Abstract
Esophageal squamous cell carcinoma (ESCC) is the most prevalent type of esophageal cancer and accumulating evidence has confirmed the role of miRNAs in ESCC. One such miRNA, miR-370, was found to be aberrantly downregulated in various human malignancies. This study showed that the expression of miR-370 was significantly lower in ESCC tissues and cell lines, and miR-370 functioned as a tumor suppressor in ESCC. Moreover, this is the first report that showed miR-370 suppresses cell proliferation and tumor growth by directly targeting Pim family kinases 1 (PIM1). Furthermore, alpinumisoflavone, a naturally occurring flavonoid, could inhibit tumor growth of ESCC by targeting miR-370/PIM1 signaling.
Keywords: ESCC, miR-370, PIM1, alpinumisoflavone
Introduction
Esophageal cancer is the sixth leading cause of cancer-related deaths worldwide [1]. Adenocarcinoma is the most prevalent type of esophageal cancer in the western countries, while 90% of the esophageal cancers in the region stretching from northern Iran to North-Central China are known to arise from the squamous cells [2]. Despite the use of multiple therapeutic strategies, esophageal squamous cell carcinoma (ESCC) has been associated with a low 5-year survival rate of 15-20% [3]. Therefore, novel therapeutic targets and agents are urgently needed.
MicroRNAs (miRNAs), a family of small noncoding RNAs, function as oncogenes or tumor suppressors and regulate various cellular processes, such as differentiation, proliferation, apoptosis, migration and invasion by repressing the expression of target genes [4]. Accumulating evidence has shown that miR-370 is commonly downregulated in various human malignancies, including ovarian cancer [5], gastric cancer [6], liver cancer [7] and non-small cell lung cancer [8], and plays a regulatory role in the proliferation of various cancerous cells, cell differentiation, apoptosis, and chemosensitivity. However, its role in the development of ESCC has not yet been explored.
Pim family kinases, a family of small, constitutively active, and highly evolutionarily conserved serine/threonine-specific kinases, includes three members, PIM1, PIM2, and PIM3. As oncogenes, PIMs are found to be aberrantly highly expressed in both solid tumors and hematological malignancies [9]. Particularly, PIM1 has been reported to play regulatory roles in cellular proliferation, cell cycle, apoptosis, and metabolism in human malignancies [9]. Overexpression of PIM1 protein has been implicated in the tumorigenesis of ESCC [10]. Moreover, PIM1 expression was associated with lymphatic metastasis of ESCC, highlighting its potential as a therapeutic target for ESCC.
Alpinumisoflavone (AIF) is one of the major active ingredients of a traditional Chinese medicine Derris eriocarpa, which is widely distributed mainly over Yunnan, Guangxi and Guizhou in China. Previous studies have found that AIF has numerous pharmacological activities, including atheroprotective [11], estrogenic [12], and anti-bacterial [13]. Recent studies have identified AIF as a potential effective antineoplastic drug [13,14]. However, the mechanisms by which AIF exerts anti-tumor effects remain unknown. The present study showed that miR-370 was downregulated in ESCC tissue and correlated with TNM stage. Ectopic overexpression of miR-370 in ESCC cell lines led to the suppression of proliferation and apoptosis induction by directly targeting PIM1. Furthermore, AIF could inhibit proliferation and induce apoptosis in vitro and in vivo through regulation of miR-370/PIM1 signaling.
Materials and methods
Patient information and specimen collection
Sixty-three ESCC patients from the General Surgery Department and Digestive Disease Department, Qingdao University Affiliated Hospital (Qingdao, China) and the First Affiliated Hospital of Chongqing Medical University (Chongqing China), between January 2013 and December 2013, were included in this study. All patients who had received radiotherapy, chemotherapy, or other esophageal surgery before esophagectomy were excluded. ESCC and matched normal esophageal tissues (5 cm away from the tumor lesion according to the NCCN guideline of esophageal cancer) were collected immediately after resection, treated with RNAlater reagent (TaKaRa, Dalian China), and stored at -80°C until use. This study was approved by the Ethics Committee of Qingdao University Affiliated Hospital and the First Affiliated Hospital of Chongqing Medical University, and a written consent form was signed by each patient.
Cell lines and cultures
Human esophageal immortalized cell line, Het-1A, was purchased from the American Type Culture Collection (Manassas, VA) and cultured in bronchial epithelial basal medium with growth supplements (Clonetics, Allendale, NJ). Human ESCC cell lines EC9706 and KYSE30 were purchased from the Chinese Academy of Medical Science (Beijing, China) and maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10 units/mL penicillin and streptomycin (Hyclone, USA) and 10% fetal bovine serum (FBS; Hyclone, Logan, UT). Cells were maintained in a 5% CO2 humidified incubator at 37°C.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Tiangen Biotech, Beijing, China), and miRNAs were obtained using the miRcute miRNA isolation kit (DP501) (Tiangen Biotech, Beijing, China) from cultured cells or human tissues. MiR-370 expression was quantified by real-time PCR with a TaqMan probe (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, cDNA was obtained by High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA), and qRT-PCR was performed using a TaqMan PCR kit and the ABI 7500 system (Thermo Fisher Scientific, Waltham, MA). The relative expression of miR-370 in cells and tissues was normalized to that of U6. For PIM1 mRNA detection, the primer was synthesized based on published sequence [15]. First-strand cDNA was reverse transcribed from 1 μg total RNA using the Super M-MLV Reverse transcriptase (BioTeke Co., Beijing, China). PCR reaction solution included SYBR GREEN mastermix (Solarbio Co., Beijing, China), forward primer, reverse primer, and 10 ng template cDNA. GADPH was used as an internal control for normalization. PCR results were analyzed using the comparative ΔCt method (ABPrism software, Applied Biosystems, Foster City, CA).
Western blot
Proteins were isolated by lysing frozen tissues in RIPA buffer (Sigma, St. Louis, MO) and cells in lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors (Sigma, St Louis, MO). The protein concentration was measured by the BCA protein assay kit (Beyotime, Shanghai, China). Extracted proteins were separated on SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Proteins were probed with specific antibodies using a standard protocol. Specific primary antibodies against PIM1 and β-actin were purchased from Abcam (Shanghai, China). The secondary antibodies used in this study were goat anti-rabbit IgG-HRP, goat anti-mouse IgG-HRP and donkey anti-goat IgG-HRP (Beyotime Institute of Biotechnology, Shanghai, China). Signals were detected using chemiluminescent substrate (KPL, Guildford, UK) and the blot intensities were quantified using BandScan software (Glyko, Novato, CA).
Construction of reporter plasmids and luciferase assay
The reporter plasmid was constructed as previously described [16]. Briefly, a fragment containing PIM1 3’UTR was amplified by PCR from human genomic DNA utilizing specific primers and then inserted into a pGL3 vector (Promega, Madison, WI) downstream the stop codon of firefly-luciferase reporter gene, thus resulting in the pGL3-3’UTR/PIM1 construct. For the luciferase assay, 293 T recipient cells were transiently co-transfected with 0.2 μg of pGL3-3’UTR/PIM1 constructs, 0.02 μg of pRL-TK-Renilla luciferase reporter plasmids (Promega, Madison, WI) containing the Renilla-luciferase for normalization, and 5 pmol of miR-370 overexpression construct or control. At 24 hours after transfection, the cells were lysed and the luciferase activity was measured with a luminometer using the dual-luciferase reporter assay system, according to the manufacturer’s instructions.
MiR-370 knockdown or overexpression
The lentiviral constructs miR-370 mimic and miR-con, and miR-370 inhibitor were obtained from Qiagen (Gene Copoeia, Rockville, MD). Cells were seeded into a 96-well plate, incubated overnight, and then transfected with miR-370 mimic and miR-con, or miR-370 inhibitor according to the manufacturer’s instructions. The transfection efficiency was confirmed by qPCR analysis.
PIM1 knockdown
The shRNA oligos targeting PIM1 gene were obtained from GenePharma (Shanghai, China). PIM1 targeting shRNA sequence and one scramble sequence as the control were inserted into the plasmid vector pGCsi-H1. The resulting plasmids were named pGCsi-H1-PIM1 and -control, respectively. For transfection, cells in logarithmic growth phase were seeded in a 6-well plate and transfected with the constructed plasmids as per the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Transfected cells were incubated for another 48 hours and the knockdown was verified by western blot.
PIM1 overexpression
PIM1 was overexpressed by transfection with an expression construct using Lipofectamine 3000 reagent (Invitrogen, Grand Island, NY), according to the manufacturer’s instructions. PIM1 overexpressing vector was constructed as previously described using a plasmid vector pGCsi-H1 [17]. Cells transfected with an empty vector were used as controls. At 48 hours after transfection, the cells were rinsed, resuspended in fresh culture media and the overexpression was verified by western blot.
Cell proliferation assay
Cell proliferation was determined by MTT assay (Sigma, MO, USA). Briefly, 5×103 cells/well were plated in 96-well plates. After treatment, 20 µl of MTT solution (5 mg/ml in PBS) was added to each well and incubated for 2 hours. MTT formazan was dissolved in 150 µl of isopropanol and the absorbance was measured at 595 nm with an ELISA reader (Tecan Group Ltd, Männedorf, Switzerland).
Colony formation
Cells suspended in RPMI-1640 agarose medium were seeded in each well of a 6-well plate over a layer of solidified RPMI-1640 agarose medium. Cultures were maintained for 14 days without fresh medium feeding at 37°C in a humidified atmosphere of 95% air and 5% CO2. Then, colonies with over 50 cells were enumerated, stained with crystal violet and photographed using a digital camera (Nikon DXM1200, Tokyo, Japan).
Apoptosis analysis
Apoptosis was evaluated by flow cytometry using a FITC Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ) according to the manufacturer’s instructions. Briefly, 1×106 cells/ml were stained with annexin V-FITC and propidium (PI) for 15 minutes in the dark before analysis with a flow cytometer (Beckman Coulter Inc., Miami, FL).
Xenograft models and immunohistochemistry analysis
The animal study was performed according to the regulations of the State Food and Drug Administration (SFDA) of China on Animal Care, and the study protocol was approved by the Medical Ethics Committee of our hospital. Female 5-week-old BALB/c nude mice were bought from the Experimental Animal Center of Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). Approximately 1×106 KYSE30 cells were suspended in 100 μl PBS and subcutaneously inoculated into the right side of the posterior flank of mice. Tumor diameters were measured every other day. The mice were sacrificed at 30 days after inoculation, and solid tumors were removed and measured. Tumor volume was calculated using the equation: V=A×B2/2 (mm3), where A is the largest diameter and B is the perpendicular diameter. The primary tumors were excised and analyzed by immunohistochemistry and TUNEL staining. Briefly, apoptotic cells in tumor tissue were measured by the TUNEL apoptosis detection kit (Beyotime, Shanghai, China) as per the manufacturer’s protocol. Three equal-sized fields were randomly chosen and the mean number of green fluorescence-positive cells was counted. Immunohistochemistry was performed using mouse anti-human PIM1 and cleaved caspase-3 for detecting tumor cell proliferation and apoptosis in the paraffin sections, respectively.
Statistical analysis
The data are presented as mean ± SD (Standard Deviation) and represent the results of three separate experiments, each conducted in quadruplicate unless otherwise stated. All statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Values are presented as the mean ± SD. Comparisons were performed by one-way ANOVA followed by Dunnett’s t-test. Clinical characteristics were compared with the Chi-square test. Any difference with a P value <0.05 was defined as statistically significant.
Results
MiR-370 is downregulated in ESCC tissue and correlates with clinicopathological stage of ESCC
To examine the role of miR-370 in the development of ESCC, qRT-PCR analysis was used to determine the expression of miR-370 in ESCC tissues. As shown in Figure 1A, miR-370 was significantly downregulated in ESCC tissue samples as compared to the matched distant normal tissues. To further investigate the clinicopathological significance of miR-370 expression in ESCC patients, the mean fold decrease of miR-370 expression in ESCC samples was taken as the cut-off point of low and high expression of miR-370. MiR-370 level was significantly negatively associated with ESCC TNM stage and pathologic grade (Figure 1B and 1C), but not with other clinicopathological data, such as age, gender, tumor location, and tumor length (Table 1).
Figure 1.
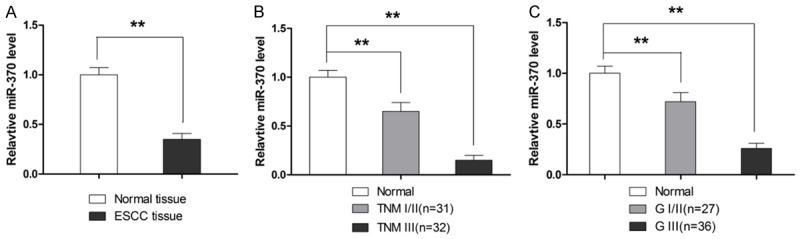
MiR-370 level in ESCC tissues. A. MiR-370 expression is lower in ESCC tissue as compared to adjacent normal tissue. B. MiR-370 expression is associated with TNM stage. C. MiR-370 expression is associated with pathological stage. **P<0.01.
Table 1.
Correlation between miR-370 expression and clinicopathological features of ESCC patients
| Characteristics | MiR-370 expression | P value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age (years) | |||
| Over 60 | 21 | 17 | 0.5723 |
| Less than 60 | 12 | 13 | |
| Gender | |||
| Male | 32 | 20 | 0.3249 |
| Female | 5 | 6 | |
| Tumor localization | |||
| Upper third | 3 | 5 | 0.7351 |
| Middle third | 13 | 16 | |
| Lower third | 9 | 17 | |
| Tumor length (cm) | |||
| <3 | 11 | 12 | 0.1008 |
| 3-5 | 12 | 10 | |
| >5 | 4 | 14 | |
| Pathological | |||
| GI | 3 | 9 | 0.0017 |
| GII | 12 | 10 | |
| GIII | 24 | 5 | |
| TNM | |||
| I | 6 | 12 | 0.0213 |
| II | 10 | 11 | |
| III | 18 | 6 | |
MiR-370 functions as a tumor suppressor in ESCC cells
The role of miR-370 in ESCC was studied in two ESCC cell lines EC9706 and KYSE30. As shown in Figure 2A, the expression of miR-370 in both cell lines was significantly lower as compared to human esophageal immortalized Het-1A cells. Then miR-370 mimic and inhibitor as well as negative control (NC) were transfected into ESCC cells to investigate the effect of miR-370 on cell viability. As shown in Figure 2B, miR-370 mimic significantly increased the expression of miR-370, while miR-370 inhibitor markedly decreased the level of miR-370. MTT assay showed that overexpression of miR-370 significantly inhibited the proliferation of ESCC cells as compared to the NC cells, while miR-370 inhibitor enhanced the proliferation of both tested cell lines (Figure 2C). The anti-proliferative effect of miR-370 was also confirmed by colony formation assay, as shown in Figure 2D. Next, we examined whether miR-370 induces apoptosis in ESCC cells. As shown in Figure 2E, miR-370 overexpression significantly increased the number of apoptotic cells, while miR-370 inhibitor resulted in decreased apoptotic cell population. The apoptosis-inducing role of miR-370 was also confirmed since its overexpression significantly elevated the levels of activated caspase-3, the key marker for apoptosis (Figure 2F).
Figure 2.
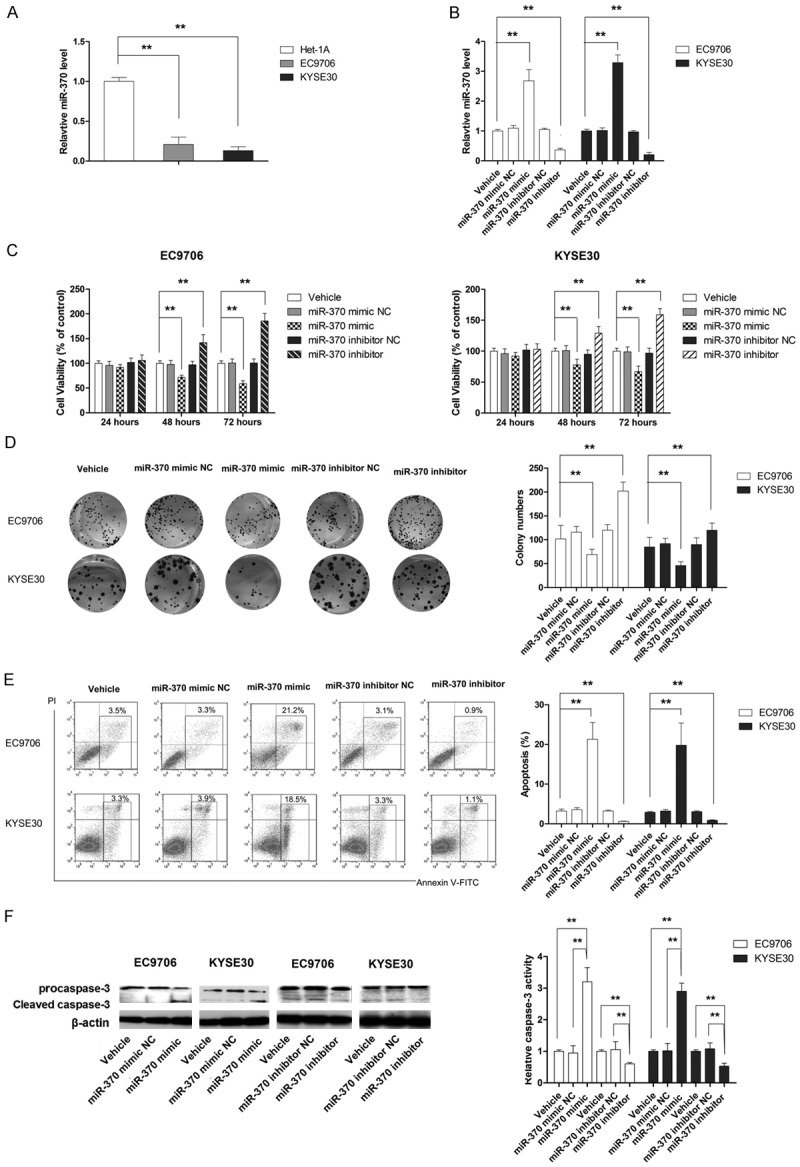
MiR-370 functions as a tumor suppressor in ESCC cells. A. MiR-370 is significantly downregulated in ESCC cells. B. The level of miR-370 is manipulated using miR-370 mimic and miR-370 inhibitor. C. MiR-370 mimic and inhibitor affected the viability of ESCC cells. D. Mi-370 mimic suppresses cell proliferation, while miR-370 inhibitor promotes cell growth. E. MiR-370 mimic induces apoptosis, while miR-370 inhibitor suppresses apoptosis in ESCC cells. F. MiR-370 mimic activates caspase-3, while miR-370 inhibitor suppresses caspase-3 activation in ESCC cells. **P<0.01.
PIM1 is directly targeted by miR-370
MiRNAs are known to influence gene expression through post-transcriptional mechanisms. Therefore, two computational algorithms, TargetScan [18] and microRNA. org [19], were used in combination to search for potential targets that mediate the anti-tumor effect of miR-370 in ESCC. Both algorithms predicted an interaction between miR-370 and the target sites in PIM1 3’-UTR, as shown in Figure 3A. The association between miR-370 expression and mRNA and protein levels of PIM1 was further examined in the tissues of 20 randomly chosen patients from our hospital. According to Pearson’s correlation scatter plots analysis, an inverse correlation between the protein or mRNA levels of PIM1 and miR-370 was observed in ESCC tissues, which indicated that miR-370 might affect the stability of PIM1 mRNA in addition to regulating its expression post-transcription (Figure 3B and 3C). To further examine the regulating mechanism of miR-370 on PIM1, the mRNA and protein expressions of PIM1 were measured in cells transfected with miR-370 mimic and miR-370 inhibitor. As shown Figure 3D and 3E, overexpression in ESCC cells resulted in significant decrease of both mRNA and protein expression of PIM1. In contrast, knockdown of miR-370 was associated with significantly higher expression of PIM1 mRNA and protein, suggesting that miR-370 might degrade PIM1 mRNA as well as suppress protein translation. Next, luciferase assay was used to determine whether miR-370 regulates the expression of PIM1 by directly targeting the 3’-UTR of PIM1. As shown in Figure 3F, miR-370 mimic significantly decreased the luciferase reporter activity, while miR-370 inhibitor significantly increased the reporter activity as compared to controls, indicating that PIM1 was directly targeted by miR-370. Taken together, our findings showed that miR-370 regulated the expression of PIM1 at both the transcript and protein levels.
Figure 3.
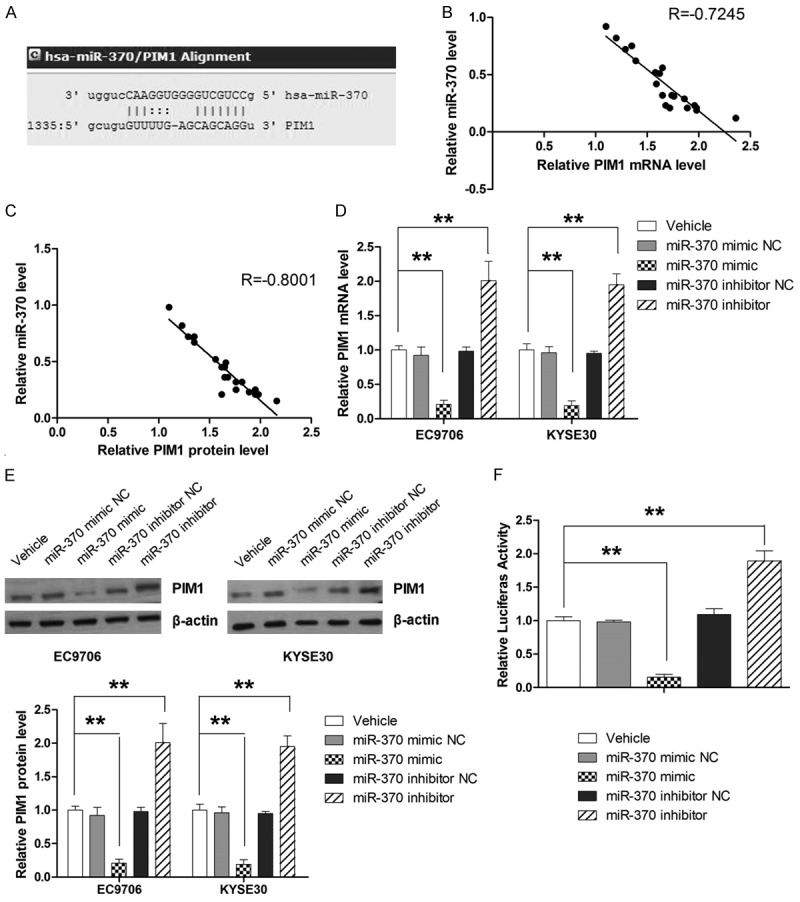
MiR-370 directly targets the PIM1 gene. A. Schematic illustration of the hypothesized duplexes formed from interactions between the PIM1 3’-UTR binding sites and miR-370. B. Inverse correlation between miR-370 and PIM1 mRNA level in ESCC tissues. C. Inverse correlation between miR-370 and PIM1 protein level in ESCC tissues. D. Quantitative analysis of PIM1 mRNA levels after transfection with miR-370 mimic and miR-370 inhibitor. E. Quantitative analysis of PIM1 protein levels after transfection with miR-370 mimic and miR-370 inhibitor. F. Luciferase activity is increased by miR-370 inhibitor and suppressed by miR-370 mimic. **P<0.01.
MiR-370 functions as a tumor suppressor by targeting PIM1 in ESCC cells
To verify the role of PIM1 in the anti-tumor effect of miR-370, its expression was manipulated in EC9706 and KYSE30 cells using shRNA or overexpression plasmid. As shown in Figures 4A and 5A, the expression of PIM1 was efficiently suppressed by shRNA, while the level of PIM1 protein was markedly increased in cells transfected with the overexpression plasmid. Next, the effect of PIM1 knockdown on cell proliferation and apoptosis was examined in cells transfected with miR-370 inhibitor. As shown in Figure 4B-E, the promoting effect of miR-370 inhibitor on cell proliferation as well as the suppressing effect of miR-370 inhibitor on apoptosis was almost completely abrogated by PIM1 shRNA. Moreover, ectopic overexpression of PIM1 significantly attenuated the suppression of proliferation and apoptosis induced by miR-370 mimic (Figure 5B-E). Our results provided strong evidence that miR-370 functioned as a tumor suppressor by targeting PIM1 in ESCC.
Figure 4.
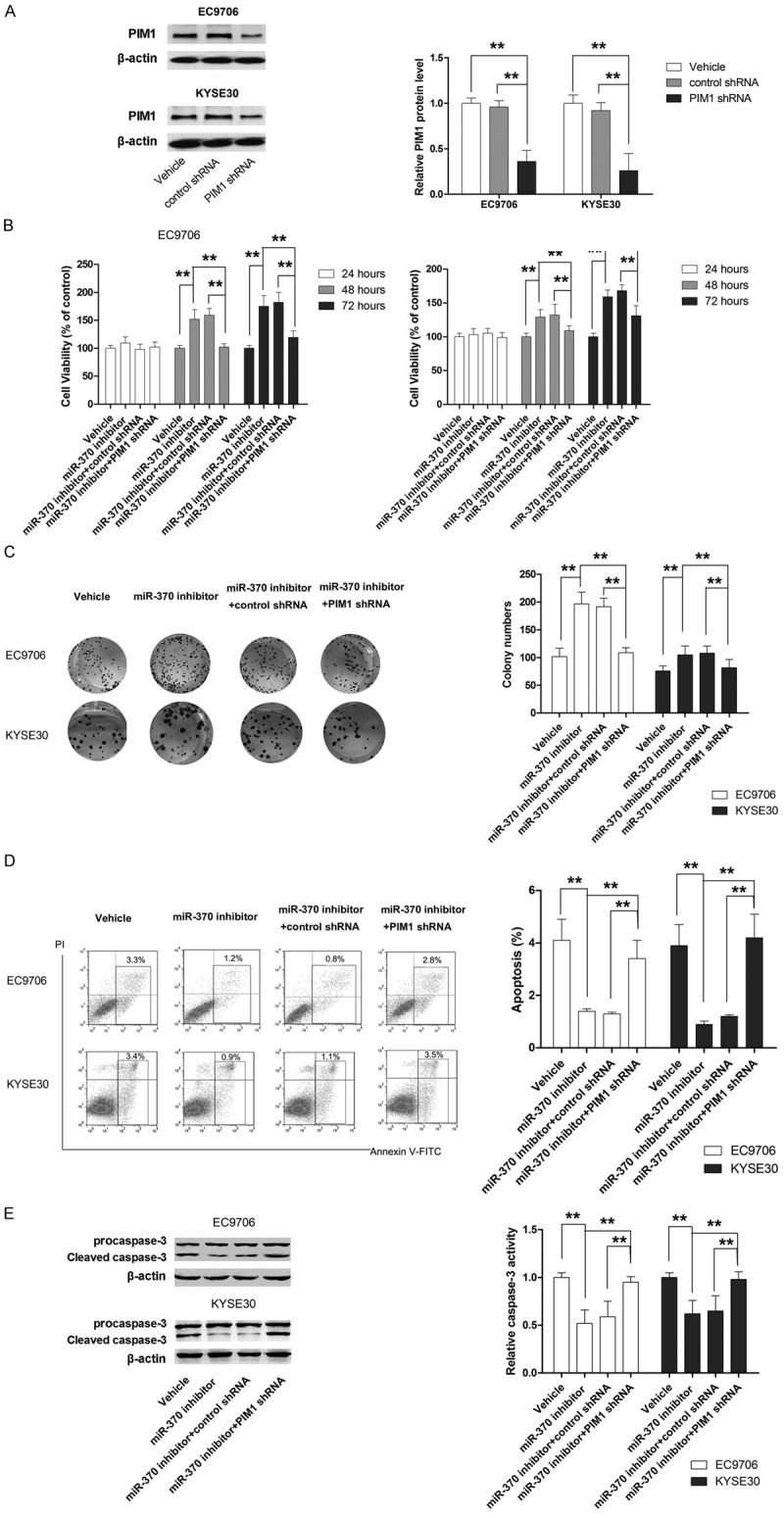
PIM1 shRNA impairs the pro-growth effect of miR-370 inhibitor in ESCC cells. A. PIM1 expression is suppressed by targeted shRNA. B. Knockdown of PIM1 in cells transfected with miR-370 inhibitor results in loss of cell viability. C. Knockdown of PIM1 in cells transfected with miR-370 inhibitor leads to growth inhibition. D. Knockdown of PIM1 in cells transfected with miR-370 inhibitor results in increased apoptosis. E. Knockdown of PIM1 in cells transfected with miR-370 inhibitor results in caspase-3 activation. **P<0.01.
Figure 5.
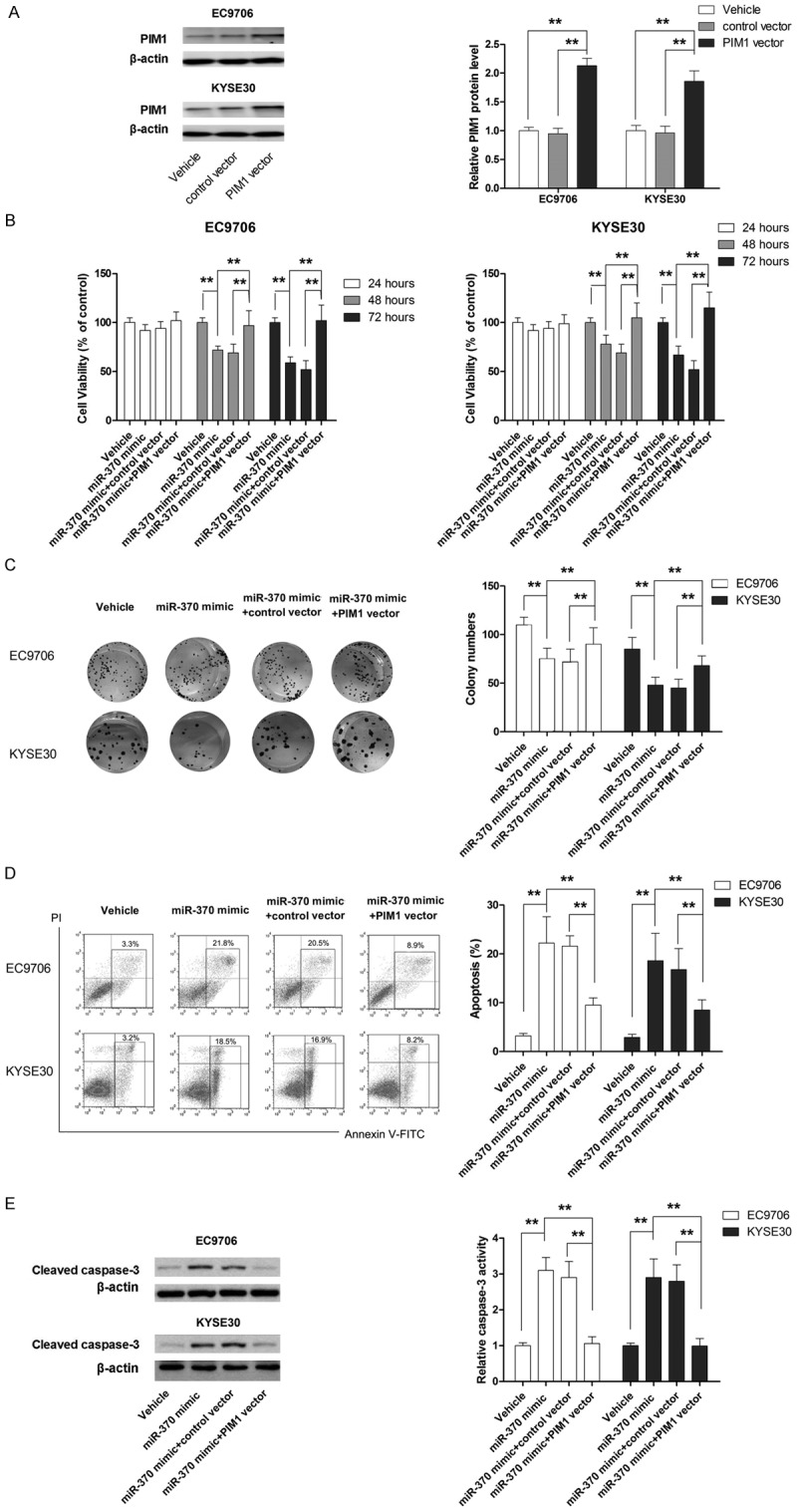
PIM1 overexpression compromises the anti-tumor effect of miR-370 mimic in ESCC cells. A. PIM1 expression is increased by PIM1 vector. B. Overexpression of PIM1 in cells transfected with miR-370 mimic leads to impaired anti-proliferative effect. C. Overexpression of PIM1 in cells transfected with miR-370 mimic leads to increased cell growth. D. Overexpression of PIM1 in cells transfected with miR-370 mimic leads to apoptosis inhibition. E. Overexpression of PIM1 in cells transfected with miR-370 mimic correlates with decreased caspase-3 activity. **P<0.01.
AIF induces apoptosis in EC9706 and KYSE30 cells
As shown in Figure 6A and 6B, AIF treatment led to a dose-dependent loss of viability in EC9706 and KYSE30 cells, although the two cell lines displayed different sensitivities to AIF. Additionally, AIF at 10 and 20 μM significantly induced apoptosis in both cell lines (Figure 6C and 6D).
Figure 6.
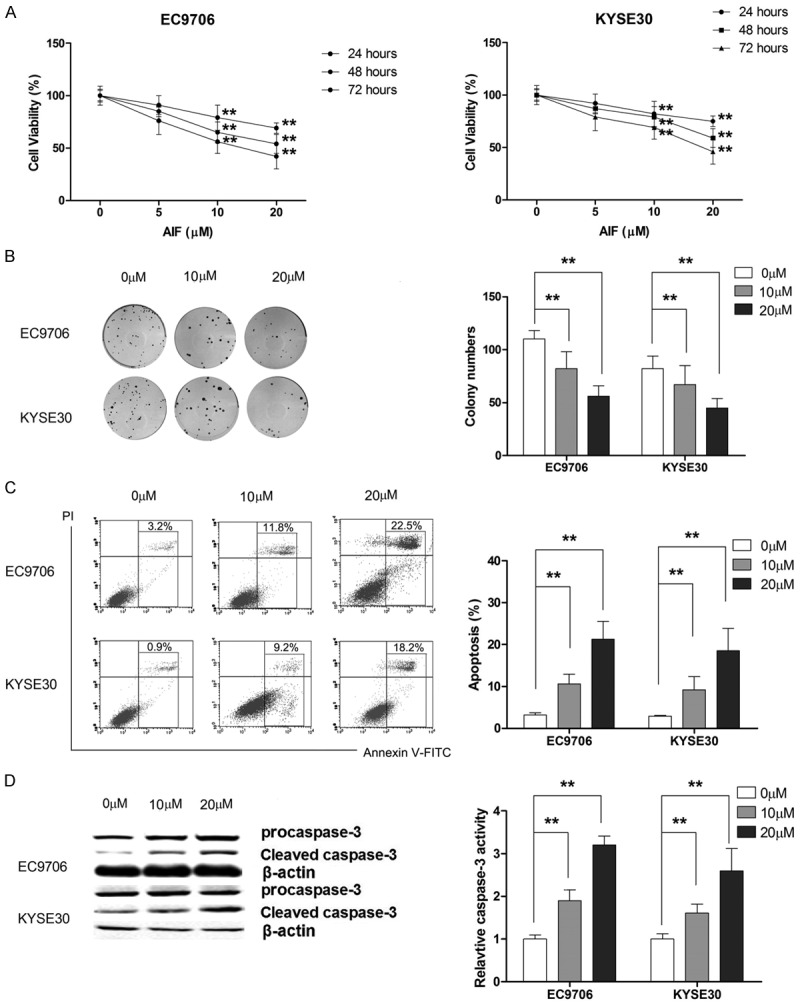
AIF exerts anti-tumor effect in ESCC cells. A. AIF dose-dependently inhibits the proliferation of ESCC cells. B. AIF suppresses cell growth in a dose-dependent manner. C. AIF induces apoptosis in ESCC cells. D. AIF activates caspase-3 in a dose-dependent manner. **P<0.01.
AIF induces apoptosis in ESCC cells by modulating miR-370/PIM1 signaling
To further investigate the mechanism by which AIF exerted its anti-tumor effect, we examined its effect on the expression of miR-370 and PIM1. As shown in Figure 7A-C, AIF dose-dependently increased the expression of miR-370 and repressed the mRNA and protein expression of PIM1. Next, we examined whether AIF exerted its apoptosis-inducing effect by targeting miR-370/PIM1 signaling. Our results showed that miR-370 inhibitor or PIM1 overexpression reversed the loss of cell viability caused by AIF (Figure 7D and 7E). Correspondingly, miR-370 inhibitor or PIM1 overexpression significantly rescued apoptosis and caspase-3 activation induced by AIF in both EC9706 and KYSE30 cells (Figure 7F and 7G). Taken together, our findings suggested that AIF exerted apoptosis-inducing effect by targeting miR-370/PIM1 signaling.
Figure 7.
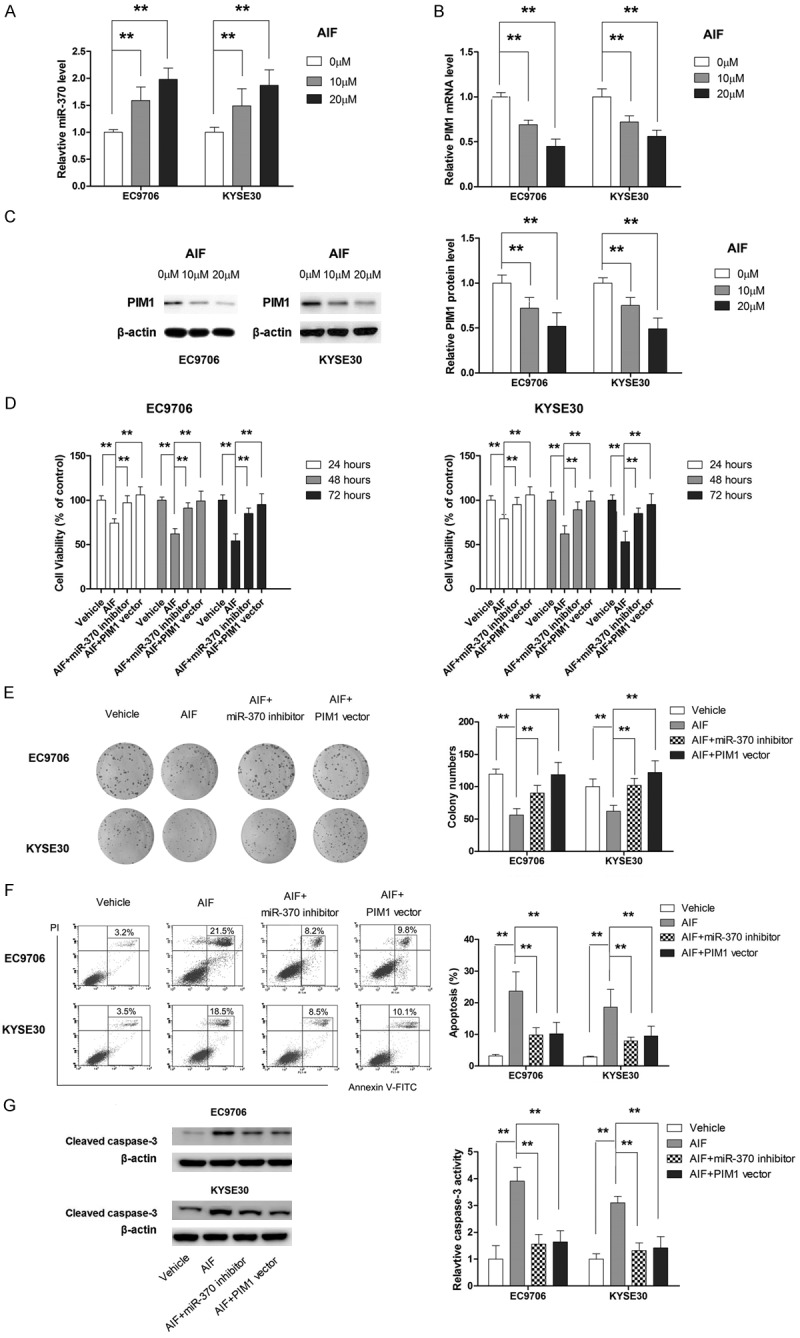
AIF upregulates miR-370 and represses the expression of PIM1. A. AIF dose-dependently increases the expression level of miR-370. B. AIF dose-dependently suppresses the mRNA level of PIM1. C. AIF dose-dependently represses the protein level of PIM1. D. MiR-370 inhibitor or PIM1 overexpression attenuates the anti-proliferative effect of AIF. E. MiR-370 inhibitor or PIM1 overexpression significantly abrogates the anti-growth effect of AIF. F. MiR-370 inhibitor or PIM1 overexpression compromises the apoptosis-inducing effect of AIF. G. MiR-370 inhibitor or PIM1 overexpression impairs the suppressing effect of AIF on invasion. **P<0.01.
AIF exerts anti-tumor effect in vivo
The anti-tumor effect of AIF was evaluated in the xenograft model of ESCC. As shown Figure 8A and 8B, AIF significantly suppressed tumor growth in a dose-dependent manner without affecting the body weight of the animal model. Similar to the observation in tumor growth, TUNEL assay and immunohischemistry assay of cleaved caspase-3 showed that AIF treatment resulted in apoptosis in vivo (Figure 8C and 8D). Moreover, tumor growth inhibition by AIF correlated with decreased expression of PIM1 and upregulation of miR-370 in tissues (Figure 8E and 8F), suggesting that AIF inhibited tumor growth by inducing apoptosis through modulation of miR-370/PIM1 signaling.
Figure 8.
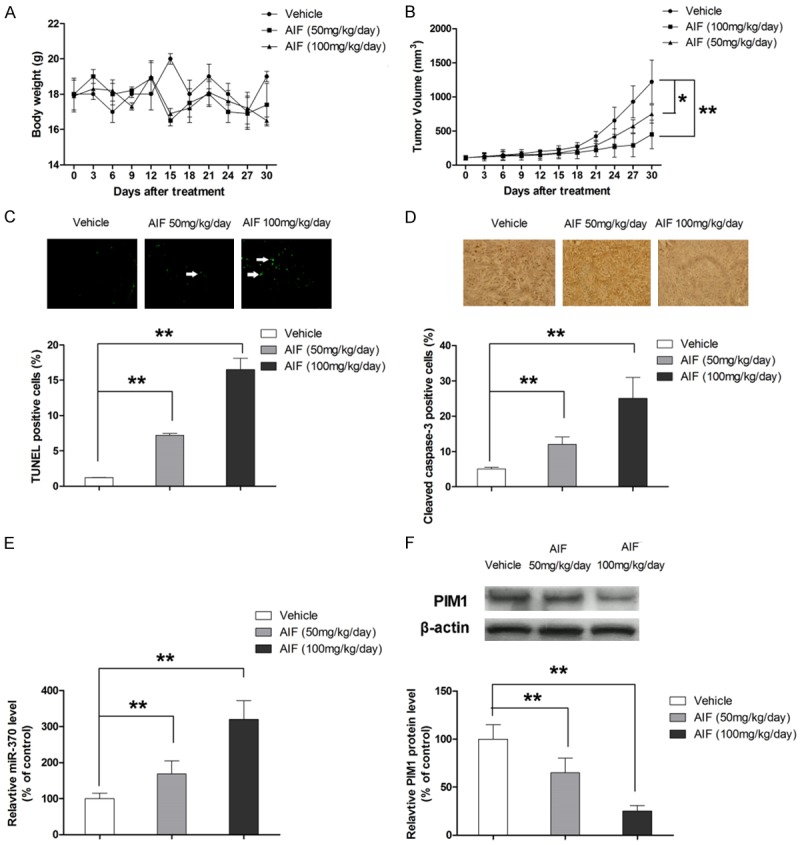
AIF exerts anti-tumor effect in xenograft model of ESCC. A. AIF treatment does not change the body weight of the animal model. B. AIF suppresses tumor growth in a dose-dependent manner. C. AIF induces apoptosis in vivo. D. AIF activates caspase-3 in vivo. E. AIF elevates the expression of miR-370 in tumor tissue. F. AIF suppresses the expression of PIM1 in tumor tissue. *P<0.05, **P<0.01.
Discussion
Although accumulating evidence has demonstrated that miR-370 dysregulation occurs in various types of cancer, its functional role in cancer remains controversial. MiR-370 may play a key role in tumorigenesis and progression by acting as an oncogene or a tumor suppressor gene. For example, miR-370 was downregulated and functioned as a tumor suppressor gene in laryngeal squamous cell carcinoma, Helicobacter pylori-induced gastric carcinoma, and acute myeloid leukemia [6,20,21]. In cholangiocarcinoma, ectopic overexpression of miR-370 was reported to exert an anti-proliferative effect [22]. In hepatocellular carcinoma cells, miR-370 suppressed metastasis by inhibiting migration and invasion [23]. Moreover, restoration of miR-370 was found to resensitize endometrioid ovarian cancer cells to cisplatin [5]. In contrast, numerous studies have demonstrated that miR-370 acts as an oncogenic miRNA. Cao et al. have found that miR-370 overexpression led to enhanced proliferation rate, upregulation of cyclin-dependent kinase inhibitors, and downregulation of cyclin D1 in the Wilms’ tumor G401 cell line, suggesting a pro-proliferative role of miR-370 [24]. In human prostate and gastric cancers, miR-370 was upregulated and functioned as an oncogene by directly regulating FOXO1 [25,26]. A recent study by Sim et al. also identified miR-370 as an oncogenic miRNA in breast cancer [27]. These studies suggested that miR-370 can function as an oncogene or tumor suppressor depending on its key target genes, which may be cell specific. To our knowledge, the clinical significance of miR-370 and its functional role in ESCC have not been investigated. In the present study, the expression of miR-370 was first examined in ESCC tissues and compared with matched distant normal tissues. Our findings revealed that miR-370 was significantly downregulated in ESCC tissues. Next, we established the correlation between the expression pattern of miR-370 and clinicopathological features in ESCC patients, and found that miR-370 was negatively associated with aggressive ESCC. Moreover, ectopic overexpression of miR-370 inhibited proliferation and induced apoptosis, while miR-370 inhibitor promoted cell proliferation and suppressed apoptosis in ESCC cells in vitro. Collectively, our results suggested that miR-370 functioned as a tumor suppressor in ESCC.
Given its regulatory role in various biological activities of cancer cells, PIM1 has been considered a promising target for anticancer drug discovery [9]. Overexpression of PIM1 protein has been implicated in the tumorigenesis of ESCC [10]. Moreover, PIM1 expression was associated with lymphatic metastasis of ESCC, highlighting its potential as a therapeutic target for ESCC. The present study showed that silencing PIM1 expression using shRNA inhibited cell proliferation and induced apoptosis in ESCC cells, whereas overexpressing PIM1 had opposite effects, thus validating the role of PIM1asan oncogene in ESCC. In addition to proliferation and apoptosis, PIM1 is also involved in other physiological behaviors of cancer cells. In hepatocellular carcinoma, PIM1 was involved in cell metabolism and promoted tumor progression by enhancing glycolysis [17]. In nasopharyngeal carcinoma, breast cancer and melanoma, PIM1 has been found to promote cell migration and invasion, highlighting its role in metastasis [15,28,29]. Therefore, by targeting PIM1, miR-370 might also play a key role in cell metabolism and metastasis, although additional data is needed.
Flavonoids, a group of important naturally occurring compounds found in several edible fruits, vegetables and medicinal plants, have been reported to protect against cardiovascular diseases, suppress cancer progression, lower the risk of diabetes, improve cognitive functions following stroke and help in neurodegenerative disorders [30,31]. Interestingly, numerous flavonoids exert anti-cancer effects through modulating miRNAs. For example, quercetin, a flavonoid enriched in onions, apples, tea, and red wine, has been reported to suppress proliferation and invasive behavior by upregulating miR-146a in human breast cancer cells [32]. A later study in pancreatic ductal adenocarcinoma cells (MIA PaCa-2, Capan-1, and S2-013) has also shown that quercetin upregulated miR-142-3p and thus inhibited cell proliferation by reducing the expression of heat-shock protein 70 (HSP70), which is a direct target of miR-142-3p [33]. Another important flavonoid compound, genistein, modulated the expression of miR-23b, miR-27a and miR-223 in various cancerous cells [34-36]. This study showed that AIF inhibited proliferation and induced apoptosis in ESCC through upregulating tumor suppressor miR-370 and subsequently repressing the expression of PIM1. To our knowledge, this is the first experimental evidence showing that AIF could exert anti-tumor effect through modulating miRNA. Moreover, our findings highlight that miRNA signaling pathway might play a key role in regulating response of cancer cells to flavonoids.
In summary, the current study provides novel evidence that miR-370 functions as a tumor suppressor in ESCC and correlates with cancer development of ESCC. This is the first report that miR-370 regulates cell proliferation and apoptosis through directly targeting PIM1. Moreover, our results showed that AIF exerts anti-tumor effect on ESCC by inhibiting cell proliferation and inducing apoptosis via up-regulation of miR-370 and suppression of PIM1 signaling.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31470570, 81603337 and 81473384), Chongqing Natural Science Foundation (No. cstc2014jcyjA80013), Chongqing Creative Program for Graduate Students (CYS15155) and Science Foundation of Chongqing Education Commission (No. kj1400534).
Disclosure of conflict of interest
None.
References
- 1.Sakai NS, Samia-Aly E, Barbera M, Fitzgerald RC. A review of the current understanding and clinical utility of miRNAs in esophageal cancer. Semin Cancer Biol. 2013;23:512–521. doi: 10.1016/j.semcancer.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Kim T, Grobmyer SR, Smith R, Ben-David K, Ang D, Vogel SB, Hochwald SN. Esophageal cancer--the five year survivors. J Surg Oncol. 2011;103:179–183. doi: 10.1002/jso.21784. [DOI] [PubMed] [Google Scholar]
- 4.Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen XP, Chen YG, Lan JY, Shen ZJ. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancer chemosensitivity to cDDP by negatively regulating ENG. Cancer Lett. 2014;353:201–210. doi: 10.1016/j.canlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, Chen C, Jia J. FoxM1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 7.Sun G, Hou YB, Jia HY, Bi XH, Yu L, Chen DJ. MiR-370 promotes cell death of liver cancer cells by Akt/FoxO3 a signalling pathway. Eur Rev Med Pharmacol Sci. 2016;20:2011–2019. [PubMed] [Google Scholar]
- 8.Chen T, Gao F, Feng S, Yang T, Chen M. MicroRNA-370 inhibits the progression of non-small cell lung cancer by downregulating oncogene TRAF4. Oncol Rep. 2015;34:461–468. doi: 10.3892/or.2015.3978. [DOI] [PubMed] [Google Scholar]
- 9.Narlik-Grassow M, Blanco-Aparicio C, Carnero A. The PIM family of serine/threonine kinases in cancer. Med Res Rev. 2014;34:136–159. doi: 10.1002/med.21284. [DOI] [PubMed] [Google Scholar]
- 10.Liu HT, Wang N, Wang X, Li SL. Overexpression of Pim-1 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2010;102:683–688. doi: 10.1002/jso.21627. [DOI] [PubMed] [Google Scholar]
- 11.Mvondo MA, Njamen D, Kretzschmar G, Imma Bader M, Tanee Fomum S, Wandji J, Vollmer G. Alpinumisoflavone and abyssinone V 4’-methylether derived from erythrina lysistemon (fabaceae) promote HDL-cholesterol synthesis and prevent cholesterol gallstone formation in ovariectomized rats. J Pharm Pharmacol. 2015;67:990–996. doi: 10.1111/jphp.12386. [DOI] [PubMed] [Google Scholar]
- 12.Mvondo MA, Njamen D, Tanee Fomum S, Wandji J. Effects of alpinumisoflavone and abyssinone V-4’-methyl ether derived from erythrina lysistemon (fabaceae) on the genital tract of ovariectomized female Wistar rat. Phytother Res. 2012;26:1029–1036. doi: 10.1002/ptr.3685. [DOI] [PubMed] [Google Scholar]
- 13.Kuete V, Mbaveng AT, Nono EC, Simo CC, Zeino M, Nkengfack AE, Efferth T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2016;23:856–863. doi: 10.1016/j.phymed.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Namkoong S, Kim TJ, Jang IS, Kang KW, Oh WK, Park J. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-kappaB pathways in lung tumor cells. Biol Pharm Bull. 2011;34:203–208. doi: 10.1248/bpb.34.203. [DOI] [PubMed] [Google Scholar]
- 15.Deng D, Wang L, Chen Y, Li B, Xue L, Shao N, Wang Q, Xia X, Yang Y, Zhi F. MicroRNA-124-3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci. 2016;107:899–907. doi: 10.1111/cas.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Song Q, Cai Y, Wang P, Wang M, Zhang D. RLIP76-dependent suppression of PI3K/AKT/Bcl-2 pathway by miR-101 induces apoptosis in prostate cancer. Biochem Biophys Res Commun. 2015;463:900–906. doi: 10.1016/j.bbrc.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Leung CO, Wong CC, Fan DN, Kai AK, Tung EK, Xu IM, Ng IO, Lo RC. PIM1 regulates glycolysis and promotes tumor progression in hepatocellular carcinoma. Oncotarget. 2015;6:10880–10892. doi: 10.18632/oncotarget.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 19.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yungang W, Xiaoyu L, Pang T, Wenming L, Pan X. miR-370 targeted FoxM1 functions as a tumor suppressor in laryngeal squamous cell carcinoma (LSCC) Biomed Pharmacother. 2014;68:149–154. doi: 10.1016/j.biopha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Zeng J, Zhou M, Li B, Zhang Y, Huang T, Wang L, Jia J, Chen C. The tumor suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer. 2012;11:56. doi: 10.1186/1476-4598-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An F, Yamanaka S, Allen S, Roberts LR, Gores GJ, Pawlik TM, Xie Q, Ishida M, Mezey E, Ferguson-Smith AC, Mori Y, Selaru FM. Silencing of miR-370 in human cholangiocarcinoma by allelic loss and interleukin-6 induced maternal to paternal epigenotype switch. PLoS One. 2012;7:e45606. doi: 10.1371/journal.pone.0045606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu WP, Yi M, Li QQ, Zhou WP, Cong WM, Yang Y, Ning BF, Yin C, Huang ZW, Wang J, Qian H, Jiang CF, Chen YX, Xia CY, Wang HY, Zhang X, Xie WF. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology. 2013;58:1977–1991. doi: 10.1002/hep.26541. [DOI] [PubMed] [Google Scholar]
- 24.Cao X, Liu D, Yan X, Zhang Y, Yuan L, Zhang T, Fu M, Zhou Y, Wang J. Stat3 inhibits WTX expression through up-regulation of microRNA-370 in Wilms tumor. FEBS Lett. 2013;587:639–644. doi: 10.1016/j.febslet.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012;7:e45825. doi: 10.1371/journal.pone.0045825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, Li Q, Zhang L. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521–526. doi: 10.1016/j.biopha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Sim J, Ahn H, Abdul R, Kim H, Yi KJ, Chung YM, Chung MS, Paik SS, Song YS, Jang K. High MicroRNA-370 expression correlates with tumor progression and poor prognosis in breast cancer. J Breast Cancer. 2015;18:323–328. doi: 10.4048/jbc.2015.18.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rang Z, Yang G, Wang YW, Cui F. miR-542-3p suppresses invasion and metastasis by targeting the proto-oncogene serine/threonine protein kinase, PIM1, in melanoma. Biochem Biophys Res Commun. 2016;474:315–320. doi: 10.1016/j.bbrc.2016.04.093. [DOI] [PubMed] [Google Scholar]
- 29.Jie W, He QY, Luo BT, Zheng SJ, Kong YQ, Jiang HG, Li RJ, Guo JL, Shen ZH. Inhibition of Pim-1 attenuates the proliferation and migration in nasopharyngeal carcinoma cells. Asian Pac J Trop Med. 2012;5:645–650. doi: 10.1016/S1995-7645(12)60132-1. [DOI] [PubMed] [Google Scholar]
- 30.Jones QR, Warford J, Rupasinghe HP, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol Sci. 2012;33:602–610. doi: 10.1016/j.tips.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31:206–238. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- 32.Tao SF, He HF, Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol Cell Biochem. 2015;402:93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 33.MacKenzie TN, Mujumdar N, Banerjee S, Sangwan V, Sarver A, Vickers S, Subramanian S, Saluja AK. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol Cancer Ther. 2013;12:1266–1275. doi: 10.1158/1535-7163.MCT-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, Miele L, Sarkar FH, Xia J, Wang Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. 2013;14:1150–1156. doi: 10.2174/13894501113149990187. [DOI] [PubMed] [Google Scholar]
- 35.Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F, Wang Z, Wang Z. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr Pharm Des. 2014;20:5348–5353. doi: 10.2174/1381612820666140128215756. [DOI] [PubMed] [Google Scholar]
- 36.Avci CB, Susluer SY, Caglar HO, Balci T, Aygunes D, Dodurga Y, Gunduz C. Genistein-induced mir-23b expression inhibits the growth of breast cancer cells. Contemp Oncol (Pozn) 2015;19:32–35. doi: 10.5114/wo.2014.44121. [DOI] [PMC free article] [PubMed] [Google Scholar]


