Abstract
Histone deacetylase inhibitors (HDIs) are a new class of drugs which affect the activity of HDACs resulting in changed of acetylation in many proteins. HDIs can induce differentiation, cell growth arrest, apoptosis, inhibit proliferation and angiogenesis in cancer, whereas normal cells are comparatively resistant to the action of HDIs. The aim of this study was to investigate the combined effect of a well-known cytostatic agent-cisplatin (CDDP) and a histone deacetylase inhibitors-either suberoylanilide hydroxamic acid (SAHA, vorinostat) or valproic acid (VPA), on the proliferation of lung cancer cells, as well as induction of apoptosis and inhibition of the cell cycle progression. The anti-proliferative activity of VPA or SAHA used alone, or in combination with CDDP were determined by means of MTT test. The type of pharmacologic interactions between HDAC inhibitors and CDDP was assessed using isobolographic analysis. We observed additive interactions for the CCDP with SAHA, as well as for the CDDP with VPA combinations with respect to their anti-proliferative effects on three different lung cancer cell lines (A549, NCI-H1563 and NCI-H2170). Such additive effects were observed regardless of the histologic type (adenocarcinoma or squamous cell carcinoma) and sensitivity for the drugs applied. Combination treatment also augmented the induction of apoptosis and cell cycle perturbation mediated by CDDP alone, thereby enhancing anti-cancer effect of tested drugs. In conclusion, the combined therapy of HDIs and CDDP may be a promising therapeutic tool in the treatment of lung cancer.
Keywords: Isobolographic analysis, histone deacetylase inhibitors (HDIs), valproic acid (VPA), suberoylanilide hydroxamid acid (SAHA), cisplatin (CDDP), lung cancer
Introduction
Lung carcinoma is the most frequent cancer cause of death worldwide (26% of all female and 28% of all male cases) [1-3]. In the current treatment for many tumors, including lung cancer, cisplatin (CDDP) is a widely used drug. CDDP blocks cell division, inhibits DNA replication and induces apoptosis [4,5]. This routine chemotherapy is limited due to serious adverse effects and the development of cisplatin resistance [6,7]. Thereby, new therapeutic strategies, including targeted therapy, are being looked for. Histone deacetylase inhibitors (HDIs) are new promising cytostatic agents that interfere with the activity of histone deacetylases (HDACs) [8]. The sodium salt of 2-propylpentanoic acid (VPA, valproic acid) for decades has been used for treatment of epilepsy and bipolar disorders [9]. It has also been shown that VPA can suppress neoplastic cell growth and induce apoptosis in vitro, and in vivo [8,10]. VPA binds to the catalytic pocket of lysine HDACs forming complexes with Zn2+ via its carboxyl group and inhibit their activity. VPA-induced inhibition of HDACs result in hyperacetylation of histone N-terminal tails, which gives a negative charge and decreases their affinity for DNA, leading to decondensation of chromatin and transcriptional activation [11-15]. There are several mechanisms, which could be responsible for anti-cancer action of VPA, often depending on target cancer cell types. For example, VPA induces cell cycle arrest due to increase of p21, p27 cell cycle inhibitors and decrease in cyclin D1 expression [16-18]. It was also reported, that VPA treatment resulted in down-regulation of anti-apoptotic protein survivin [19] and SMAD4 transcription factor [20].
Suberoylanilide hydroxamic acid (SAHA, vorinostat, Zolizna®) is the first HDI approved by FDA for the treatment of cutaneous T-cell lymphoma. It has also been shown that SAHA has anticancer effect in lung cancer cells [21,22]. Like VPA, SAHA seems to induce the expression of many factors involved in apoptosis, differentiation and growth suppression as demonstrated in several cancer cell lines, including A549 lung cancer cells [8,21]. It was also reported that, SAHA’s inhibition of HDACs suppresses telomerase activity by decreasing the expression of telomerase reverse transcriptase via epigenetic regulation mechanism in adenocarcinoma A549 lung cells [8,23,24]. In other cancer cell types, SAHA downregulated cell cycle-associated factors that modulate the G1/S transition, including cyclin D1 and cdc25a, and the G2/M transition (cyclin B1, Plk1, Aurora kinase A) in breast cancer cells [25], inhibited HIF-1α expression in liver cancer-derived cell lines [26], upregulated MHC class I-related chain molecules A and B expression through miR-17-92 cluster, thereby enhancing the sensitivity of hepatoma to natural killer cell-mediated lysis [27], or triggered autophagy through the downregulation of AKT-MTOR signaling in glioblastoma stem cells [28].
Parallel to the development and introduction of new types of chemotherapeutics, the combination of HDIs with other anticancer drugs has been considered as a new promising strategy in cancer treatment [29]. In our study, we examined the anti-proliferative activity of VPA and SAHA used alone, or in combination with CDDP using isobolographic protocol in A549, NCI-H1563 and NCI-H2170 lung cancer cell lines, supplemented by apoptosis induction and cell cycle inhibition analyses.
Materials and methods
Cell lines
All non-small lung cancer cell lines: A549 human adenocarcinoma (ATCC® CCL185™), NCI-H1563 human adenocarcinoma (ATCC® CRL5875™), and NCI-H2170 human squamous cell carcinoma (ATCC® CRL5928™) were obtained from the American Type Culture Collection (Manassas, VA). A549 cell line was grown in DMEM/F12 culture medium (Sigma, St Louis, MO, USA), NCI-H1563 and NCI-H2170 were maintained in RPMI1640 (Sigma) culture medium with additional 1 mM sodium pyruvate and 2.5 g/L glucose (Sigma). All cells were cultured in medium supplemented with 10% fetal bovine serum (FBS), streptomycin (100 µg/mL) plus penicillin (100 IU/mL) (Sigma). Mycoplasma-free cultures were kept in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Drugs
CDDP and VPA were purchased from Sigma (St. Louis, MO, USA) and dissolved in phosphate buffered saline (PBS) with Ca2+ and Mg2+ as 1 mg/ml, and 100 mM stock solution, respectively. SAHA was purchased from Cayman Chemical (San Diego, CA, USA), and was diluted in dimethyl sulfoxide (DMSO) at 10 mM as stock solution. The drugs were dissolved to the respective concentration with culture medium before use.
Cell viability assay
Cancer cells were plated on 96-well microplates at a density of 1 × 104 cells/ml (A549, NCIH2170) and 4 × 104 cells/ml (NCIH1563). The cell viability was determined using the MTT assay as we described previously [30].
Isobolographic analysis of interactions
The percent inhibition of cell viability per dose of CDDP, SAHA or VPA administered alone and the dose-response relationship curves (DRRCs) for each investigated drug in A549, NCI-H1563 and NCI-H2170 cell lines measured by the MTT assay were fitted using log-probit linear regression analysis according to Litchfield and Wilcoxon [31]. Subsequently, from the respective linear equations the median inhibitory con-centrations (IC50s) of CDDP, SAHA or VPA administered alone were calculated. Next, the test for parallelism of DRRCs for CDDP and SAHA or VPA based on the log-probit analysis was used, as described in detail in our previous studies [32,33]. Isobolographic interactions between CDDP and SAHA or VPA were analyzed according to the methodology described by Grabovsky and Tallarida [34], Tallarida [35,36] and Luszczki [32]. Based upon the IC50 values denoted experimentally for the drugs administered alone, median additive inhibitory concentrations of the mixture of CDDP with SAHA or VPA-i.e., concentrations of the mixture, which theoretically should inhibit cell viability in 50% (IC50 add) were calculated from two equations of additivity presented by Tallarida [32,33]. The evaluation of the experimentally-derived IC50 mix at the fixed-ratio of 1:1 was based upon the concentration of the mixture inhibiting 50% of cell viability in investigated cell lines measured by the MTT assay. Finally, to determine the separate concentrations of CDDP and SAHA or VPA in the mixture, the IC50 mix values were multiplied by the respective proportions of drugs (denoted for additive mixture). Further details regarding these concepts have been published elsewhere [32,35,36].
Cell cycle analysis by flow cytometry
To assess cell cycle distribution, lung cancer cell lines were seeded on 6-well plates (Nunc) at a density of 0.5 × 105/ml. Next day cell lines were treated with different concentrations of VPA, SAHA and CDDP alone or in combination (SAHA/CDDP and VPA/CDDP) for 48 hours and then fixed (for 48 h) in ice-cold 80% ethanol at -20°C. After fixation, the treated cells were stained with propidium iodide utilizing the PI/RNase Staining Buffer (BD Biosciences) according to the manufacturer’s instructions as we described previously [30].
Analysis of apoptosis
Examined cell lines were seeded on 6-well plates (Nunc) at a density of 0.5 × 105/ml and then treated with VPA, SAHA and CDDP alone or in combination (SAHA/CDDP and VPA/CDDP) for 48 hours. The measurement of apoptosis was conducted according to the manufacturer’s instructions of PE Active Caspase-3 Apoptosis Kit (BD Pharmingen) as we described previously [30].
Statistical analysis
The IC50 and IC50 mix values for CDDP, SAHA and VPA administered alone or in combination at the fixed-ratio of 1:1 were calculated by computer-assisted log-probit analysis according to Litchfield and Wilcoxon [32]. The experimentally-derived IC50 mix values for the mixture of CDDP with SAHA or VPA were statistically compared with their respective theoretical additive IC50 add values by the use of unpaired Student’s t-test, according to Tallarida [37].
Results were analyzed using GraphPad Prism software (one-way ANOVA; Tukey post-hoc testing). Statistical differences were considered relevant at P<0.05. Findings were presented as mean ± standard deviation of the mean (± SD).
Results
VPA, SAHA and CDDP decrease proliferation of the A549, NCI-H1563, NCI-H2170 lung cancer cell lines
The antiproliferative activity of VPA, SAHA and CDDP was assessed in a variety of lung cancer cell lines using the MTT assay. All cancer cells were exposed to either culture medium (control) or increasing concentrations of VPA: [A549 (16.619-1661.6 μg/ml; equivalent to 0.1-10 mM), NCI-H1563 (16.619-1994.28 μg/ml; 0.1-12 mM), NCI-H2170 (16.619-830.95; 0.1-5 mM)], SAHA: A549, NCI-H1563 (0.02643-2.643 μg/ml; 0.1-10 μM), NCI-H2170 (0.02643-1.3215 μg/ml; 0.1-5 μM) and CDDP: A549, NCI-H2170 (0.01-5 μg/ml), NCI-H1563 (0.01-10 μg/ml) for 96 h. We have shown a decrease of proliferation of all cancer cell lines after VPA, SAHA, and CDDP treatment in the dose-dependent manner (Figure 1A). IC50 values for all investigated cell lines were established and depicted in Table 1. NCI-H2170 was the most sensitive cell line both to VPA, SAHA, and CDDP treatment. NCI-H1563 was the least sensitive cell line to all drugs treatment (Tables 1, 2 and 3).
Figure 1.
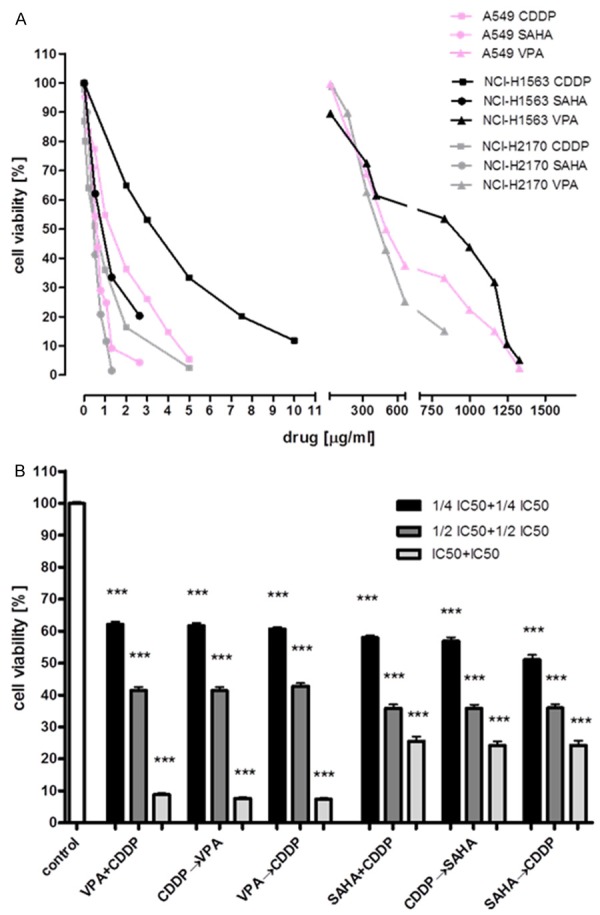
The anti-proliferative effects of HDIs and CDDP against studied lung cancer cell lines. Inhibition of the proliferation of A549, NCI-H1563, NCI-H2170 cancer cells measured by the MTT assay (A). Anticancer activity of HDIs and CDDP occurs in order of treatment independent manner. A549 cell line was treated with both HDIs and CDDP (VPA + CDDP; SAHA + CDDP) in culture medium in doses of 1/4 IC50 + 1/4 IC50, 1/2 IC50 + 1/2 IC50, IC50 + IC50. Next, A549 cell line was treated with CDDP in culture medium and after 1 hour one of HDIs was added to medium containing CDDP (CDDP→VPA; CDDP→SAHA). Parallel, A549 cell line was treated with one of HDIs in medium and after 1 hour CDDP was added to medium containing VPA (VPA→CDDP; SAHA→CDDP). The same doses were used (B).
Table 1.
Anti-proliferative effects of CDDP, SAHA and VPA administered singly in three lung cancer cell lines (A549, NCIH-1563, NCI-H2170) measured in vitro by the MTT assay
| Cell line | Drug | IC50 (μg/ml) | n | CFP | q/p | S.R. | f ratio S.R. | Parallelism* |
|---|---|---|---|---|---|---|---|---|
| A549 | CDDP | 1.184 ± 0.333 | 64 | 1.126 (q) | - | - | - | - |
| A549 | SAHA | 0.560 ± 0.069 | 80 | 2.301 (p1) | 0.489 | 2.265 | 1.495 | NP |
| A549 | VPA | 485.6 ± 87.95 | 64 | 1.424 (p2) | 0.791 | 1.763 | 1.523 | NP |
| NCI-H1563 | CDDP | 2.989 ± 0.577 | 75 | 5.237 (q) | - | - | - | - |
| NCI-H1563 | SAHA | 0.723 ± 0.169 | 80 | 0.626 (p1) | 8.366 | 1.346 | 1.559 | P |
| NCI-H1563 | VPA | 847.2 ± 203.0 | 104 | 2.104 (p2) | 2.489 | 1.726 | 1.835 | P |
| NCI-H2170 | CDDP | 0.350 ± 0.123 | 80 | 0.538 (q) | - | - | - | - |
| NCI-H2170 | SAHA | 0.460 ± 0.052 | 80 | 2.891 (p1) | 0.186 | 4.542 | 1.607 | NP |
| NCI-H2170 | VPA | 415.7 ± 51.87 | 64 | 2.218 (p2) | 0.243 | 4.567 | 1.604 | NP |
Results are presented as median inhibitory concentrations (IC50 values in μg/ml ± S.E.M.) of CDDP, SAHA and VPA administered singly with respect to their anti-proliferative effects in three cancer cell lines (A549, NCI-H1563, NCI-H2170) measured in vitro by the MTT assay. n-total number of items used at concentrations whose expected anti-proliferative effects ranged between 4 and 6 probits (16% and 84%); CFP-(q and p) curve-fitting parameters; q/p-ratio of q and p values; S.R.-slope function ratio (SCDDP/SSAHA, SCDDP/SVPA); f ratio S.R.-factor for slope function ratio. Test for parallelism of two dose-response relationship curves (DRRCs) was performed according to Litchfield and Wilcoxon (1949). It this case, if the slope function ratio (S.R.) value is higher than the factor for slope function ratio (f ratio S.R.) value, the examined two DRRCs are not parallel to each other. Otherwise, the examined two DRRCs are parallel to one another (Litchfield and Wilcoxon 1949). NP-not parallel; P-parallel;
All detailed calculations required to test the parallelism of two DRRCs were presented in the Appendix to the paper by Luszczki and Czuczwar (2006).
Table 2.
Type I isobolographic analysis of interactions (for non-parallel DRRCs) between CDDP and SAHA or VPA at the fixed-ratio combination of 1:1 in two cancer cell lines (A549 and NCI-H2170) measured in vitro by the MTT assay
| Cell line | Combination | IC50 mix (μg/ml) | n mix | #IC50 add (μg/ml) | n add | &IC50 add (μg/ml) | n add |
|---|---|---|---|---|---|---|---|
| A549 | CDDP + SAHA | 0.529 ± 0.174 | 80 | 0.660 ± 0.197 | 140 | 1.085 ± 0.285 | 140 |
| A549 | CDDP + VPA | 213.1 ± 55.10 | 64 | 223.5 ± 78.93 | 124 | 263.2 ± 84.36 | 124 |
| NCI-H2170 | CDDP + SAHA | 0.387 ± 0.061 | 80 | 0.190 ± 0.150 | 156 | 0.620 ± 0.136 | 156 |
| NCI-H2170 | CDDP + VPA | 222.3 ± 28.05 | 80 | 112.6 ± 54.85 | 140 | 303.2 ± 87.49 | 140 |
Results are presented as median inhibitory concentrations (IC50 values in μg/ml ± S.E.M.) for two-drug mixtures, determined either experimentally (IC50 mix) or theoretically calculated (IC50 add) from the equations of additivity (Tallarida 2006, 2007), blocking proliferation in 50% of tested cells in two cancer cell lines (A549 and CRL5928) measured in vitro by the MTT assay. n mix-total number of items used at those concentrations whose expected anti-proliferative effects ranged between 16% and 84% (i.e., 4 and 6 probits) for the experimental mixture; n add-total number of animals calculated for the additive mixture of the drugs examined (n add = n -CDDP + n -SAHA - 4) or (n add = n -CDDP + n -VPA - 4);
IC50 add value calculated from the equation for the lower line of additivity;
IC50 add value calculated from the equation for the upper line of additivity.
Statistical evaluation of data was performed with unpaired Student’s t-test according to Tallarida (2000). There was no statistical difference between the IC50 mix and IC50 add values with unpaired Student’s t-test and thus, the analyzed interactions were additive in two cancer cell lines (A549 and NCI-H2170) as measured by the MTT assay in vitro.
Table 3.
Type I isobolographic analysis of interactions (for parallel DRRCs) between CDDP and SAHA or VPA at the fixed-ratio combination of 1:1 in a cancer cell line (NCI-H1563) measured in vitro by the MTT assay
| Cell line | Combination | IC50 mix (μg/ml) | n mix | IC50 add (μg/ml) | n add |
|---|---|---|---|---|---|
| NCI-H1563 | CDDP + SAHA | 1.768 ± 0.254 | 64 | 1.856 ± 0.373 | 151 |
| NCI-H1563 | CDDP + VPA | 455.1 ± 46.79 | 80 | 425.1 ± 101.8 | 175 |
Results are presented as median inhibitory concentrations (IC50 values in μg/ml ± S.E.M.) for two-drug mixtures, determined either experimentally (IC50 mix) or theoretically calculated (IC50 add) from the equations of additivity (Tallarida 2006, 2007), blocking proliferation in 50% of tested cells in a cancer cell line (NCI-H1563) measured in vitro by the MTT assay. n mix-total number of items used at those concentrations whose expected anti-proliferative effects ranged between 16% and 84% (i.e., 4 and 6 probits) for the experimental mixture; n add-total number of animals calculated for the additive mixture of the drugs examined (n add = n -CDDP + n -SAHA - 4) or (n add = n -CDDP + n -VPA - 4); Statistical evaluation of data was performed with unpaired Student’s t-test according to Tallarida (2000). There was no statistical difference between the IC50 mix and IC50 add values with unpaired Student’s t-test and thus, the analyzed interactions between drugs were additive in a cancer cell line (NCI-H1563) as measured by the MTT assay in vitro.
In the next experiments we examined if the order of treatment will affect the cytotoxicity of a mixture of HDIs with CDDP. A549 cells were treated with combinations of various doses of VPA/CDDP and SAHA/CDDP in culture medium for 96 hours in three diverse orders: 1) - VPA (or SAHA) and CDDP were added to the cell cultures at the same time; 2) the cells were pre-incubated for 1 hour with CDDP, followed by addition of VPA (or SAHA); 3) or treated in opposite order-first incubated with VPA (or SAHA) for one hour, then with CDDP. Regardless of the order of compounds (HDIs, CDDP) added in the 1 hour interval to the cell cultures, we did not observe changes in the inhibition of cellular proliferation (Figure 1B).
Anti-proliferative action of SAHA administered singly and in combination with CDDP in the A549 cell line
CDDP administered alone produced anti-proliferative effects in the A549 cells. The equation of DRRC for CDDP (y = 1.4459 x + 4.8937; Figure 2A), allowed the determination of the IC50 value for CDDP, which was 1.184 ± 0.333 μg/ml (Table 1). Similarly, SAHA administered alone produced anti-proliferative effects on A549 cells. The equation of DRRC for SAHA (y = 2.9717 x + 5.7478; Figure 2A) allowed the calculation of its IC50 value that amounted to 0.560 ± 0.069 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and SAHA revealed that the DRRCs of both compounds were non-parallel to each other (Table 1; Figure 2A). In this case, the combination of CDDP with SAHA at the fixed-ratio of 1:1 produced the anti-proliferative effects in the A549 cells and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 1.1037 x + 5.3049; Figure 2A) was 0.529 ± 0.174 μg/ml (Table 2).
Figure 2.
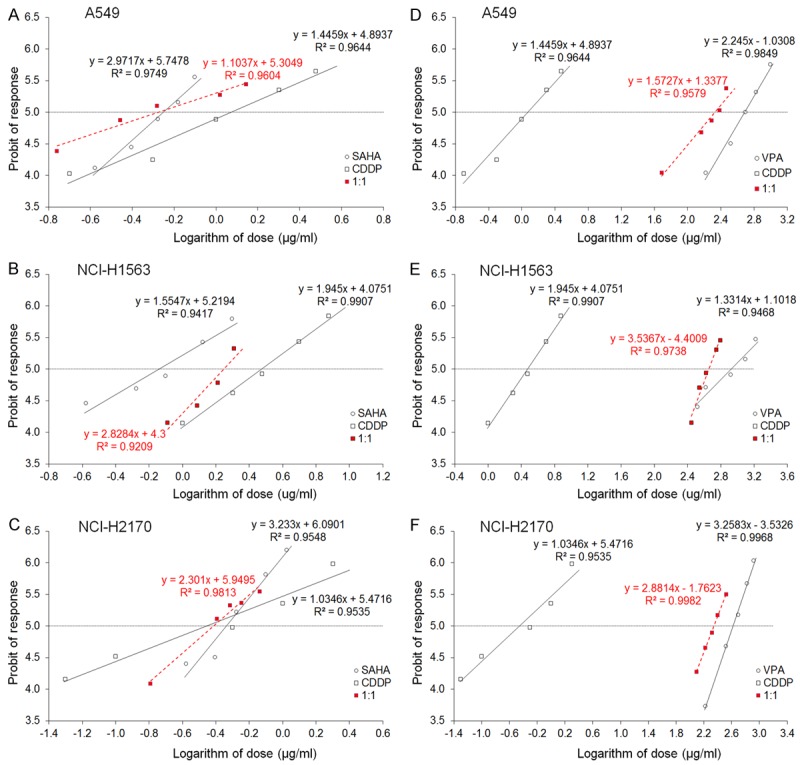
A-F. Log-probit dose-response relationship curves (DRRCs) for CDDP, SAHA and VPA administered alone, and in combinations at the fixed-ratio of 1:1 (in red), illustrating the anti-proliferative effects of the drugs in three cancer cell lines (A549, NCI-H1563 and NCI-H2170) measured in vitro by the MTT assay (A-F). Doses of CDDP, SAHA and VPA administered separately and the mixture of the drugs at the fixed-ratio combination of 1:1 (in red) were transformed into logarithms, whereas the anti-proliferative effects produced by the drugs in three cancer cell lines (A549, NCI-H1563 and NCI-H2170) measured in vitro by the MTT assay were transformed into probits according to Litchfield and Wilcoxon (1949). Linear regression equations of DRRCs are presented on the graph; where y-is the probit of response, and x-is the logarithm (to the base 10) of a drug dose, R2-coefficient of determination. Test for parallelism revealed that the experimentally determined DRRCs for CDDP, SAHA and VPA (administered alone) in two cancer cell lines (A549 and NCI-H2170) are not parallel to one another. Only the DRRCs for CDDP, SAHA and VPA (administered alone) in the cancer cell line (NCI-H1563) are parallel to each other (for more details see Table 1).
Anti-proliferative action of VPA administered singly and combined with CDDP in the A549 cell line
VPA administered alone reduced the proliferation of A549 cells. The equation of DRRC for VPA (y = 2.245 x - 1.0308; Figure 2D) allowed the calculation of its IC50 value that amounted to 485.6 ± 87.95 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and VPA revealed that the DRRCs of both compounds were non-parallel to each other (Table 1; Figure 2B). In this case, the combination of CDDP with VPA at the fixed-ratio of 1:1 produced the anti-proliferative effects in the A549 cells and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 1.5727 x + 1.3377; Figure 2D) was 213.1 ± 55.10 μg/ml (Table 2).
Anti-proliferative action of SAHA administered singly and combined with CDDP in the NCI-H1563 cell line
CDDP administered alone produced anti-proliferative effects on NCI-H1563 cells. The equation of DRRC for CDDP (y = 1.945 x + 4.0751; Figure 2B), allowed the determination of the IC50 value for CDDP, which was 2.989 ± 0.577 μg/ml (Table 1). Similarly, SAHA administered alone produced anti-proliferative effects on NCI-H1563 cells. The equation of DRRC for SAHA (y = 1.5547 x + 5.2194; Figure 2B) allowed the calculation of its IC50 value that amounted to 0.723 ± 0.169 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and SAHA revealed that the DRRCs of both compounds were parallel to each other (Table 1; Figure 2B). In this case, the combination of CDDP with SAHA at the fixed-ratio of 1:1 produced the anti-proliferative effects on NCI-H1563 cells and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 2.8284 x + 4.3; Figure 2B) was 1.768 ± 0.254 μg/ml (Table 3).
Anti-proliferative action of VPA administered singly and combined with CDDP in the NCI-H1563 cell line
The anti-proliferative effect in the NCI-H1563 cells by VPA administered alone was observed. The equation of DRRC for VPA (y = 1.3314 x + 1.1018; Figure 2E) allowed the calculation of its IC50 value that amounted to 847.2 ± 203.0 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and VPA revealed that the DRRCs of both compounds were parallel to each other (Table 1; Figure 2E). In this case, the combination of CDDP with VPA at the fixed-ratio of 1:1 produced the anti-proliferative effects in the NCI-H1563 cell line and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 3.5367 x - 4.4009; Figure 2E) was 455.1 ± 46.79 μg/ml (Table 3).
Anti-proliferative action of SAHA administered singly and combined with CDDP in the NCI-H2170 cell line
CDDP administered alone produced anti-proliferative effects on NCI-H2170 cells. The equation of DRRC for CDDP (y = 1.0346 x + 5.4716; Figure 2C), allowed the determination of the IC50 value for CDDP, which was 0.350 ± 0.123 μg/ml (Table 1). Similarly, SAHA administered alone produced anti-proliferative effects on NCI-H2170 cells. The equation of DRRC for SAHA (y = 3.233 x + 6.0901; Figure 2C) allowed the calculation of its IC50 value that amounted to 0.460 ± 0.052 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and SAHA revealed that the DRRCs of both compounds were non-parallel to each other (Table 1; Figure 2C). In this case, the combination of CDDP with SAHA at the fixed-ratio of 1:1 produced the anti-proliferative effects on NCI-H2170 cells and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 2.301 x + 5.9495; Figure 2C) was 0.387 ± 0.061 μg/ml (Table 2).
Anti-proliferative action of VPA administered singly and combined with CDDP to the NCI-H2170 cell line
The anti-proliferative effect in the NCI-H2170 cells by VPA administered alone was observed. The equation of DRRC for VPA (y = 3.2583 x - 3.5326; Figure 2F) allowed the calculation of its IC50 value that amounted to 415.7 ± 51.87 μg/ml (Table 1). The test for parallelism of DRRCs between CDDP and VPA revealed that the DRRCs of both compounds were non-parallel to each other (Table 1; Figure 2F). In this case, the combination of CDDP with VPA at the fixed-ratio of 1:1 produced the anti-proliferative effects in the NCI-H2170 cell line and the IC50 mix value calculated from the DRRC for the mixture of both compounds (y = 2.8814 x - 1.7623; Figure 2F) was 222.3 ± 28.05 μg/ml (Table 2).
Isobolographic analysis of interactions between CDDP and SAHA in the A549 cells
The isobolographic analysis of interaction for non-parallel DRRCs revealed that the mixture of CDDP with SAHA at the fixed-ratio of 1:1 exerted additive interaction in the A549 cells (Figure 3A). The experimentally derived IC50 mix value for this fixed-ratio combination was 0.529 ± 0.174 μg/ml, whereas the additively calculated IC50 add values were 0.660 ± 0.197 μg/ml (for the lower IC50 add) and 1.085 ± 0.285 μg/ml (for the upper IC50 add; Table 2). Thus, the IC50 mix value did not significantly differ from the IC50 add values (Table 2; Figure 3A).
Figure 3.
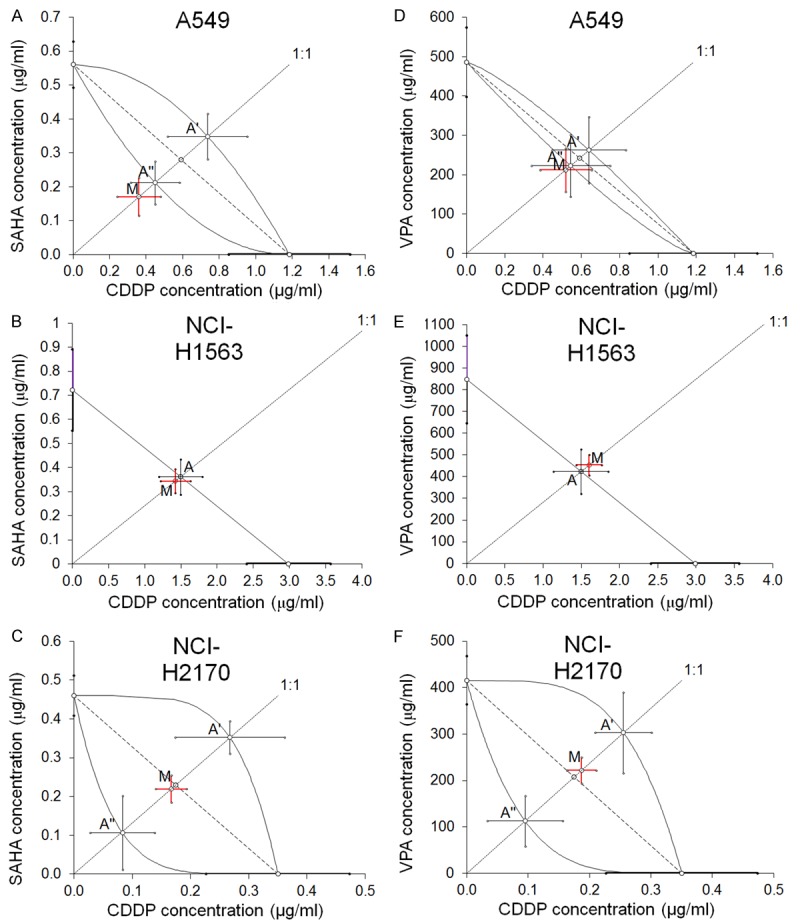
A-F. Isobolograms showing additive interactions between CDDP, SAHA and VPA with respect to their anti-proliferative effects on three cancer cell lines (A549, NCI-H1563 and NCI-H2170) measured in vitro by the MTT assay. The median inhibitory concentrations (IC50) for CDDP, SAHA and VPA are plotted graphically on the X- and Y-axes, respectively. The solid lines on the X and Y axes represent the S.E.M. for the IC50 values for the studied drugs administered alone. The lower and upper isoboles of additivity represent the curves connecting the IC50 values for CDDP and SAHA or VPA administered alone. The dotted line starting from the point (0, 0) corresponds to the fixed-ratio of 1:1 for the combination of CDDP with SAHA or VPA. The diagonal dashed line connects the IC50 for CDDP and SAHA or VPA on the X- and Y-axes. The points A’ and A” (A, C, D, F) depict the theoretically calculated IC50 add values for both, lower and upper isoboles of additivity. The point A (B, E) depicts the theoretically calculated IC50 add value. The point M represents the experimentally-derived IC50 mix value for total dose of the mixture expressed as proportions of CDDP and SAHA or VPA that produced a 50% anti-proliferative effect (50% isobole) in three cancer cell lines (A549, NCI-H1563 and NCI-H2170) measured in vitro by the MTT assay. On the graph, the S.E.M. values are presented as horizontal and vertical error bars for every IC50 value. The experimentally-derived IC50 mix value is placed close to the points: A” (A, D), A (B, E) and within the area bounded by two lines of additivity (C, F), indicating additive interaction between CDDP and SAHA or VPA in three cancer cell lines (A549, NCI-H1563 and NCI-H2170) measured in vitro by the MTT assay.
Isobolographic analysis of interaction between CDDP and VPA in the A549 cells
The isobolographic analysis of interaction for non-parallel DRRCs revealed that the mixture of CDDP with VPA at the fixed-ratio of 1:1 exerted additive interaction on A549 cells (Figure 3D). The experimentally derived IC50 mix value for this fixed-ratio combination was 213.1 ± 55.10 μg/ml, whereas the additively calculated IC50 add values were 223.5 ± 78.93 μg/ml (for the lower IC50 add) and 263.2 ± 84.36 μg/ml (for the upper IC50 add; Table 2). Thus, the IC50 mix value did not significantly differ from the IC50 add values (Table 2; Figure 3D).
Isobolographic analysis of interaction between CDDP and SAHA in the NCI-H1563 cells
The isobolographic analysis of interaction for parallel DRRCs revealed that the mixture of CDDP with SAHA at the fixed-ratio of 1:1 exerted additive interaction on NCI-H1563 cells (Figure 3B). The experimentally derived IC50 mix value for this fixed-ratio combination was 1.768 ± 0.254 μg/ml, whereas the additively calculated IC50 add value was 1.856 ± 0.373 μg/ml (Table 3). Thus, the IC50 mix value did not significantly differ from the IC50 add value (Table 3; Figure 3B).
Isobolographic analysis of interaction between CDDP and VPA in the NCI-H1563 cells
The isobolographic analysis of interaction for parallel DRRCs revealed that the mixture of CDDP with VPA at the fixed-ratio of 1:1 exerted additive interaction in the NCI-H1563 cell line (Figure 3E). The experimentally derived IC50 mix value for this fixed-ratio combination was 455.1 ± 46.79 μg/ml, whereas the additively calculated IC50 add value was 425.1 ± 101.8 μg/ml (Table 3). Thus, the IC50 mix value did not significantly differ from the IC50 add value (Table 3; Figure 3E).
Isobolographic analysis of interaction between CDDP and SAHA in the NCI-H2170 cells
The isobolographic analysis of interaction for non-parallel DRRCs revealed that the mixture of CDDP with SAHA at the fixed-ratio of 1:1 exerted additive interaction NCI-H2170 cells (Figure 3C). The experimentally derived IC50 mix value for this fixed-ratio combination was 0.387 ± 0.061 μg/ml, whereas the additively calculated IC50 add values were 0.190 ± 0.150 μg/ml (for the lower IC50 add) and 0.620 ± 0.136 μg/ml (for the upper IC50 add; Table 2). Thus, the IC50 mix value did not significantly differ from the IC50 add values (Table 2; Figure 3C).
Isobolographic analysis of interaction between CDDP and VPA in the NCI-H2170 cells
The isobolographic analysis of interaction for non-parallel DRRCs revealed that the mixture of CDDP with VPA at the fixed-ratio of 1:1 exerted additive interaction in the NCI-H2170 cell line (Figure 3F). The experimentally derived IC50 mix value for this fixed-ratio combination was 222.3 ± 28.05 μg/ml, whereas the additively calculated IC50 add values were 112.6 ± 54.85 μg/ml (for the lower IC50 add) and 303.2 ± 87.49 μg/ml (for the upper IC50 add; Table 2). Thus, the IC50 mix value did not significantly differ from the IC50 add values (Table 2; Figure 3F).
Effects of HDIs/CDDP on cell cycle arrest
In order to estimate changes in the cell cycle progression, the most sensitive lung cancer cell line A549 was incubated with SAHA, VPA and CDDP alone or in combination. According to data obtained from isobolographic analysis we choose the drug combination ratio 1.4 (70% IC50) and 2.4 (120% IC50). The effect of FACS analysis of PI-stained cells indicated that treatment with SAHA of both concentrations ratio of 70% IC50 and 120% IC50 did not induce significant changes in the cell cycle progression in comparison to control. Incubation with CDDP led to accumulation of cells in the G2 phase, simultaneously reducing the number of cells in G1 phase, in dose-dependent manner. Despite relatively high concentrations of VPA (1.4 and 2.4) there was observed a marginal increase of cells in G1 phase. Incubation of A549 cells with HDIs and CDDP applied together showed a very clear tendency to maintain or improve the effect induced by CDDP alone (Figure 4A, 4B).
Figure 4.
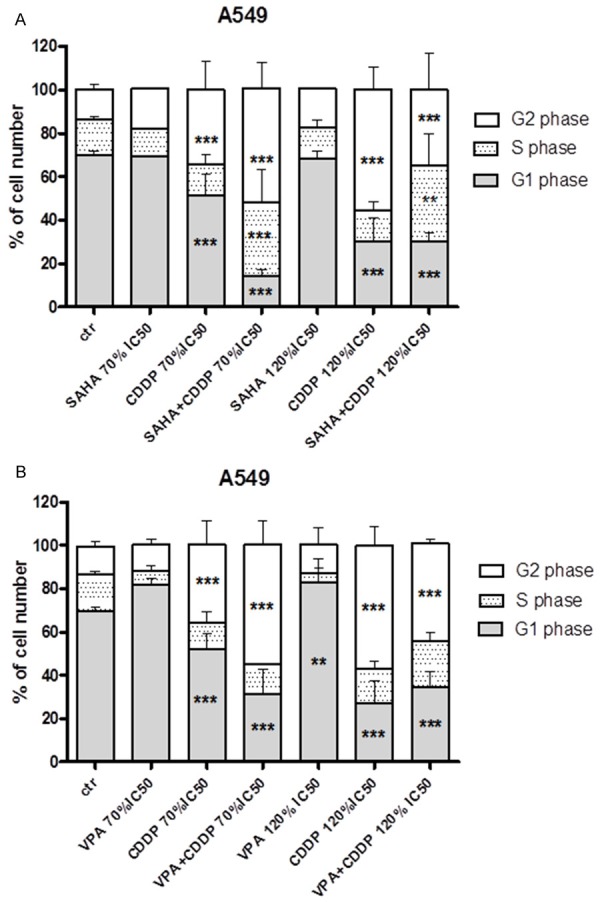
A, B. Effects of HDIS and CDDP on cell cycle arrest in A549 cells. Lung cancer cell line was incubated for 48 h with HDIs and CDDP alone or in combination in concentration 1.4 (70% IC50) and 2.4 (120% IC50) respectively. Data was analyzed by flow cytometry and results are expressed as mean ± SD of three separate experiments (n = 6 per concentration, **P<0.01, ***P<0.001 versus the control, one-way ANOVA test).
Treatment of HDIs and CDDP induced apoptosis
The exposure of analyzed lung cancer cell line A549 to SAHA and VPA in combination with CDDP after 48h cased dose-dependent activation of caspase-3. VPA treatment alone at concentrations of 1.4 (70% IC50) induced slight increase of apoptosis, whereas other concentrations of VPA and SAHA caused no statistical changes in the number of active caspase-3-positive cells relative to control. However, when the A549 cells were incubated only with CDDP, cellular apoptosis was induced at higher concentrations. Finally, as shown at Figures 5A, 5B, 6A-F, 7A-F and Table 4, the exposure of the A549 cells to HDIs and CDDP caused evident increase in the degree of apoptosis at all analyzed concentrations.
Figure 5.
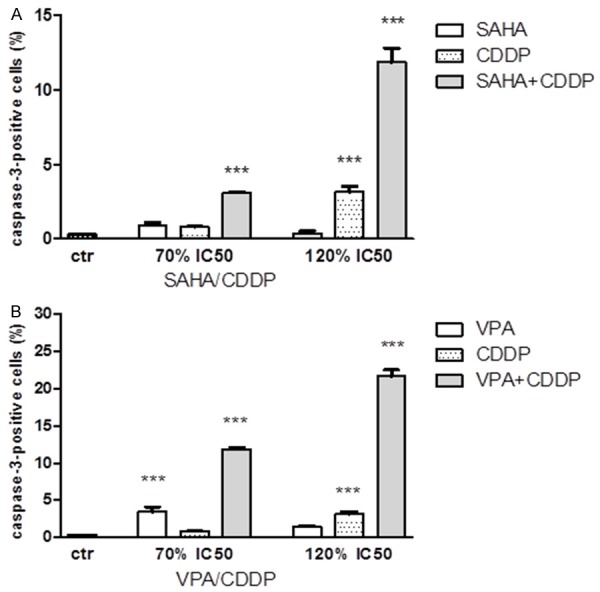
A, B. Effects of HDIs and CDDP on caspase-3 activation in A549 cells. Induction of apoptosis by SAHA or VPA and CDDP in concentrations 1.4 (70% IC50) and 2.4 (120% IC50) alone or in combinations after 48 h exposure. Data was analyzed by flow cytometry and results are expressed as mean ± SD of three separate experiments (n = 6 per concentration, **P<0.01, ***P<0.001 versus the control, one-way ANOVA test).
Figure 6.
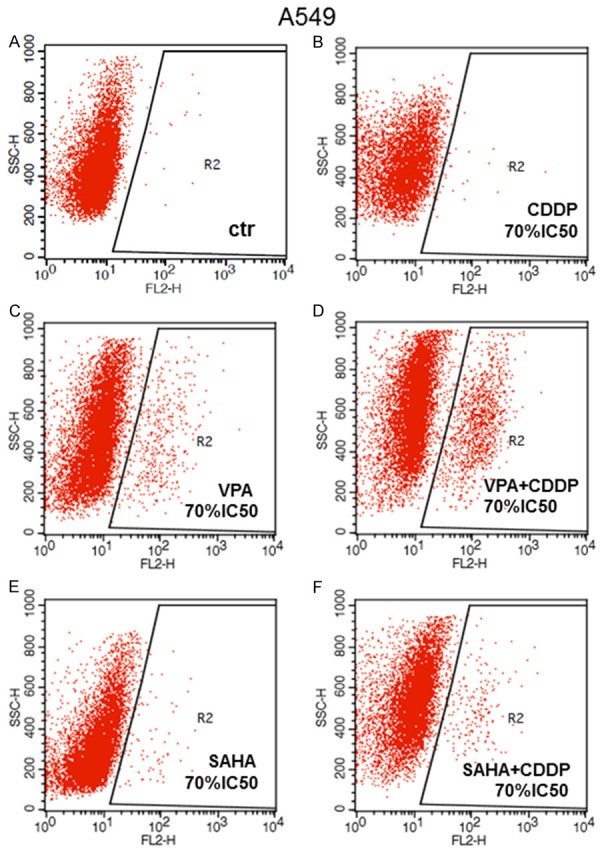
A-F. Detection of apoptotic cells. Induction of apoptosis by SAHA or VPA and CDDP alone or in combinations. A549 cell line was cultivated for 48 hours with dose 1.4 = 70% IC50 of active compounds and their mixtures and analyzed by flow cytometry. Symbol R1 indicates the number of all cells, R2-cells with active caspase-3. Data was analyzed by flow cytometry.
Figure 7.
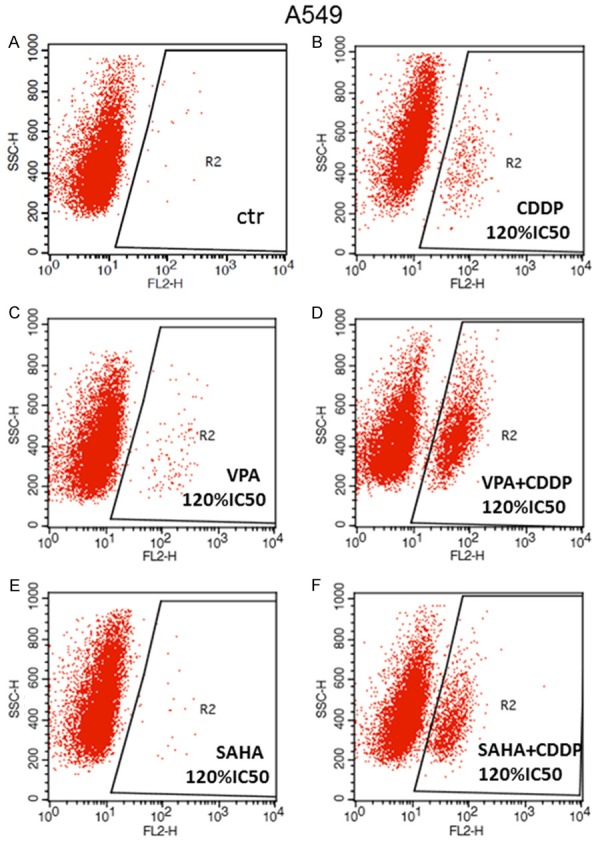
A-F. Detection of apoptotic cells. Induction of apoptosis by SAHA or VPA and CDDP alone or in combinations. A549 cell line was cultivated for 48 hours with dose 2.4 = 120% IC50) of active compounds and their mixtures and analyzed by flow cytometry. Symbol R1 indicates the number of all cells, R2-cells with active caspase-3. Data was analyzed by flow cytometry.
Table 4.
Detection of apoptotic cells
| A549 | Region | Events | % Gated | % Total | X Mean | X Geo Mean | Y Mean | Y Geo Mean |
|---|---|---|---|---|---|---|---|---|
| Ctr | R1 | 9237 | 100.00 | 92.37 | 8.42 | 7.27 | 438.56 | 417.68 |
| R2 | 17 | 0.18 | 0.17 | 154.21 | 120.98 | 618.82 | 588.02 | |
| CDDP 70% IC50 | R1 | 5951 | 100.00 | 59.51 | 9.05 | 6.27 | 463.55 | 443.41 |
| R2 | 26 | 0.44 | 0.26 | 156.55 | 64.74 | 418.65 | 404.21 | |
| VPA 70% IC50 | R1 | 9626 | 100.00 | 96.26 | 15.00 | 8.78 | 469.60 | 428.63 |
| R2 | 404 | 4.20 | 4.04 | 141.39 | 101.77 | 453.67 | 409.01 | |
| VPA+CDDP 70% IC50 | R1 | 9553 | 100.00 | 95.53 | 29.80 | 12.92 | 591.59 | 557.49 |
| R2 | 1260 | 13.19 | 12.60 | 155.83 | 127.96 | 532.78 | 502.44 | |
| SAHA 70% IC50 | R1 | 8860 | 100.00 | 88.60 | 9.50 | 7.36 | 311.01 | 278.37 |
| R2 | 76 | 0.86 | 0.76 | 88.89 | 64.58 | 336.67 | 300.38 | |
| SAHA+CDDP 70% IC50 | R1 | 7551 | 100.00 | 75.51 | 15.15 | 9.80 | 531.33 | 504.17 |
| R2 | 191 | 2.53 | 1.91 | 163.22 | 120.11 | 492.93 | 471.69 | |
| CDDP 120% IC50 | R1 | 9723 | 100.00 | 97.23 | 13.80 | 10.38 | 557.91 | 537.33 |
| R2 | 335 | 3.45 | 3.35 | 100.21 | 87.73 | 445.07 | 420.25 | |
| VPA 120% IC50 | R1 | 9614 | 100.00 | 96.14 | 10.66 | 7.62 | 399.43 | 374.29 |
| R2 | 119 | 1.24 | 1.19 | 191.94 | 153.40 | 363.56 | 334.57 | |
| VPA+CDDP 120% IC50 | R1 | 9754 | 100.00 | 97.54 | 20.06 | 8.60 | 440.97 | 421.18 |
| R2 | 2332 | 23.91 | 23.32 | 66.30 | 59.62 | 461.03 | 442.99 | |
| SAHA 120% IC50 | R1 | 9585 | 100.00 | 95.85 | 9.24 | 7.97 | 484.04 | 458.75 |
| R2 | 22 | 0.23 | 0.22 | 146.17 | 117.48 | 464.23 | 427.33 | |
| SAHA+CDDP 120% IC50 | R1 | 9678 | 100.00 | 96.78 | 14.61 | 9.86 | 433.49 | 413.62 |
| R2 | 1173 | 12.12 | 11.73 | 56.64 | 49.96 | 391.34 | 374.33 |
A549 lung cancer cell line was incubated with active agents alone or in mixture of them with doses 1.4 = 70% IC50 and 2.4 = 120% IC50 for 48 h. Data was analyzed by flow cytometry.
Discussion
Routine methods of treatment of lung cancer include chemotherapy with taxanes, gemcitabine, irinotecan, pemetrexed, as well as cisplatin and its analogs. Efficiency of CDDP is limited due to its toxicity to normal cells, and consequently a number of adverse effects, low therapeutic index, as well as development of CDDP resistance [6,7,38].
Thereby, new active compounds which selectively and effectively eliminate lung cancer cells, and additionally could enhance anticancer properties of currently used chemotherapeutics without destroying healthy tissue, are of great importance. HDIs, to some extent, can fulfill these criteria. VPA is generally well tolerated by patients, whereas SAHA treatment resulted in anemia, anorexia, hyperglycemia, thrombo cytopenia, fatigue and nausea. Despite these limitations, VPA and SAHA have been approved by FDA for the treatment of different types of cancer [39]. Additionally, combinational therapies of HDIs and routinely used chemotherapeutics, as demonstrated in several cancer cells types, such as the concomitant administration of VPA and CDDP increased the sensitivity of melanoma [40], ovarian [41] and neuroblastoma [42] cells, whereas SAHA and CDDP displayed cytotoxic effects in vitro in platinum-resistant ovarian cancer cells [43], and in oral squamous cell carcinoma in vitro [44].
In the present study, we show that both VPA and SAHA enhance the cytotoxicity of CDDP in A549, NCI-1563 and NCI-H2170 lung cancer cell lines. To assess precise type of drug-drug interaction we applied isobolographic analysis, which is a valuable method used to analyze and characterize the type of interaction effects in combined treatment in vitro and in vivo [45], and allows to evaluate whether the two active agents could make an effective combination, which improve the therapy, regardless of the mechanism of drug doses action. Synergistic interaction analyzed on the basis of this protocol permits the use of lower doses of components of the mixture and thus allows to reduce adverse effects [45-47].
Based on the isobolographic analysis we have shown the addition in the direction of synergism between CDDP and VPA or CDDP and SAHA treatments to A549, NCI-H1563 and NCI-H2170 lung cancer cells. Incomplete synergism of HDIs and CDDP may result from possible masking molecular mechanisms of both active compounds. It has been demonstrated that HDIs can induce cell cycle arrest and apoptosis, by modulating genes expression through precise epigenetic mechanisms [48,49]. CDDP intercalating with DNA, thereby unspecific inhibiting expression of several genes, exert similar mechanism of action on cellular level, resulting in cancer cells death, which could affect drug-drug interactions observed in our studies. Almost identical observations were presented in our published investigation on breast cancer cell lines [30], and larynx cancer cells [accepted manuscript, Journal of Cancer], showing that enhancement of the CCDP-mediated cytotoxicity by HDIs seems to be general phenomenon, which is not cell type specific and occurs regardless of the histologic origin of cancer cells. Moreover, combined treatment does not affect much normal cells [accepted manuscript, Journal of Cancer].
Similar to our results, it has been reported that HDIs tested in combination with CDDP on numerous cancer cells, including malignant pleural mesothelioma (MPM) and lung adenocarcinoma (ADCA) cells, showed much better antitumor effect as compared with results obtained when the drugs were used alone [29]. Combined treatment of trichostatin A (TSA), an HDAC inhibitor and CDDP produced synergistic effect of inhibition on A549 cells [50]. However, these drug-drug interactions were estimated by simple type of analysis, and not by extensive isobolographic method, used in our research, and very rare in other studies. Especially, combined treatment of VPA and CDDP could be regarded of great interests for the cancer patients. VPA, already used for a long time for the treatment of epilepsy, and the concentrations achieved in patients plasma (1-1.3 mM) during clinical trials [39] are also effective against cancer cells in vitro, as presented in our present and earlier studies [30]. Additive value of such (CDDP/VPA) combined treatment, resulting in their synergistic action, and possible reduction of CDDP doses for patient treatment, could prompt this drug combination into the clinical trials. No clinical trials of SAHA/CDDP or VPA/CDDP combined treatment in lung cancer have been reported to date [51]. Until now available results from clinical trials shows remarkable beneficiary effect of SAHA and paclitaxel and carboplatin treatment in patients with advanced-stage NSCLC, resulting in 34% response rate with carboplatin + paclitaxel + vorinostat (SAHA) comparing to 12.5% with carboplatin + paclitaxel + placebo, and a tendency to increase in median progression-free survival and overall survival in the vorinostat (SAHA) treated group [52]. In contrast, SAHA applied together with gefitinib displayed no clinical benefit and did not improve progression free survival for NLSC-bearing patients [53].
Thereby, combined treatment of HDI/CDDP could have some advances in comparison to other types of combined chemotherapy, especially that HDI/CDDP applied together affects NSLC cells proliferation regardless of the histologic type-adenocarcinoma (A549) or squamous cell carcinoma (NCI-H2170) at similar level, and that the anti-cancer effect was evident in more sensitive (A549, NCI-H2170) and less sensitive (NCI-H1563) lung cancer cell lines, as demonstrated in our present study.
In conclusion, these results show that the combined treatment of CDDP and HDIs can be used to more successfully eliminate lung carcinoma cells, indicating a promising new drugs combinations for lung cancer treatment.
Acknowledgements
The authors thank Professor Adolfo Rivero-Müller for critical comments and proofreading. The study was supported by Medical University of Lublin DS440/2014-2015, DS440/2016-2017 and MNmb510/2012-2014 grants.
Disclosure of conflict of interest
None.
References
- 1.Malvezzi M, Arfe A, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2011. Ann Oncol. 2011;22:947–956. doi: 10.1093/annonc/mdq774. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0. [DOI] [PubMed] [Google Scholar]
- 5.Takahara PM, Rosenzweig AC, Frederick CA, Lippard SJ. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature. 1995;377:649–652. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- 6.Viet CT, Dang D, Achdjian S, Ye Y, Katz SG, Schmidt BL. Decitabine rescues cisplatin resistance in head and neck squamous cell carcinoma. PLoS One. 2014;9:e112880. doi: 10.1371/journal.pone.0112880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Cai XW, Zhu ZF, Yu W, Liu Q, Feng W, Xue MC, Fu XL. Full-dose pemetrexed plus cisplatin combined with concurrent thoracic radiotherapy for previously untreated advanced nonsquamous non-small cell lung cancer. Anticancer Drugs. 2015;26:456–463. doi: 10.1097/CAD.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 8.Petta V, Gkiozos I, Strimpakos A, Syrigos K. Histones and lung cancer: are the histone deacetylases a promising therapeutic target? Cancer Chemother Pharmacol. 2013;72:935–952. doi: 10.1007/s00280-013-2223-9. [DOI] [PubMed] [Google Scholar]
- 9.Blaheta RA, Cinatl J Jr. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22:492–511. doi: 10.1002/med.10017. [DOI] [PubMed] [Google Scholar]
- 10.Noro R, Miyanaga A, Shimokawa T, Kuribayashi H, Mizutani H, Minegishi Y, Okano T, Seike M, Soeno C, Kataoka K, Matsuda K, Yoshimura A, Gemma A. The anticancer effect of histone deacetylase inhibitors and combination with the cytotoxic agents in lung cancer cells: biological analyses for future clinical application. J Nippon Med Sch. 2009;76:44–46. doi: 10.1272/jnms.76.44. [DOI] [PubMed] [Google Scholar]
- 11.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 12.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 13.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Wolffe AP, Hayes JJ. Chromatin disruption and modification. Nucleic Acids Res. 1999;27:711–720. doi: 10.1093/nar/27.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka M, Smith MM. Functional consequences of histone modifications. Curr Opin Genet Dev. 2003;13:154–160. doi: 10.1016/s0959-437x(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 16.Dodurga Y, Gundogdu G, Tekin V, Koc T, Satiroglu-Tufan NL, Bagci G, Kucukatay V. Valproic acid inhibits the proliferation of SHSY5Y neuroblastoma cancer cells by downregulating URG4/URGCP and CCND1 gene expression. Mol Biol Rep. 2014;41:4595–4599. doi: 10.1007/s11033-014-3330-3. [DOI] [PubMed] [Google Scholar]
- 17.Sidana A, Wang M, Shabbeer S, Chowdhury WH, Netto G, Lupold SE, Carducci M, Rodriguez R. Mechanism of growth inhibition of prostate cancer xenografts by valproic acid. J Biomed Biotechnol. 2012;2012:180363. doi: 10.1155/2012/180363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan CP, Hamid S, Hor SY, Zain RB, Ismail SM, Wan Mustafa WM, Teo SH, Saunders N, Cheong SC. Valproic acid: growth inhibition of head and neck cancer by induction of terminal differentiation and senescence. Head Neck. 2012;34:344–353. doi: 10.1002/hed.21734. [DOI] [PubMed] [Google Scholar]
- 19.Shah RD, Jagtap JC, Mruthyunjaya S, Shelke GV, Pujari R, Das G, Shastry P. Sodium valproate potentiates staurosporine-induced apoptosis in neuroblastoma cells via Akt/survivin independently of HDAC inhibition. J Cell Biochem. 2013;114:854–863. doi: 10.1002/jcb.24422. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Zheng Y, Huang Z, Wang M, Zhang Y, Wang Z, Jin X, Xia Q. Role of SMAD4 in the mechanism of valproic acid’s inhibitory effect on prostate cancer cell invasiveness. Int Urol Nephrol. 2014;46:941–946. doi: 10.1007/s11255-013-0609-6. [DOI] [PubMed] [Google Scholar]
- 21.Seo SK, Jin HO, Lee HC, Woo SH, Kim ES, Yoo DH, Lee SJ, An S, Rhee CH, Hong SI, Choe TB, Park IC. Combined effects of sulindac and suberoylanilide hydroxamic acid on apoptosis induction in human lung cancer cells. Mol Pharmacol. 2008;73:1005–1012. doi: 10.1124/mol.107.041293. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu N, Kawamata N, Takeuchi S, Yin D, Chien W, Miller CW, Koeffler HP. SAHA, a HDAC inhibitor, has profound anti-growth activity against non-small cell lung cancer cells. Oncol Rep. 2006;15:187–191. [PubMed] [Google Scholar]
- 23.Li CT, Hsiao YM, Wu TC, Lin YW, Yeh KT, Ko JL. Vorinostat, SAHA, represses telomerase activity via epigenetic regulation of telomerase reverse transcriptase in non-small cell lung cancer cells. J Cell Biochem. 2011;112:3044–3053. doi: 10.1002/jcb.23229. [DOI] [PubMed] [Google Scholar]
- 24.Han S, Fukazawa T, Yamatsuji T, Matsuoka J, Miyachi H, Maeda Y, Durbin M, Naomoto Y. Anti-tumor effect in human lung cancer by a combination treatment of novel histone deacetylase inhibitors: SL142 or SL325 and retinoic acids. PLoS One. 2010;5:e13834. doi: 10.1371/journal.pone.0013834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson AK, Welsh J, Taylor T, Roy S, Wang WL, Tenniswood M. Comparative effects of histone deacetylase inhibitors on p53 target gene expression, cell cycle and apoptosis in MCF-7 breast cancer cells. Oncol Rep. 2012;27:849–853. doi: 10.3892/or.2011.1590. [DOI] [PubMed] [Google Scholar]
- 26.Hutt DM, Roth DM, Vignaud H, Cullin C, Bouchecareilh M. The histone deacetylase inhibitor, vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PLoS One. 2014;9:e106224. doi: 10.1371/journal.pone.0106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, Tian Z, Zhang C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer. 2015;112:112–121. doi: 10.1038/bjc.2014.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiao MT, Cheng WY, Yang YC, Shen CC, Ko JL. Suberoylanilide hydroxamic acid (SAHA) causes tumor growth slowdown and triggers autophagy in glioblastoma stem cells. Autophagy. 2013;9:1509–1526. doi: 10.4161/auto.25664. [DOI] [PubMed] [Google Scholar]
- 29.Gueugnon F, Cartron PF, Charrier C, Bertrand P, Fonteneau JF, Gregoire M, Blanquart C. New histone deacetylase inhibitors improve cisplatin antitumor properties against thoracic cancer cells. Oncotarget. 2014;5:4504–4515. doi: 10.18632/oncotarget.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wawruszak A, Luszczki JJ, Grabarska A, Gumbarewicz E, Dmoszynska-Graniczka M, Polberg K, Stepulak A. Assessment of interactions between cisplatin and two histone deacetylase inhibitors in MCF7, T47D and MDA-MB-231 human breast cancer cell lines-an isobolographic analysis. PLoS One. 2015;10:e0143013. doi: 10.1371/journal.pone.0143013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litchfield JT Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 32.Luszczki JJ. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: a practical application. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:105–114. doi: 10.1007/s00210-007-0144-z. [DOI] [PubMed] [Google Scholar]
- 33.Luszczki JJ, Czuczwar SJ. Biphasic characteristic of interactions between stiripentol and carbamazepine in the mouse maximal electroshock-induced seizure model: a three-dimensional isobolographic analysis. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:51–64. doi: 10.1007/s00210-006-0100-3. [DOI] [PubMed] [Google Scholar]
- 34.Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther. 2004;310:981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- 35.Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 36.Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacol Ther. 2007;113:197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallarida RJ. Drug synergism and dose-effect data analysis. CRC Press; 2000. [Google Scholar]
- 38.Stathopoulos GP. Cisplatin: process and future. J BUON. 2013;18:564–569. [PubMed] [Google Scholar]
- 39.Nervi C, De Marinis E, Codacci-Pisanelli G. Epigenetic treatment of solid tumours: a review of clinical trials. Clin Epigenetics. 2015;7:127. doi: 10.1186/s13148-015-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chodurek E, Orchel A, Gruchlik A, Aleksander E, Golabek K, Dzierzewicz Z. Valproic acid enhances cisplatin cytotoxicity in melanoma cells. Acta Pol Pharm. 2012;69:1298–1302. [PubMed] [Google Scholar]
- 41.Lin CT, Lai HC, Lee HY, Lin WH, Chang CC, Chu TY, Lin YW, Lee KD, Yu MH. Valproic acid resensitizes cisplatin-resistant ovarian cancer cells. Cancer Sci. 2008;99:1218–1226. doi: 10.1111/j.1349-7006.2008.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaheta RA, Michaelis M, Natsheh I, Hasenberg C, Weich E, Relja B, Jonas D, Doerr HW, Cinatl J Jr. Valproic acid inhibits adhesion of vincristine-and cisplatin-resistant neuroblastoma tumour cells to endothelium. Br J Cancer. 2007;96:1699–1706. doi: 10.1038/sj.bjc.6603777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong PS, Wang XQ, Lin HS, Chan SY, Ho PC. Synergistic effects of suberoylanilide hydroxamic acid combined with cisplatin causing cell cycle arrest independent apoptosis in platinum-resistant ovarian cancer cells. Int J Oncol. 2012;40:1705–1713. doi: 10.3892/ijo.2012.1354. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H. Enhancement of cisplatin cytotoxicity by SAHA involves endoplasmic reticulum stress-mediated apoptosis in oral squamous cell carcinoma cells. Cancer Chemother Pharmacol. 2009;64:1115–1122. doi: 10.1007/s00280-009-0969-x. [DOI] [PubMed] [Google Scholar]
- 45.Teuschler L, Klaunig J, Carney E, Chambers J, Conolly R, Gennings C, Giesy J, Hertzberg R, Klaassen C, Kodell R. Support of science-based decisions concerning the evaluation of the toxicology of mixtures: a new beginning. Regul Toxicol Pharmacol. 2002;36:34–39. doi: 10.1006/rtph.2002.1570. [DOI] [PubMed] [Google Scholar]
- 46.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 47.Jagdev S, Coleman R, Shipman C, Rostami-H A, Croucher P. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84:1126. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noureen N, Rashid H, Kalsoom S. Identification of type-specific anticancer histone deacetylase inhibitors: road to success. Cancer Chemother Pharmacol. 2010;66:625–633. doi: 10.1007/s00280-010-1324-y. [DOI] [PubMed] [Google Scholar]
- 49.Botrugno OA, Santoro F, Minucci S. Histone deacetylase inhibitors as a new weapon in the arsenal of differentiation therapies of cancer. Cancer Lett. 2009;280:134–144. doi: 10.1016/j.canlet.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Jiang SJ, Shang B, Jiang HJ. Effects of histone deacetylase inhibitor trichostatin A combined with cisplatin on apoptosis of A549 cell line. Thorac Cancer. 2015;6:202–208. doi: 10.1111/1759-7714.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. https://clinicaltrials.gov/ct2/results?term=histone+deacetylase+inhibitors++and+CDDP&pg=1.
- 52.Ramalingam SS, Maitland ML, Frankel P, Argiris AE, Koczywas M, Gitlitz B, Thomas S, Espinoza-Delgado I, Vokes EE, Gandara DR, Belani CP. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han JY, Lee SH, Lee GK, Yun T, Lee YJ, Hwang KH, Kim JY, Kim HT. Phase I/II study of gefitinib (Iressa(R)) and vorinostat (IVORI) in previously treated patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;75:475–483. doi: 10.1007/s00280-014-2664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


