Abstract
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies. The current study is designed to explore the role of physcion, a major active ingredient in several traditional herbal medicinal plants, for the treatment of HCC. HCC cell lines, SMMC7721 and HepG2, were treated with physcion and its apoptosis-inducing effect was examined. Both in vitro and in vivo results from the present study demonstrated that physcion treatment resulted in apoptotic cell death in HCC cells via upregulation of miR-370. Furthermore, our findings showed that the physcion modulated the level of miR-370 through AMPK/Sp1/DNMT1 signaling. Taken together, these results showed that physcion exerts anti-tumor effect against HCC, which may be a potential agent for the adjunct chemotherapy.
Keywords: Physcion, apoptosis, miR-370, DNMT1
Introduction
Hepatocellular carcinoma (HCC), one of the most common and aggressive malignancies, is the second most leading cause of cancer-related deaths in males and the sixth in females due to the intrahepatic metastases and high-risk recurrence [1,2]. Moreover, the incidence and mortality of HCC have been increasing in developing countries, such as China, in the last decade [3]. According to the statistics, 355,595 new cases were diagnosed, and 322,416 patients with HCC deceased in China in 2011 [4]. Owing to the unsatisfactory therapeutic benefits provided by mainstream treatment such as surgical resection and chemotherapy, the discovery of novel agents has become a promising approach to improve the prognosis of HCC patients.
MicroRNAs (miRNAs), a class of small non-coding RNAs with a length of 21-25 nucleotides, play a major role in a variety physiological activities. These activities in the cells include proliferation, development, apoptosis, and differentiation through post-transcriptionally regulated expression of their target genes’ mRNA [5]. Accumulating evidence has shown that the aberrant upregulation or downregulation of miRNAs correlates with the development and prognosis of different human malignancies including HCC [6]. For instance, aberrantly low expression of miR-148b has been found to correlate with poor outcome in HCC [7]. Sun et al. reported that the elevated level of miR-522 predicts poor prognosis in patients with HCC [8]. In HCC cells, miR-370 exhibits the potential to suppress metastasis by inhibiting migration and invasion [9]. Furthermore, another study by Sun et al. also reported that increasing miR-370 expression promotes cell death of liver cancer cells in vitro [10]. However, the underlying mechanism through which miR-370 affects the biological behavior of HCC cells is not entirely understood.
Physcion, an active ingredient in medicinal plant Radix et Rhizoma Rhei [11], has been used as a laxative, hepatoprotective, anti-inflammatory, and anti-microbial agent [11-14]. Recently, physcion has also been reported to induce apoptosis [15-18], block cell cycle progression [16], and suppress the metastatic potential of cancer cells [19]. However, the role of physcion in HCC has not been investigated. In the present study, our results revealed that physcion induces apoptosis in HCC cell lines by upregulating miR-370. Moreover, we also demonstrated that the physcion exerts a regulatory effect on miR-370 by modulating the AMPK/Sp1/DNMT1 signaling.
Materials and methods
Cell lines and cultures
Human HCC cells SMMC7721 and HepG2 were purchased from American Type Culture Collection (Manassas, VA, USA). The immortalized liver cell line L02 was obtained from Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 4 mM/L-glutamine, 3.7 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) in a 5% CO2 humidified incubator at 37°C.
Quantitative real-time PCR (qRT-PCR)
TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) was utilized to extract total RNA from cultured cells. miR-370 expression was quantified by real-time PCR using a TaqMan Probe (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, cDNA was obtained by High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) and qRT-PCR was performed using a TaqMan PCR kit and the ABI 7500 System (Thermo Fisher Scientific). The relative expression of miR-370 in the cells and tissues was normalized to that of U6. For mRNA expression of Sp1 and DNMT1, the specific primers were obtained from Synbio Tech (Beijing, China) and purchased from Sino Biological Inc. (Beijing, China), respectively. The relative mRNA expression of Sp1 and DNMT1 was normalized to that of GADPH. PCR results were analyzed using the comparative ΔCt method (ABPrism software, Applied Biosystems, Foster City).
Cell viability test
CCK-8 assay was performed to determine the cell viability (WST-8 Cell Counting Kit-8, Beyotime, Shanghai, China) according to the manufacturer’s instructions. Briefly, cells at a density of 1×105 cells/mL were seeded in culture plates and maintained for 24 or 48 h. Next, the CCK-8 solution was added to the culture medium, and the cells were incubated for an additional 1 h before the viable cell count was determined by measuring absorbance at 450 nm (Tecan Group Ltd, Männedorf, Switzerland).
Flow cytometry analysis
Cell apoptosis was evaluated by flow cytometry using an FITC-Annexin V Apoptosis Kit (BD Pharmingen, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Briefly, the cells at a density of 1×106 cells/mL were stained with annexin-V-FITC and propidium (PI) for 15 min in the dark before analysis with a flow cytometer (Beckman Coulter Inc., Miami, FL, USA).
Hoechst staining
The cells were washed with PBS and fixed before staining with 1 μmol/L Hoechst 33258 (Sigma-Aldrich, St Louis, MO, USA) for 10 min before the examination.
Caspase-3, caspase-8, and caspase-9 activity analysis
Following treatment, the cells were collected and lysed with lysis buffer. Next, an equivalent of 50 μg extracted protein was analyzed using specific colorimetric assay kit (Ray Biotech, Guangzhou, China) to examine the activity of caspase-3, caspase-8, and caspase-9.
Mitochondrial membrane potential (MMP) analysis
The effect of physcion on MMP was examined using fluorochrome dye JC-1 following the standard protocol. Briefly, HCC cells were incubated with different concentrations of physcion for 48 h before cells were harvested and incubated with JC-1. After staining, the excessive dye was removed by gentle rinsing with PBS and analyzed by then flow cytometry to quantify MMP.
Sp1 and DNMT1 overexpression
Overexpressing plasmids for DNMT1 and Sp1 were transfected using Lipofectamine 3000 reagent (Invitrogen, Grand Island, NY, USA), respectively. DNMT1 overexpressing plasmid construct was purchased from Life Technologies, Inc. (Rockville, MD, USA) and Sp1 overexpressing vector were constructed by our group as described previously [20]. 48 h post transfection, the expression level of both proteins was verified by Western blot.
Transfection of siRNAs targeting DNMT1 or Sp1 in HCC cells
The siRNA oligos targeting Sp1 and DNMT1 were obtained from Seebio Biotech (Shanghai, China) and Santa Cruz Biotech (Santa Cruz, CA, USA), respectively. siRNA sequence and one scramble sequence as control were inserted into the plasmid vector pGCsi-H1, independently. For transfection, the cells in logarithmic growth phase were seeded in a 6-well plate and transfected with the respective constructs following the manufacture’s protocol (Invitrogen). The transfected cells were incubated for 48 h, and the knockdown was verified by Western blot.
miR-370 knockdown or overexpression
The lentiviral constructs miR-370 mimic and miR-370 and miR-370 inhibitor were obtained from Qiagen (Dusseldorf, Germany). Cells were seeded into each well of a 96-well plate, incubated overnight, and then transfected with miR-370 mimic and miR-con, or miR-370 inhibitor according to the manufacturer’s instructions. The transfection efficiency was confirmed by qPCR analysis.
Western blotting analysis
Proteins were isolated from tissues by lysing the frozen tissues in RIPA buffer (Sigma). Proteins were extracted from cells by lysis buffer (Beyotime) containing protease inhibitors (Sigma). The protein concentration was measured by the BCA protein assay kit (Beyotime). The extracted total protein extracts were resolved on SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride membranes (PVDF; Millipore, Billerica, MA, USA). The membranes were probed with specific antibodies following the standard protocol. The second antibodies used in this study were goat anti-rabbit IgG-HRP, goat anti-mouse IgG-HRP, and donkey anti-goat IgG-HRP (Beyotime). The immunoreactive signals were detected using a chemiluminescent substrate (KPL, Guildford, UK) and the intensities were quantified using BandScan software (Glyko, Novato, CA, USA).
Anti-tumor effect in xenograft animal model
Four-week-old male nude mice (BALB/c, nu/nu; SPF laboratory animal center of the People’s Hospital of Wuhai, Inner Mongolia, China) were housed under pathogen-free conditions. All the animal experiments were approved by the People’s Hospital of Wuhai. A total of 5×105 HepG2 cells were injected I.P. into the animals. Tumor volumes were calculated as 1/2 a2b (a and b are the short and long tumor axes, respectively). After euthanasia, the tumors were harvested for further analysis. The primary tumors were excised and analyzed by HE and TUNEL staining. Briefly, TUNEL detection analysis of apoptotic cells in tumor tissue was performed by the TUNEL Apoptosis Detection Kit (Beyotime) as per the manufacturer’s directions. Three equal-sized fields were randomly selected, and the mean number of green fluorescence-positive cells was counted. The expression of miR-370 was determined by qRT-PCR, whereas the expression of Sp1, DNMT1, p-AMPK, and t-AMPK was determined by Western blot.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Data were presented as mean ± SD. Statistical comparisons were performed by one-way ANOVA followed by Dunnett’s t-test. The difference with a P-value <0.05 was defined as statistically significant.
Results
Physcion reduces the viability of HCC cells
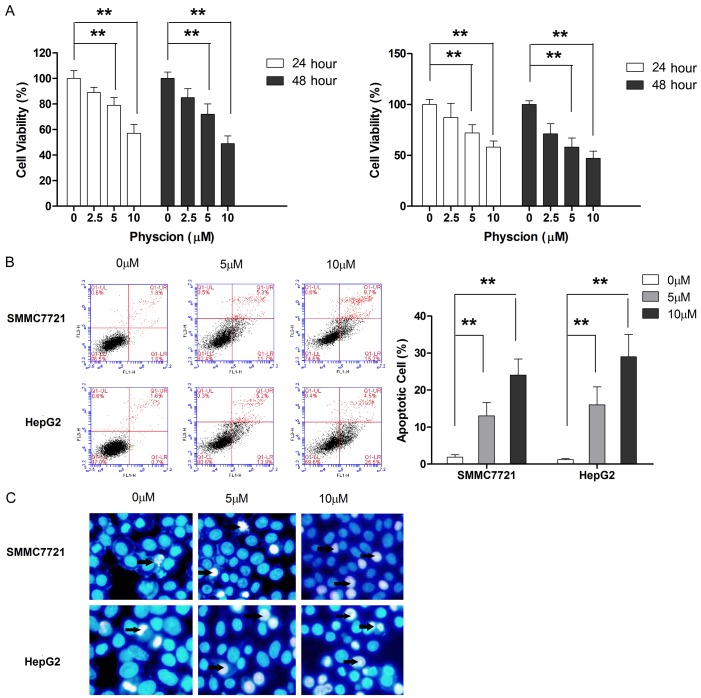
To investigate the anti-tumor effect of physcion, both HCC cell lines were challenged with an increasing dosage of physcion for 24 and 48 h. At a dose of 5 μM, physcion treatment did not cause any change in the cell viability in both cell lines at both times points (24 and 48 h). However, when the dose was increased to 10 μM, a slight decrease in the number of viable cells was observed at 24 h, and a profound reduction was noticed at 48 h, although the sensitivity of both cell lines to physcion was different (Figure 1A). In the case of 20 μM, the cell viability of both cell lines was further decreased.
Figure 1.
Physcion suppresses cell growth and induces apoptosis in HCC cells. A. The cell viability was measured by CCK-8 kit. B. Apoptotic cell population was determined by flow cytometry following treatment with physcion for 48 hours. C. The morphological change of apoptotic cells were examined by Hochest staining following treatment with physcion for 48 hours. **P<0.01.
Physcion induces apoptosis in mitochondria-dependent pathway
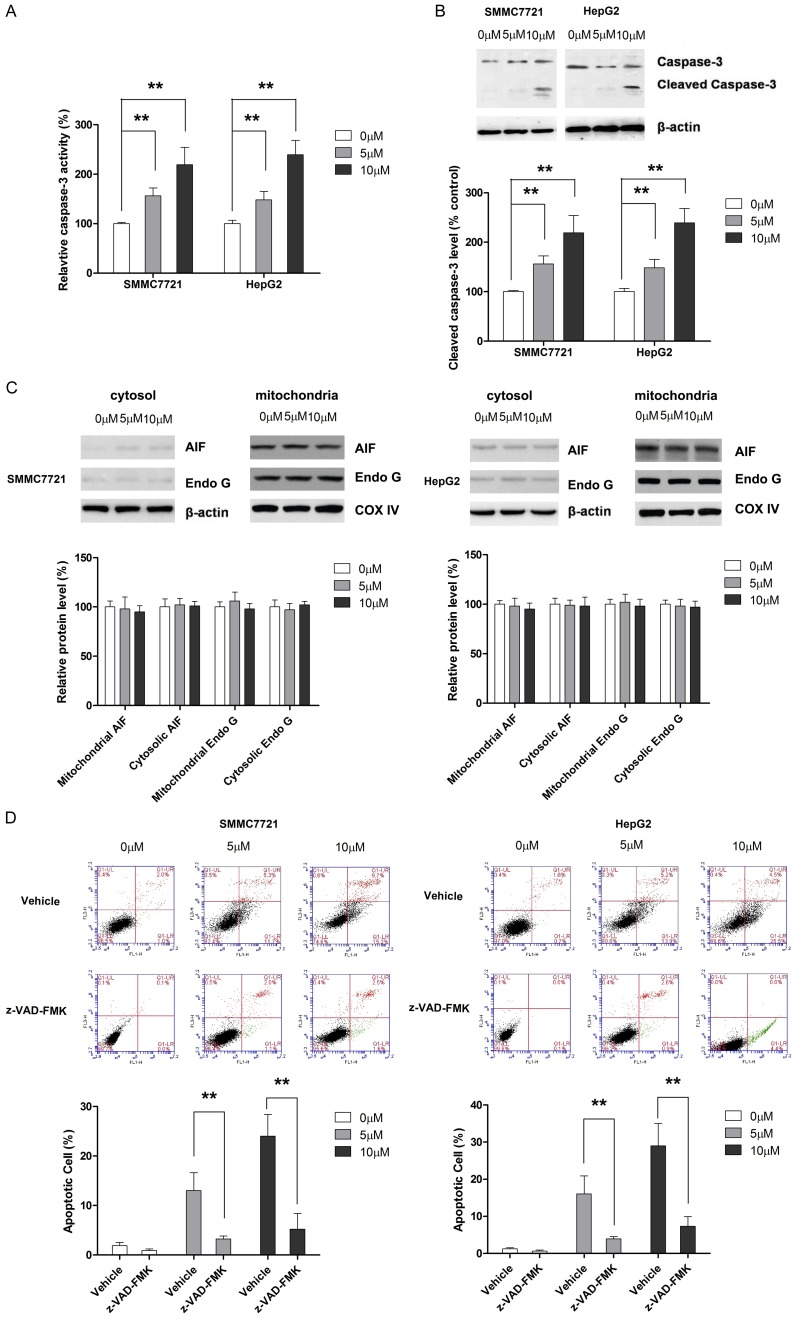
In the subsequent experiments, we investigated the involvement of apoptosis induction in the anti-tumor effect of physcion. As shown in Figure 1B, flow cytometric analysis revealed that physcion was able to increase the apoptotic proportion in both cell lines. In addition, the apoptosis-inducing effect of physcion was also supported by Hoechst staining (Figure 1C). Given the crucial role of caspase-3 in apoptosis, the effect of physcion on caspase-3 activity was examined. As shown in Figure 2A and 2B, physcion treatment led to a dose-dependent increase caspase-3 activation. Moreover, the effect of physcion on the release of two crucial mediators in caspase-independent apoptosis pathway, AIF and Endo G, from the mitochondria to the cytosol was examined. As shown in Figure 2C, even 20 μM physcion neither affected the mitochondrial nor the cytosolic level of AIF and Endo G. The apoptosis in tumor cells is known to occur via caspase-independent pathway [21]; thus, a caspase inhibitor (Z-VAD-FMK) was used to fully appreciate the apoptosis-inducing effect of physcion. As shown in Figure 2D, Z-VAD-FMK treatment significantly abolished the apoptosis-inducing effect of physcion in both HCC cell lines. Taken together, our results excluded the possibility of physcion inducing apoptosis by a caspase-independent pathway.
Figure 2.
Physcion induces apoptosis in caspase-dependent manner in HCC cells. HCC cells were treated with physcion for 48 hours before assay were performed. A. Physcion treatment correlates with dose-dependent increase in caspase-3 activity. B. Cleaved caspase-3 level is elevated in cells treated by physcion. C. AIF and Endo G are not involved in the physcion-mediated apoptosis. D. Caspase inhibitor z-VAD-FMK significantly abrogates the apoptosis-inducing effect of physcion in HCC cells. **P<0.01.
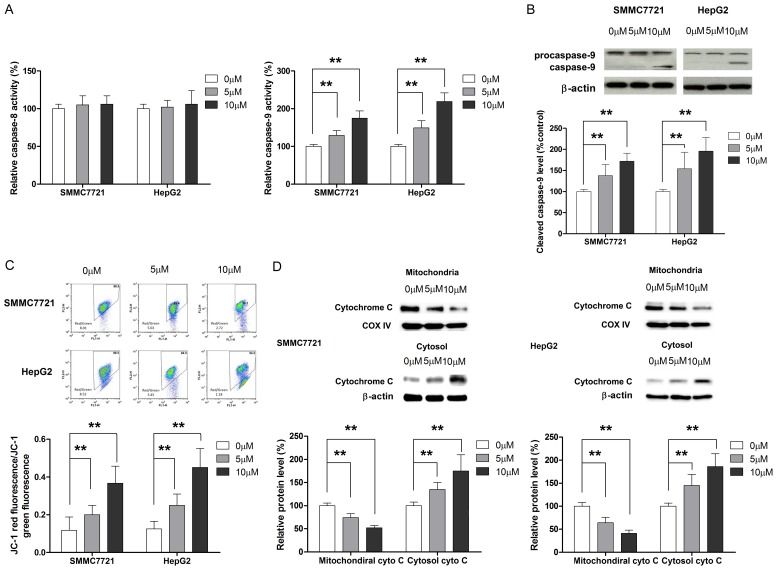
Apoptosis may occur either through intrinsic or extrinsic pathway [22], which is characterized by the activation of caspase-8 and caspase-9, respectively. Hence, the effect of physcion on caspase-8 and caspase-9 activity was assessed by ELISA and Western blot. As shown in Figure 3A and 3B, physcion treatment resulted in dose-dependent changes in the activity of caspase-9 in HCC cells. On the other hand, any remarkable changes in the activity of capase-8 were triggered by physcion, irrespective of the dose. Furthermore, the assessment of the effect of physcion on MMP and release of cytochrome C from mitochondria to cytosol confirmed that physcion-induced apoptosis in a mitochondria-dependent manner (Figure 3C and 3D).
Figure 3.
Mitochondria mediate the physcion-inducing apoptosis in HCC cells. A. Physcion treatment results in dose-pendent increase in caspase-9 activity while does not affect the activity of caspase-8. B. Cleaved caspase-9 level is elevated in cells treated by physcion. C. Loss of mitochondrial membrane potential is observed in cells treated with physcion. D. Release of cytochrome C from mitochondria to cytosol is found in cells treated with physcion. **P<0.01.
Upregulation of miR-370 mediates physcion-induced apoptosis in HCC cells
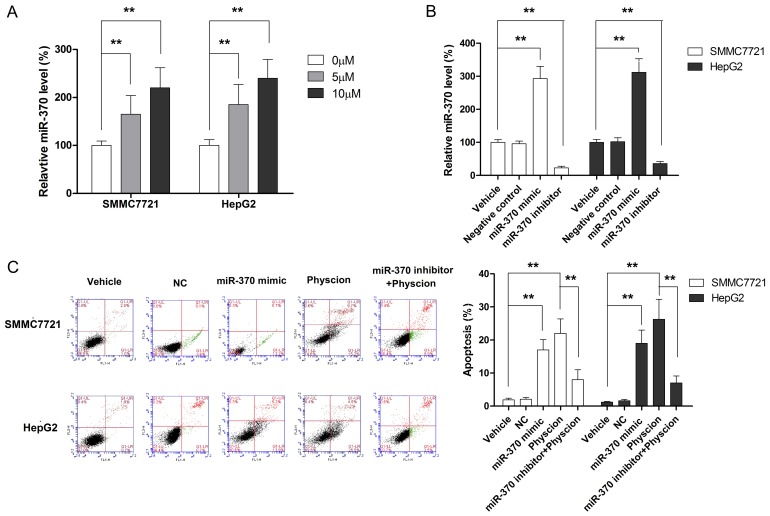
The role of miR-370 in apoptosis of cancerous cells has been documented previously [23,24]. In the present study, we examined whether miR-370 was involved in physcion-induced apoptosis in HCC cells. As shown in Figure 4A, physcion treatment resulted in a dose-dependent increase in the level of miR-370. To further elucidate the role of miR-370 in physcion-induced apoptosis, both HCC cell lines were transfected with miR-370 mimic, miR-370 inhibitor, or negative control (NC). As seen in Figure 4B, miR-370 mimic significantly increased the expression of miR-370 while miR-370 inhibitor transfection was associated with a marked decreased in the level of miR-370. HCC cells transfected with miR-370 mimic served as a positive control and demonstrated the effect of miR-370 on cell apoptosis, showing that the upregulation of miR-370 was associated with a significant increase in the apoptotic cell population (Figure 4C). On the other hand, the miR-370 inhibitor was found to significantly abolish the physcion-induced apoptosis in both cell lines. Collectively, our results suggested that physcion induced apoptosis in HCC cells occurs through the upregulation of miR-370.
Figure 4.
MiR-370 mediates the apoptosis-inducing effect of physcion in HCC cells. A. Physcion treatment is associated with increased miR-370 level. B. The expression of miR-370 is manipulated with miR-370 mimic and inhibitor. C. Repressing miR-370 level by miR-370 inhibitor significantly decreases the apoptotic percentage of cells treated with physcion. **P<0.01.
Physcion regulates the expression of miR-370 through Sp1/DNMT1 signaling
It is well-established that epigenetic regulation plays a critical role in the regulation of miRNAs [25]. DNMT1, one of the main enzymes responsible for setting up and maintaining DNA methylation patterns in eukaryotic cells, has been found to be involved in the regulation of miRNAs, including miR-370, in tumor cells [26,27]. Therefore, we examined whether physcion modulated the level of miR-370 through DNMT1. As observed in Figure 5A, physcion treatment led to a decrease in both mRNA and protein level of DNMT1. Sp1, a transcription factor, acts as an upstream signaling molecule to modulate the expression of DNMT1 in response to several naturally occurring factors [28,29]. In the current study, we also found that physcion suppresses the expression of Sp1, suggesting that it might regulate the level of DNMT1 through Sp1 (Figure 5B). Moreover, our results showed that physcion suppressed the expression of Sp1 through the activation of AMPK (Figure 5C and 5D), and AMPK/Sp1 signaling was responsible for the downregulated level of DNMT1 by physcion (Figure 5E). To confirm the involvement of AMPK/Sp1/DNMT1 in the regulation of physcion on miR-370, the expression of DNMT1 and Sp1 was manipulated using targeting shRNA or overexpressing vector and AMPK inhibitor compound C and activator AICAR. Our findings illustrated in Figure 5F clearly showed that physcion modulated the level of miR-370 through AMPK/Sp1/DNMT1 signaling. Next, we examined the role of AMPK/Sp1/DNMT1 signaling in physcion-induced apoptosis of HCC cells. The findings in Figure 5G suggested that physcion induced the mitochondrial apoptosis by upregulating miR-370 through AMPK/Sp1/DNMT1 signaling.
Figure 5.
Physcion modulates the level of miR-370 through AMPK/Sp1/DNMT1 signaling. A. Physcion represses the expression of DNMT1 at both mRNA and protein level. B. Physcion dose-dependently suppresses the protein level of Sp1. C. Physcion activates AMPK in dose-dependent manner. D. Physcion suppresses Sp1 expression by activating AMPK. E. AMPK/Sp1 signaling mediates the repressing effect of physcion on DNMT1. F. Physcion modulates miR-370 level through AMPK/Sp1/DNMT1 signaling. G. AMPK/Sp1/DNMT1 signaling is involved in physcion-induced apoptosis of HCC cells. **P<0.01.
Physcion suppresses the tumor growth in vivo
In order to substantiate the anti-tumor effect of physcion in vivo, a xenograft animal model was established by inoculating the nude mice with HepG2 cells. When the tumors reached a volume of about 100 mm3, mice were administered various dosages of physcion (40 mg and 20 mg/kg/day) by I.P. injection while mice in the control group were injected with saline. As shown in Figure 6A and 6B, physcion at both 20 and 40 mg/kg/day significantly suppressed the tumor growth and induced apoptosis. Our findings also revealed that the anti-tumor effect of physcion was associated with a significantly increased level of miR-370 (Figure 6C). In addition, a marked increase in AMPK phosphorylation and a prominent decrease in DNMT1 and Sp1 expression in tumor tissues were also observed (Figure 6D). Taken together, these findings support our in vitro results that apoptosis induced by physcion correlated with miR-370 upregulation by modulating the AMPK/Sp1/DNMT1 signaling.
Figure 6.
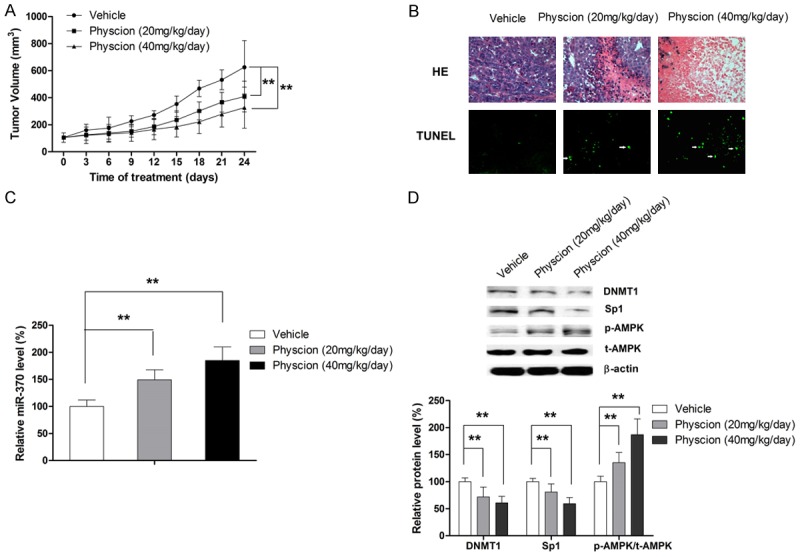
Physcion exerts anti-tumor effect in vivo. A. Physcion suppresses the tumor growth. B. Physcion induces apoptosis in tumor tissues. C. Physcion increases the level of miR-370 in tumor tissue. D. In vivo results confirms the involvement of AMPK/Sp1/DNMT1 signaling in the anti-tumor effect of physcion in xenograft model. **P<0.01.
Discussion
As a natural anthraquinone derivative isolated from a medicinal plant rhubarb and the marine-derived fungus Microsporum sp., physcion was firstly reported to exert a cytotoxic effect in colon cancer and leukemia cells [30]. Since then, the apoptosis-inducing effect of physcion has been documented in human breast cancer cells, cervical carcinoma cells, colon cancer cells, and nasopharyngeal carcinoma cells [16-18,31]. Moreover, several molecule targets, including EMMPRIN [18], 6-Phosphogluconate dehydrogenase [32] and Sp1 [31], have been identified. In the current study, the anti-tumor effect of physcion was confirmed in HCC in vitro and in vivo, and our results clearly demonstrated that physcion is a potent apoptosis-inducing agent. Moreover, we also provided experimental evidence that physcion induces apoptosis via mitochondrial pathway by targeting miR-370, which is mediated by AMPK/Sp1/DNMT1 signaling.
miR-370 has been found to be aberrantly expressed in a variety of human malignancies; however, contradictory results on the role of miR-370 in the development and progression of human malignancies have been proposed. Clinical and pre-clinical evidence points out that miR-370 could act either as an oncogene or a tumor suppressor gene. For instance, aberrantly low expression of miR-370 has been detected in both solid tumor and hematological malignancies [33-35], suggesting that miR-370 functions as a tumor suppressor. The in vitro study with cholangiocarcinoma cells also demonstrated that transfection with miR-370 mimic suppressed the cell growth [36]. Furthermore, the refurbishment of miR-370 was found to be able to resensitize the cancer cells towards chemotherapy [37,38]. Conversely, miR-370 has also been observed to act as an oncogenic miRNA in cancer. The ectopic overexpression of miR-370 in the Wilms tumor G401 cell line was found to correlate with enhanced proliferation rate, indicating the oncogenic role of miR-370 [39]. Moreover, miR-370 can promote cell growth in human prostate and gastric cancers by directly regulating FOXO1 [40,41]. The association between high miR-370 expression and poor outcome in breast cancer patients has also been established [42]. In HCC cells, miR-370 displayed the potential to suppress metastasis by inhibiting migration and invasion [9]. A recent study by Sun et al. reported that increasing miR-370 expression promotes cell death of liver cancer cells in vitro [10]. In agreement with these previous studies, the present study showed that miR-370 mediated the apoptosis-inducing effect of physcion in HCC, further supporting the role of miR-370 as a tumor suppressor in HCC.
The epigenetic instability has been found to play a major role in the development and progression of cancer [43], since the epigenetic alterations in cancer lead to the silencing of certain tumor suppressor genes through promoter methylation [44]. In addition to proteins, the epigenetic regulation also plays a pivotal role in the regulation of miRNAs [25]. This phenomenon renders DNA methyltransferases (DNMTs), the catalyzing enzyme responsible for transferring methylcytosine nucleotides to the first five carbon atoms in DNA using S-methionine as a methyl donor, as critical regulatory effectors for miRNAs [45]. Among the DNMT family, DNMT1 is most studied and has been found to be involved in the tumorigenesis and cancer progression [46]. In the case of HCC, aberrantly increased expression of DNMT1 promotes the malignant progression of HCCs and correlates with both recurrence and poor outcome in HCC patients [47]. DNMT1 has also been reported to mediate the hypermethylation of induced p16-INK4A gene promoter by hepatitis B virus x protein (HBx) [48]. In the present study, we showed that DNMT1 acted as an upstream signaling molecule to modulate the expression of miR-370 in HCC, highlighting that DNMT1 may affect the biological activities of HCC cells by modulating miRNAs and supporting the role of DNMT1 as a potential therapeutic target of HCC.
Sp1, one of the first transcription factors to be identified in mammalian cells, plays a regulatory role in cellular processes by modulating the expression of various genes [49]. Since Sp1 is known to bind to GC-rich motifs of several gene promoters and induces growth of cancerous cells [50], it has been considered as a promising target for cancer therapy [51]. Recent findings of the presence of GC-rich motifs in the DNMT1 gene promoter suggested that DNMT1 could be a downstream target of Sp1 [52,53], and some natural compounds have been found to regulate the expression of DNMt1 through Sp1 [29,54]. In line with the previous studies, our findings also pointed out that physcion modulated the expression of DNMT1 through repressing Sp1. Moreover, we also showed that physcion suppressed the expression of Sp1 by enhancing the phosphorylation of AMPK. A recent study with nasopharyngeal carcinoma cells demonstrated that physcion modulated Sp1 expression by activating ROS/miR-27a signaling [31]. Altogether, we may postulate that more than one axis structure is responsible for the regulatory effect of physcion on Sp1.
In summary, the current study revealed that physcion induces apoptosis in HCC cell lines by upregulating miR-370. Also, physcion exerts a regulatory effect on miR-370 through the modulation of AMPK/Sp1/DNMT1 signaling pathway.
Acknowledgements
This project was supported by the Wuhai Science and Technology Project (Nos. 2016150305000002, 2016150305000001) and Inner Mongolia Science Foundation.
Disclosure of conflict of interest
None.
References
- 1.Vilarinho S, Taddei T. Therapeutic strategies for hepatocellular carcinoma: new advances and challenges. Curr Treat Options Gastroenterol. 2015;13:219–234. doi: 10.1007/s11938-015-0049-8. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Tang W. Perspectives on using des-gamma-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr. 2013;2:227–231. doi: 10.3978/j.issn.2304-3881.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo TT, Zheng RS, Zhang SW, Zeng HM, Chen WQ. Incidence and mortality of liver cancer in China in 2011. Chin J Cancer. 2015;34:508–513. doi: 10.1186/s40880-015-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebrant A, Floor S, Saiselet M, Antoniou A, Desbuleux A, Snyers B, La C, de Saint Aubain N, Leteurtre E, Andry G, Maenhaut C. miRNA expression in anaplastic thyroid carcinomas. PLoS One. 2014;9:e103871. doi: 10.1371/journal.pone.0103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong SG, Lee WH, Kodo K, Wu JC. MicroRNA-mediated regulation of differentiation and trans-differentiation in stem cells. Adv Drug Deliv Rev. 2015;88:3–15. doi: 10.1016/j.addr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziari K, Zarea M, Gity M, Fayyaz AF, Yahaghi E, Darian EK, Hashemian AM. Downregulation of miR-148b as biomarker for early detection of hepatocellular carcinoma and may serve as a prognostic marker. Tumour Biol. 2016;37:5765–5768. doi: 10.1007/s13277-015-3777-4. [DOI] [PubMed] [Google Scholar]
- 8.Shi YH, Qi BB, Liu XB, Ding HM. Upregulation of miR-522 is associated with poor outcome of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2016;20:3194–3198. [PubMed] [Google Scholar]
- 9.Xu WP, Yi M, Li QQ, Zhou WP, Cong WM, Yang Y, Ning BF, Yin C, Huang ZW, Wang J, Qian H, Jiang CF, Chen YX, Xia CY, Wang HY, Zhang X, Xie WF. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology. 2013;58:1977–1991. doi: 10.1002/hep.26541. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Hou YB, Jia HY, Bi XH, Yu L, Chen DJ. MiR-370 promotes cell death of liver cancer cells by Akt/FoxO3a signalling pathway. Eur Rev Med Pharmacol Sci. 2016;20:2011–2019. [PubMed] [Google Scholar]
- 11.Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–46. doi: 10.1016/s0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao YL, Wang JB, Zhou GD, Shan LM, Xiao XH. Investigations of free anthraquinones from rhubarb against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic Clin Pharmacol Toxicol. 2009;104:463–469. doi: 10.1111/j.1742-7843.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, Das Sarma M, Patra A, Hazra B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn. , Rubia cordifolia Linn. and Lantana camara Linn. J Pharm Pharmacol. 2010;62:1158–1166. doi: 10.1111/j.2042-7158.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- 14.Tamokou Jde D, Tala MF, Wabo HK, Kuiate JR, Tane P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J Ethnopharmacol. 2009;124:571–575. doi: 10.1016/j.jep.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 15.Almeida AP DT, Singburaudom N, Lima R, Vasconcelos MH, Pinto M. The in vitro anticancer activity of the crude extract of the sponge-associated fungus eurotium cristatum and its secondary metabolites. Journal of Natural Pharmaceuticals. 2010;1:25. [Google Scholar]
- 16.Hong JY, Chung HJ, Bae SY, Trung TN, Bae K, Lee SK. Induction of cell cycle arrest and apoptosis by physcion, an anthraquinone isolated from rhubarb (rhizomes of rheum tanguticum), in MDA-MB-231 human breast cancer cells. J Cancer Prev. 2014;19:273–278. doi: 10.15430/JCP.2014.19.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesekara I, Zhang C, Van Ta Q, Vo TS, Li YX, Kim SK. Physcion from marine-derived fungus microsporum sp. induces apoptosis in human cervical carcinoma heLa cells. Microbiol Res. 2014;169:255–261. doi: 10.1016/j.micres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Gao H, Han Y, Ye J, Xie J, Wang C. Physcion induces mitochondria-driven apoptosis in colorectal cancer cells via downregulating EMMPRIN. Eur J Pharmacol. 2015;764:124–133. doi: 10.1016/j.ejphar.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Han YT, Chen XH, Gao H, Ye JL, Wang CB. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcription factor SOX2. Acta Pharmacol Sin. 2016;37:264–275. doi: 10.1038/aps.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wang Z, Yang H. EMMPRIN, SP1 and microRNA-27a mediate physcion 8-O-beta-glucopyranoside-induced apoptosis in osteosarcoma cells. Am J Cancer Res. 2016;6:1331–1344. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zhou H, Xu M, Gao Y, Deng Z, Cao H, Zhang W, Wang Q, Zhang B, Song G, Zhan Y, Hu T. Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol Cancer. 2014;13:59. doi: 10.1186/1476-4598-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng D, Wang L, Chen Y, Li B, Xue L, Shao N, Wang Q, Xia X, Yang Y, Zhi F. MicroRNA-124-3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci. 2016;107:899–907. doi: 10.1111/cas.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou Q, Lin Q, Cheng D, Liao Q, Zheng L, Gong Y. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012;586:3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Datta J, Kutay H, Nasser MW, Nuovo GJ, Wang B, Majumder S, Liu CG, Volinia S, Croce CM, Schmittgen TD, Ghoshal K, Jacob ST. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Yie Y, Zhao S, Tang Q, Zheng F, Wu J, Yang L, Deng S, Hann SS. Ursolic acid inhibited growth of hepatocellular carcinoma HepG2 cells through AMPKalpha-mediated reduction of DNA methyltransferase 1. Mol Cell Biochem. 2015;402:63–74. doi: 10.1007/s11010-014-2314-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Wu J, Zheng F, Tang Q, Yang L, Li L, Wu W, Hann SS. beta-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKalpha signalling pathways in human lung cancer cells: the role of Sp1. J Cell Mol Med. 2015;19:630–641. doi: 10.1111/jcmm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge HM, Song YC, Shan CY, Ye YH, Tan RX. New and cytotoxic anthraquinones from Pleospora sp. IFB-E006, an endophytic fungus in Imperata cylindrical. Planta Med. 2005;71:1063–1065. doi: 10.1055/s-2005-864190. [DOI] [PubMed] [Google Scholar]
- 31.Pang MJ, Yang Z, Zhang XL, Liu ZF, Fan J, Zhang HY. Physcion, a naturally occurring anthraquinone derivative, induces apoptosis and autophagy in human nasopharyngeal carcinoma. Acta Pharmacol Sin. 2016;37:1623–1640. doi: 10.1038/aps.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou L, Hitosugi T, Zhang L, Zhang S, Seo JH, Xie J, Tucker M, Gu TL, Sudderth J, Jiang L, Mitsche M, DeBerardinis RJ, Wu S, Li Y, Mao H, Chen PR, Wang D, Chen GZ, Hurwitz SJ, Lonial S, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Lei Q, Brat DJ, Ye K, Boggon TJ, He C, Kang S, Fan J, Chen J. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17:1484–1496. doi: 10.1038/ncb3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yungang W, Xiaoyu L, Pang T, Wenming L, Pan X. miR-370 targeted FoxM1 functions as a tumor suppressor in laryngeal squamous cell carcinoma (LSCC) Biomed Pharmacother. 2014;68:149–154. doi: 10.1016/j.biopha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, Chen C, Jia J. FoxM1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zeng J, Zhou M, Li B, Zhang Y, Huang T, Wang L, Jia J, Chen C. The tumor suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer. 2012;11:56. doi: 10.1186/1476-4598-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An F, Yamanaka S, Allen S, Roberts LR, Gores GJ, Pawlik TM, Xie Q, Ishida M, Mezey E, Ferguson-Smith AC, Mori Y, Selaru FM. Silencing of miR-370 in human cholangiocarcinoma by allelic loss and interleukin-6 induced maternal to paternal epigenotype switch. PLoS One. 2012;7:e45606. doi: 10.1371/journal.pone.0045606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen XP, Chen YG, Lan JY, Shen ZJ. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancer chemosensitivity to cDDP by negatively regulating ENG. Cancer Lett. 2014;353:201–210. doi: 10.1016/j.canlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Zhou M, Zeng J, Wang X, Guo Q, Huang T, Shen H, Fu Y, Wang L, Jia J, Chen C. MiR-370 sensitizes chronic myeloid leukemia K562 cells to homoharringtonine by targeting forkhead box M1. J Transl Med. 2013;11:265. doi: 10.1186/1479-5876-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao X, Liu D, Yan X, Zhang Y, Yuan L, Zhang T, Fu M, Zhou Y, Wang J. Stat3 inhibits WTX expression through up-regulation of microRNA-370 in Wilms tumor. FEBS Lett. 2013;587:639–644. doi: 10.1016/j.febslet.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012;7:e45825. doi: 10.1371/journal.pone.0045825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, Li Q, Zhang L. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521–526. doi: 10.1016/j.biopha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Sim J, Ahn H, Abdul R, Kim H, Yi KJ, Chung YM, Chung MS, Paik SS, Song YS, Jang K. High MicroRNA-370 expression correlates with tumor progression and poor prognosis in breast cancer. J Breast Cancer. 2015;18:323–328. doi: 10.4048/jbc.2015.18.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer-a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 44.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 45.Brenner C, Fuks F. DNA methyltransferases: facts, clues, mysteries. Curr Top Microbiol Immunol. 2006;301:45–66. doi: 10.1007/3-540-31390-7_3. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Yang X, Davidson NE. Role of DNA methylation and histone acetylation in steroid receptor expression in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:183–192. doi: 10.1023/a:1011308707512. [DOI] [PubMed] [Google Scholar]
- 47.Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, Hirohashi S. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105:527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 48.Zhu YZ, Zhu R, Shi LG, Mao Y, Zheng GJ, Chen Q, Zhu HG. Hepatitis B virus X protein promotes hypermethylation of p16(INK4A) promoter through upregulation of DNA methyltransferases in hepatocarcinogenesis. Exp Mol Pathol. 2010;89:268–275. doi: 10.1016/j.yexmp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Dynan WS, Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983;32:669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- 50.Chai G, Li L, Zhou W, Wu L, Zhao Y, Wang D, Lu S, Yu Y, Wang H, McNutt MA, Hu YG, Chen Y, Yang Y, Wu X, Otterson GA, Zhu WG. HDAC inhibitors act with 5-aza-2’-deoxycytidine to inhibit cell proliferation by suppressing removal of incorporated abases in lung cancer cells. PLoS One. 2008;3:e2445. doi: 10.1371/journal.pone.0002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Zou WX, Chang KS. Inhibition of Sp1 functions by its sequestration into PML nuclear bodies. PLoS One. 2014;9:e94450. doi: 10.1371/journal.pone.0094450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Shen Q, Zhang D, Mei X, Ran W, Xu Y, Yu G. Functional groups determine biochar properties (pH and EC) as studied by two-dimensional C NMR correlation spectroscopy. PLoS One. 2013;8:e65949. doi: 10.1371/journal.pone.0065949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sontag RL, Weber TJ. Ectopic ERK expression induces phenotypic conversion of C10 cells and alters DNA methyltransferase expression. BMC Res Notes. 2012;5:217. doi: 10.1186/1756-0500-5-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao S, Wu J, Tang Q, Zheng F, Yang L, Chen Y, Li L, Hann SS. Chinese herbal medicine Xiaoji decoction inhibited growth of lung cancer cells through AMPKalpha-mediated inhibition of Sp1 and DNA methyltransferase 1. J Ethnopharmacol. 2016;181:172–181. doi: 10.1016/j.jep.2016.01.041. [DOI] [PubMed] [Google Scholar]