Abstract
VGLL4 is a transcriptional repressor that interacts with transcription factors TEADs and inhibits YAP-induced overgrowth and tumorigenesis. VGLL4 protein was dramatically reduced in various types of human cancers. But how VGLL4 protein is post-transcriptional regulated is poorly understood. In this study, we identify deubiquitinating enzyme USP11 as a novel VGLL4 interactor. We reveal that the USP domain of USP11 and the N-terminal region of VGLL4 are required for mutual binding. USP11 controls VGLL4 protein stability by promoting its deubiquitination. Furthermore, our results show that knockdown of USP11 promotes cell growth, migration, and invasion in a YAP-dependent manner. Together, our results suggest that USP11 may exert its tumor suppressor role by modulating VGLL4/YAP-TEADs regulatory loop.
Keywords: USP11, VGLL4, deubiquitination, TEADs, Hippo pathway
Introduction
The Hippo pathway is a signaling cascade conserved from Drosophila melanogaster to mammals. Defects in Hippo pathway regulation have been linked to uncontrolled cell proliferation and tumorigenesis [1]. Two homologous oncoproteins, YAP and TAZ, are the major downstream targets inhibited by the Hippo signaling network [1]. YAP/TAZ interact with the TEA domain transcription factors (TEADs) to promote cell proliferation and inhibit apoptosis [1,2].
Vestigial-like (VGLL) proteins are transcription coactivators and they are named after the Drosophila transcription coactivator vestigial (Vg), which is a master regulator of wing development [3]. There are four VGLL proteins named VGLL1-4 in humans [3]. Among them, VGLL4 has been demonstrated as a novel negative regulator of YAP-TEADs transcriptional complex through direct competition with YAP for binding to TEADs [4]. VGLL4 has also been shown to be an important tumor suppressor, and its expression is dramatically reduced in various types of human tumors, including lung, gastric cancer and esophageal squamous cell carcinoma [4-6]. VGLL4 suppressed lung cancer growth and progression via direct competition with YAP in forming the complex with TEADs through two TDU domains [4]. Importantly, a recent study clearly showed that a peptide mimicking the function of VGLL4 potently suppressed gastric cancer cell growth in vitro and in de novo mouse model, suggesting that disruption of YAP-TEADs interaction by a VGLL4-mimicking peptide may be a promising therapeutic strategy against YAP-driven human cancers [5]. Furthermore, another study showed that VGLL4 expression decrease was accompanied with miR-222 expression increase in gastric cancer tissues and that miR-222 directly targets VGLL4 and VGLL4 responsible for the role of miR-222 in gastric cell lines [7]. However, little is known about how VGLl4 protein is post-transcriptional regulated in cells.
Posttranslational modification of proteins by covalent attachment of ubiquitin via E1-E2-E3 ubiquitin ligation enzyme cascades controls many essential cellular processes [8]. The E3 ubiquitin ligases select substrates for ubiquitin conjugation, which is reversed by the action of deubiquitinating enzymes (DUB) [9]. USP11 is a DUB that belongs to one of the USP family and harbors two internal ubiquitin-like (UBL) domains and an N-terminal domain present in ubiquitin-specific proteases (DUSP) [9]. In the present study, USP11 is identified as a novel VGLL4 interactor. USP11 increases VGLL4 protein stability by promoting its deubiquitination. Moreover, we show that knockdown of USP11 promotes cell growth, migration, and invasion in a YAP-dependent manner. These findings reveal that USP11 is a novel regulator of Hippo pathway. Modulation of USP11/VGLL4/YAP-TEADs regulatory loop may have potential therapeutic benefits on human tumors.
Materials and methods
Cell culture and transfection
786-O and 293T cells were obtained from the American Type Culture Collection (ATCC). 786-O cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). 293T cells were cultured in DMEM medium supplemented with 10% FBS. All transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Expression constructs
The USP11 and VGLL4 cDNAs were purchased from Genechem and subcloned into pCMV-FLAG or pCMV-Myc expression vectors, respectively. USP11 and VGLL4 mutants were generated by PCR or ligation-mediated PCR, and subcloned into pCMV-FLAG expression vectors. All the constructs were confirmed by DNA sequencing.
RNA interference
Non-specific control siRNA and siRNAs for human USP11 and YAP were purchased from GenePharma. siRNA transfection of cells was performed following the manufacturer’s instructions. The siRNA oligos sequences for USP11 are: si-USp11#1: 5’-CCGTGATGATATCTTCGTCTA-3’. si-USP11#3: 5’-CCGTGACTACAACAACTCCTA-3’. si-YAP: 5’-GACAUCUUCUGGUCAGAGA-3’. The sequence of negative control is: si-Control: 5’-ACAGACUUCGGAGUACCUG-3’.
Antibodies
Commercially available antibodies for Western Blotting were as follows: VGLL4 (SAB1410022-; Sigma), USP11 (10244-1-AP; Proteintech), Myc (9E10; Sigma), FLAG (M2; Sigma), HA (MM5-101R; Millipore), and Actin (AC-74; Sigma).
Immunoprecipitation
To immunoprecipitate the ectopically expressed Flag-tagged proteins, transfected cells were lysed 24 h post-transfection in BC100 buffer. The whole-cell lysates were immunoprecipitated with the monoclonal anti-Flag antibody-conjugated M2 agarose beads (Sigma) at 4°C overnight. After three washes with Flag lysis buffer, followed by two washes with BC100 buffer, the bound proteins were eluted using Flag-Peptide (Sigma)/BC100 for 3 h at 4°C. The eluted material was resolved by SDS-PAGE. To immunoprecipitate the endogenous proteins, cells were lysed with 1× cell lysis buffer (Cell Signaling), and the lysate was centrifuged. The supernatant was precleared with protein A/G beads (Sigma) and incubated with indicated antibodies overnight. Thereafter, protein A/G beads were applied, all at 4°C. After 2 h of incubation, pellets were washed five times with lysis buffer and resuspended in sample buffer and analyzed by SDS-PAGE.
Immunofluorescence
Cells were cultured on coverslips in 24-well plates were fixed in 4% paraformaldehyde for 10 min and were permeabilized in 0.2% Triton X-100 solution for 5 min. The coverslips were blocked with 2% BSA plus 5% goat serum for 1 h, and subsequently incubated with primary antibodies against Flag or Myc, which was followed by sequential incubation with fluorescent secondary antibodies (Alexa 488 goat anti-mouse, Alexa 555 goat anti-rabbit; Invitrogen). Finally, cells were counterstained with DAPI to reveal the nuclei. Fluorescence images were captured and processed using a fluorescence microscope.
Western blot
Cell lysates or immunoprecipitates were subjected to SDS-PAGE and proteins were transferred to nitrocellulose membranes (GE Healthcare). The membranes were blocked in Tris-buffered saline (TBS, pH 7.4) containing 5% non-fat milk and 0.1% Tween-20, washed twice in TBS containing 0.1% Tween-20, and incubated with primary antibody for 2 h and followed by secondary antibody for 1 h at room temperature. The proteins of interest were visualized using ECL chemiluminescence system (Santa Cruz).
Quantitative RT-PCR
Total RNA was isolated from transiently transfected cells using the TRIzol reagent (Tiangen), and cDNA was reversed-transcribed using the Superscript RT kit (TOYOBO), according to the manufacturer’s instructions. PCR amplification was performed using the SYBR Green PCR master mix Kit (TOYOBO). All quantization was normalized to the level of endogenous control GAPDH. The primer sequences for SYBR green are as follows: USP11_fwd: TGGAAGGCGAGGATTATGTGC-3’; USP11_rev: GCA GCA GCT CTT GAG GCA GGT TG-3’. VGLL4_fwd: 5’-AAC TGC AAC CTC TCG CAC TG-3’; VGLL4_rev: 5’-GCT CGG GCT CCT TGT AAT TCT-3’.
CCK-8 assay
Cell proliferation rate was determined using Cell Counting Kit-8 (CCK-8) according to the manufacturer’s protocol (Vazyme, CCK-8 Cell Counting Kit (A311)). Briefly, the mock or siRNAs transfected cells were seeded onto 96-well plates at a density of 1000 cells per well. During a 7-d culture periods, 10 μl of the CCK-8 solution was added to cell culture once a day, and incubated for 1 h. The resulting color was assayed at 450 nm using a microplate absorbance reader (Bio-Rad). Each assay was carried out in triplicates.
Cell migration and invasion assay
Cell migration was assessed using 24-well Transwell unit with polycarbonate membrane (pore size, 8 um) (Corning) according to the manufacturer’s protocol. The membrane was coated with Matrigel basement membrane matrix (1 μg/μl) (BD Bioscience) for 24 h. Then Cells (0.5∼2.5×104) were seeded into the upper chamber in a serum-free medium. The lower chamber was filled with a medium containing 10% FBS. After 24 h of incubation, the cells in the upper chamber were removed, and the cells were fixed in 4% paraformaldehyde, stained with crystal violet for 20 min. After being washed with water three times. Digital images were obtained from the membranes, and cell areas were selected using Scan Scope CS system (Aperio Technologies). The migrating cells were quantified in five randomly selected fields in each membrane, and the average value was defined as a migration or invasion index on three independent membranes. For invasion, the membranes utilized were Matrigel-coated invasion chambers (BD Biosciences) that were pre-hydrated in serum-free medium.
Results
VGLL4 interacts with USP11 in cells
To understand how VGLL4 is regulated in cells, we searched for VGLL4-associated proteins in cells. FLAG-VGLL4 was used to immunoprecipitate potential associated proteins from 293T cells. Mass spectrometry analysis identified several peptides derived from USP11, suggesting that USP11 might form a complex with VGLL4 (data not shown). To verify that USP11 is a bona fide VGLL4 interactor, we first examined whether USP11 can interact with VGLL4 in cells. FLAG-USP11 and Myc-VGLL4 constructs were co-expressed in 293T cells. Cells were subsequently harvested for co-immunoprecipitation (Co-IP) with the anti-FLAG antibody. As shown in Figure 1A, Myc-VGLL4 was immunoprecipitated by FLAG-USP11, suggesting an exogenous interaction between these two proteins. In addition, a reciprocal Co-IP assay performed using anti-Myc antibody indicated that Myc-VGLL4 was able to immunoprecipitate FLAG-USP11 (Figure 1B). We also found that the catalytically deficient mutant of USP11 CA (C275A/C283A) also efficiently interacted with VGLL4 similar as USP11 WT, suggesting the catalytic activity of USP11 is not required for VGLL4 binding (Figure 1A and 1B). Next, we investigated whether endogenous VGLL4 and USP11 can interact with each other. Immunoprecipitation using the anti-VGLL4 antibody was performed using cell lysates prepared from 786-O cells. As shown in Figure 1C, endogenous USP11 was immunoprecipitated by VGLL4, suggesting an endogenous interaction between these two proteins. Similarly, endogenous VGLL4 was immunoprecipitated by USP11 (Figure 1D). To investigate whether USP11 co-localizes with VGLL4 in vivo, 786-O cells were transfected with FLAG-VGLL4 and/or Myc-USP11, immunostained and visualized by confocal microscopy. As shown in Figure 1D, USP11 and VGLL4 were co-localized in the nucleus. Taken together, these results indicate that USP11 forms a complex with VGLL4 in cells.
Figure 1.
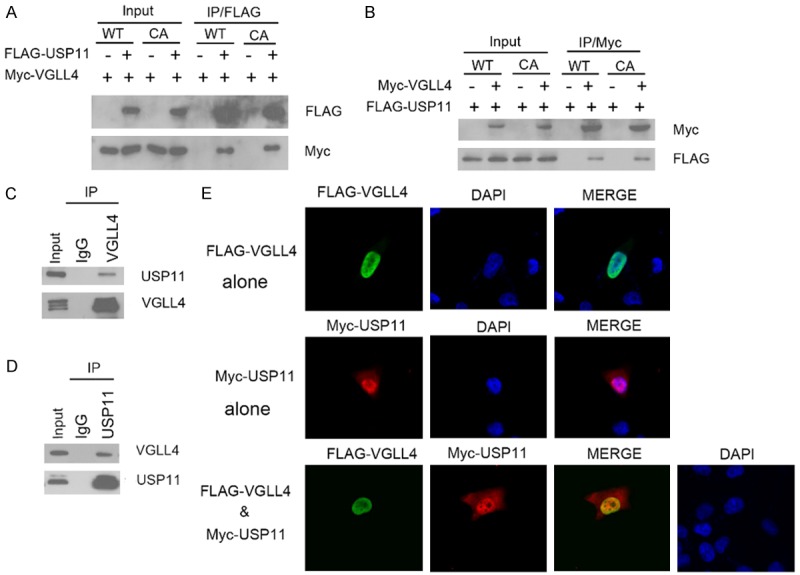
USP11 interacts with VGLL4 in cells. A. 293T cells were co-transfected with FLAG-USP11 (WT or CA mutant) and Myc-VGLL4 constructs. After 24 h, cell lysates were prepared for Co-IP with anti-FLAG antibody and Western blotting. B. 293T cells were as well co-transfected with FLAG-USP11 (WT or CA mutant) and Myc-VGLL4 constructs. After 24 h, cell lysates were for Co-IP with anti-Myc antibody and Western blotting. C. After treated with 20 µM MG132 for 4 h, 786-O cell lysates were prepared for Co-IP with anti-VGLL4 antibody and WB analyses with indicated antibodies. D. After treated with 20 µM MG132 for 4 h, 786-O cell lysates were prepared for Co-IP with anti-USP11 antibody and WB analyses with indicated antibodies. E. 786-O cells were transfected with Myc-USP11 and/or FLAG-VGLL4 constructs. Cells were fixed 48 h after transfection and subjected to immunofluorescence analysis.
Identification of the mutual-binding regions of VGLL4 and USP11
To gain more insight into the VGLL4-USP11 interaction, we determined which region of VGLL4 mediated its interaction with USP11. As shown in Figure 2A, VGLL4 contains a nuclear export signal (NES), a novel conserved sequence (NCS), and two tandem TONDU (TDU) domains. Fragments carrying different subsets of these domains were tested for their binding capability towards USP11. As shown in Figure 2B, Co-IP assay showed that all fragments, except fragment (151-293 aa), were able to bind to USP11. Therefore, we concluded that the N-terminal region of VGLL4 (1-151 aa), is required for its interaction with USP11. We next determined the region of USP11 that binds to VGLL4. We generated USP11 deletion mutants and tested their ability to interact with VGLL4 by Co-IP assay. The results showed that three fragments containing the C-terminal region of USP11, but not others, were able to immunoprecipitate Myc-VGLL4 (Figure 2C and 2D), suggesting that the C-terminal region (700-921aa) of VGLL4 is responsible for binding to VGLL4.
Figure 2.
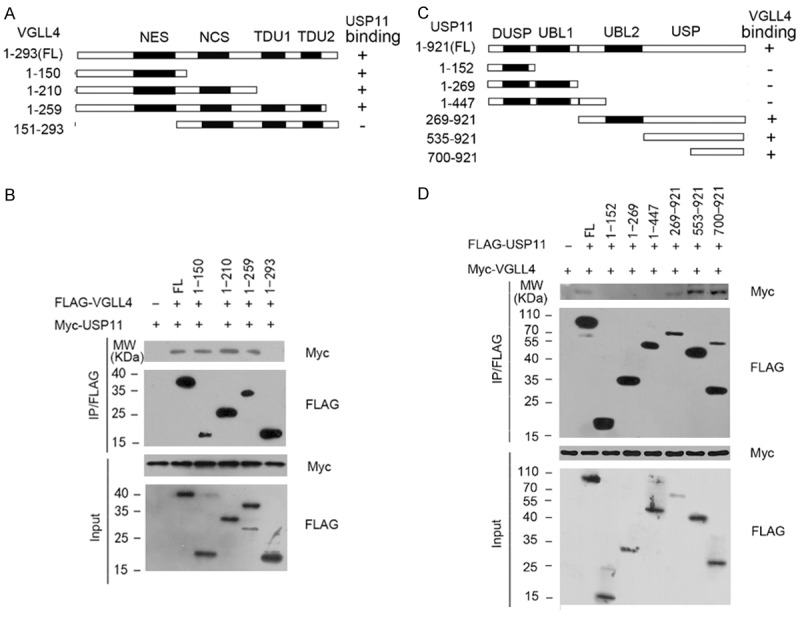
Delineation of the domains mediating the mutual interactions between USP11 and VGLL4. A. Schematic representation of VGLL4 truncation showed in the study. NLS, nuclear localization signal. NCS, novel conserved sequence. The ability of each VGLL4 deletion mutants to bind to USP11 is indicated (+: binding, -: no binding). B. 293T cells were co-transfected with Myc-USP11 and the indicated FLAG-VGLL4 or deletion mutants, cell lysates were prepared and mmunoprecipitated with anti-FLAG antibody, followed by WB analyses with indicated antibodies. C. Schematic presentation of USP11 domains and deletion mutants. The ability of each USP11 deletion mutants to bind to VGLL4 is indicated. D. 293T cells were co-transfected with Myc-VGLL4 and the indicated FLAG-USP11 or deletion mutants, cell lysates were prepared and immunoprecipitated with anti-FLAG antibody, followed by WB analyses with indicated antibodies.
USP11 stabilizes VGLL4 by deubiquitination
Since USP11 is a deubiquitinating enzyme, we explored whether USP11 can deubiquitinate and stabilize VGLL4 protein. As shown in Figure 3A, ectopic expression of USP11-WT, but not the USP11 CA mutant (catalytic inactive), increased the co-expressed VGLL4 protein level in a dose-dependent manner, indicating that its deubiquitinase activity is required for promoting VGLL4 stabilization. Moreover, ectopic expression of USP11-WT, but not the USP11 CA mutant in 786-O cells, resulted in an increase of the protein level of endogenous VGLL4 (Figure 3B). Next, we depleted the endogenous USP11 by two specific siRNAs in 786-O cells, and observed that VGLL4 protein was markedly down-regulated (Figure 3C). To exclude the possibility that VGLL4 protein down-regulation resulted from transcriptional down-regulation, we performed qRT-PCR assay to measure the mRNA level of VGLL4 and USP11 in USP11-depleted 786-O cells. In contrast to the significant decrease in USP11 mRNA levels, VGLL4 mRNA level in USP11-depleted 786-O cells stayed at a level similar to that of the control cells (Figure 3D), suggesting that the effect of USP11 on VGLL4 is not mediated through the up-regulation of VGLL4 mRNA expression. To determine whether USP11 increases VGLL4 by extending its half-life, the protein level of VGLL4 were monitored after treatment with the protein synthesis inhibitor cycloheximide (CHX). In the absence of de novo protein synthesis, the half-life of endogenous VGLL4 protein was shorter in the USP11-depleted cells than that in control cells (Figure 3E and 3F), further suggesting that USP11 regulates VGLL4 protein level at the post-translational level.
Figure 3.
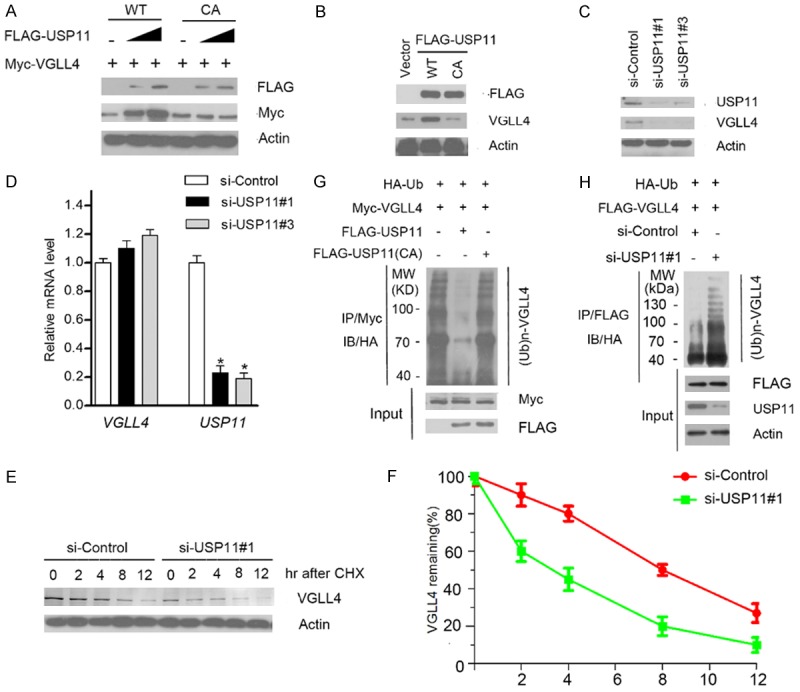
USP11 stabilizes VGLL4 by deubiquitination. (A) 293T cells were transfected with Myc-VGLL4 and increasing amounts of FLAG-USP11-WT or CA mutant constructs. 24 h after transfection, cells were harvested for WB analyses. (B) 786-O cells were transfected with FLAG-USP11-WT or CA mutant constructs. 24 hr after transfection, cells were harvested for WB analyses. (C) 786-O cells were transfected with the negative control or two independent USP11 siRNAs, respectively. 48 h after transfection, cells were harvested for WB analyses. (D) qRT-PCR measurement of the mRNA levels of USP11 and VGLL4 in USP11-depleted cells. GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown (*, P < 0.05). (E, F) 786-O cells were transfected with the negative control or USP11 siRNAs. 48 h after transfection, cells were collected at various times after cycloheximide (CHX) treatment and then were subjected to WB analyses (E). The relative intensities of VGLL4 were first normalized to the intensities of actin and then to the value of the 0-h time point (F). (G) HA-Ub and Myc-VGLL4 along with FLAG-USP11-WT or CA mutant constructs were co-transfected into 293T cells. 24 h after transfection, cells were treated with 20 μM MG132 for 6 h. Myc-VGLL4 protein was immunoprecipitated with anti-Myc antibody. The ubiquitinated forms of VGLL4 were analyzed by WB with anti-HA antibody. (H) USP11 was depleted by siRNAs in 293T cells transiently expressing HA-Ub and FLAG-VGLL4. Cells were treated with 20 μM MG132 for 6 h. FLAG-VGLL4 protein was immunoprecipitated and subjected to WB analyses.
To further determine that USP11 promotes VGLL4 stabilization through the regulation of VGLL4 deubiquitination, HA-ubiquitin and FLAG-VGLL4 constructs were co-expressed with USP11-WT or CA mutant in293T cells. As shown in Figure 3G, ectopically expressing USP11-WT, but not the CA mutant, markedly reduced the VGLL4 poly-ubiquitination. Conversely, depletion of USP11 using siRNAs increased VGLL4 ubiquitination (Figure 3H), indicating that USP11 is responsible for VGLL4 deubiquitination. Taken together, these data demonstrate that the deubiquitinase USP11 maintains VGLL4 protein steady-state level through promoting VGLL4 deubiquitination in cells.
Knockdown of USP11 promotes cell growth, migration, and invasion in a YAP-dependent manner
Accumulating evidence showed that VGLL4 expression was downregulated in various types of human cancers [4-6]. Mechanically, VGLL4 acts as a transcriptional repressor that interacts with TEADs and inhibits YAP-induced overgrowth and tumorigenesis [9]. To determine the functional significance of USP11-mediated VGLL4 protein stabilization, we examined the effect of reduced USP11 levels on cell proliferation in 786-O cells transfected with control siRNAs or two independent USP11 siRNAs, respectively. As shown in Figure 4A, USP11 depletion resulted in a marked increase of 786-O cell growth. In contrast, co-depletion of USP11 and YAP significantly reduced 786-O cell growth compared with depletion of USP11 only (Figure 4A). Moreover, cell migration and invasion were determined by transwell assay. Similar to cell growth assay, we found that USP11 depletion resulted in a marked increase of 786-O cell migration and invasion, but these effects were reversed by co-depletion of YAP (Figure 4B). Thus, these results suggest that VGLL4 may play negative roles in cell growth, migration, and invasion at least in part, by regulating Hippo-YAP pathway.
Figure 4.
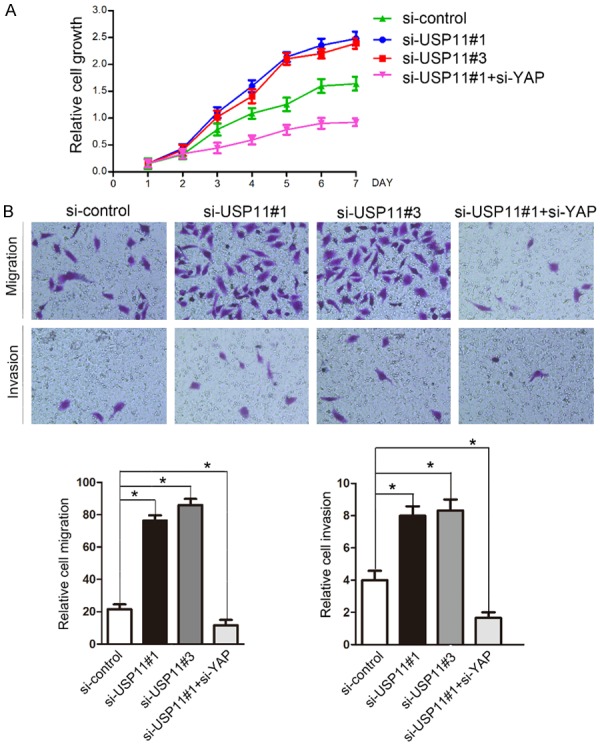
Knockdown of USP11 promotes cell growth, migration, and invasion in a YAP-dependent manner in 786-O cells. A. 786-O cells were transfected with control or indicated siRNAs. After 48 h, the cell growth was measured by CCK-8 assay at indicated days. These results show data from at least three independent experiments. B. 786-O cells were transfected with control or indicate siRNAs. Representative images of cell migration and invasion assays are shown on the upper panel, and the quantitative analysis is shown on the right panel of each figure. The mean values (S.D.) of three independent experiments are shown (*, P < 0.05). Migration and invasion assays were performed using Transwell chambers without or with Matrigel as described under Materials and Methods.
Discussion
Here, we identify VGLL4 deubiquitination, which is regulated by the deubiquitinating enzyme USP11, as a new regulatory layer of the Hippo pathway. Ubiquitination has been shown to play a critical role in regulating YAP/TAZ activity through regulation of many core Hippo pathway components. Several ubiquitin ligase complexes have been implicated in this process. For example, YAP/TAZ protein stability are controlled by a phosphodegron recognized by the F-box protein β-TrCP and ubiquitinated by the SCF/CRL1 (β-TrCP) E3 ubiquitin ligase [10]. Negative regulation of the Hippo pathway component LATS1 by E3 ubiquitin ligase ITCH is sufficient to promote epithelial-mesenchymal transition (EMT) and tumorigenicity [11]. However, the potential roles of deubiquitinating enzymes (DUBs) as regulators of Hippo pathway activity remain poorly understood. However, a recent study showed that a DUB, USP9x deubiquitinates Angiomotin (AMOT) and Angimotin-like proteins, resulting in protein stabilization and lower YAP/TAZ activity [12].
In addition to VGLL4, USP11 binds, deubiquitinates and stabilizes diverse substrates. USP11 enhances PML stability to control Notch-induced malignancy in brain tumors [13]. USP11 interacts with p53 and stabilizes p53 by promoting its deubiquitination, and downregulation of USP11 dramatically attenuated p53 induction in response to DNA damage signal [14]. USP11 plays an important role in the downregulation of TNFα-mediated NF-κB activation through modulating IKKα stability [15]. Moreover, USP11 modulates Polycomb regulation of the INK4a tumor suppressor [16]. Taken together, these studies and ours collectively shows that USP11 may exert its tumor suppresser roles in by regulating multiples signaling pathway such as p53, NF-κB, Notch and Hippo.
Our current results raise several immediate issues. First, the E3 ubiquitin ligase of VGLL4 remains to be identified. This E3 ubiquitin ligase would be expected to have an oncogenic function by targeting VGLL4 for ubiquitination-dependent degradation. Second, an interesting aspect of our work that needs further investigation is the physiological conditions that dynamically regulates VGLL4 protein stability. A recent study showed that Notch signal induces transcription factor Hey1 to bind to USP11 promoters and downregulate USP11 mRNA level [13]. It is interesting to investigate whether VGLL4 protein was downregulated through a Notch/Hey1-dependent mechanism. Moreover, previous study showed that cell density-dependent activation of the Hippo pathway decreases YAP protein stability [17]. Thus we need to investigate Whether USP11-mediated VGLL4 stabilization was potentiated at high cell density. Finally, since USP11 mRNA were downregulated in some types of cancers, such as glioma, it will be helpful to check the correlation of USP11 and VGLl4 expression in human cancer tissues.
Disclosure of conflict of interest
None.
References
- 1.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 2.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther. 2013;14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, Li F, Chen H, Zhou Z, Zhang L, Ji H. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, Wang X, Guo T, Li P, Zhao Y, Ji H, Zhang L, Zhou Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Yao F, He J, Lv B, Fang W, Zhu W, He G, Chen J, He J. Downregulation of VGLL4 in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1289–1297. doi: 10.1007/s13277-014-2701-7. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Yu N, Wang J, Xi H, Lu W, Xu H, Deng M, Zheng G, Liu H. miR-222/VGLL4/YAP-TEAD1 regulatory loop promotes proliferation and invasion of gastric cancer cells. Am J Cancer Res. 2015;5:1158–1168. [PMC free article] [PubMed] [Google Scholar]
- 8.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 9.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salah Z, Melino G, Aqeilan RI. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 2011;71:2010–2020. doi: 10.1158/0008-5472.CAN-10-3516. [DOI] [PubMed] [Google Scholar]
- 12.Thanh Nguyen H, Andrejeva D, Gupta R, Choudhary C, Hong X, Eichhorn PJ, Loya AC, Cohen SM. Deubiquitylating enzyme USP9x regulates hippo pathway activity by controlling angiomotin protein turnover. Cell Discov. 2016;2:16001. doi: 10.1038/celldisc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu HC, Lin YC, Liu CH, Chung HC, Wang YT, Lin YW, Ma HI, Tu PH, Lawler SE, Chen RH. USP11 regulates PML stability to control Notch-induced malignancy in brain tumours. Nat Commun. 2014;5:3214. doi: 10.1038/ncomms4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke JY, Dai CJ, Wu WL, Gao JH, Xia AJ, Liu GP, Lv KS, Wu CL. USP11 regulates p53 stability by deubiquitinating p53. J Zhejiang Univ Sci B. 2014;15:1032–1038. doi: 10.1631/jzus.B1400180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun W, Tan X, Shi Y, Xu G, Mao R, Gu X, Fan Y, Yu Y, Burlingame S, Zhang H, Rednam SP, Lu X, Zhang T, Fu S, Cao G, Qin J, Yang J. USP11 negatively regulates TNFalpha-induced NF-kappaB activation by targeting on IkappaBalpha. Cell Signal. 2010;22:386–394. doi: 10.1016/j.cellsig.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maertens GN, El Messaoudi-Aubert S, Elderkin S, Hiom K, Peters G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 2010;29:2553–2565. doi: 10.1038/emboj.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]


