology, and organismal development’ and (ii) ‘cellular assembly and organization, nervous system development and function, and tissue morphology’ (Fig. 4). IPA analysis of the top scoring physiological system functions also revealed up‐regulation of functions pertaining to nervous system function, as well as CREB and cAMP signalling, at 8 vs. 14 weeks (Table 6). Enrichment analysis of GO categories also revealed an up‐regulation of functions related to nervous system function and synaptic transmission.
Figure 4. Top IPA generated networks up‐regulated in 8‐ vs. 14‐week old wheel running rats.
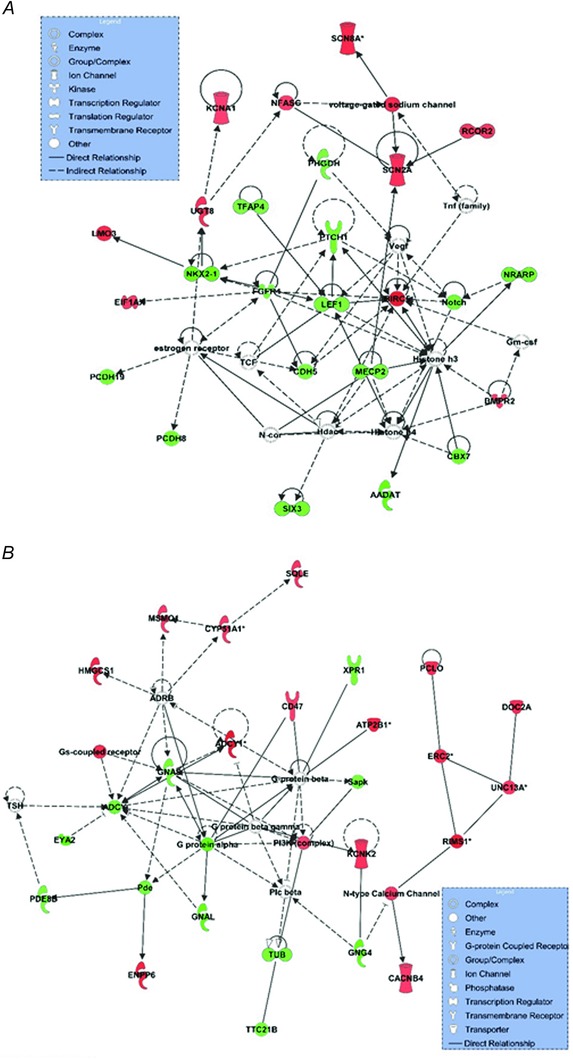
Top associated networks as defined by IPA for NAc mRNAs differently expressed in 8‐ and 14‐week‐old wheel running rats included (A) ‘nervous system and function, organ morphology, and organismal development’ (Focus molecules: 24/35; Score: 35) and (B) ‘cellular assembly and organization, nervous system development and function, and tissue morphology’ (Focus molecules: 23/35; Score: 35). Nodes represent genes/molecules. Shading is proportional to fold change size (red, up‐regulated; green, down‐regulated). Direct and indirect relationships are denoted with solid and dashed lines, respectively. White nodes denote network members that were not altered in the network. Lines ending in an arrow or blunt end indicate the known direction of molecular activation or inhibition, respectively.
Table 6.
Top scoring IPA biological functions and GO terms identified as up‐regulated in 8‐ vs. 14‐week‐old running rats
| Term | Number of molecules | P value | Z score/ Q value |
|---|---|---|---|
| IPA | |||
| Top physiological system development and functions | |||
| Nervous system development and function | 135 | 4.71 × 10–3 to 2.14 × 10–10 | NA |
| Tissue development | 139 | 4.44 × 10–3 to 2.14 × 10–10 | NA |
| Behaviour | 70 | 3.04 × 10–3 to 9.19 × 10–8 | NA |
| Top nervous system development and function categories | |||
| Neuritogenesis | 43 | 9.64 × 10–10 | 2.258 |
| Development of neurons | 53 | 2.14 × 10–10 | 2.201 |
| Morphogenesis of neurons | 29 | 1.00 × 10–6 | 2.023 |
| Long‐term potentiation | 19 | 6.14 × 10–6 | 2.004 |
| Branching of neurites | 16 | 5.63 × 10–4 | 1.748 |
| Select activated canonical pathways associated with nervous system development and function | |||
| CREB signalling in neurons | 10 | 0.003 | 2.121 |
| Dopamine‐DARPP32 feedback in cAMP signalling | 7 | 0.04 | 0.816 |
| cAMP‐mediated signalling | 11 | 0.006 | 0.302 |
| GO | |||
| Top biological processes | |||
| GO:0007268–synaptic transmission | 20 | 6.6 × 10–5 | 1.0 × 10–3 |
| GO:0019226–transmission of nerve impulse | 20 | 7.1 × 10–6 | 6.0 × 10–3 |
| GO:0007267–cell‐cell signalling | 23 | 4.9 × 10–4 | 1.5 × 10–1 |
| Top cellular components | |||
| GO:0044459–plasma membrane part | 62 | 1.9 × 10–5 | 5.1 × 10–3 |
| GO:0045202–synapse | 18 | 8.9 × 10–5 | 7.9 × 10–3 |
| GO:0030054–cell junction | 22 | 1.4 × 10–4 | 9.6 × 10–3 |
| Top molecular functions | |||
| GO:0000287–magnesium ion binding | 17 | 4.4 × 10–3 | 8.9 × 10–1 |
| GO:0046873–metal ion transmembrane transporter activity | 13 | 1.0 × 10–2 | 9.2 × 10–1 |
| GO:0009055–electron carrier activity | 10 | 1.2 × 10–2 | 8.6 × 10–1 |
For IPA generated data, the Z score is used to reflect the predicted activation state of a given network or pathway. For GO generated data, the far right column displays Q values. For GO terms, only terms inclusive of at least 10 molecules per function were included in for analysis. NA, not applicable.
Cadm4 and Cdk5‐associating molecules inherently decrease from 8 weeks to 14 weeks and are correlated with running
Following the aforementioned IPA and GO analysis, we analysed our RNA‐seq data for mRNAs that (i) associate with the IPA and GO predicted up‐regulated networks and (ii) have been previously associated with a low voluntary running phenotype (Roberts et al. 2014). In doing so, we identified Cadm4 as a transcript differentially expressed between 8‐ and 14‐week‐old running rats (P < 0.01) (Fig. 5 A) that (i) associates with the predicted up‐regulated networks and (ii) whose expression in the NAc is intrinsically greater, and correlated with running behaviour, in rats selectively bred for high vs. low voluntary running (Roberts et al. 2014). Follow‐up qRT‐PCR between sedentary 8‐ and 14‐week rats revealed that Cadm4 mRNA expression decreases from 8 weeks to 14 weeks of age independent of wheel running (P = 0.026) (Fig. 5 B). Cadm4 mRNA was also highly correlated with wheel running distance (r = 0.84, P < 0.001) (Fig. 5 C). Additionally, western blot analysis confirmed that Cadm4 protein was increased at 8 vs. 14 weeks, independent of wheel running (F 1,24 = 12.10, P < 0.01) (Fig. 5 D).
Figure 5. Cadm4 mRNA and protein are up‐regulated at 8‐ vs. 14‐weeks of age independent of wheel running.
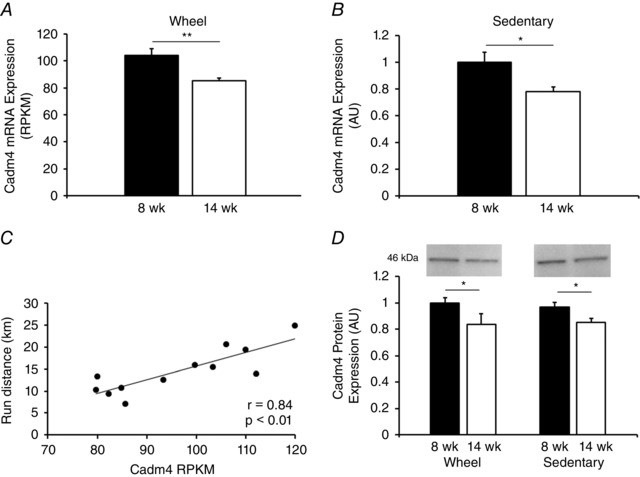
Cadm4 mRNA is up‐regulated at 8 vs. 14 weeks of age in wheel running (A) and sedentary (B) rats. Note the units in (A) and (B) differ as a result of (A) being determined by RNA‐seq and (B) by qRT‐PCR. Cadm4 mRNA is strongly correlated with running distance during the final week of the study (C). Similarly, Cadm4 protein decreases from 8 to 14 weeks of age independent of wheel running (data normalized to the 8‐week‐old wheel running group) (D). * P < 0.05, ** P < 0.01, *** P < 0.001.
Other studies suggest that Cdk5 is a downstream regulator of Cadm signalling pathways and promotes synaptic formation and specialization (Samuels et al. 2007; Lai & Ip, 2009). Given the role of Cdk5 in modifying cocaine‐induced locomotor activity (Taylor et al. 2007), we next assessed other transcripts potentially critical for Cdk5 function. Of these transcripts, mRNA expression of the Cdk5 regulatory subunit Cdk5r2 (p39) was decreased in 8‐ vs. 14‐week‐old wheel running (P < 0.01) (Fig. 6 A) and sedentary (P < 0.01) (Fig. 6 B) rats. p39 mRNA was also strongly correlated with running distance (r = 0.71, P < 0.01) (Fig. 6 C). Follow‐up western blot analysis revealed an age‐dependent decrease in p39 protein from 8 weeks to 14 weeks that was independent of wheel running (F 1,24 = 9.54, P < 0.01) (Fig. 6 D). Assessment of a homologous Cdk5 regulatory subunit Cdk5r1 (p35) by western blotting revealed no differences in p35 between 8 and 14 weeks (F 1,24 = 0.052, P = 0.81) or by wheel status (F 1,24 = 0.25, P = 0.62) (Fig. 6 E). However, western blotting showed an increase in p25 (the cleavage product of p35) in wheel running vs. sedentary rats (F 1,24 = 20.98, P < 0.01) suggesting increased Cdk5 activity with wheel running (Fig. 6 F).
Figure 6. Cdk5‐associated mRNA and protein differences in 8‐ vs. 14‐week old wheel running and sedentary rats.
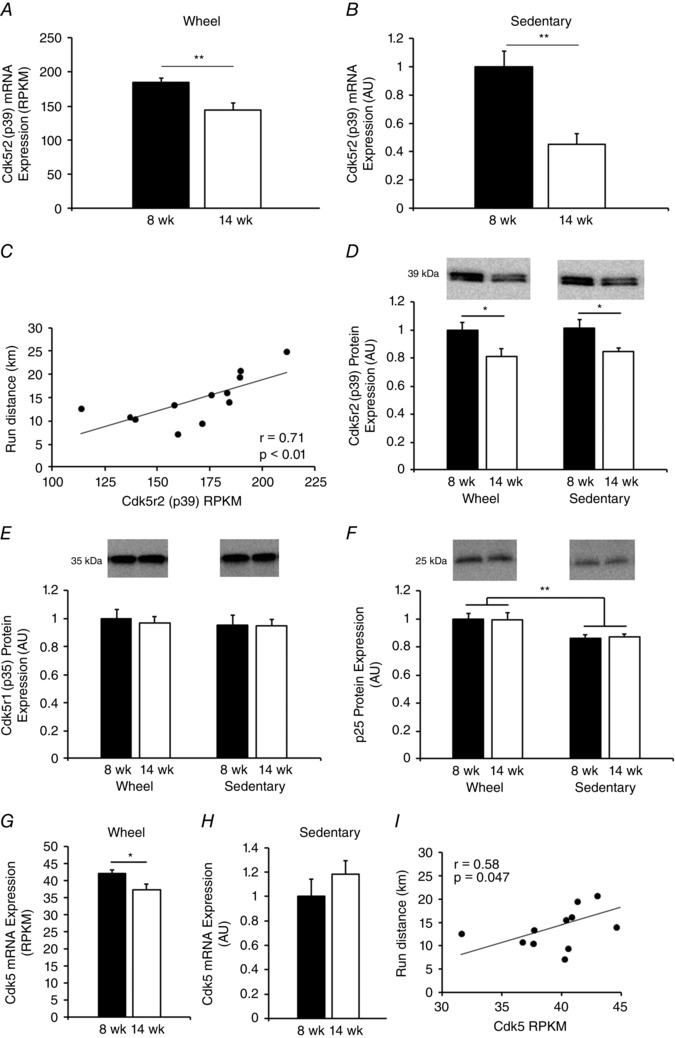
Cdk5r2 (p39) mRNA is higher at 8 vs. 14 weeks of age in wheel running (A) and sedentary (B) rats. Note the units in (A) and (B) differ as a result of (A) being determined by RNA‐seq and (B) by qRT‐PCR. p39 mRNA is strongly correlated with running distance during the final week of the study (C). Similarly, p39 protein decreases from 8 to 14 weeks of age independent of wheel running (data normalized to the 8‐week‐old wheel running group) (D). Wheel running and age had no effect on Cdk5r1 (p35) protein level (E); however, wheel running increased protein expression of the p35 cleavage product p25 (F). Cdk5 mRNA was modestly but significantly greater in 8‐ vs. 14‐week‐old wheel running rats (G) but was not different between 8‐ and 14‐week‐old sedentary rats (H). Cdk5 mRNA was significantly correlated with running distance during the final week of the study (I). * P < 0.05, ** P < 0.01, *** P < 0.001.
Finally, analysis of Cdk5 mRNA expression revealed a decrease between 8‐ and 14‐week wheel running (P < 0.05) but not sedentary rats (P = 0.34) (Fig. 6 G and H). Cdk5 mRNA was also significantly correlated with wheel running distance (r = 0.58, P = 0.047) (Fig. 6 I). Together, these results suggest that there is an age‐related decline in select molecules controlling Cdk5 function, therefore possibly influencing/modulating wheel running behaviour, and that Cdk5 function may be enhanced by, and/or possibly regulate, wheel running, as hypothesized previously (Werme et al. 2002).
Neuronal maturation and dendritic density
Given the resultant IPA and GO gene networks and functions, we hypothesized that a decrease in MSN number may be associated with the decrease in wheel running observed between 8 and 14 weeks. Darpp‐32 protein is predominantly expressed in differentiated striatal MSNs, comprising 95% of neurons in the NAc (Arlotta et al. 2008), which we used as a marker of NAc MSN content in the present study. Western blotting showed no differences in the levels of Darpp‐32 protein in 8‐ vs. 14‐week‐old rats (F 1,24 = 1.48, P = 0.24) or in wheel vs. sedentary rats (F 1,24 = 0.06, P = 0.81) (Fig. 7 A).
Figure 7. Dendritic spine density is increased in 8‐ vs. 14‐week old wheel running and sedentary rats.
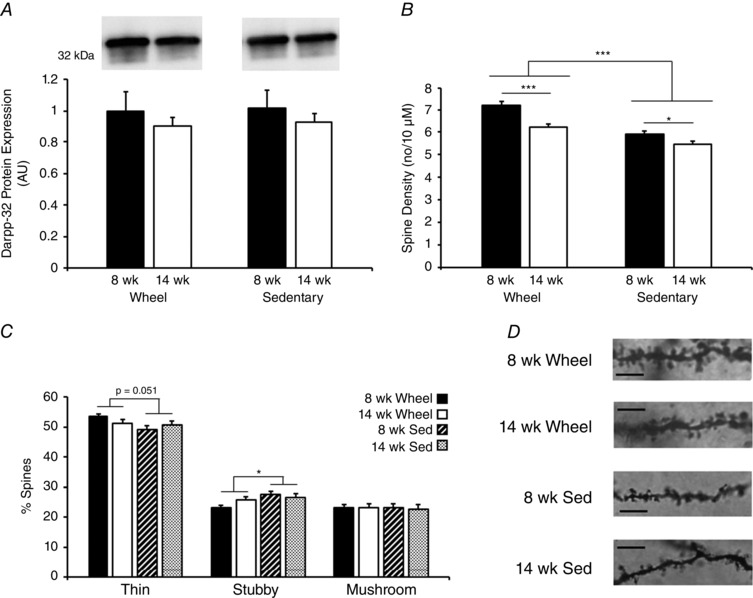
A, Darrp‐32 protein was not different between any of the four experimental groups assessed. B, analysis of dendritic spine density showed that wheel running increased spine density, whereas increases in age from 8 to 14 weeks were associated with a decrease in dendritic spine density. C, analysis of spine type showed that wheel running decreased the percentage of stubby spines and tended to increase the percentage of thin spins. D, representative images for the four experimental groups assessed. Scale bar = 5 μm. * P < 0.05, ** P < 0.01, *** P < 0.001.
Additionally, we hypothesized that decreases in dendritic spine density were associated with age‐related declines in wheel running. Assessment of NAc MSNs showed that the total number of dendritic spines was greater at 8 weeks compared to 14 weeks (F 1,282 = 24.28, P < 0.001) and was increased by wheel running (F 1,282 = 51.12, P < 0.001) (Fig. 7 B). We also observed a trend for an age × wheel status interaction (F 1,282 = 3.48, P = 0.063). No differences in dendritic spine density where observed between NAc subregions (data not shown). Post hoc analysis found a modest, but significant, decrease in dendritic spine density in sedentary rats from 8 to 14 weeks, suggesting that dendritic spine density in the NAc inherently decreases from 8 to 14 weeks of age (P = 0.031). Analysis of spine type composition showed that wheel running reduced the percentage of stubby spines (F 1,282 = 5.94, P = 0.015) at the same time as tending to increase the percentage of thin spines (F 1,282 = 3.84, P = 0.051) (Fig. 7 C).
Intra‐NAc roscovitine injection decreases voluntary wheel running
Total running distance was significantly reduced following intra‐NAc infusion of roscovitine at 40 nmol/0.5 μl (F 2,42 = 4.93, P = 0.024) and 80 nmol/0.5 μl (F 2,48 = 19.41, P > 0.001) (Fig. 8 A and C). Post hoc analysis revealed total running over the 120 min test session was decreased compared to baseline after 40 and 80 nmol roscovitine on both days 1 and 5 of drug injection (P < 0.05). Additionally, following 80 nmol roscovitine injection, running distance was decreased in each 30 min interval recorded during the 120 min trial on both days 1 and 5 (P < 0.05) (Fig. 8 A).
Figure 8. Intra‐NAc roscovitine infusion decreases voluntary wheel running.
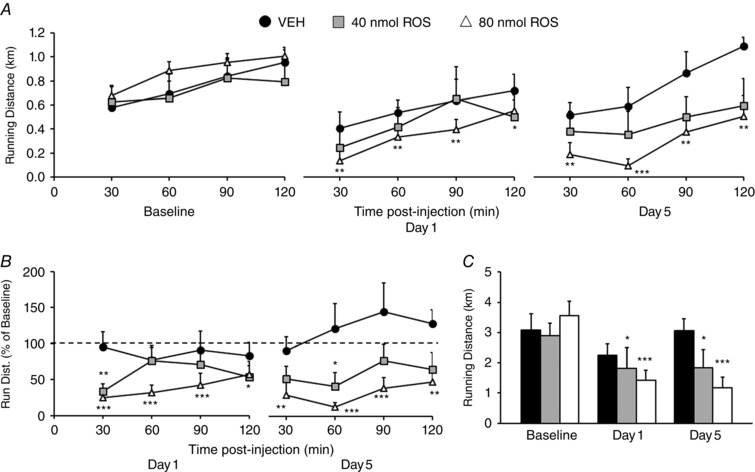
A, running distance (km) shown in 30 min increments for baseline injection and on the first and fifth nights of vehicle (black) or 40 nmol (grey) or 80 nmol (white) roscovitine (ROS) injection. B, percentage running distance on the first and fifth nights of vehicle or drug injection compared to baseline injection values. Note: two outlier points (±2 SD) were removed from this analysis (not shown). C, total running distance over the 120 min test session for the baseline injection and for the first and fifth night of vehicle or drug injection. All data were analysed with repeated‐measures ANOVA. * P < 0.05, ** P < 0.01, *** P < 0.001.
A repeated‐measures ANOVA examining of the effect of roscovitine on percentage change in running distance from baseline showed significant decreases in running after 40 nmol (F 2,41 = 5.58, P = 0.016) and 80 nmol roscovitine (F 2,46 = 37.07, P < 0.001) after the exclusion of two outlier points (> ± 2 SD) (Fig. 8 B). Post hoc analysis revealed decreased percentage running at 0–30 and 30–60 min post‐injection following 40 nmol infusion on days 1 and 5, respectively (P < 0.05). Similarly, following 80 nmol roscovitine injection, percentage running from baseline was decreased in each 30 min interval recorded during the 120 min trial on both days 1 and 5 (P < 0.05).
Discussion
In the present study, our two aims were: (i) to analyse molecular transducers in the NAc that may initiate and are associated with decreases in voluntary wheel running in young rats and (ii) to assess how the inhibition of Cdk5, a molecule highlighted in our first aim, impacts wheel running behaviour. We report that decreasing levels of voluntary wheel running between 8 and 14 weeks are associated with declines in (i) MSN dendritic spine density and (ii) gene networks critical for synaptic communication and neuron development, and also that (iii) several transcripts central to these functions are highly correlated with wheel running distance. Additionally, declines in key mRNAs and proteins related to the above functions and dendritic spine density were observed in analysis in age‐matched sedentary rats, suggesting decrements in these functions may be the result age, rather than from the reduction in physical activity. Finally, pharmacological inhibition of Cdk5, a molecule central to some of the functions described above, dose‐dependently decreased wheel running. Taken together, these findings suggest, for the first time, that decreases in molecules associated with Cdk5 function in the NAc may regulate the initial decrease in wheel running behaviour that begins after 8 weeks of age.
Human physical inactivity is strongly association with increased chronic disease and mortality later in life (Booth et al. 2012). Surprisingly, few studies, to our knowledge, have investigated any of the underlying biological mechanisms occurring during youth that may account for early reductions in physical activity. Furthermore, effective public health‐related strategies to combat physical inactivity are relatively non‐existent. Intriguingly, our observation that wheel running is greatest at 8 weeks of age in female, Wistar rats matches our previous publications in male rats given wheel access at 4, rather than 6 weeks of age, suggesting that inherent changes at 8 weeks mediate running behaviour in both males and females (Ruegsegger et al. 2016).
Factors associated with MSN structure are associated with age‐dependent reductions in voluntary wheel running
Relationships between enhanced striatal MSN architecture and wheel running behaviour have been previously established (Roberts et al. 2014). In the present study, bioinformatics using IPA and GO revealed extensive up‐regulation of gene networks with functions in neuron development, dendritic spine density, and cAMP signalling in 8‐ vs. 14‐week‐old wheel running rats; however, these results could represent alterations to reduced levels of wheel running, rather than age‐induced adaptations. The extensive up‐regulation of mRNAs which have nervous system function included ∼22% of all transcripts meeting our bioinformatics‐filtering criteria. Additionally, several of the top up‐regulated mRNAs at 8 weeks have functions specific to nervous system function, as described in the results. Similarly, one transcript only expressed at 8 weeks, Arhgef7, has been implicated in the assembly of functional synapses (Zhang et al. 2003; Zhang et al. 2005), further suggesting neural plasticity and function is higher in 8‐ compared to 14‐week‐old wheel running rats (data not shown). These findings are similar to those of a previous study by Roberts et al. (2014), who reported that high intrinsic levels of mRNAs indicative of increased neuronal maturation and synaptic function in the NAc are associated with high voluntary running distances. Thus, assuming that NAc mRNA expression is an indicator of molecular transducers that are associated with voluntary wheel running behaviour, we posit that these identified transcripts provide candidates that may ‘predict’ running behaviour, and therefore begin to offer a neuromolecular basis for declining physical activity seen between 8 and 14 weeks of age.
The above led us to identify molecules located at the synapse. Specifically, cell adhesion molecule 4 (Cadm4) and Cadm2 were identified as being higher at 8 vs. 14 weeks, and they had positive, strong correlations between their transcript levels and running distance. Cadm4 has been shown to promote synaptogenesis (Tanabe et al. 2013) and Cadm4 can drive the assembly of glutamatergic synapses (Biederer et al. 2002). Lower levels of Cadm4 protein were previously associated with a low voluntary running phenotype (Roberts et al. 2014). Importantly, the overexpression of the Cadm family member Cadm1 in mice promotes an increase in excitatory synapse number and synaptic plasticity, whereas loss of Cadm1 results in fewer excitatory synapses (Robbins et al. 2010). Therefore, given that synapse formation within the NAc has been attributed to addictive‐like behaviours (Russo et al. 2010), we posit that the ∼20% decreases in Cadm4 mRNA and protein levels could decrease the formation of specific synapses between NAc neurons and other brain regions (e.g. the ventral tegmental area, prefrontal cortex and hippocampus); an effect that may influence the reduced running behaviour observed in 14‐week‐old rats. Furthermore, this decrease in Cadm4 mRNA and protein appears to be independent of running, and provides a potential mechanism by which age may influence the decline in wheel running between 8 and 14 weeks. Although a major limitation of the present study is the lack of RNA‐seq on age‐matched sedentary rats, the decrease in Cadm4 mRNA by qRT‐PCR in the sedentary group supports a hypothesis that similar decreases in mRNAs related to synaptic function and neural plasticity decrease independent of wheel running occur between 8 and 14 weeks of age; however, this remains to be assessed in future studies.
Previous reports suggesting that Cadm proteins regulate Cdk5 signalling pathways led us to assess Cdk5 mRNA expression in 8‐ and 14‐week‐old wheel running and sedentary rats (Samuels et al. 2007; Lai & Ip, 2009). However, the results for Cdk5 were not completely confirmatory. Although we observed a correlation between Cdk5 mRNA and running distance in NAc, Cdk5 mRNA was only lower at 14 weeks of age, compared to 8 weeks of age, in the voluntary wheel running group. In addition, literature indicates that Cdk5 protein without its co‐factors does not exhibit enzyme activity. Cdk5 co‐factors Cdk5r2 (p39) (Cai et al. 1997) and Cdk5r1 (p35) (Lew et al. 1994; Tsai et al. 1994) are indispensable for Cdk5 activity. They have been shown to have similar affinity and ability to activate Cdk5 (Tang et al. 1995). One of these, Cdk5r2 (p39) was decreased at 14 weeks, compared to 8 weeks, with respect to both its mRNA and protein levels, independent of wheel running. In addition, p39 in the wheel running group was strongly positively correlated with running distance. Concerning the other Cdk5 co‐factor, no differences were noted for p35. However, wheel running increased protein expression of its cleavage product p25, suggesting that wheel running increases p35 activity. Immunohistochemical localization analysis suggests that p39 and p35 could have different functional roles in regulating Cdk5 activity, particularly in the basal ganglia (Honjyo et al. 1999). Furthermore, Wu et al. (2000) demonstrated that Cdk5 activity is ∼40% less in the striatum at 6 months compared to 3 months age despite no difference in Cdk5 protein level, and this decrease was attributed to decrements in p35 and p39 expression. Together with data from the present study, these findings suggest the notion that the age‐dependent declines in p39 in the striatum may drive age‐related declines in Cdk5 activity.
To provisionally summarize the above, the finding that p39 is decreased by age is particularly interesting given that Cdk5 was hypothesized to mediate increases in running behaviour associated with increased expression of its transcription factor ΔFosB. Wheel running increases ΔFosB expression, probably increasing Cdk5 activity (Werme et al. 2002). Thus, it is possible that p39 influences age‐related changes in Cdk5 activity, whereas p35/25 influences exercise‐induced changes in Cdk5 activity. Finally, although Cadm and other synaptic proteins, as well as p39 and/or p35, probably influence Cdk5 signalling by independent mechanisms, taken together, these findings highlight the possibility of Cdk5 representing a promising target molecule in the regulation of age‐related declines in voluntary physical activity.
Dendritic spine density in MSNs was measured to assess whether structural differences could begin to explain the aforementioned gene and network differences. Intriguingly, we observed significant increases in spine density with wheel running. To our knowledge, the present study is the first to show increases in dendritic spines in the NAc in response to wheel running. A precedence for increased spine density exists. Wheel running has been shown to increase dendritic spine density in the hippocampus (Stranahan et al. 2007; Stranahan et al. 2009). Taken together with the findings of the present study, this justifies an assessment of how physical activity influences dendritic density in additional brain regions. Other studies have shown that increases in dendritic spine density in dopaminoceptive NAc MSNs are associated with long lasting addictive behaviours, and increases in dendritic spine density in the NAc have been observed in response to cocaine (Norrholm et al. 2003; Lee et al. 2006).
We also observed subtle changes in dendritic spine composition following wheel running. Wheel access tended to increase, and to significantly decrease the percentage of thin and stubby dendritic spines, respectively. Although comprising a non‐significant trend (P = 0.051), the increase in thin spines following wheel running is intriguing given their important functions in excitatory synaptic activity and in dictating rapid responses to the changes imposed by salient stimuli (Bourne & Harris, 2007). Similarly, LaPlant et al. (2010) demonstrated that chronic cocaine use increased the percentage of thin spines in the NAc. Hence, wheel running produces alterations in the quality of dendritic spine density in the NAc in a manner similar to that for better‐characterized addictive behaviours.
We also observed significant reductions in dendritic spine density in 14‐ vs. 8‐week‐old sedentary rats. Anderson (1982) observed higher dendritic densities in the medial preoptic area of the hypothalamus, at 55 days (∼8 weeks of age) compared to 75 days (∼11 weeks of age) in rats. Similar to the NAc, the medial preoptic area of the hypothalamus is highly responsive to dopamine (Hull et al. 1995). Likewise, a recent study by Madison et al. (2012) reported a ∼20% reduction in dendritic spine density in the MSNs for 13‐ to 16‐week‐old mice, further supporting the plasticity of the MSNs at relatively young ages.
Cdk5 has been implicated in many biochemical processes that influence structural and functional changes in synaptic plasticity (Cheung et al. 2006). Injection of the Cdk5 inhibitor roscovitine into the NAc reduces dendritic spine density in the NAc (Norrholm et al. 2003), further highlighting Cdk5 as a candidate target that could mediate age‐dependent reductions physical activity. These differences in dendritic density in the present study were independent of differences in the amount of mature NAc MSNs, as assessed by Darpp‐32 western blotting. However, the ∼8% drop in Darpp‐32 protein between 8 and 14 weeks is comparable to the 5–10% rate of decline in the number of dopaminergic neurons in the NAc and striatum per decade in humans (Naoi & Maruyama, 1999).
Cdk5 inhibition dose‐dependently decreases wheel running
To expound upon the data generated in the first experiments, we tested our notion that pharmacological inhibition of Cdk5 in the NAc core would decrease voluntary running. To our knowledge, this is the first study to observe a dose–response decrease in wheel running following infusion of the Cdk5 inhibitor roscovitine into the NAc core. The novel response to roscovitine presents important evidence that Cdk5 function in the NAc core is at least partially necessary for chronic adaptations that influence the motivation for voluntary wheel running; however, voluntary wheel running is probably under polygenic control. This observation also extends our findings from the first experiment by potentially localizing a site of action for these changes to the NAc core. Taken together, the above findings add to the growing body of literature suggesting that Cdk5 activity in the NAc may be an important modulator of reward‐related behaviours, as well as highlight the neurochemical parallels between wheel running behaviour and drug addiction.
Dopamine neurotransmission is important to multiple reward‐related processes in addition to those involved in drug addiction. In the striatum, Cdk5 controls dopamine neurotransmission through the regulation of the presynaptic components of dopamine synthesis and release (Chergui et al. 2004; Kansy et al. 2004; Moy & Tsai, 2004) and Cdk5 appears to interface dopamine signalling with several downstream targets considered to mediate reward‐related behaviour including PKA and DARPP‐32 (Bibb, 2003). Several studies have shown that both agonism and antagonism of dopamine signalling in the NAc reduces voluntary wheel running (Knab et al. 2012; Roberts et al. 2012). Cdk5 is a natural, negative‐regulator of dopamine signal transduction (Bibb et al. 1999). Rats find voluntary wheel running rewarding (Brene et al. 2007) and the inhibition of Cdk5 could promote a ‘substitution of reward’ by replacing the reward derived through wheel running with the reward derived through potential increases in dopamine signalling. Similarly, drug intake studies blocking mesolimbic dopamine receptors have shown increased nicotine and cocaine intake (Corrigall & Coen, 1991; Arnold & Roberts, 1997), which suggests that decreasing NAc dopamine may promote reward‐seeking behaviour and vice versa. Importantly, reductions in running following roscovitine infusion occurred on the both the first and fifth night of injection. Acute roscovitine treatment evokes dopamine release (Chergui et al. 2004), suggesting that roscovitine‐induced alterations in dopamine may decrease wheel running via hedonic substitution, rather than Cdk5‐induced plasticity. The notion that Cdk5 influences wheel running by altering dopamine action is also similar to findings suggesting that Cdk5 inhibition in the NAc enhances the behavioural responses to cocaine in a manner dependent of dopamine signalling in the NAc (Benavides et al. 2007; Taylor et al. 2007). Interestingly, although behavioural responses to drugs of abuse are often enhanced following roscovitine treatment, we observed a reduction in wheel running behaviour, suggesting that the neuromolecular mechanisms controlling voluntary wheel running and behavioural responses to drugs of abuse are similar but not identical.
Our finding that Cdk5 inhibition decreases wheel running also extends previous research by Werme et al. (2002), who show that ∆FobB overexpression in dynorphin neurons in the striatum increases the wheel running distance, to suggest that Cdk5, a ∆FobB target gene, may mediate the influences of ∆FobB on wheel running. Additionally, when extending these results to age‐related changes, the considerable decreases between 7 and 14 weeks of age in cAMP production in the NAc and striatum parallel the declines in locomotor activity (Andersen, 2002), further suggesting that decreases in PKA‐cAMP signalling between 8 and 14 weeks may influence wheel running. Our results are also in agreement with the analysis by Benavides et al. (2007) who used Cdk5 conditional knockout mice to show that, after acquisition of running behaviour, Cdk5 conditional knockout mice displayed dramatically reduced steady‐state levels of voluntary wheel running compared to controls. Our findings extend such findings to suggest that the loss of Cdk5 activity, as well as expression, mediates running behaviour; however, further work is needed to identify more precisely how changes in Cdk5 activity/expression alter voluntary wheel running.
Our use of the Cdk5 inhibitor roscovitine is limited by the possibility of non‐specific pharmacological effects and its inability to localize the effects of Cdk5 inhibition to either presynaptic or postsynaptic compartments. An additional limitation is that inhibition of Cdk5 catalytic activity may not affect the described structural or activity independent roles of the protein (Hawasli et al. 2007).
Conclusions
In the present study, we show that age‐related decreases in Cadm4, p39 and MSN dendritic density are associated with age‐related decreases in voluntary running, and that repeated infusion of the Cdk5 inhibitor roscovitine decreases wheel running. Our data provide guidance for future investigations aiming to determine how synaptic plasticity, dopaminergic signalling and Cdk5 function in the NAc impact voluntary wheel running. Given the epidemic levels of physical inactivity, significant efforts should be taken to unravel the neuromolecular mechanisms underlying the biological motivation for voluntary physical activity.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
G.N.R. outlined and performed all experiments, helped procure funding, performed bioinformatics and drafted the manuscript. F.W.B. procured funding, outlined experiments, and draft the manuscript. R.G.T., and T.E.C. critically assisted with RNA‐seq bioinformatics and helped draft the manuscript. K.B.G. performed Golgi staining analysis. G.N.R., R.G.T., and T.E.C. assisted in tissue collection, data analysis, and/or writing of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Partial funding for this project was obtained from grants awarded to FWB and GNR by the College of Veterinary Medicine at the University of Missouri, AHA 16PRE2715005 (GNR), and the University of Missouri Life Sciences Fellowship Program (GNR). This project was also supported by funds procured through the College of Veterinary Medicine's Development Office.
This is an Editor's Choice article from the 1 January 2017 issue.
References
- Anantharaman‐Barr HG & Decombaz J (1989). The effect of wheel running and the estrous cycle on energy expenditure in female rats. Physiol Behav 46, 259–263. [DOI] [PubMed] [Google Scholar]
- Andersen SL (2002). Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD). Behav Brain Res 130, 197–201. [DOI] [PubMed] [Google Scholar]
- Anderson CH (1982). Changes in dendritic spine density in the preoptic area of the female rat at puberty. Brain Res Bull 8, 261–265. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y & Macklis JD (2008). Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 28, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM & Roberts DC (1997). A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57, 441–447. [DOI] [PubMed] [Google Scholar]
- Bal M, Leitz J, Reese AL, Ramirez DM, Durakoglugil M, Herz J, Monteggia LM & Kavalali ET (2013). Reelin mobilizes a VAMP7‐dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron 80, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR & Bibb JA (2007). Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci 27, 12967–12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacherjee V, Mukhopadhyay P, Singh S, Johnson C, Philipose JT, Warner CP, Greene RM & Pisano MM (2007). Neural crest and mesoderm lineage‐dependent gene expression in orofacial development. Differentiation 75, 463–477. [DOI] [PubMed] [Google Scholar]
- Bibb JA (2003). Role of Cdk5 in neuronal signaling, plasticity, and drug abuse. Neurosignals 12, 191–199. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr. , Nairn AC & Greengard P (1999). Phosphorylation of DARPP‐32 by Cdk5 modulates dopamine signalling in neurons. Nature 402, 669–671. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET & Sudhof TC (2002). SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297, 1525–1531. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK & Laye MJ (2012). Lack of exercise is a major cause of chronic diseases. Compr Physiol 2, 1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J & Harris KM (2007). Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17, 381–386. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L & Werme M (2007). Running is rewarding and antidepressive. Physiol Behav 92, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XH, Tomizawa K, Tang D, Lu YF, Moriwaki A, Tokuda M, Nagahata S, Hatase O & Matsui H (1997). Changes in the expression of novel Cdk5 activator messenger RNA (p39nck5ai mRNA) during rat brain development. Neurosci Res 28, 355–360. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu Z, Gong S, Wu X, Taylor WL, Williams RW, Matta SG & Sharp BM (2011). Genome‐wide gene expression profiling of nucleus accumbens neurons projecting to ventral pallidum using both microarray and transcriptome sequencing. Front Neurosci 5, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P & Greengard P (2004). Cyclin‐dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci USA 101, 2191–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Fu AK & Ip NY (2006). Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron 50, 13–18. [DOI] [PubMed] [Google Scholar]
- Corrigall WA & Coen KM (1991). Selective dopamine antagonists reduce nicotine self‐administration. Psychopharmacology (Berl) 104, 171–176. [DOI] [PubMed] [Google Scholar]
- den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, Spector TD, Wareham NJ & Loos RJ (2013). Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr 98, 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N, Beil T, Strahle U & Rastegar S (2015). Differential expression of id genes and their potential regulator znf238 in zebrafish adult neural progenitor cells and neurons suggests distinct functions in adult neurogenesis. Gene Expr Patterns 19, 1–13. [DOI] [PubMed] [Google Scholar]
- Festing MF (1977). Wheel activity in 26 strains of mouse. Lab Anim 11, 257–258. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC & Bibb JA (2007). Cyclin‐dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci 10, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH & Driscoll M (2002). Stochastic and genetic factors influence tissue‐specific decline in ageing C. elegans . Nature 419, 808–814. [DOI] [PubMed] [Google Scholar]
- Heruth DP, Gibson M, Grigoryev DN, Zhang LQ & Ye SQ (2012). RNA‐seq analysis of synovial fibroblasts brings new insights into rheumatoid arthritis. Cell Biosci 2, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjyo Y, Kawamoto Y, Nakamura S, Nakano S & Akiguchi I (1999). Immunohistochemical localization of CDK5 activator p39 in the rat brain. Neuroreport 10, 3375–3379. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS & Matuszewich L (1995). Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci 15, 7465–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK (2000). Age‐related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc 32, 1623–1629. [DOI] [PubMed] [Google Scholar]
- Jakubczak LF (1969). Effects of age and activity restriction on body weight loss of rats. Am J Physiol 216, 1081–1083. [DOI] [PubMed] [Google Scholar]
- Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J, Conway EM, Robinson ML & Altura RA (2005). Essential role for survivin in early brain development. J Neurosci 25, 6962–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LC, Bellingham WP & Ward LC (1990). Sex differences in voluntary locomotor activity of food‐restricted and ad libitum‐fed rats. Implications for the maintenance of a body weight set‐point. Comp Biochem Physiol A Comp Physiol 96, 287–290. [DOI] [PubMed] [Google Scholar]
- Kansy JW, Daubner SC, Nishi A, Sotogaku N, Lloyd MD, Nguyen C, Lu L, Haycock JW, Hope BT, Fitzpatrick PF & Bibb JA (2004). Identification of tyrosine hydroxylase as a physiological substrate for Cdk5. J Neurochem 91, 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M & Sarna S (1981). Cigarette smoking, use of alcohol, and leisure‐time physical activity among same‐sexed adult male twins. Prog Clin Biol Res 69, 37–46. [PubMed] [Google Scholar]
- Kirkwood TB & Finch CE (2002). Ageing: the old worm turns more slowly. Nature 419, 794–795. [DOI] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Hamilton AT, Gulledge AA & Lightfoot JT (2009). Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res 204, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Hamilton AT & Lightfoot JT (2012). Pharmacological manipulation of the dopaminergic system affects wheel‐running activity in differentially active mice. J Biol Regul Homeost Agents 26, 119–129. [PMC free article] [PubMed] [Google Scholar]
- Knab AM & Lightfoot JT (2010). Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci 6, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO & Ip NY (2009). Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol 19, 275–283. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd , Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH & Nestler EJ (2010). Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13, 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Fabsitz R, Meyer JM, Sholinsky P, Ramakrishnan V & Goldberg J (1997). Familial determinants of moderate and intense physical activity: a twin study. Med Sci Sports Exerc 29, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN & Katzmarzyk PT (2012). Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC & Greengard P (2006). Cocaine‐induced dendritic spine formation in D1 and D2 dopamine receptor‐containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA 103, 3399–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J & Leinwand LA (2002). Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol 92, 2245–2255. [DOI] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T & Wang JH (1994). A brain‐specific activator of cyclin‐dependent kinase 5. Nature 371, 423–426. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A & Kleeberger SR (2004). Genetic influence on daily wheel running activity level. Physiol Genomics 19, 270–276. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR & Leamy LJ (2008). Quantitative trait loci for physical activity traits in mice. Physiol Genomics 32, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M & Bowman AB (2012). Disease‐toxicant interactions in manganese exposed Huntington disease mice: early changes in striatal neuron morphology and dopamine metabolism. PLoS ONE 7, e31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH, Rogina B, Montooth KL & Helfand SL (2003). Conditional tradeoffs between aging and organismal performance of Indy long‐lived mutant flies. Proc Natl Acad Sci USA 100, 3369–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M & Yadid G (2015). Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci 35, 8042–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL & Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Moy LY & Tsai LH (2004). Cyclin‐dependent kinase 5 phosphorylates serine 31 of tyrosine hydroxylase and regulates its stability. J Biol Chem 279, 54487–54493. [DOI] [PubMed] [Google Scholar]
- Naoi M & Maruyama W (1999). Cell death of dopamine neurons in aging and Parkinson's disease. Mech Ageing Dev 111, 175–188. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR & Greengard P (2003). Cocaine‐induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin‐dependent kinase‐5. Neuroscience 116, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, McCall JG & Will MJ (2010). Basolateral amygdala opioids contribute to increased high‐fat intake following intra‐accumbens opioid administration, but not following 24‐h food deprivation. Pharmacol Biochem Behav 97, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998). The Rat Brain in Stereotaxic Coordinates, 4th edn Academic Press, San Diego, CA. [Google Scholar]
- Peters A & Kaiserman‐Abramof IR (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127, 321–355. [DOI] [PubMed] [Google Scholar]
- Pitts GC (1984). Body composition in the rat: interactions of exercise, age, sex, and diet. Am J Physiol Regul Integr Comp Physiol 246, R495–R501. [DOI] [PubMed] [Google Scholar]
- Potter KA, Kern MJ, Fullbright G, Bielawski J, Scherer SS, Yum SW, Li JJ, Cheng H, Han X, Venkata JK, Khan PA, Rohrer B & Hama H (2011). Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 59, 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Sudhof TC, Stein V & Biederer T (2010). SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron 68, 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Brown JD, Company JM, Oberle LP, Heese AJ, Toedebusch RG, Wells KD, Cruthirds CL, Knouse JA, Ferreira JA, Childs TE, Brown M & Booth FW (2013). Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. Am J Physiol Regul Integr Comp Physiol 304, R1024–R1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ & Booth FW (2012). Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav 105, 661–668. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Toedebusch RG, Wells KD, Company JM, Brown JD, Cruthirds CL, Heese AJ, Zhu C, Rottinghaus GE, Childs TE & Booth FW (2014). Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J Physiol 592, 2119–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger GN, Speichinger KR, Manier JB, Younger KM, Childs TE & Booth FW (2016). Hypothalamic Npy mRNA is correlated with increased wheel running and decreased body fat in calorie‐restricted rats. Neurosci Lett 618, 83–88. [DOI] [PubMed] [Google Scholar]
- Ruegsegger GN, Toedebusch RG, Will MJ & Booth FW (2015). Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology 97, 171–181. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC & Nestler EJ (2010). The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustemeyer SM, Lamberson WR, Ledoux DR, Wells K, Austin KJ & Cammack KM (2011). Effects of dietary aflatoxin on the hepatic expression of apoptosis genes in growing barrows. J Anim Sci 89, 916–925. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hsueh YP, Shu T, Liang H, Tseng HC, Hong CJ, Su SC, Volker J, Neve RL, Yue DT & Tsai LH (2007). Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron 56, 823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak CT, Tapp PD, Zicker SC, Murphey HL, Muggenburg BA, Head E, Cotman CW & Milgram NW (2003). Locomotor activity rhythms in dogs vary with age and cognitive status. Behav Neurosci 117, 813–824. [DOI] [PubMed] [Google Scholar]
- Song HK, Hong SE, Kim T & Kim do H (2012). Deep RNA sequencing reveals novel cardiac transcriptomic signatures for physiological and pathological hypertrophy. PLoS ONE 7, e35552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D & Gould E (2007). Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 17, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG & Mattson MP (2009). Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Fujita E, Hayashi YK, Zhu X, Lubbert H, Mezaki Y, Senoo H & Momoi T (2013). Synaptic adhesion molecules in Cadm family at the neuromuscular junction. Cell Biol Int 37, 731–736. [DOI] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O & Wang JH (1995). An isoform of the neuronal cyclin‐dependent kinase 5 (Cdk5) activator. J Biol Chem 270, 26897–26903. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ & Bibb JA (2007). Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor‐activating and incentive‐motivational effects of cocaine. Proc Natl Acad Sci USA 104, 4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T & McDowell M (2008). Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40, 181–188. [DOI] [PubMed] [Google Scholar]
- Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M & Sirard J (2002). Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc 34, 350–355. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Jr Caviness VS, ., Chae T & Harlow E (1994). p35 is a neural‐specific regulatory subunit of cyclin‐dependent kinase 5. Nature 371, 419–423. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ & Brene S (2002). Delta FosB regulates wheel running. J Neurosci 22, 8133–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Cioe JD, Previsich N & Kolb B (1977). The variability of the interaural line vs the stability of bregma in rat stereotaxic surgery. Physiol Behav 19, 719–722. [DOI] [PubMed] [Google Scholar]
- Wolff‐Hughes DL, Bassett DR & Fitzhugh EC (2014). Population‐referenced percentiles for waist‐worn accelerometer‐derived total activity counts in U.S. youth: 2003–2006 NHANES. PLoS ONE 9, e115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2010). Global Recommendations on Physical Activity for Health. WHO, Geneva. [PubMed] [Google Scholar]
- Wu DC, Yu YP, Lee NT, Yu AC, Wang JH & Han YF (2000). The expression of Cdk5, p35, p39, and Cdk5 kinase activity in developing, adult, and aged rat brains. Neurochem Res 25, 923–929. [DOI] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H & Horwitz AF (2003). Synapse formation is regulated by the signaling adaptor GIT1. J Cell Biol 161, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S & Horwitz AF (2005). A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci 25, 3379–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LQ, Cheranova D, Gibson M, Ding S, Heruth DP, Fang D & Ye SQ (2012). RNA‐seq reveals novel transcriptome of genes and their isoforms in human pulmonary microvascular endothelial cells treated with thrombin. PLoS ONE 7, e31229. [DOI] [PMC free article] [PubMed] [Google Scholar]


