Abstract
Integrin αvβ3 (ITG) is highly expressed in various cancers and is considered a major target for anti-angiogensis cancer therapy. The single chain fragment variable of which (ScFv αvβ3) has been reported to inhibit tumor growth both in vitro and in vivo. Here, we conjugated cdGIGPQc which can exclusively bind to NSCLC cells according to our previous study synthesized by SPPS with ScFv αvβ3 expressed in E. coli BL21 (DE3) to develop a novel lung cancer specific targeted drug. Specific cell targeting of cdGIGPQc-ScFv was assessed in parallel with the single ScFv and a control nonspecific peptide-ScFv through immunofluorescence and flow cytometry while the αvβ3-binding property was examined by Western blot. Our results showed that cdGIGPQc-ScFv retained both the lung cancer-binding activity of cdGIGPQc and the antigen-recognizing ability of ScFv αvβ3 in vitro. CCK8 assays and in animal experiments suggested that cdGIGPQc-ScFv possessed a superior antitumor effect than ScFv and nonspecific peptide-ScFv both in vitro and vivo. Further immunohistochemical staining revealed that cdGIGPQc-ScFv retarded lung cancer growth through inhibiting tumor angiogensis and proliferation. Therefore, cdGIGPQc delivery of ScFv αvβ3 to lung cancer may be a hopeful new strategy for enhancing specific antitumor efficacy and cdGIGPQc-ScFv could be a potential drug for lung cancer targeted treatment.
Keywords: Lung cancer, cdGIGPQc, integrin avβ3, ScFv, targeted therapy
Introduction
Lung cancer has always been known as a leading cause of cancer-related death worldwide with increasing incidence and mortality [1]. Despite advances in the treatment of lung cancer, the overall disease course has remained relatively unchanged over decades. To date, comprehensive treatment of multiple subjects is still the major therapy pattern, though the overall outcome is unsatisfactory [2,3]. However, biological target therapy aiming at the process of tumorigenesis or progression has provided new approaches for the treatment which may surmount the therapeutic plateau owing to their high sensitivity and specificity [4-6].
Angiogenesis, a prerequisite and indispensable process in the proliferation, invasion and metastasis of tumors, is regarded as a symbol and therapeutic target of tumor evolution [7-9]. Integrin family plays an important role in cancer-related angiogenesis [10,11], and its particular expression pattern promotes the malignant behavior of lung cancer [12]. One of the subtypes alphavbeta3 (ITG αvβ3) is a primary component of the vascular cell adhesion receptor and contributes fundamentally to invasive angiogenesis [13,14], whose aberrant overexpression and activation in various cancers promotes tumor growth [15], hence recognized as a key target for anti-angiogenic therapy [16-19]. The antagonists of ITG αvβ3 can selectively induce apoptosis of angiogenic blood vessels especially proliferating vascular cells and promote regression of human tumors, leaving existing quiescent blood vessels unaffected [19]. Being the monoclonal antibodies (mAbs) of ITG αvβ3, LM609 had great anti-angiogenesis and proliferation inhibition effects both in vitro and in vivo [19], and the humanized version named as Vitaxin undergoing phase II clinical trial, was well tolerable in clinical patients with metastatic cancer [20,21], whereas without objective anti-tumor responses [21,22]. It may be the low local drug concentrations caused by large molecule dimensions, or the lack of selectivity and specificity to cancer cell that limited the anti-tumor efficacy. On the other hand, the ScFv format of ITG αvβ3 antibody has been developed as an alternative to Vitaxin and proved to have good therapeutic potential of inhibiting cancer cell growth both in vitro and vivo [23]. Unlike full length mAbs, ScFvs are recombinant antibody fragments just composed of connected variable light (VL) and variable heavy (VH) while retaining the original antigen-binding capacity and biological function [24]. They have lower production cost, better tumor microcirculation penetration and less immunogenicity due to the lack of Fc portion [25,26]. However, the low binding affinity and fast clearance of small-size ScFvs has become another obstacle to take [27]. To achieve the sustained release, many efforts have been made such as adding small targeting agent [28].
Our preliminary study had screened a novel small molecular peptide cdGIGPQc using the “one-bead one-peptide” combinatorial library, which was able to specially bind lung cancer cells by recognizing the human integrin α3β1 abnormally expressed on the tumor cell surface [29]. All about this specific peptide had been applied for national invention patents (CN 101486754, CN 101638429, CN 102643331A, CN 102641511A), laying the foundation for new therapy method targeted lung cancer. In this study, we conjugated cdGIGPQc with ScFv αvβ3 for targeting lung cancer cells to achieve selective killing. cdGIGPQc was supposed to enhance the binding specificity of ScFv to lung cancers and the recombinant protein was expected to characterized by more specific tumor targeting and faster tumor penetration. Our results demonstrated that cdGIGPQc-ScFv αvβ3 retained both the targeting characteristic of cdGIGPQc and antiangiogenic effect of ScFv αvβ3 to suppress tumor progression both in vitro and vivo.
Materials and methods
Cell culture
A549 human lung adenocarcinoma cell and L78 human lung adenosquamous carcinoma cell were obtained from ATCC (American type culture collection). A549 and L78 cells were maintained at 37°C in a 5% CO2 atmosphere with RPMI-1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin.
Polypeptide synthesis
Small molecular cdGIGPQc (cd) and cNAQAEQc (non-related, cN ) are two short peptides composed of eight amino acids, inside which “c” represents D-cysteine, “d” represents Daspartic acid, “G” represents L-glycine, “I” represents L-isoleucine, “P” represents L-proline, “Q” represents L-glutamine, “N” represents L-asparagine, “A” represents L-alanine, “E” represents L-glutamic acid, respectively. They were synthesized by solid-phase peptide synthesis (SPPS) technology (by Abgent Biotechnology Co., Ltd). The compounds were analyzed by electrospray ionization mass spectrometry (ESI-MS) and purified by high performance liquid chromatography (HPLC).
Construction of the ScFv expression vector
The DNA sequence of anti-human integrin αvβ3 ScFv was obtained from reference [30] and synthesized by Abgent Biotechnology Co., Ltd (Suzhou, China). The ScFv DNA fragment with 5’-EcoRI, 3’-XhoI restriction site was then inserted into vector pET-28a. The transfer vector plasmid pET-28a-ScFv was confirmed by restriction analysis.
Expression and purification of ScFv fragments
The recombinant plasmid pET-28a-ScFv was introduced into the escherichia coli BL21 (DE3) as host strain. Single colonies were inoculated into LB medium with Kanamycin the next day and incubated over night at 37°C under shaking (240 rpm). When the value of optical density at 600 nm (OD600) became 0.6-0.8, maintained induction was achieved by adding isopropy-β-D-thiogalactoside (IPTG) to a final concentration of 0.5 mM. After 3 to 4 h induction, the thalli were harvested by 6500 rpm centrifugation of 5 min and dissolved in bacterial lysate buffer. The supernatant and precipitation were collected separately by ultrasonic decomposition and centrifuge (13000 rpm, 15 min). Next, the precipitation was thoroughly dissolved in 1× binding buffer (20 mM imidazole, 0.5 M NaCl, 20 mM Tris, 8 M Urea, PH 7.9), sonicated, and centrifuged at 13000 rpm, 4°C for 15 min. Then the supernatant of ScFv was filtrated and purified by Ni-NTA Agarose before analyzed via polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie Blue staining.
Combination of cdGIGPQc-ScFv
The connection of cdGIGPQc and ScFv was accomplished by using thiols inside of molecules, mediated through the coupling agent SMCC [Succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-1 carboxylate]. Briefly, the purified protein ScFv and SMCC were put together in the proportion of 1:2 to make up a reaction system and reacted at room temperature for 6 h. ScFv modified with SMCC was collected after redundant SMCC was removed by dialysis. Subsequently, dissolved peptide was added into the solution for another reaction at room temperature for 4 h. Redundant peptide was removed via dialysis, then recombinant cdGIGPQc-ScFv (cd-ScFv) was obtained and verified by SDS-PAGE. As a contrast, cNAQAEQc-ScFv (cN-ScFv) was prepared using the same method.
Cell-binding assay of cdGIGPQc-scFv
To explore whether cdGIGPQc-scFv bound lung cancer cells as well as cdGIGPQc, immunofluorescence technique was utilized as follow. Firstly, A549 and L78 cells were seeded evenly in 96-well plates at 5,000 cells per well for a night to achieve adherence. After fixed in 4% paraformaldehyde for 30 min and blocked with 5% BSA for 1 h, they were incubated with 100 μL FITC-cd-ScFv (15 μg/mL) or FITC-cN-ScFv (15 μg/mL) in the dark at 4°C overnight. The unbound fluorescent antibodies were removed by washing three times with PBS and the nuclei were labeled with DAPI (1:200, GeneCopoeia, Guangzhou, China). After three washes with PBS, the cells were observed under the fluorescence microscope. Pictures were merged by Image J software. The same volume of PBS and FITC-cdGIGPQc (1 μg/mL) were used as negative and positive control, respectively. Experiments were performed more than three times.
Furthermore, flow cytometry was next used to quantified the binding ability of the recombined protein to lung cancer cells. After washed three times with PBS, 2×106 A549 and L78 cells were resuspended and incubated with FITC-cd-ScFv (15 μg/mL) or FITC-cN-ScFv (15 μg/mL) in 100 μl PBS at room temperature for 20 min, while the cells treated with FITC-cdGIGPQc and PBS were regarded as positive and negative controls. The results were then analyzed by BD FACSVerseTM and FlowJo software.
The αvβ3-binding property of cdGIGPQc-scFv
The binding abilities of ScFv and cdGIGPQc-ScFv to human integrin αvβ3 were checked by Western Blot assay. Briefly, the human integrin αvβ3 proteins (Chemicon, USA) were subjected to 10% SDS-PAGE and then electrically transferred in constant current of 100 mA for 116 min to a 0.45 μm PVDF membrane. After blocked with 5% BSA for 2 h, the PVDF membrane was incubated with protein ScFv and cdGIGPQc-scFv as primary antibodies at 4°C overnight, followed by second antibody HRP-conjugated anti-His tag antibody (1:2000, cwbiotech, Beijing, China) at room temperature for 1 h. At the same time, primary antibody anti-αvβ3 mAb (1:1000, Chemicon, USA) was used as positive control, followed by HRP-conjugated goat anti-mouse IgG (1:2000, Bioss, Beijing, China) as second antibody. After washed with TBST, the target proteins were detected by adding HRP substrate (Millipore Corporation, Billerica, USA) which consisted of equal volumes of Luminol Reagent and Peroxide Solution, and visualized using Bio-Rad ChemiDoc XRS+.
CCK8 assay
The anti-tumor growth effects of cdGIGPQc-ScFv and cNAQAEQc-ScFv in vitro were tested via measuring the quantity of living cells using the Cell Counting Kit-8 (CCK8) (DOJINDO, Japan) according to the manufacturer’s instruction. Briefly, A549 and L78 cells were seeded in 96-well plates (5,000 cells/well). After 24 h, the culture medium was replaced with serum-free medium for the next 12 h. Then, the cells were incubated with serial dilutions of cdGIGPQc-scFv and cNAQAEQc-scFv from 50 pM to 5 μM for 24 h. Equimolar ScFv was used as controls. After washed with PBS, the cells were incubated with CCK8 solution for 1 to 4 h at 37°C until the optical density values at 450 nm (OD450) were read from microplate reader. The relative inhibition rates of tumor cell growth and the half-maximal inhibitory concentration (IC50) were calculated by SPSS 20.0. Experiments were repeated three times.
Animal experiments
Male nude BALB/c mice (nu/nu) of 4 weeks were purchased from the Laboratory Animal Center, Medical School of Sun Yat-sen University, Guangdong, China. It took mice approximately one week to adapt to new specific pathogen free conditions before receiving lung cancer cells. A549 and L78 cells in logarithmic growth phase were harvested and injected subcutaneously into the back of each mouse at 5×106 cells in 200 μl PBS. After implantation, tumor size was regularly assessed every three days using vernier caliper. About one weeks later, when the average diameter of tumor reached 5-8 mm, mice were randomly divided into cd-scFv, cN-scFv, ScFv, PBS groups (n=6). 10 μg cd-scFv and equimolar amounts of cN-scFv and ScFv in 100 μl PBS were intravenously injected three times per mouse on days 0, 4, 8 referring to previous reports [23], and the mice treated with equal volume of PBS were used as negative control. We then followed the mice for three weeks and routinely checked the general condition including appearance, activity and signs of toxicity. Tumor volume was calculated by the formula: V=(length × width2)/2. The last tumor weight was measured using electronic analytical balance.
Histopathology and immunohistochemistry
At the end of the experiment, harvested tumors were formalin fixed and then paraffin embedded. We performed hematoxylin-eosin staining (H&E staining) to verify the tumor tissue excised from the xenograft models and to check the effect of drug on vital organs, including heart, lung, liver, kidney and spleen. Next, paraffin-embedded tumor specimens were cut into about 4 µm-thick section for immunohistochemistry analysis. The sections were stained by CD31 (Santa Cruz, lnc) and Ki67 (MXB, lnc), followed by corresponding secondary antibodies. The results were observed under fluorescent microscopy and then analyzed with Image-Pro Plus software.
Statistical analysis
Data were presented as mean ± SD. Statistical analysis of cytotoxicity assay was performed using one-way ANOVA with Statistical Product and Service Solutions 20.0 (SPSS). For animal experiment, tumor growth curve diagram and histogram were draw using GraphPad Prism 6.02 (Graphpad Software). p value <0.05 was considered statistically significant.
Results
Construction and analysis of cdGIGPQc-ScFv
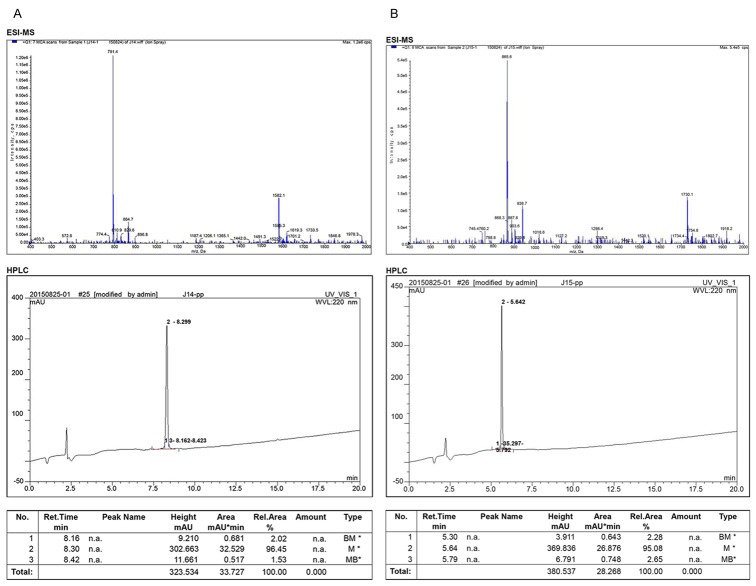
Small-molecule peptide cdGIGPQc possessing the binding specificity to lung cancer cells, along with non-related and nonspecific peptide cNAQAEQc, were synthesized by SPPS. The products were identified and purified by ESI-MS and HPLC. The purity of cdGIGPQc and cNAQAEQc were 96.45% and 95.08% respectively, shown as prominent peak in the HPLC report (Figure 1).
Figure 1.
Synthesis and analysis of small molecular peptides. Polypeptides were artificially synthesized using SPPS method and the structure of cdGIGPQc (A) and cNAQAEQc (B) were analyzed by ESI-MS and HPLC, respectively.
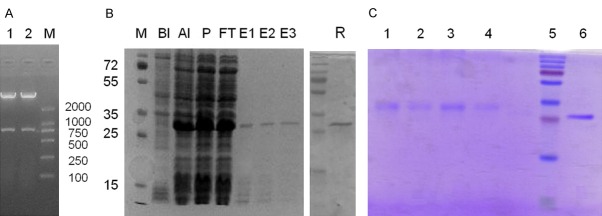
The synthesized DNA and the corresponding amino acid sequences of αvβ3 ScFv were present in Table 1. To obtain purified ScFv protein, the recombinant plasmid pET-28a-ScFv was confirmed by restriction analysis (Figure 2A) before transferred into E. coli BL21 (DE3). Kanamycin resistant colonies were selected for further culture, and then the ScFv fragments in the supernatant were purified with Ni-NTA Agarose. After purification, the ScFv protein migrated as a single band of 26.5 KDa when demonstrated by SDS-PAGE and Coomassie Blue staining (Figure 2B).
Table 1.
The synthesized DNA sequence and the amino acid sequence of ScFv αvβ3
| DNA sequence | CAGGTGCAGTTACAGCAGTCTGGTGCTGAATTAGCCCGTCCGGGTACAAGCGTTAAGTGGAG |
| TTGTCGCACGTCAGGCTATACCTTTACGAGCTTTGGCATTGCCTGGGTTAAACAGCGCTCTGG | |
| CCAGGGCTTAGAGTGGATCGGCGAAATCTTTCCTCGCTCAGGCAATACTTATTATAACGAAAA | |
| GTTCACGGGCAAAGCCACACTGACCGCCGATACAAGTAGCTCAACCGCATATATGGAACTGC | |
| GTAGCCTGACGAGTGAAGATTCTGCTGTGTATTTTTGTGCGACATATACGAGCTATGGTGCGA | |
| TGGATTATTGGGGTCAGGGCACCAGTGTGACCGTGTCTTCAGGTGGCGGTGGTAGCGGTGGT | |
| GGCGGTTCAGGCGGCGGTGGTTCAGAAATCGTGTTAACACAGAGTCCGACAACGATGGCCG | |
| CGTCACGCGGCGAAAAAATTACAATCACATGTAGTGCTAATAGTTCAATCTCTAGTAATCATC | |
| TGCATTGGTATCAGCAGAAAAGTGGCTTTTCTCCTAAATTTCTGATCTATCGCACCTCTATCTT | |
| AGCAAGCGGCGTTCCTGCACGCTTTTCTGGCAGCGGCTCAGGCACCTCTTATAGTTTAACCA | |
| TTGATACGATGGAAGCCGAAGATGTTGCCACATATTATTGCCAGCAGGGCTCAAGTAAACCGT | |
| ATACGTTTGGCGGTGGGACAAAACTGGAAATTAAACGCGCCCATCATCATCATCATCATtaa | |
| Amino acid sequence | QVQLQQSGAELARPGTSVKWSCRTSGYTFTSFGIAWVKQRSGQGLEWIGEIFPRSGNTYYNEKFTGKATLTADTSSSTAYMELRSLTSEDSAVYFCATYTSYGAMDYWGQGTSVTVSSGGGGSGGGGSGGGGSEIVLTQSPTTMAASRGEKITITCSANSSISSNHLHWYQQKSGFSPKFLIYRTSILASGVPARFSGSGSGTSYSLTIDTMEAEDVATYYCQQGSSKPYTFGGGTKLEIKRAHHHHHH |
Figure 2.
Expression of ScFv and purification of cdGIGPQc-ScFv. The recombinant plasmid pET-28a-ScFv was transformed into the E. coli BL21 (DE3). A: The recombinant plasmid pET-28a-ScFv confirmed by restriction analysis before transformation. B: After induction, purified ScFv protein extracted from bacteria was separated on a SDS-PAGE gel and stained with Coomassie Blue. Lane M, protein molecular mass markers (KDa); Lane BI, bacterial proteins before induction; Lane AI, bacterial proteins after induction; Lane p, the precipitation after sonication; Lane FT, the filtered proteins; Lane E1, E2, E3, the purified ScFv protein; Lane R, the renatured ScFv protein. C: SDS-PAGE analysis of cdGIGPQc-ScFv and cNAQAEQc-ScFv. Lane 1, 2, cNAQAEQc-ScFv; Lane 3, 4, cdGIGPQc-ScFv; Lane 5, protein molecular mass markers; Lane 6, the ScFv protein.
Peptide cdGIGPQc and ScFv were connected through SMCC and the compound was analyzed via SDS-PAGE. Recombinant cdGIGPQc-ScFv presented a molecule weight slightly larger than ScFv (Figure 2C). And ScFv fused with cNAQAEQc was prepared in the same way, which shared a similar molecule size as cdGIGPQc-ScFv.
Binding ability of cdGIGPQc-ScFv to lung cancer cells in vitro
The particular binding property of cdGIGPQc-scFv to lung cancer cells was analyzed through immunofluorescence staining and flow cytometry. Figure 3A, 3C represented the results of A549 cells, while Figure 3B, 3D represented L78’s. The fluorescent images demonstrated that FITC-cd-ScFv was able to bind A549 and L78 lung cancer cells like the positive control FITC-cdGIGPQc, with an apparently higher affinity compared to FITC-cN-ScFv (Figure 3A, 3B). Consistently in flow cytometry assays, cells treated with FITC-cd-ScFv (15 μg/mL) exhibited a similar high positive rate as FITC-cdGIGPQc by more than 95%, whereas FITC-cN-ScFv treatment came out with a much lower positive rate as well as fluorescence intensity (Figure 3C, 3D). Taken all together, these results indicated that the fusion of cdGIGPQc and ScFv did not affect the lung cancer cell binding characteristic of cdGIGPQc agent, but enhanced the binding capacity of ScFv to lung cancer cells.
Figure 3.
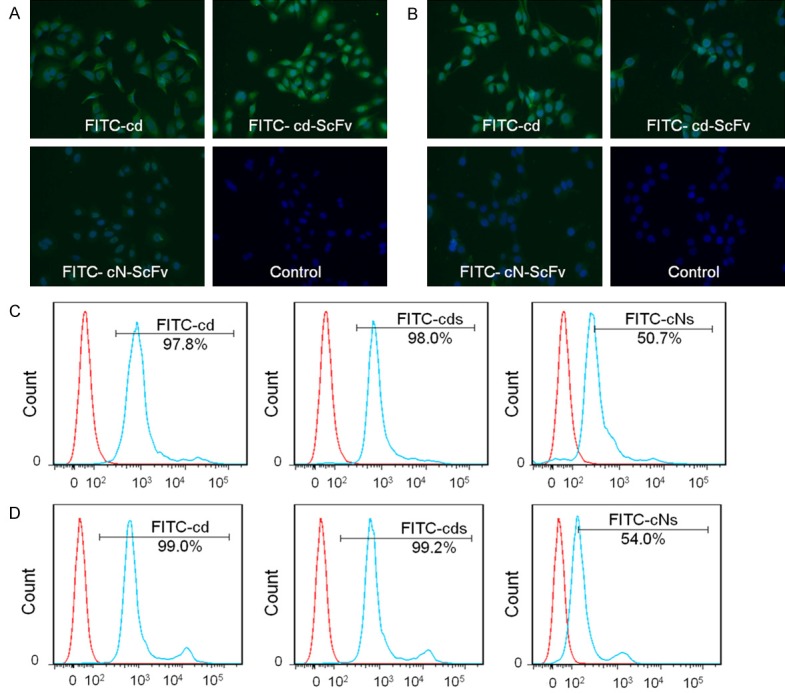
Binding ability of cdGIGPQc-ScFv to lung cancer cells in vitro. Fluorescence microscope imaging was used to detect the binding of FITC-cdGIGPQc-ScFv and FITC-cNAQAEQc-ScFv to A549 cells (A) and L78 cells (B), respectively. Scale bar: 100 μm. The binding affinity of cdGIGPQc-ScFv and cNAQAEQc-ScFv to A549 (C) and L78 cells (D) was examined by flow cytometry. Histograms were generated from FlowJo software. The blue lines represented lung cancer cells treated with FITC-cdGIGPQc, FITC-cd-ScFv or FITC-cN-ScFv, while the red lines represented the negative control.
ITG αvβ3-binding activity of ScFv and cdGIGPQc-ScFv in vitro
We next used Western Blot assay to examine the integrin αvβ3-binding ability of ScFv and cdGIGPQc-ScFv in vitro. Primarily, human integrin αvβ3 proteins mixed with loading buffer were electrophoresed to 10% SDS-PAGE and transferred to a PVDF membrane. As primary antibodies, both ScFv and cdGIGPQc-ScFv were able to recognize integrin αvβ3 through antigen-antibody reaction, resulting in identical protein bands with the mAb of αvβ3 (Figure 4A). These results clearly declared that the reformed ScFv fragment did not lose the original antigen-binding ability of its parent full-length αvβ3 monoclonal antibody, which was neither destroyed for the combination of cdGIGPQc and ScFv.
Figure 4.
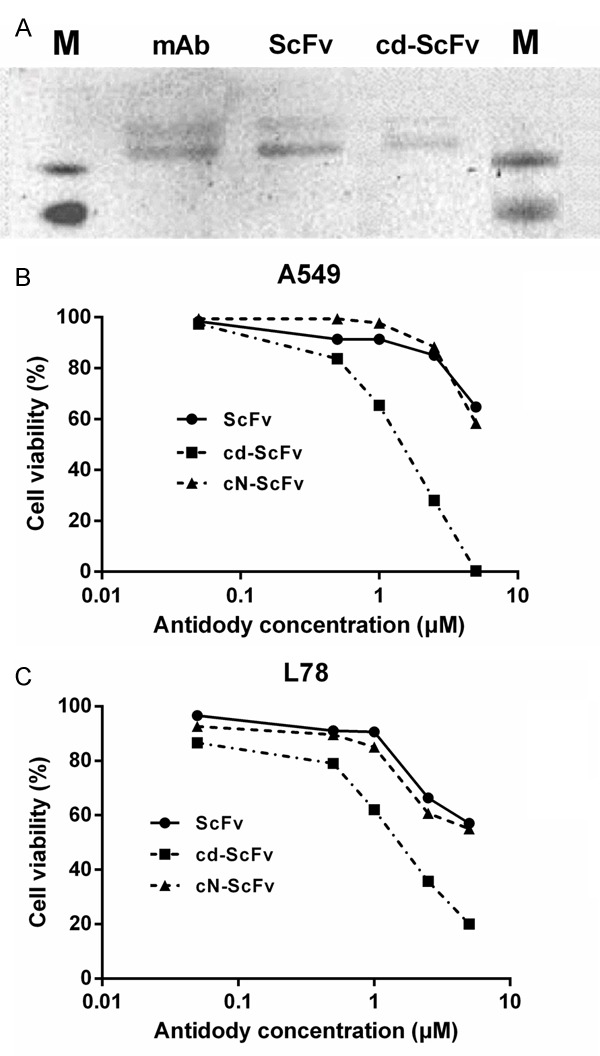
Antigen-binding ability and tumor growth inhibition of cdGIGPQc-ScFv in vitro. A: The antigen-specific binding of ScFv and cd-ScFv was analyzed via Western blot analysis. After human ITG αvβ3 was imprinted, ScFv and cd-ScFv detected a protein band similar to that by positive control mAb, as primay atibodies. B, C: Human lung adenocarcinoma cell A549 and human lung adenosquamous carcinoma cell L78 were incubated with indicated concentrations of cdGIGPQc-ScFv, cNAQAEQc-ScFv or control ScFv for 24 h and the cell proliferation was checked by CCK8 assay. cdGIGPQc-ScFv showed a superior antitumor effect than ScFv and cNAQAEQc-ScFv. Data were from one of independent triplicate experiments.
Inhibition of cancer cell proliferation by cdGIGPQc-ScFv in vitro
The cytotoxicity of cdGIGPQc-ScFv in vitro was evaluated via CCK8 assays. Figure 4B and 4C represented the results in A549 and L78 cells respectively, as one of the three repeated experiments. The lower IC50 values of cdGIGPQc-ScFv calculated by SPSS meant less viable cells after treatment, thereby indicating a more significant tumor suppression. Under the same concentration, cdGIGPQc-ScFv inhibited tumor cell proliferation more dramatically than ScFv and cNAQAEQc-ScFv, while the semblable dose-dependent curves of ScFv and cNAQAEQc-ScFv suggested a similar inhibitory effect between them two (Figure 4B, 4C). It’s clear that cdGIGPQc-ScFv could efficiently inhibit proliferation of A549 and L78 cells at micromolar concentrations. When the concentration increased to 5 μM, cdGIGPQc-ScFv was able to kill almost all the NSCLC cells after 24 h of treatment.
Inhibition of tumor growth by cdGIGPQc-ScFv in vivo
We chose A549 and L78 cells to establish two nude-mice xenograft models of lung cancer and evaluate the anti-tumor effect of cd-ScFv in vivo. When the average tumor volume was 100 mm3, the same amounts of ScFv, cd-ScFv and cN-ScFv were given for three times. After two weeks of treatment, the tumor volumes in ScFv, cd-ScFv and cN-ScFv groups, especially the cd-ScFv, were significantly decreased compared with the PBS group in both A549 model (Figure 5A) and L78 model (Figure 5D), as Figure 5B and 5E represented the tumor growth curves of A549 and L78 xenograft. The last average tumor weights of different groups in the A549 cell xenograft model were 982.9 ± 111 mg (PBS group), 636.3 ± 30.42 mg (ScFv group), 429.9 ± 85.32 mg (cd-ScFv group) and 631.1 ± 141.07 mg (cN-ScFv group) (Figure 5C), respectively. Analogously in the L78 xenograft model, the last tumor weights were 530.8 ± 72.50 mg (PBS group), 250.1 ± 28.68 mg (ScFv group), 185.8 ± 48.99 mg (cd-ScFv group) and 305.5 ± 100.45 mg (cN-ScFv group) (Figure 5F). In a word, ScFv and cN-ScFv showed a similar anti-tumor growth activity, while cd-ScFv retarded tumor growth more remarkably. Moreover, notable ruptures with small tumor volume were only observed in cd-ScFv group. All the above implied that conjugating with lung cancer-special peptide cdGIGPQc instead of other non-special peptide (like cNAQAEQc) did enhance the antitumor efficacy of ScFv, considering both the smaller volume and lower weight of tumor. Unlike conventional chemotherapy, there was no obvious change in mice weight or general activity when treated with cd-ScFv, suggesting a good tolerance in vivo.
Figure 5.
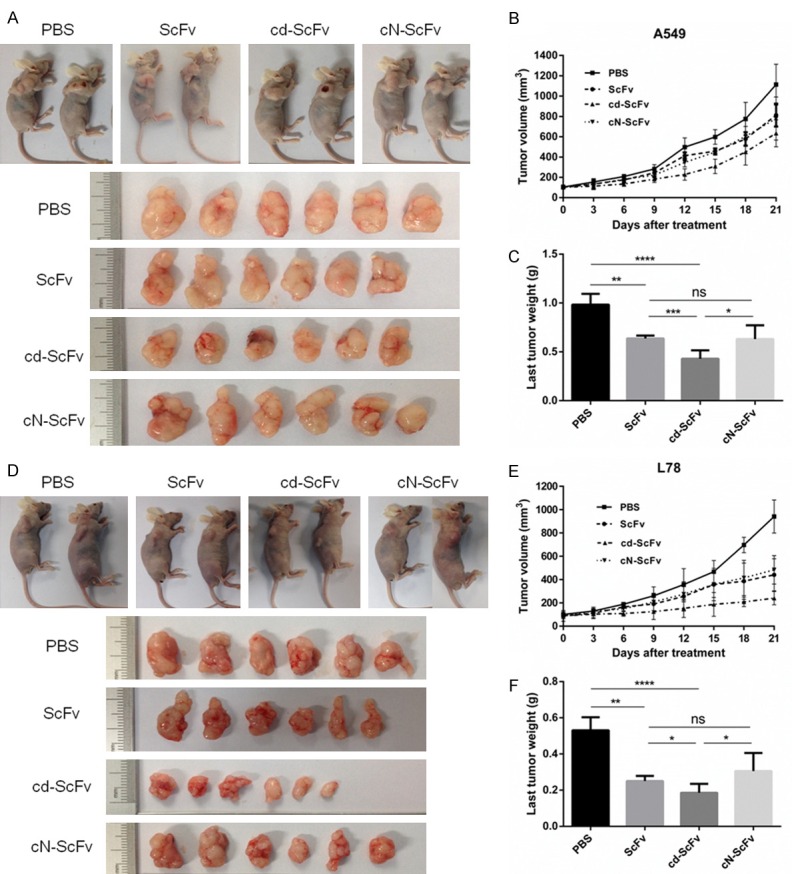
The antitumor efficacy of cdGIGPQc-ScFv in vivo. Inhibitory effects of cdGIGPQc-ScFv were evaluated in murine xenograft models of A549 cell (A) and L78 cell (D). When average tumor volume reached 100 mm3, mice were randomly divided into four group (n=6), equal amount of cdGIGPQc-ScFv, cNAQAEQc-ScFv and ScFv were injected i.v. every four days for three times. Mice treated with PBS served as negative control. (B, E) The tumor volume was regularly measured using caliper and calculated by the formula: V=(length × width2)/2. (C, F) The last tumor weight was obtained by analytical balance. Results were shown as mean ± SD. Statistical analyses were performed using unpaired t test. *, P<0.05.
High efficiency and low-toxicity of cdGIGPQc-ScFv
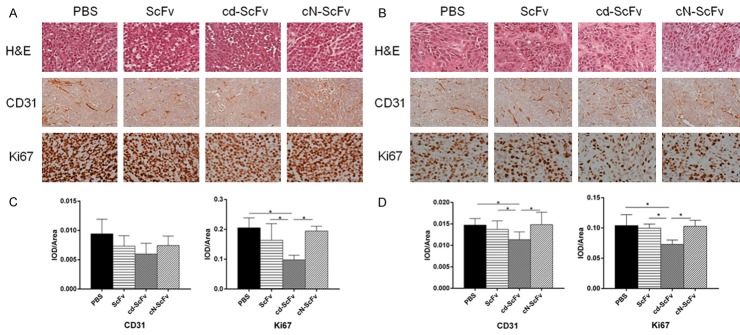
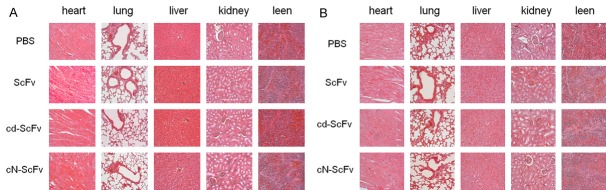
The H&E staining visually demonstrated the tumor nature of A549 and L78 xenograft, and for immunochemistry, CD31 was supposed to reflect the tumor angiogenesis while Ki67 represented tumor cell proliferation (Figure 6A, 6B). The IOD/Area values of CD31 and Ki67 were significantly lower in the cd-ScFv group compared with any other groups (Figure 6C, 6D). The simultaneously decreased trend of CD31 and Ki67 further verified the antitumor mechanism of cd-ScFv. These studies proved that cd-ScFv can decrease the tumor microvessel density and suppress cell proliferation more effectively than ScFv and cN-ScFv which indicated a better antitumor effect. As exhibited by Figure 7, there was no obvious influence or toxicity of cd-ScFv on major organs and only mild inflammation was seen in a few individuals, therefore implying a low animal toxicity.
Figure 6.
Representative images of H&E staining and Immunohistochemistry of xenografted tumor. (A, B) Histological features of tumor specimens and representative photomicrographs of anti-CD31 and anti-Ki67 immunostaining on A549 (A) and L78 (B) xenograft after the mice were treated with ScFv, cdGIGPQc-ScFv, cNAQAEQc-ScFv for 2 weeks. Scale bar: 100 μm. (C, D) The IOD/Area of anti-CD31 and anti-Ki67 positivity in images of A549 (C) and L78 (D) xenograft was analyzed and calculated with Image-Pro Plus analysis software. At least 9 images from each group were used for quantification and statistical analysis. Statistical analyses were performed using unpaired t test. *, P<0.05.
Figure 7.
Representative images of H&E staining of major organs. After treatments, the influence of drugs on normal vital organs harvested from A549 (A) and L78 (B) xenograft was observed with H&E staining.
Discussion
ScFvs have been widely used in the research work of solid tumors like nasopharyngeal [31], lung [32], gastric [33], colon [34], bladder [35], ovarian [36], and prostate cancers [37]. Since ITG αvβ3 is considered the most pivotal target for angiogensis, ScFv αvβ3 had been produced to be an available alternative to Vitaxin which had been proven to be effective in human breast carcinoma cells [23,30]. In this study, we performed CCK8 assays and animal experiments to verify the tumor inhibition capacity of ScFv αvβ3. Nevertheless, considering the small size of ScFv, the lower binding affinity and faster systemic clearance might lead to a short half-life period and limit the use in vivo [27]. In addition, lacking of tumor-specificity is likely to result in a relative low concentration on one particular tumor. In order to overcome the intrinsic limitations and enhance the actual effects, single ScFv has been engineered to form multifunctional multimers [38,39], or to combine with small targeting agent [28].
It had been reported that biodegradable peptide could achieve both specific cell targeting and selective release of cytotoxic cargo [40]. And we had previously selected a small molecular peptide cdGIGPQc that exclusively bound NSCLC cells which might serve as specific targeted deliver. Therefore, we initiated to conjugate cdGIGPQc with ScFv αvβ3 attempting to apply it in lung cancer therapy, which may moderately increase the molecular weight and enhance the lung cancer binding specificity. Herein, cdGIGPQc can be synthesized via SPPS with high quality as proved by ESI-MS and HPCL analysis. For the conjugation, we found that cdGIGPQc and ScFv αvβ3 can be simply connected by sulfhydryl, while retaining both the NSCLC cell-binding ability of cdGIGPQc and the antitumor effect of ScFv αvβ3. As demonstrated by immunofluorescent cell staining and flow cytometry, cdGIGPQc-ScFv had a more vigorous NSCLC cell targeting and binding affinity than the single ScFv and cNAQAEQc-ScFv (nonspecial peptide-ScFv). Our study also confirmed that the tumor inhibitory effect of ScFv was enhanced both in vitro and in vivo after conjugating to cdGIGPQc. The synchronous change of microvessel density and tumor cell proliferation indicated by IHC revealed that cdGIGPQc-ScFv retard tumor growth through inhibiting angiogenesis. About the unique early tumor ruptures among the mice treated with cd-ScFv, it was speculated that cdGIGPQc-ScFv significantly cut off the nutrient supply of blood vessel infiltration by blocking integrin αvβ3, leading to a failure of supporting even when the tumor was small. The little influence on normal vital organs also implied a good tolerance and low toxicity. Whereas given an experimental concentration, the therapeutic superiority of cd-ScFv in murine xenograft wasn’t so significant as in vitro, therefore it deserves more investigation about the optimal concentration and better drug-given method.
In conclusion, we first constructed and characterized the recombinant cdGIGPQc-ScFv αvβ3 that provided great lung cancer-special binding activity and antitumor efficacy both in vitro and murine model. The productive simplicity, high efficacy and low toxicity of cdGIGPQc-ScFv make it convincible and feasible for clinical use. Therefore, it may be a potential new targeted drug for lung cancer treatment. Based on this success of employing the NSCLC cell specific small molecular peptide as a drug deliver, next attempt to make it an agent in other multifunctional drugs or Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T) might whipping up a storm in the field of solid tumor therapy, especially in lung cancers.
Acknowledgements
This work was supported by Science and Technology Planning Project of Guangdong Province, China Grant (2013B021800146) and by Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
Disclosure of conflict of interest
None.
References
- 1.Zhi XY, Zou XN, Hu M, Jiang Y, Jia MM, Yang GH. Increased lung cancer mortality rates in the Chinese population from 1973-1975 to 2004-2005: an adverse health effect from exposure to smoking. Cancer. 2015;121(Suppl 17):3107–3112. doi: 10.1002/cncr.29603. [DOI] [PubMed] [Google Scholar]
- 2.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis KL, Goyal RK, Able SL, Brown J, Li L, Kaye JA. Real-world treatment patterns and costs in a US Medicare population with metastatic squamous non-small cell lung cancer. Lung Cancer. 2015;87:176–185. doi: 10.1016/j.lungcan.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Martinez P, Martinez-Marti A, Navarro A, Cedres S, Felip E. Molecular targeted therapy for early-stage non-small-cell lung cancer: will it increase the cure rate? Lung Cancer. 2014;84:97–100. doi: 10.1016/j.lungcan.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 6.Umemura S, Tsuchihara K, Goto K. Genomic profiling of small-cell lung cancer: the era of targeted therapies. Jpn J Clin Oncol. 2015;45:513–519. doi: 10.1093/jjco/hyv017. [DOI] [PubMed] [Google Scholar]
- 7.Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 8.Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour angiogenesis and angiogenic inhibitors: a review. J Clin Diagn Res. 2015;9:E1–E5. doi: 10.7860/JCDR/2015/12016.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9:498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 10.Ruegg C, Dormond O, Foletti A. Suppression of tumor angiogenesis through the inhibition of integrin function and signaling in endothelial cells: which side to target? Endothelium. 2002;9:151–160. doi: 10.1080/10623320213635. [DOI] [PubMed] [Google Scholar]
- 11.Demircioglu F, Hodivala-Dilke K. Alphavbeta3 Integrin and tumour blood vessels-learning from the past to shape the future. Curr Opin Cell Biol. 2016;42:121–127. doi: 10.1016/j.ceb.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Takahashi T, Nakamura S, Koike K, Ariyoshi Y, Takahashi T, Ueda R. Alterations of integrin expression in human lung cancer. Jpn J Cancer Res. 1993;84:168–174. doi: 10.1111/j.1349-7006.1993.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark RA, Tonnesen MG, Gailit J, Cheresh DA. Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 15.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Tan H, Dong X, Xu Z, Shi C, Han X, Jiang H, Krissansen GW, Sun X. Antisense integrin alphaV and beta3 gene therapy suppresses subcutaneously implanted hepatocellular carcinomas. Dig Liver Dis. 2007;39:557–565. doi: 10.1016/j.dld.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 18.Bello L, Lucini V, Giussani C, Carrabba G, Pluderi M, Scaglione F, Tomei G, Villani R, Black PM, Bikfalvi A, Carroll RS. IS20I, a specific alphavbeta3 integrin inhibitor, reduces glioma growth in vivo. Neurosurgery. 2003;52:177–185. 185–186. doi: 10.1097/00006123-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 20.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 21.Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16:125–132. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- 22.Patel SR, Jenkins J, Papadopolous N, Burgess MA, Plager C, Gutterman J, Benjamin RS. Pilot study of vitaxin--an angiogenesis inhibitor-in patients with advanced leiomyosarcomas. Cancer. 2001;92:1347–1348. doi: 10.1002/1097-0142(20010901)92:5<1347::aid-cncr1456>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Wang C, Li C, Zhang X, Zhang B, Mi Z, An X, Tong Y. Production and characterization of a humanized single-chain antibody against human integrin alphav beta3 protein. J Biol Chem. 2011;286:24500–24507. doi: 10.1074/jbc.M110.211847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padiolleau-Lefevre S, Alexandrenne C, Dkhissi F, Clement G, Essono S, Blache C, Couraud J, Wijkhuisen A, Boquet D. Expression and detection strategies for an scFv fragment retaining the same high affinity than Fab and whole antibody: implications for therapeutic use in prion diseases. Mol Immunol. 2007;44:1888–1896. doi: 10.1016/j.molimm.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Monnier P, Vigouroux R, Tassew N. In vivo applications of single chain Fv (Variable domain) (scFv) fragments. Antibodies. 2013;2:193–208. [Google Scholar]
- 27.Liang H, Li X, Chen B, Wang B, Zhao Y, Zhuang Y, Shen H, Zhang Z, Dai J. A collagen-binding EGFR single-chain Fv antibody fragment for the targeted cancer therapy. J Control Release. 2015;209:101–109. doi: 10.1016/j.jconrel.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, Guo Y, Derick L, Xu Y. A novel specific small molecule peptide for non-small cell lung cancer cell A549. Progress in Biochemistry Biophysics. 2007;34:1080–1085. [Google Scholar]
- 29.Wang C, Hou LH, Zhang YM, Li JM, Liao ZL, Du GX, Chen W, Sun QH, Tong YG. [Construction and expression of anti-human integrin alphavbeta3 scFv] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004;20:159–162. [PubMed] [Google Scholar]
- 30.He XJ, Li GC, Zhu JG, Li YH, Zhou GH. [Construction and screening of human anti-idiotypic single chain antibodies of nasopharyngeal carcinoma] . Ai Zheng. 2004;23:124–129. [PubMed] [Google Scholar]
- 31.Tamura M, Yan H, Zegarra-Moro O, Edl J, Oursler S, Chard-Bergstrom C, Andrews G, Kanehira T, Takekoshi S, Mernaugh R. Specific single chain variable fragment (ScFv) antibodies to angiotensin II AT(2) receptor: evaluation of the angiotensin II receptor expression in normal and tumor-bearing mouse lung. J Mol Histol. 2008;39:351–358. doi: 10.1007/s10735-008-9172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhikui L, Changcun G, Yongzhan N, Fengtian H, Xingling R, Shujun L, Zheyi H, Ying H, Xin W, Daiming F. Screening and identification of recombinant anti-idiotype antibodies against gastric cancer and colon cancer monoclonal antibodies by a phage-displayed single-chain variable fragment library. J Biomol Screen. 2010;15:308–313. doi: 10.1177/1087057109360252. [DOI] [PubMed] [Google Scholar]
- 33.Coelho V, Dernedde J, Petrausch U, Panjideh H, Fuchs H, Menzel C, Dubel S, Keilholz U, Thiel E, Deckert PM. Design, construction, and in vitro analysis of A33scFv:CDy, a recombinant fusion protein for antibody-directed enzyme prodrug therapy in colon cancer. Int J Oncol. 2007;31:951–957. [PubMed] [Google Scholar]
- 34.Tsai YS, Shiau AL, Chen YF, Tsai HT, Lee HL, Tzai TS, Wu CL. Enhancement of antitumor immune response by targeted interleukin-12 electrogene transfer through antiHER2 single-chain antibody in a murine bladder tumor model. Vaccine. 2009;27:5383–5392. doi: 10.1016/j.vaccine.2009.06.079. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Su P, Lin C, Li H, Cheng J, Shi D. Ribosome display and selection of human anti-placental growth factor scFv derived from ovarian cancer patients. Protein Pept Lett. 2010;17:585–590. doi: 10.2174/092986610791112729. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69:1128–1141. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016;11:353–361. doi: 10.1007/s11523-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231:177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 39.Dal Corso A, Caruso M, Belvisi L, Arosio D, Piarulli U, Albanese C, Gasparri F, Marsiglio A, Sola F, Troiani S, Valsasina B, Pignataro L, Donati D, Gennari C. Synthesis and biological evaluation of RGD peptidomimetic-paclitaxel conjugates bearing lysosomally cleavable linkers. Chemistry. 2015;21:6921–6929. doi: 10.1002/chem.201500158. [DOI] [PubMed] [Google Scholar]